Abstract
Selenium (Se) and silicon (Si) have been demonstrated to mitigate the adverse effects of cadmium (Cd) stress on the growth of various higher plants. However, the distinct mechanisms by which Se and Si, when applied to soil, alleviate the toxicity of Artemisia argyi under Cd stress remain unclear. This study employed A. argyi as the experimental material and simulated a Cd stress environment in soil pot experiments by adding CdCl2·2.5H2O at concentrations of 4 mg kg−1 and 10 mg kg−1. Exogenous Se in the form of Na2SeO3 (2 mg kg−1) or Si in the form of Na2SiO3 (20 mg kg−1) was applied simultaneously. After 90 days of combined exposure, the study investigated the differential mechanisms by which Se and Si application influenced Cd uptake by roots, its translocation within aboveground and underground tissues, and the antioxidant system of A. argyi under two levels of Cd stress. The results indicate that under Cd stress conditions, the application of Se significantly promotes the growth of A. argyi. Under both Cd concentration treatments, the application of Se or Si alone markedly reduces the Cd content in the aboveground parts of A. argyi. This reduction may be attributed to alterations in the subcellular distribution of Cd, decreased Cd content in organelles, and increased Cd sequestration in cell walls and soluble components. Furthermore, the application of Se or Si in soil can enhance the content of osmoregulatory substances, chlorophyll, and plant chelating agents in A. argyi leaves while reducing the levels of malondialdehyde (MDA) and reactive oxygen species (ROS), particularly at high Cd concentrations. The findings suggest that the alleviation of Cd toxicity by exogenous Se is primarily due to its role in inhibiting Cd uptake and translocation to shoots, as well as strengthening Cd compartmentalization in root cell walls and enhancing the antioxidant defense system. In contrast, exogenous Si primarily mitigates Cd toxicity by restricting Cd translocation from underground to aboveground plant tissues.
1. Introduction
In recent decades, rapid industrialization and anthropogenic activities have led to severe soil degradation and heavy metal (HM) pollution [1]. Among HM contaminants, cadmium (Cd) is one of the most hazardous, ranking first among soil inorganic pollutants [2]. The sources of Cd in soil can be broadly classified into natural and anthropogenic factors. In the natural environment, volcanic eruptions, forest fires, and marine spray release substantial amounts of Cd into the atmosphere, which subsequently enter the soil as fallouts. Anthropogenic sources primarily include mining and smelting activities, industrial emissions, and related processes. The impact of natural pollution on ecosystems is relatively minor; however, anthropogenic pollution is typically concentrated in industrial, mining, and agricultural areas, where Cd concentrations are higher and pose greater threats to ecosystems and human health, potentially leading to severe health problems [3]. The concentration of Cd in soil varies considerably depending on the degree and location of contamination. The background Cd concentration in Chinese soil has been reported to be 0.3 mg kg−1 [4].
The high mobility of Cd in soil–plant systems renders plants highly susceptible to excessive Cd accumulation in roots and leaves [5]. Excessive Cd concentrations in plants not only hinder growth, development, and physiological and biochemical processes but may also result in plant mortality under severe conditions [6]. Cd-induced stress reduces the transpiration rate, causes stomatal closure, disrupts calcium (Ca) signaling, impairs photosynthesis, and ultimately suppresses plant growth [7]. Furthermore, Cd exposure induces excessive production of reactive oxygen species (ROS) in plant tissues, leading to structural damage in cellular components, including chloroplasts, mitochondria, the Golgi apparatus, and vacuole [8]. The major ROS species toxic to proteins, carbohydrates, photosynthetic pigments, and cell membranes include superoxide (O2•−), singlet oxygen (1O2), hydroxyl radicals (HO−), and hydrogen peroxide (H2O2) [9]. Additionally, excessive Cd accumulation in plants enters the human body through the food chain, contributing to comorbidities such as cancer and kidney diseases [10]. These risks highlight the urgent need for environmentally sustainable and effective strategies to reduce Cd toxicity and accumulation in plants [7]. Compared with conventional physical or chemical remediation methods, phytoremediation is considered more efficient, safer, and environmentally sustainable [11]. Studies have reported that certain mineral fertilizers, including Se, Si, Ca, and phosphorus (P), can reduce Cd uptake and accumulation in crops, enhance plant defense mechanisms, and alleviate Cd toxicity [12]. This ultimately leads to reduced Cd concentrations in edible plant parts, offering a cost-effective and practical approach to green agricultural production and food safety [12,13].
Selenium is an essential nutrient for humans and animals, playing a crucial role in the synthesis of selenoproteins, which are involved in reproduction, thyroid hormone metabolism, DNA synthesis, and protection against oxidative damage and infections [14]. However, Se is not considered an essential element for plants [15]. At high Se concentrations, plant biomass can increase significantly, suggesting that exogenous Se application may have a beneficial effect on crop yield enhancement [16]. In recent years, Se has been widely applied as a basal fertilizer or foliar spray to increase crop productivity [17]. Additionally, it has been shown to mitigate various biotic and abiotic stresses, including HM toxicity (Cd, As, Pb), drought, and pest infestations [18,19,20]. Exogenous Se can counteract the deleterious effects of Cd on plants by improving photosynthetic efficiency, regulating mineral element homeostasis, reducing Cd accumulation, enhancing antioxidant capacity, and alleviating Cd-induced stress. In Brassica napus L., exogenous Se mitigates Cd-induced degradation of chloroplast membranes, restores thylakoid and stromal lamella structures, increases chloroplast size, and enhances membrane fluidity [21]. In Solanum lycopersicum L., Se application under Cd stress restores normal growth and increases the fresh and dry weight of roots, fruits, and leaves [22]. This growth enhancement is primarily attributed to the reduced activity of antioxidant enzymes such as catalase (CAT) and peroxidase (POD). Moreover, exogenous Se decreases the total extractable Cd and Cd content in various chemical forms within plant tissues [23]. Additionally, studies have demonstrated that Se reduces the expression of genes involved in Cd translocation in Oryza sativa L., thereby limiting Cd movement from roots to shoots and ultimately reducing Cd contamination in edible plant parts [5,24]. In summary, Se plays a pivotal role in enhancing plant resistance to HM toxicity by stabilizing the antioxidant defense system and inhibiting Cd accumulation and translocation within plants [24].
Silicon is the second most abundant element on the Earth’s surface after oxygen. However, most silicates and oxides of this fundamental element are not readily absorbed by plants [25]. The presence of Si enhances the strength and rigidity of plant epidermis, vascular structures, and tissues, thereby regulating plant physiological activities to protect against abiotic stress [26]. It reduces Cd migration and environmental risks by converting exchangeable Cd into carbonate-bound and residual Cd [27]. Numerous studies have demonstrated that Si application enhances plant growth, mineral absorption, and gas exchange properties while mitigating oxidative stress and organic acid accumulation in various plant species through the scavenging of ROS [28,29]. Moreover, exogenous Si exerts a protective effect in plants by restricting the absorption and translocation of HM [30]. Si can enhance the antioxidant capacity of plants, enabling them to withstand metal-induced stress. The maintenance of endogenous antioxidant levels contributes to the stabilization of chloroplast structure Photosystem II (PSII), thereby increasing pigment concentration. Similar effects have been reported for Fe, Zn, Al, Mn, and Cd [31]. Exogenous Si enhances plant antioxidant enzyme activity, thereby reducing lipid peroxidation (evidenced by decreased H2O2 levels and malondialdehyde (MDA) concentration). The effect of exogenous Si on alleviating Cd-induced oxidative damage is more pronounced in Cd-tolerant varieties than in Cd-sensitive varieties [32].
When plants experience abiotic stress, in addition to inducing antioxidant enzyme activity, the levels of certain non-enzymatic antioxidant compounds also change. Among these, glutathione (GSH) and phytochelatins (PCs) serve as primary chelating agents for Cd. Glutathione is the predominant non-protein thiol in plants and plays a crucial role in maintaining cellular redox balance. Phytochelatins are GSH-derived peptides with high thiol content that bind HM ions to form stable chelates [33]. Previous studies have demonstrated that the metabolic levels of GSH, PCs, and metallothioneins (MTs) in plant cells are closely associated with plant Cd tolerance [34]. Exogenous Si primarily enhances the production of GSH and PCs, facilitating the sequestration of PC–Cd complexes into vacuoles and promoting Cd compartmentalization into cell walls and vacuoles by regulating the relative expression of OsNramp1 (which mediates the transmembrane influx of Cd2+ and Mn2+ in rice) and OsHMA3 (which transports Cd2+ from the cytoplasm into vacuoles), thereby alleviating Cd toxicity in rice roots. In summary, Si effectively reduces Cd uptake and accumulation in plants by mitigating oxidative stress [34].
Artemisia argyi, an aromatic perennial herb belonging to the family Asteraceae, is widely distributed across temperate, cold-temperate, and subtropical regions of Asia, Europe, and North America. In China, it is predominantly found in the northeastern, northern, eastern, southern, and southwestern provinces [35]. According to relevant literature, cultivating mugwort on HM-contaminated soil demonstrates a certain enrichment capacity for HM [36]. Recent studies have shown that HM pollution in A. argyi is relatively severe, with a high incidence of Cd contamination. In some regions, Cd concentrations in A. argyi samples exceed the permissible limit [37]. A. argyi exhibits an ability to accumulate HM; however, only when Cd concentrations exceed 50 mg kg−1 does the fresh and dry weight of its roots decline significantly [38].
According to the Risk Control Standards for Soil Environment Quality and Soil Pollution in Agricultural Land (GB 15618-2018) [4], the risk screening and intervention values for Cd pollution in agricultural soils with a pH greater than 7.5 are 0.6 mg kg−1 and 4 mg kg−1, respectively. In most cases, the Cd concentration in soil remains below 10 mg kg−1. The Cd treatment levels used in this study (4 mg kg−1 and 10 mg kg−1) correspond to the concentration ranges specified for soil pollution risk control in agricultural land and are consistent with levels detected in contaminated soils. These treatment concentrations were selected to represent typical conditions in polluted environments, thereby enhancing the practical relevance of this study. In this experiment, we hypothesized that Se and Si treatments tackle Cd stress in A. argyi. By measuring various physiological indicators, Cd content in aboveground and underground tissues, Cd content in different subcellular components, and mineral element concentrations aimed to elucidate the physiological regulatory mechanisms by which exogenous Se and Si alleviate Cd stress in A. argyi, thereby providing theoretical support for understanding its tolerance mechanisms to Cd stress.
2. Materials and Methods
The experiments were conducted at the Northwest A&F University Water-Saving Agriculture in Arid Areas Industry Research Institute, Shaanxi, China. Seedlings were cultivated outdoors. Se concentrations were established based on the findings of Zhao, Y. et al. [39], and Si concentrations were determined according to the results of Huang, F. et al. [40], followed by an expansion within a specific range.
2.1. Experimental Materials and Potted Plant Experiments
Soil samples were collected from the upper layer (0–20 cm) of a test field at Northwest A&F University (34°17′ N, 108°04′ E). The soil type used in the experiments was classified as dark loess (Cambisol). The properties were as follows: Cd was measured to be 0.15 mg kg−1 using atomic absorption spectroscopy [41]; the organic matter content was determined to be 7.69 g kg−1 using potassium dichromate oxidation external heating method; the total N was measured to be 0.57 g kg−1 using the Kjeldahl method; The total p content was determined to be 0.66 g kg−1 using the acid dissolution molybdenum antimony colorimetric method; the total K content was determined to be 17.2 g kg−1 using flame photometry; and pH was determined to be 8.1 using the electrode potential method [42].
Rhizomes of A. argyi were collected from Qichun County, Hubei Province, China. The plant material, provided by Hubei Duanyang Qiai Technology Co., Ltd., (Huanggang, China) was taxonomically identified as A. argyi.
A completely randomized design was employed. Cd (0, 4, and 10 mg kg−1), Se (0 and 2 mg kg−1), and Si (0 and 20 mg kg−1) were applied in the form of CdCl2·2.5H2O, Na2SeO3, and Na2SiO3, respectively, and thoroughly mixed with 6.0 kg of soil before being placed in plastic pots (25 cm in diameter and 20 cm in height). There were three pots by treatment, replicated thrice. No additional fertilizers were applied. The soil was equilibrated for two weeks while maintaining its maximum water-holding capacity. Rhizomes were planted at 5 cm long, weighing 1.5 g, planted on 10 April 2024, one plant per pot. All treatments were watered as needed for 90 days. Treated plants were sampled after 90 days and immediately preserved for further analysis.
This study employed a completely randomized design with seven treatment groups: (1) Cd0: without Cd, Se, or Si; (2) Cd4: amendment with Cd (4.0 mg kg−1 Cd); (3) Cd4Se2: amendment with Cd and Se (4.0 mg kg−1 Cd and 2.0 mg kg−1 Se); (4) Cd4Si20: amendment with Cd and Si (4.0 mg kg−1 Cd and 20 mg kg−1 Si); (5) Cd10: amendment with Cd (10.0 mg kg−1 Cd); (6) Cd10Se2: amendment with Cd and Se (10.0 mg kg−1 Cd and 2.0 mg kg−1 Se); (7) Cd10Si20: amendment with Cd and Si (10.0 mg kg−1 Cd and 20 mg kg−1 Si); (8) Se2: without Cd or Si; (9) Si20: without Cd or Se.
2.2. Growth Index Measurement
After 90 days the aboveground height and fresh weight of the roots, stems, and leaves were measured in all treatment groups. Samples were then dried at 65 °C until a constant weight was achieved, and the dry weights of roots, stems, and leaves were recorded.
2.3. Cd Accumulation, Transport Coefficient, and Enrichment Coefficient Calculation
Dried roots, stems, and leaves were ground into a fine powder. A 0.5000 g sample was weighed and digested with 5 mL of nitric acid using a microwave digester (MA165-001 Multiplap-41 FC2, Milestone, Italy). The solution was subsequently concentrated to 1–2 mL by heating at 130 °C, followed by cooling at room temperature. The digestion tube was rinsed multiple times with deionized water, and the final solution volume was adjusted to 25 mL. A graphite furnace atomic absorption spectrophotometer (AA-6880; Shimadzu, Japan) was used for Cd quantification.
The translocation factor (TF) was calculated as follows [38]:
where Croot represents the Cd concentration in the roots of A. argyi (mg kg−1), and Caboveground plant organs represents the Cd concentrations in stems and leaves of A. argyi (mg kg−1).
TF = Caboveground plant organs/Croot
2.4. Determination of Cd Subcellular Distribution
The determination of Cd subcellular distribution was based on the method described by Jia, H.L. et al. [43], with slight modifications. Plant tissues were homogenized in a pre-cooled extraction buffer containing 250 mmol L−1 sucrose, 50 mmol L−1 Tris-HCl (pH 7.5), and 1.0 mmol L−1 dithiothreitol. The homogenate was centrifuged at 300× g for 15 min, and the resulting precipitate was designated as the “cell wall fraction,” primarily composed of cell walls. The supernatant was further centrifuged at 2000× g for 30 min, yielding a sediment and supernatant, referred to as the “organelle-containing fraction” and the “soluble fraction,” respectively. Cd content was analyzed in different cellular components, and Cd quantification and sample digestion methods were as described in Section 2.3.
2.5. Determination of Se and Si Content in Plant Tissues
The determination of Se content in plant tissues was conducted in accordance with the national food safety standard of the People’s Republic of China for the determination of multiple elements in food (GB 5009.268-2016) [44]. A 0.1 g dry sample was weighed and soaked overnight in a polytetrafluoroethylene (PTFE) digestion vessel with 5 mL of nitric acid. The vessel was then sealed, enclosed within a stainless steel jacket, and placed in a constant-temperature drying oven. The temperature was maintained at 80 °C for 2 h, increased to 120 °C for an additional 2 h, and then further raised to 160 °C for 4 h. Following natural cooling to room temperature inside the oven, the vessel was opened, and the acid was evaporated to near dryness. The residue was transferred into a 25 mL volumetric flask, and the digestion vessel and lid were rinsed three times with a small amount of 1% nitric acid solution. The rinsing solutions were combined into the volumetric flask, diluted to volume with 1% nitric acid, mixed thoroughly, and set aside for analysis. A reagent blank test was conducted simultaneously. The Se concentration in the samples was determined using inductively coupled plasma mass spectrometry (NexION® 1000 ICP-MS, PerkinElmer, Waltham, MA, USA).
The determination of Si content in plant tissues was performed using the Si molybdenum blue colorimetric method, as described by Hua, H.X. et al. [45]. A 5 mL aliquot of 4 mol L−1 NaOH was placed in a nickel crucible, heated, and evaporated on an electric heating plate. A 0.1 g finely ground, air-dried sample of A. argyi was accurately weighed and sprinkled onto the NaOH in the nickel crucible. The crucible was then covered and placed on a regulated electric heating plate for ashing before being transferred to a high-temperature electric furnace, where it was melted at 700–720 °C for 10 min. After cooling, the residue was washed with distilled water into a 50 mL volumetric flask containing 5 mL of 4 mol L−1 HCl and brought to volume to obtain the Si test solution. A 1 mL aliquot of the test solution was diluted to approximately 10 mL, and 2,6-dinitrophenol indicator was added. The solution was adjusted to a slight yellow color using 0.1 mol L−1 NaOH and 0.05 mol L−1 H2SO4, mixed thoroughly, and allowed to stand at room temperature for 15 min. Subsequently, 2.5 mL of 0.3 mol L−1 H2SO4 and 2.5 mL of 5% ammonium molybdate solution were added, shaken well, and left at room temperature for 10 min. Following this, 2.5 mL of 5% oxalic acid solution and 2.5 mL of 15 g L−1 ascorbic acid solution were added sequentially, diluted to volume, and analyzed spectrophotometrically (T6 New Century, Pgeneral, Beijing, China) at 700 nm after 20 min. A blank test was conducted simultaneously.
2.6. Photosynthesis Measurement
A miniature plant leaf photosynthetic pigment content detector (HPL-A, China) was used to measure photosynthetic pigments. Fresh, undamaged leaves from the same position on A. argyi plants were selected for measurement. The middle section between the leaf edge and the main vein of each A. argyi leaf was chosen, and Soil Plant Analysis Development (SPAD) values were recorded at ten points symmetrically along the main vein, including the base, middle, and tip of the leaf.
Fresh leaf samples (0.5 g) were placed in a 10 mL tube containing 20 mL of 95% ethanol and allowed to extract for 24 h. Absorbance values at 470 nm, 665 nm, and 649 nm were measured using a spectrophotometer (UV-5200, Shanghai METASH Instruments Co., Ltd., Shanghai, China). Chlorophyll and carotenoid concentrations were calculated using standard equations:
Chlorophyll a content (Ca, mg L−1): Ca = 13.95 × A665 − 6.88 × A649
Chlorophyll b content (Cb, mg L−1): Cb = 24.96 × A649 − 7.32 × A665
Total chlorophyll content: Ca + Cb.
2.7. Determination of Osmoregulatory Substances
The concentrations of proline, soluble protein, soluble sugar, and reducing sugar in A. argyi leaves were determined as osmoregulatory indicators. The free proline content was measured using the ninhydrin method. The soluble protein content was determined using Coomassie Brilliant Blue G-250. The soluble sugar concentration was assessed using the anthrone method, while the reducing sugar content was quantified using the 3,5-dinitrosalicylic acid method [46].
2.8. Determination of MDA, GSH, and PCs
The MDA content was determined using the thiobarbituric acid (TBA) reaction method, with absorbance recorded at 532 nm and 600 nm. The GSH content in A. argyi leaves was measured using a detection kit (A006-1-1, Nanjing Jiancheng Bioengineering Research Institute, Nanjing, China). The non-protein thiol (NPT) content in A. argyi leaves was determined using a detection kit (BC1430, Solarbio LIFE SCIENCE, Beijing, China). All reagents, samples, and standards were prepared according to the manufacturer’s protocol, and calculations were performed as per the instructions. The content of root PCs was derived by subtracting the GSH content from the NPT content [43].
2.9. Determination of Hydrogen Peroxide and Superoxide Anion Content
The concentration of hydrogen peroxide (H2O2) in A. argyi leaves was determined using a detection kit (A064-1-1, Nanjing Jiancheng Bioengineering Research Institute, Nanjing, China). O2•− content was measured using a detection kit (A052-1-1, Nanjing Jiancheng Bioengineering Research Institute, Nanjing, China). All reagents, samples, and standards were prepared according to the manufacturer’s protocol, and calculations were performed following the instructions.
2.10. Determination of Total Phenolic and Flavonoid Content
The total phenolic content in plant tissues was determined using the Folin–Ciocalteu reagent spectrophotometric method [47]. The total flavonoid content was measured using the NaNO2-Al(NO3)3-NaOH colorimetric method [48].
2.11. Statistical Analysis
The mean and standard deviation (SD) were calculated using WPS Office (version 12.1.0). One-way analysis of variance (ANOVA) was performed in R to determine statistical significance, and means were compared using the least significant difference (LSD) test at a significance level of p = 0.05. Pearson correlation coefficients were obtained using R (version 4.2.2). Data in bar charts are presented as means, with error bars representing SD. Results are expressed as means ± SD.
3. Results
3.1. Growth Changes
The effects of exogenous Se and Si on various growth traits of A. argyi are shown in Figure 1. Increasing Cd concentrations reduced the biomass of A. argyi. However, the application of exogenous Se promoted leaf and stem fresh weights, while exogenous Si increased plant height. Under Cd10 stress, Se application increased the fresh and dry weights of leaves. Under Cd10 stress, the exogenous application of Se increased the fresh and dry weight of A. argyi leaves. Specifically, the Cd4Se2 treatment group exhibited a 7.7% increase in leaf fresh weight compared to the Cd4 treatment group, while the fresh weight of leaves in the Cd10Se2 treatment group increased by 5.48% compared to the Cd10 treatment group. Furthermore, the dry leaf weight in the Cd10Se2 treatment group increased by 10.8% relative to the Cd10 treatment group (Figure 1b). No significant impact was observed on the fresh and dry weights of the stem and root (Figure 1c,d). Under varying Cd stress conditions, the external application of Si increased the height of A. argyi plants by 2.86% and 2.18% under Cd4 and Cd10 stress conditions, respectively (Figure 1a). In addition, the addition of exogenous Si reduced the biomass of A. argyi (Figure 1).
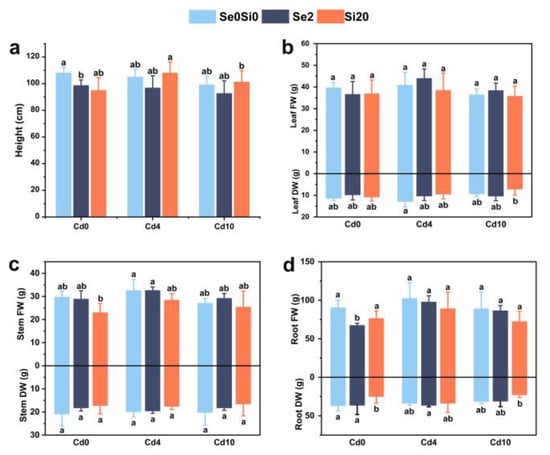
Figure 1.
Effects of Se and Si on the growth parameters of A. argyi under Cd stress. (a) Plant height; (b) fresh and dry leaf weight; (c) fresh and dry stem weight; (d) fresh and dry root weight. Lowercase letters in the bar graph indicate significant differences among treatments at p < 0.05.
Under Cd stress, the application of appropriate Se concentrations promoted the growth of A. argyi and mitigated the adverse effects of Cd on both aboveground and underground plant growth.
3.2. Cd Contamination and Se, Si Content in A. argyi
The ability of Se and Si to mitigate Cd accumulation in different parts of A. argyi was assessed by analyzing Cd content in aboveground and underground tissues under different Cd stress levels following exogenous application of Se and Si. Under Cd4 stress conditions, the addition of exogenous Se reduced the Cd content in the aboveground part of A. argyi, with the Cd4Se2 treatment group exhibiting the most pronounced effect (Figure 2). Compared with the Cd4 treatment group, Se and Si application reduced Cd content in aboveground tissues by 29.98% and 0.43%, respectively. The Cd10Si20 treatment group exhibited the lowest aboveground Cd content, with a 39.86% reduction compared with the Cd10 treatment group. Under Cd10 stress conditions, exogenous Se decreased Cd content in both aboveground and underground tissues. Although Si application reduced Cd accumulation in aboveground tissues, it resulted in a 39.86% decrease in Cd content in underground tissues compared to the Cd10 treatment group (Figure 2b–d).
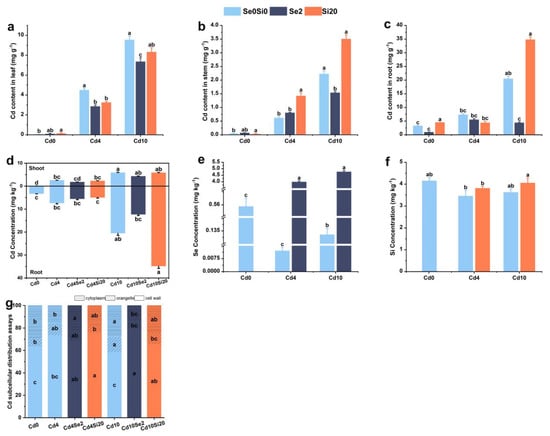
Figure 2.
Effects of Se and Si on Cd content in different parts of A. argyi under Cd stress. (a) Cd content in leaves; (b) Cd content in stems; (c) Cd content in roots; (d) Cd content in aboveground and underground parts; (e) Se content in leaves; (f) Si content in leaves; (g) Cd subcellular distribution assays. Lowercase letters in the bar graph indicate significant differences among treatments at p < 0.05.
Following Se and Si treatments, Se and Si contents in leaves were higher than those in the Cd4 and Cd10 treatment groups (Figure 2e,f). Additionally, the application of Se and Si reduced the TF of Cd-stressed plants, with the exception of the Cd4Si20 treatment group, which exhibited an increased TF value compared to the Cd4 treatment group. Under Cd4 stress, the lowest TF value was observed in the Cd4Se2 treatment group, whereas under Cd10 stress, the Cd10Si20 treatment group had lower TF values (Table 1). Analysis of Se and Si content in A. argyi leaves revealed that TF decreased with the addition of Se under both low and high Cd levels. At low Cd levels, TF increased with the addition of Si, whereas at high Cd levels, TF decreased following Si application. In summary, exogenous Se reduced Cd accumulation in leaves and roots of A. argyi, whereas exogenous Si reduced Cd accumulation in leaves.

Table 1.
Cd TF of A. argyi under different treatments.
3.3. Subcellular Distribution of Cd
Given the practical value of A. argyi leaves and the observed reduction in Cd content upon Se and Si application, subcellular Cd distribution in the leaves was examined in different treatment groups (Figure 2g). The study demonstrated that Cd was predominantly sequestered in the cell wall, followed by soluble components, with the lowest accumulation in organelles. As Cd stress levels increased, Cd content in the cell wall decreased, while accumulation in organelles and soluble components increased. Under Cd4 stress conditions, the Cd4Se2 treatment group exhibited an increase in the soluble component of Cd content, while Cd content in the cell wall and organelles decreased. Compared with the Cd4 treatment group, the Cd4Si20 treatment group demonstrated an increase in Cd content in the cell wall and soluble components, alongside a decrease in organelle Cd content, thereby alleviating Cd stress in A. argyi. Under Cd10 stress conditions, exogenous Se and Si reduced Cd content in organelles and soluble components while increasing Cd content in the cell wall, with the Cd10Se2 treatment group displaying the most pronounced effect.
Exogenous Se and Si alleviated Cd stress in A. argyi through distinct mechanisms. Se application reduced Cd accumulation in organelles, thereby mitigating Cd-induced stress, whereas Si alleviated Cd stress by enhancing Cd sequestration in the cell wall.
3.4. Oxidative Stress-Related Indicators
To evaluate the effects of exogenous Se and Si on oxidative stress-related indicators in A. argyi under Cd stress, the contents of MDA, GSH, PCs, hydrogen peroxide (H2O2), and superoxide anion (O2•−) were measured (Figure 3). Compared with the control group (Cd0), MDA content increased by 62.91% and 21.35% under Cd4 and Cd10 stress conditions, respectively. Under Cd4 stress, the external application of Se and Si significantly reduced MDA content in A. argyi leaves, with the Cd4Si20 treatment group exhibiting the most pronounced effect, reducing MDA content by 35.25% compared to the Cd4 treatment group. Under Cd10 stress, the effects of exogenous Se and Si were similar to those observed under Cd4 stress, with the application of Si showing a reduction (Figure 3a).
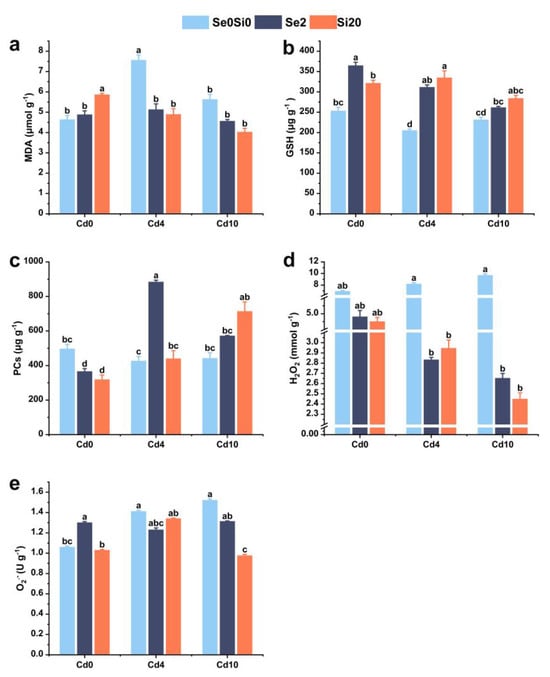
Figure 3.
Effects of Se/Si on oxidative stress indicators in A. argyi plants under Cd stress. (a) MDA content; (b) GSH content; (c) PC content; (d) H2O2 content; (e) O2•− content. Lowercase letters in the bar graph indicate significant differences among treatments at p < 0.05.
The contents of GSH and PCs in the Cd4 and Cd10 treatment groups decreased compared to the control group (Cd0), with reductions of 19.03% and 15.78% in the Cd4 treatment group, and 8.62% and 10.97% in the Cd10 treatment group, respectively (Figure 3b,c). The application of Se further increased GSH and PCs levels across all Cd stress conditions. Compared to the Cd4 and Cd10 treatment groups, GSH content increased by 51.86% and 12.97%, while PC content increased by 107% and 29.32%, respectively. Similarly, the application of Si resulted in an increase of 63.17% and 22.72% in GSH content, and an increase of 3.16% and 61.28% in PC content, under Cd4 and Cd10 stress conditions, respectively (Figure 3b,c).
Under Cd stress, the contents of H2O2 and O2•− in A. argyi increased compared to the control group (Cd0) (Figure 3d,e). H2O2 levels increased by 18.03% and 39.36% under Cd4 and Cd10 stress, respectively, while O2•− levels increased by 33.11% and 43.44% compared to the control group. Following the application of exogenous Se and Si, H2O2 content was reduced compared with the Cd stress treatment groups (Figure 3d), and O2•− content also decreased (Figure 3e). After the application of Se, H2O2 and O2•− levels decreased by 65.52% and 12.80% at low Cd levels, and by 72.65% and 13.59% at high Cd levels, respectively. Similarly, after Si application, H2O2 and O2•− levels decreased by 64.14% and 4.99% at low Cd levels, and by 74.74% and 35.76% at high Cd levels, respectively. The exogenous application of Se and Si effectively reduced reactive oxygen species levels in A. argyi, mitigating Cd-induced oxidative damage.
These results indicate that the application of exogenous Se and Si enhances oxidative stress-related defense mechanisms in A. argyi under Cd stress, thereby improving its ability to withstand Cd toxicity.
3.5. Photosynthesis
The effects of Se and Si on the photosynthetic pigments of A. argyi under Cd stress are presented in Figure 4. Under Cd stress, the SPAD value decreased as Cd levels increased, thereby negatively impacting photosynthesis in A. argyi (Figure 4a). However, under high Cd conditions, the application of exogenous Se and Si increased the SPAD value.
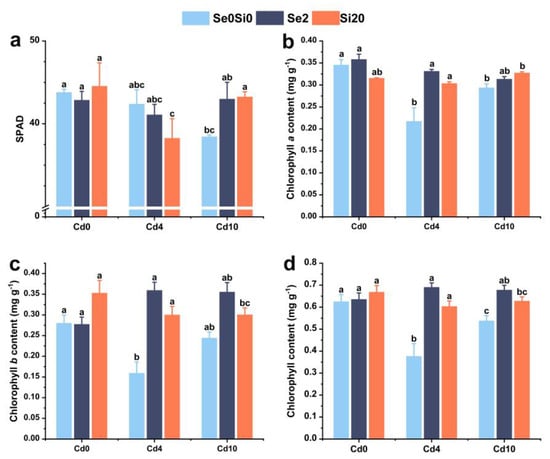
Figure 4.
Effects of Se/Si on photosynthetic parameters in A. argyi under Cd stress. (a) SPAD value; (b) chlorophyll a content; (c) chlorophyll b content; (d) total chlorophyll content. Lowercase letters in the bar graph indicate significant differences among treatments at p < 0.05.
Cd stress led to a decrease in chlorophyll a, chlorophyll b, and total chlorophyll content in A. argyi (Figure 4b–d). However, after the application of exogenous Se and Si, the contents of these photosynthetic pigments increased. Under low Cd conditions, exogenous Se application resulted in the highest levels of chlorophyll a, chlorophyll b, and total chlorophyll content. Under high Cd conditions, the application of exogenous Si increased chlorophyll a content but had no significant effect on chlorophyll b or total chlorophyll content (Figure 4b). These results suggest that both Se and Si alleviate Cd stress in A. argyi by enhancing photosynthesis, with Se exerting a more pronounced effect.
3.6. Osmotic Regulation Substances
To examine the effects of exogenous Se and Si on osmotic regulation substances in A. argyi under Cd stress, the contents of proline (Pro), soluble protein, soluble sugar, and reducing sugar were measured under different treatment conditions (Figure 5). Under Cd10 stress, Pro content in A. argyi leaves increased compared with the control group (Cd0), indicating Cd-induced damage (Figure 5a). Following the application of Se and Si, Pro content decreased. Under low Cd conditions, Se and Si application resulted in reductions of 1.79% and 10.81% in Pro content, respectively. Under high Cd conditions, reductions of 16.48% and 9.67% in Pro content were observed following Se and Si application, respectively.
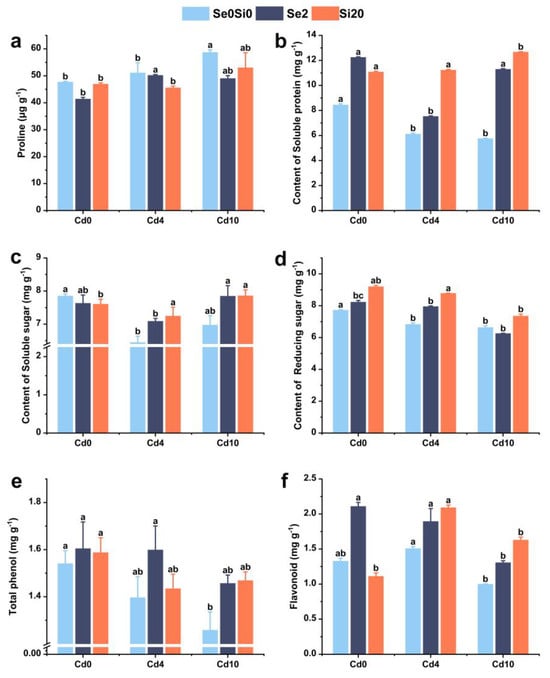
Figure 5.
Effects of Se and Si on osmotic regulators and secondary metabolites in A. argyi under Cd stress. (a) Proline content; (b) soluble protein content; (c) soluble sugar content; (d) reducing sugar content; (e) total phenolic content; (f) flavonoid content. Lowercase letters in the bar graph indicate significant differences among treatments at p < 0.05.
Soluble protein content decreased with increasing Cd stress but increased following Se and Si application. Under Cd4 stress, treatment with 2 mg kg−1 Se resulted in only a slight increase in soluble protein content, suggesting that this concentration may alleviate Cd stress through the regulation of other osmotic substances, such as Pro and soluble sugars. Treatment with 20 mg kg−1 Si further increased soluble protein content (Figure 5b). Under Cd10 stress conditions, Se and Si treatments increased soluble protein content by 96.11% and 119.95%, respectively, thereby alleviating Cd-induced damage in A. argyi (Figure 5b).
Cd stress reduced soluble sugar and reducing sugar contents in A. argyi leaves, while the application of Se and Si led to varying degrees of restoration. Under Cd4 stress, the application of 2 mg kg−1 Se increased both soluble sugar and reducing sugar contents (Figure 5c,d). Under Cd10 stress, the application of 20 mg kg−1 Si resulted in increased levels of these osmotic regulatory substances (Figure 5c,d), whereas the application of 2 mg kg−1 Se led to a slight reduction of 5.67% in reducing sugar content (Figure 5d).
In summary, the application of exogenous Se and Si effectively alleviates Cd toxicity in A. argyi by enhancing osmotic regulatory mechanisms and increasing the content of protective osmotic substances in leaves.
3.7. Total Phenolic and Flavonoid Content in A. argyi Leaves
To investigate the effects of Se and Si on the secondary metabolites of A. argyi under Cd stress, the total phenolic and flavonoid contents in A. argyi leaves were measured under different treatment conditions (Figure 5e,f). Under Cd stress, the total phenolic and flavonoid contents in A. argyi leaves exhibited a slight decrease; however, under Cd4 conditions, the flavonoid content increased by 13.58% (Figure 5f). Following the application of exogenous Se and Si, the total phenolic and flavonoid contents in A. argyi leaves demonstrated varying degrees of increase (Figure 5e).
3.8. PCA and Correlation Analysis of Growth and Physiological Indicators in A. argyi
To further examine the relationship between the growth and physiological indices of A. argyi under Cd stress alleviated by Se and Si, 27 indicators were analyzed using principal component analysis (PCA). Two principal components, PC1 (30.52%) and PC2 (21.21%), were extracted. The indicators in the Si treatment group exhibited significant differences compared to those in the Cd0 and Cd treatment groups, with Cd10Si20 showing the most pronounced difference from other treatments. Overall, both Se and Si treatments effectively alleviated the adverse effects of Cd stress on A. argyi (Figure 6).
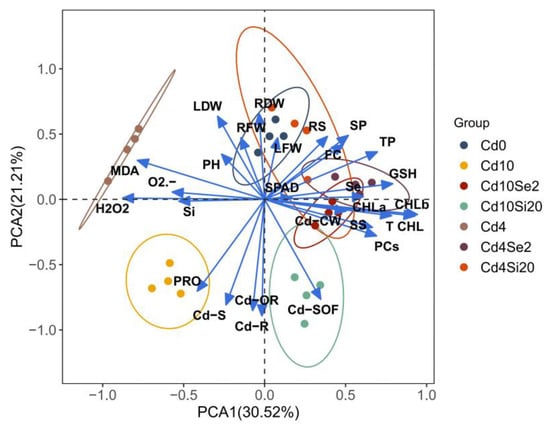
Figure 6.
PCA of A. argyi indicators under different treatments.
Additionally, correlation analysis between phenotypic data and physicochemical indicators of A. argyi under Se- and Si-mediated Cd stress alleviation indicated that Cd content was negatively correlated with osmotic regulators, chlorophyll content, and secondary metabolites but positively correlated with ROS, MDA, and Pro. Under Cd stress, Se exhibited a positive correlation with chlorophyll content, PCs, osmoregulatory substances, and secondary metabolites, while showing a negative correlation with MDA and ROS. Similarly, Si was positively correlated with SPAD values and osmoregulatory substances and negatively correlated with MDA (Figure 7).
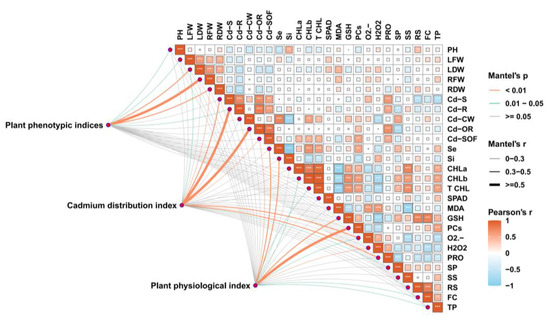
Figure 7.
Correlation analysis of phenotypic and physicochemical indices, illustrating pairwise comparisons of physicochemical indices under various treatments. The color gradient represents Pearson’s correlation coefficient (* p < 0.05, ** p < 0.01, *** p < 0.001). Plant phenotypic indices (plant height, leaf biomass, stem biomass, root biomass), Cd distribution indices (Cd content in shoot, Cd content in root, Cd content in subcellular components), and physiological indices (SPAD, chlorophyll content, osmoregulatory substances, peroxidation index) were analyzed for correlations using the Mantel test. Edge widths correspond to Mantel’s r statistic for distance correlation, with edge colors indicating statistical significance based on 999 permutations. PH, plant height; LFW, leaf fresh weight; LDW, leaf dry weight; RFW, root fresh weight; RDW, root dry weight; Cd-S, Cd content in shoot; Cd-R, Cd content in root; Cd-CW, Cd content in cell wall; Cd-OR, Cd content in organelles; Cd-SOF, Cd content in cytoplasm; Se, selenium; Si, silicon; CHLa, chlorophyll a content; CHLb, chlorophyll b content; T CHL, total chlorophyll content; MDA, malondialdehyde; GSH, glutathione; PCs, phytochelatins; O2•−, superoxide anion; H2O2, hydrogen peroxide; PRO, proline; SP, soluble protein; SS, soluble sugar; RS, reducing sugar; FC, flavonoid content; TP, total phenolic content.
4. Discussion
This study demonstrated that Cd10 stress reduced the biomass and plant height of A. argyi (Figure 1). Previous studies have shown that prolonged exposure to Cd stress exerts detrimental effects on plant physiological and biochemical processes, including inhibition of photosynthesis and respiration, disruption of nutrient balance, and interference with metabolic pathways. These disruptions ultimately inhibit plant growth and development, reducing yield and quality [49].
Selenium and Si are beneficial elements for plants, and their protective effects under Cd stress have been widely reported [50]. Both Se and Si can inhibit the entry of Cd into the soil and its subsequent absorption and translocation within plants [51]. Se has been suggested to hinder the translocation of Cd from roots to shoots by forming Cd–thiol complexes [52]. Additionally, Se may counteract Cd toxicity by reconstructing chloroplast ultrastructure and preventing chlorophyll degradation, thereby enhancing photosynthetic efficiency and maintaining plant growth [53]. Si can increase soil pH, which induces the precipitation of HM and their deposition on root surfaces, thereby reducing Cd uptake and effectively mitigating its toxicity [54].
Plant biomass and height are the most direct indicators of growth status and stress resistance. In this study, the addition of appropriate concentrations of Se promoted the growth of A. argyi and mitigated the adverse effects of Cd on both aboveground and underground plant growth (Figure 1). The findings of this study are consistent with those of Shahid, M. A. et al. [55] and Wu, Z. et al. [56], who similarly reported that Se enhanced plant growth under Cd stress conditions.
Appropriate concentrations of exogenous substances can facilitate the accumulation of HM in plants [57]. In this study, the application of Se and Si was found to significantly influence Cd content in both the aboveground and belowground parts of A. argyi (Figure 2d). Notably, exogenous Se mitigated Cd accumulation by reducing the overall Cd content in A. argyi plants, whereas exogenous Si alleviated Cd accumulation by limiting Cd uptake by the root system and its subsequent transport to the aboveground tissues (Figure 2). Previous studies have reported that Se and Si mitigate Cd accumulation in plants by forming “barriers” in different plant tissues, thereby restricting Cd translocation and accumulation [5].
Selenium has been shown to enhance the uptake and translocation of essential elements like S, Mn, Mg, Ca, and K in plants under Cd stress, thereby improving overall plant nutritional status [58]. As Cd and Se enter root cells through distinct specific transporters, no extracellular competition occurs for their absorption by roots. Thus, the antagonistic interaction between Se and Cd in plant cells has been elucidated [59]. Intracellular Cd is primarily bound to S ligands, particularly thiol groups [60]. Due to its chemical similarity, Se undergoes transformation in cells via the S metabolic pathway [61]. Studies have demonstrated that most Cd–thiol complexes are sequestered in vacuoles [51]. The soluble fraction, which includes vacuoles, plays a crucial role in Cd sequestration within roots, leading to an increased Cd concentration in the root cell wall. Vacuoles serve as the primary storage sites for excess metals to maintain metal homeostasis, while Cd binding to the cell wall may prevent its entry into plant tissues [61]. Se can undergo Cd–Se complexation, thereby reducing Cd absorption. Previous research has shown that Cd–Se binding compounds, like Cd–SeCys and Cd–SeMet, appear in the leaves and stems of corn, thereby reducing Cd toxicity. The formation of these complexes further decreases the bioavailability and mobility of Cd in plants [62].
Unlike Se, Si precipitates within the root endodermis as a robust physical barrier, commonly referred to as the “silica layer,” after being absorbed by plants. This barrier effectively restricts Cd translocation from the root system to the aerial parts, thereby reducing overall Cd accumulation in plant tissues [63]. In addition, most metals are present in the cytoplasm and cell walls, and only a limited amount accumulates in the protoplasts of plant cells. The protoplast-to-protoplast ratio can be reduced by Si treatment, which is crucial for lowering the toxicity of excess metals in key sites of cellular metabolism, like the cytoplasm and organelles [63,64]. Additionally, Gu, H. H. et al. demonstrated that Si inhibits Cd transport from stems to leaves, which may be attributed to the co-precipitation of high concentrations of Si and Cd within stems [65]. In rice (Oryza sativa L.) subjected to Cd stress, it was found that Cd co-precipitated with hemicellulose-bound Si in the cell wall, a key mechanism by which Si inhibits Cd uptake [66]. From a proteomic perspective, Si is involved in key regulatory processes, reducing Cd uptake by downregulating the expression of LCT1, an HM transport protein associated with Cd uptake at the plasma membrane, and HMA2, a protein responsible for Cd efflux from the cytoplasm. Additionally, Si upregulates PCS1, a plant phytochelatin synthase, and IRT1, an Fe transport gene, which may contribute to Cd detoxification [67]. Si has been shown to effectively reduce the absorption and translocation of Cd in tomato seedlings. Plants primarily absorb Cd through their roots, where most Cd2+ ions are fixed as insoluble complexes in root tissues, thereby limiting their movement. Only a small fraction of soluble Cd is transported to the aboveground parts of plants [68].
Photosynthesis is significantly inhibited in plants under HM stress, primarily due to its adverse effects on chlorophyll content, chloroplast structure, thylakoid membrane integrity and function, and the activity of photosynthetic carbon assimilation enzymes [24,69]. In this study, Cd treatment markedly reduced chlorophyll content and photosynthetic efficiency in A. argyi leaves (Figure 4). One of the primary pathways of Cd toxicity involves damage to photosynthetic system components, particularly light-harvesting complexes, leading to a decline in chlorophyll and carotenoid content [70]. For instance, in Salvia sclarea, high Cd concentrations cause structural damage to chloroplasts, leading to thylakoid membrane rupture and starch granule depletion, thereby impairing photosynthetic capacity [71].
Studies have indicated that exogenous Se enhances photosynthesis and increases photosynthetic pigment content, thereby promoting plant growth. Selenium may counteract Cd toxicity by reconstructing chloroplast ultrastructure and preventing chlorophyll degradation, thus enhancing the synthesis of photosynthetic products and sustaining plant growth [72]. Similarly, in this study, exogenous Se was observed to promote photosynthesis in A. argyi leaves and increase chlorophyll content under various Cd stress conditions. Additionally, exogenous Si mitigates enzymatic oxidative damage, thereby enhancing the plant’s antioxidant defense system and supporting photosynthesis [73]. The findings of this study demonstrated that exogenous Si increased chlorophyll content and SPAD values in A. argyi leaves, thereby alleviating Cd-induced damage to the photosynthetic process (Figure 4).
Heavy metal exposure often induces oxidative stress in plants, either directly or indirectly, by promoting the formation of ROS [74]. Cd toxicity disrupts ROS homeostasis, leading to excessive ROS accumulation in plant cells [75]. Reactive oxygen species such as O2•−, H2O2, and -OH accumulate in large quantities, with MDA as a final oxidation product [7]. In this study, Cd stress led to increased levels of O2•−, H2O2, and MDA in A. argyi, while the levels of GSH and PCs decreased. The application of exogenous Se and Si alleviated Cd-induced damage to the antioxidant defense system of A. argyi (Figure 3).
Studies have shown that exogenous Se promotes plant growth by enhancing cell membrane stability. Se supplementation reduces ROS production in plants, thereby preserving the structural and functional integrity of cell membranes and preventing the leakage of vital cellular components. The significant reduction in membrane lipid peroxidation in seedlings treated with Se may be attributed to decreased oxidase activity and upregulation of the antioxidant defense system, which protects plants from the deleterious effects of Cd stress [76]. Moreover, Se exerts its protective effects by inhibiting the expression of ROS-producing enzymes such as BnaRBOHC, BnaRBOHD1, BnaRBOHF1, and glycolate oxidase (BnaGLO), thereby mitigating ROS accumulation under Cd stress [77].
Previous studies have demonstrated that Si enhances antioxidant enzyme activity and increases the levels of non-enzymatic antioxidants in plants under Cd stress, thereby accelerating ROS scavenging and mitigating Cd-induced oxidative damage [78]. In this study, the application of Se and Si increased GSH and PC content while reducing H2O2, O2•−, and MDA levels, thereby alleviating Cd toxicity in A. argyi.
Proline plays a crucial role in cellular osmotic regulation, including the removal of reactive oxygen species, the protection of membrane structures, and the maintenance of redox balance. Plants mitigate the damage induced by Cd stress by promoting Pro synthesis and inhibiting its degradation [79]. Studies have demonstrated that the application of Se effectively enhances plant tolerance to Cd by stimulating the synthesis of key osmoprotectants, such as Pro and betaine, facilitating greater water uptake from the soil, and protecting plant metabolism [53]. These findings are consistent with the results of the present study, in which the addition of Se increased the accumulation of osmotic regulatory substances in A. argyi. Previous research has shown that plants under Cd stress maintain intracellular osmotic pressure balance and protect cellular structures and functions by accumulating osmoprotectants such as Pro, soluble proteins, soluble sugars, and reducing sugars. The exogenous application of Se or Si further promotes the accumulation of these osmotic regulatory substances, thereby significantly alleviating the toxic effects of Cd stress on plants [35,78]. These findings suggest that the application of Se and Si effectively mitigates Cd-induced increases in Pro content.
Secondary metabolites are derived from primary metabolites and accumulate in cells, tissues, and organs through various biosynthetic pathways. They play a critical role in plant defense against biotic and abiotic stresses [80]. The bioactive compounds in traditional Chinese medicine are often secondary metabolites, including flavonoids, alkaloids, polysaccharides, terpenes, and coumarins. These substances contribute to plant resistance against pathogens, insects, and environmental stress [81]. Therefore, these bioactive compounds in medicinal plants warrant particular attention.
In the present study, Cd stress increased the flavonoid content in certain treatment groups of A. argyi (Figure 5). This finding aligns with previous research, which reported that Cd stress significantly reduces the levels of resveratrol, total flavonoids, apigenin, and quercetin in wild chrysanthemum (Chrysanthemum indicum L.) [70]. Furthermore, our study revealed that the application of Se and Si significantly increased the concentrations of flavonoids and total phenolic compounds in A. argyi leaves, thereby enhancing the plant’s resistance to Cd toxicity. Exogenous Se has been shown to effectively elevate flavonoid and phenolic compound levels in celery (Anthriscus cereifolium) under Cd stress [82]. Additionally, metabolomic analysis has confirmed that under Cd stress, exogenous Si enhances the biosynthesis and accumulation of flavonoids (such as flavonols and quercetin) and phenolic acids (including chlorogenic acid and salicylic acid) in peas (Pisum sativum L.) [83].
5. Conclusions
The uptake of Cd by plant roots and its subsequent translocation to various tissues increase Cd toxicity in plants. The independent application of Se and Si not only promoted the growth of A. argyi but also reduced Cd absorption and accumulation by altering the Cd content in aboveground and underground parts and its distribution among subcellular components. Notably, these two elements mitigated Cd stress through distinct mechanisms. Comparative analysis indicates that Se alleviates Cd accumulation by reducing the overall Cd content in A. argyi, whereas Si mitigates Cd accumulation by restricting Cd transport from the root system to the aboveground tissues. The effect of exogenous Se in alleviating Cd stress in A. argyi was greater than that of exogenous Si. These findings enhance our understanding of the differential mechanisms underlying Cd detoxification when Se and Si are applied to A. argyi under Cd stress. Moreover, this study provides an effective and practical approach to reducing Cd accumulation in the edible parts of A. argyi.
Author Contributions
Y.Y. conducted the primary experiments, performed the data analysis, and drafted the manuscript. Y.G. contributed to validation, investigation, and formal analysis. Q.Y. was responsible for visualization and data curation. M.W. participated in visualization and resource management. W.H. supervised validation, provided oversight for project administration, acquired funding, and conceptualized the study. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by Shaanxi Provincial Science and Technology Plan Project (SXLK2022-02).
Institutional Review Board Statement
Experimental research and field studies on plants (wild), including the collection of plant material comply with the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora.
Informed Consent Statement
We all declare that manuscript reporting studies do not involve any human participants, human data, or human tissue. So, it is not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
We thank the Horticulture Science Research Center at the College of Horticulture (NWAFU), the Life Science Research Core Services (NWAFU), the Teaching and Research Core Facility at the College of Life Sciences (NWAFU), the Chemical Experiment Teaching Center at the College of Chemistry and Pharmacy (NWAFU), and the Analysis center of resources and environmental science research at the College of Natural Resources and Environment (NWAFU) for their technical support in this work.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
| A. Argyi | Artemisia argyi |
| Cd | Cadmium |
| GSH | Glutathione |
| H2O2 | Hydrogen peroxide |
| MDA | malondialdehyde |
| O2•− | Superoxide |
| PCs | Phytochelatins |
| ROS | reactive oxygen species |
| Se | Selenium |
| Si | Silicon |
References
- Yan, K.; Wang, H.; Lan, Z.; Zhou, J.; Fu, H.Z.; Wu, L.; Xu, J. Heavy metal pollution in the soil of contaminated sites in China: Research status and pollution assessment over the past two decades. J. Clean. Prod. 2022, 373, 133780. [Google Scholar] [CrossRef]
- Qin, G.; Niu, Z.; Yu, J.; Li, L.; Ma, J.; Xiang, P. Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere 2021, 267, 129205. [Google Scholar] [CrossRef]
- Afzal, B.; Yasin, D.; Naaz, H.; Sami, N.; Zaki, A.; Rizvi, M.A.; Kumar, R.; Srivastava, P.; Fatma, T. Biomedical potential of anabaena variabilis NCCU-441 based selenium nanoparticles and their comparison with commercial nanoparticles. Sci. Rep. 2021, 11, 13507. [Google Scholar] [CrossRef] [PubMed]
- GB 15618—2018; Soil Environmental Quality Risk Control Standard for Soil Contamination of Agricultural Land. Ministry of Ecology and Environment: Beijing, China, 2018.
- Wang, Z.; Wang, Y.; Lu, J.; Li, T.; Li, S.; Nie, M.; Shi, G.; Zhao, X. Silicon and selenium alleviate cadmium toxicity in artemisia selengensis turcz by regulating the plant-rhizosphere. Environ. Res. 2024, 252, 119064. [Google Scholar] [CrossRef]
- Cui, B.; Luo, H.; Yao, X.; Xing, P.; Deng, S.; Zhang, Q.; Yi, W.; Gu, Q.; Peng, L.; Yu, X.; et al. Nanosized-selenium-application-mediated cadmium toxicity in aromatic rice at different stages. Plants 2024, 13, 2253. [Google Scholar] [PubMed]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar]
- Sood, M. Reactive oxygen species (ROS): Plant perspectives on oxidative signalling and biotic stress response. Discov. Plants 2025, 2, 187. [Google Scholar] [CrossRef]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, environmental exposure, and health outcomes. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, H.; Zhao, B. Exploring the remediation potential of Hydrangea macrophylla (thunb.) Ser. In cadmium-contaminated soil by comparing cultivars and seedling age. Environ. Technol. Innov. 2024, 33, 103474. [Google Scholar]
- Sarwar, N.; Malhi, S.S.; Zia, M.H.; Naeem, A.; Bibi, S.; Farid, G. Role of mineral nutrition in minimizing cadmium accumulation by plants. J. Sci. Food Agric. 2010, 90, 925–937. [Google Scholar] [CrossRef]
- Nazar, R.; Iqbal, N.; Masood, A.; Khan, M.I.R.; Syeed, S.; Khan, N.A. Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Am. J. Plant Sci. 2012, 3, 1476–1489. [Google Scholar] [CrossRef]
- Kaur, N.; Sharma, S.; Kaur, S.; Nayyar, H. Selenium in agriculture: A nutrient or contaminant for crops? Arch. Agron. Soil Sci. 2014, 60, 1593–1624. [Google Scholar] [CrossRef]
- Yasin, M.; El-Mehdawi, A.F.; Anwar, A.; Pilon-Smits, E.A.; Faisal, M. Microbial-enhanced selenium and iron biofortification of wheat (Triticum aestivum L.)—Applications in phytoremediation and biofortification. Int. J. Phytoremediation 2015, 17, 341–347. [Google Scholar]
- Li, Z.; Tian, Y.; Wang, B.; Peng, R.; Xu, J.; Fu, X.; Han, H.; Wang, L.; Zhang, W.; Deng, Y.; et al. Enhanced phytoremediation of selenium using genetically engineered rice plants. J. Plant Physiol. 2022, 271, 153665. [Google Scholar] [CrossRef]
- Odeyemi, I.A.; Oyediran, J.A.; Ademiluyi, A.O.; Oboh, G.; Ogunsuyi, O.B. Selenium-biofortified gboma (Solanum macro-carpon L.) Vegetable-supplemented diets increased circulating selenium levels and potentiated endogenous anti-inflammatory, antioxidant and immunomodulatory properties in wistar rats. Biometals 2025. [Google Scholar]
- Ahmad, R.; Waraich, E.A.; Nawaz, F.; Ashraf, M.Y.; Khalid, M. Selenium (se) improves drought tolerance in crop plants—A myth or fact? J. Sci. Food Agric. 2016, 96, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xian, L.; Yuan, L.; Lin, Z.; Chen, X.; Wang, J.; Li, T. The use of selenium for controlling plant fungal diseases and in-sect pests. Front. Plant Sci. 2023, 14, 1102594. [Google Scholar]
- Pukacka, S.; Ratajczak, E.; Kalemba, E. The protective role of selenium in recalcitrant Acer saccharium L. Seeds subjected to desiccation. J. Plant Physiol. 2010, 168, 220–225. [Google Scholar] [PubMed]
- Nedjimi, B. The role of selenium and selenium nanoparticles in enhancing plant tolerance to cadmium stress: A sustainable approach. Discov. Plants 2025, 2, 1–22. [Google Scholar] [CrossRef]
- Khan, M.I.; Nazir, F.; Asgher, M.; Per, T.S.; Khan, N.A. Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J. Plant Physiol. 2015, 173, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.R.; Prado, E.R.; de Oliveira, R.; Santos, E.F.; Lemos, D.S.I.; Dos, R.A.; Azevedo, R.A.; Gratao, P.L. Mechanisms of cadmium-stress avoidance by selenium in tomato plants. Ecotoxicology 2020, 29, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhou, W.; Dai, H.; Cao, F.; Zhang, G.; Wu, F. Selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. J. Hazard. Mater. 2012, 235–236, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Awan, S.A.; Rizwan, M.; Ali, S.; Hassan, M.J.; Brestic, M.; Zhang, X.; Huang, L. Effects of silicon on heavy metal uptake at the soil-plant interphase: A review. Ecotoxicol. Environ. Saf. 2021, 222, 112510. [Google Scholar] [CrossRef]
- Aqeel, A.; Ahmad, Y.N.; Ullah, K.W.; Waheed, A.; Rui, W.; Ali, S.A.; Muhammad, A.; Aamir, A.; Tingquan, W. Silicon assisted ameliorative effects of iron nanoparticles against cadmium stress: Attaining new equilibrium among physiochemical parameters, antioxidative machinery, and osmoregulators of phaseolus lunatus. Plant Physiol. Biochem. 2021, 166, 874–886. [Google Scholar]
- Wang, L.; Yang, B.; Liu, Q.; Zhang, Q.; Zhao, F.; Xiao, Y.; Liao, X. Safe usage of cd-polluted paddy fields using alkaline si–rich compound amendment: Effect and mechanism. J. Environ. Manag. 2023, 335, 117547. [Google Scholar] [CrossRef]
- Tan, Y.; Liu, X.; Shen, Z.; Xiao, Y.; Zhang, Y.; Du, H.; Wu, Z.; Zhi, D.; Delgado, A.N.; Yang, Y. Effects of seed priming with different concentrations and forms of silicon on germination and growth of rice under cadmium stress. Appl. Soil Ecol. 2025, 207, 105947. [Google Scholar] [CrossRef]
- Altaf, M.M.; Yi, H.; Bashir, S.; Abbasi, S.S.; Anwar, M.; Alsahli, A.A.; Altaf, M.A.; Ahmad, P. Mitigating chromium stress in tomato plants using green-silicone nanoparticles: Enhancing cellular oxidative stress management and chromium reduction. Sci. Hortic. 2024, 338, 113635. [Google Scholar] [CrossRef]
- Javed, M.T.; Saleem, M.H.; Aslam, S.; Rehman, M.; Iqbal, N.; Begum, R.; Ali, S.; Alsahli, A.A.; Alyemeni, M.N.; Wijaya, L. Elucidating silicon-mediated distinct morpho-physio-biochemical attributes and organic acid exudation patterns of cad-mium stressed ajwain (Trachyspermum ammi L.). Plant Physiol. Biochem. 2020, 157, 23–37. [Google Scholar] [CrossRef]
- Manna, I.; Nath, S.; Mandal, P.; Paul, S. Effect of cerium oxide and silicon dioxide nanoparticles in combating heavy metal stress in plants. Nucleu 2025, 1–27. [Google Scholar] [CrossRef]
- Song, A.; Li, Z.; Zhang, J.; Xue, G.; Fan, F.; Liang, Y. Silicon-enhanced resistance to cadmium toxicity in Brassica chinensis L. Is attributed to si-suppressed cadmium uptake and transport and si-enhanced antioxidant defense capacity. J. Hazard. Mater. 2009, 172, 74–83. [Google Scholar] [CrossRef]
- Shi, Q.; Bao, Z.; Zhu, Z.; He, Y.; Qian, Q.; Yu, J. Silicon-mediated alleviation of mn toxicity in cucumis sativus in relation to activities of superoxide dismutase and ascorbate peroxidase. Phytochemistry 2005, 66, 1551–1559. [Google Scholar] [CrossRef]
- Huang, H.; Li, M.; Rizwan, M.; Dai, Z.; Yuan, Y.; Hossain, M.M.; Cao, M.; Xiong, S.; Tu, S. Synergistic effect of silicon and selenium on the alleviation of cadmium toxicity in rice plants. J. Hazard. Mater. 2021, 401, 123393. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, Y.; Guo, Y.; Pan, M.; Hao, W. Exogenous selenium enhances cadmium stress tolerance by improving physio-logical characteristics of Artemisia argyi seedlings. Sci. Rep. 2025, 15, 3450. [Google Scholar]
- Mousa, M.K.S.; Maryam, M. Assessment of phytoremediation potential of native plant species naturally growing in a heavy metal-polluted saline-sodic soil. Environ. Sci. Pollut. Res. Int. 2020, 27, 10027–10038. [Google Scholar]
- Zhao, L.; Yang, Y.; Hu, Y.; Yang, S.; Jin, H.; Wei, J.; Yang, M. Analysis of the current situation of heavy metal pollution in Chinese medicinal materials in China and countermeasures research. Chin. J. Tradit. Chin. Med. 2014, 45, 1199–1206. [Google Scholar]
- Yang, Y.; Zhao, Y.; Pan, M.; Yu, Y.; Guo, Y.; Ge, Q.; Hao, W. Physiology and transcriptome analysis of Artemisia argyi adaptation and accumulation to soil cadmium. Ecotoxicol. Environ. Saf. 2024, 278, 116397. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, C.; Wu, Z.; Liu, X.; Cai, M.; Jia, W.; Zhao, X. Selenium reduces cadmium accumulation in seed by increasing cadmium retention in root of oilseed rape (Brassica napus L.). Environ. Exp. Bot. 2018, 158, 161–170. [Google Scholar] [CrossRef]
- Huang, F.; Wen, X.H.; Cai, Y.X.; Cai, K.Z. Silicon-mediated enhancement of heavy metal tolerance in rice at different growth stages. Int. J. Environ. Res. Public Health 2018, 15, 2193. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, Y.; Yang, X.; Zhang, H.; Wang, D.; Wu, P.; Yin, A.; Gao, C. Classification and regression tree (CART) for predicting cadmium (cd) uptake by rice (Oryza sativa L.) And its application to derive soil cd threshold based on field data. Ecotoxicol. Environ. Saf. 2024, 285, 117125. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, S.; Lin, L.; Fan, Z.; Liu, X. Effects of methyl jasmonate on soil physicochemical properties and mi-crobial communities of continuous cropping strawberry. Jiangsu Agric. Sci. 2025, 6, 254–263. [Google Scholar]
- Jia, H.; Wang, X.; Shi, C.; Guo, J.; Ma, P.; Ren, X.; Wei, T.; Liu, H.; Li, J. Hydrogen sulfide decreases cd translocation from root to shoot through increasing cd accumulation in cell wall and decreasing cd(2+) influx in isatis indigotica. Plant Physiol. Biochem. 2020, 155, 605–612. [Google Scholar] [CrossRef]
- GB 5009.268-2025; National Food Safety Standard—Determination of Multiple Elements in Foods. China Standards Press: Beijing, China, 2025.
- Hua, H.; Yu, H.; Liu, D. Determination of silicon in plants by silicon molybdenum blue colorimetric method. Mod. Agric. Sci. Technol. 2013, 24, 173–174. [Google Scholar]
- Hou, X.; Ma, C.; Wang, Z.; Shi, X.; Duan, W.; Fu, X.; Liu, J.; Guo, C.; Xiao, K. Transcription factor gene TaWRKY76 confers plants improved drought and salt tolerance through modulating stress defensive-associated processes in Triticum aestivum L. Plant Physiol. Biochem. 2024, 216, 109147. [Google Scholar] [CrossRef]
- Cappellari, L.D.R.; Santoro, M.V.; Nievas, F.; Giordano, W.; Banchio, E. Increase of secondary metabolite content in mari-gold by inoculation with plant growth-promoting rhizobacteria. Appl. Soil Ecol. 2013, 70, 16–22. [Google Scholar] [CrossRef]
- Yang, J.; Kim, J.S.; Kwon, Y.S.; Seong, E.S.; Kim, M.J. Antioxidant and antiproliferative activities of Eclipta prostrata (L.) L. Extract and isolated compounds. Molecules 2023, 28, 7354. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhuang, K.; Peng, Y.; Huang, X.; Lu, Q.; Qian, M.; Liu, Y.; Chen, X.; Peng, K.; et al. Multiple in-sights into differential cd detoxification mechanisms in new germplasms of mung bean (Vigna radiata L.) And potential mitigation strategy. Plant Physiol. Biochem. 2025, 220, 109458. [Google Scholar] [CrossRef]
- Cui, J.; Liu, T.; Li, F.; Yi, J.; Liu, C.; Yu, H. Silica nanoparticles alleviate cadmium toxicity in rice cells: Mechanisms and size effects. Environ. Pollut. 2017, 228, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Kamran, M.; Rizwan, M.; Ali, S.; Parveen, A.; Malik, Z.; Wang, X. Cadmium uptake and translocation: Selenium and silicon roles in cd detoxification for the production of low cd crops: A critical review. Chemosphere 2021, 273, 129690. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, Y.; Qin, X.; Zhao, L.; Liang, X.; Xu, Y. Selenite mitigates cadmium-induced oxidative stress and affects cd uptake in rice seedlings under different water management systems. Ecotoxicol. Environ. Saf. 2019, 168, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Saffaryazdi, A.; Lahouti, M.; Ganjeali, A.; Bayat, H. Impact of selenium supplementation on growth and selenium accumu-lation on spinach (Spinacia oleracea L.) Plants. Not. Sci. Biol. 2012, 4, 95–100. [Google Scholar] [CrossRef]
- Li, L.; Zheng, C.; Fu, Y.; Wu, D.; Yang, X.; Shen, H. Silicate-mediated alleviation of pb toxicity in banana grown in pb-contaminated soil. Biol. Trace Elem. Res. 2012, 145, 101–108. [Google Scholar] [CrossRef]
- Shahid, M.A.; Balal, R.M.; Khan, N.; Zotarelli, L.; Liu, G.D.; Sarkhosh, A.; Fernandez-Zapata, J.C.; Martinez, N.J.; Gar-cia-Sanchez, F. Selenium impedes cadmium and arsenic toxicity in potato by modulating carbohydrate and nitrogen metabolism. Ecotoxicol. Environ. Saf. 2019, 180, 588–599. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, S.; Zhao, J.; Wang, F.; Du, Y.; Zou, S.; Li, H.; Wen, D.; Huang, Y. Comparative responses to silicon and selenium in relation to antioxidant enzyme system and the glutathione-ascorbate cycle in flowering chinese cabbage (Brassica campestris L. ssp. chinensis var. utilis) under cadmium stress. Environ. Exp. Bot. 2017, 133, 1–11. [Google Scholar]
- Ghuge, S.A.; Nikalje, G.C.; Kadam, U.S.; Suprasanna, P.; Hong, J.C. Comprehensive mechanisms of heavy metal toxicity in plants, detoxification, and remediation. J. Hazard. Mater. 2023, 450, 131039. [Google Scholar] [CrossRef]
- Giri, J. Glycinebetaine and abiotic stress tolerance in plants. Plant Signal. Behav. 2011, 6, 1746–1751. [Google Scholar] [CrossRef]
- Wan, Y.; Yu, Y.; Wang, Q.; Qiao, Y.; Li, H. Cadmium uptake dynamics and translocation in rice seedling: Influence of different forms of selenium. Ecotoxicol. Environ. Saf. 2016, 133, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Dalcorso, G.; Farinati, S.; Maistri, S.; Furini, A. How plants cope with cadmium: Staking all on metabolism and gene ex-pression. J. Integr. Plant Biol. 2008, 50, 1268–1280. [Google Scholar] [CrossRef]
- Ernst, W.H.; Krauss, G.J.; Verkleij, J.A.; Wesenberg, D. Interaction of heavy metals with the sulphur metabolism in angio-sperms from an ecological point of view. Plant Cell Environ. 2008, 31, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Sahito, J.H.; Ma, C.; Zhang, J.; Li, J.; Zhao, J.; Mu, L.; Zhang, Y.; Gishkori, Z.; Ding, D.; Zhang, X.; et al. Selenium and its mechanisms mitigate cadmium toxicity in plants: Promising role and future potentials. Ecotoxicol. Environ. Saf. 2025, 300, 118422. [Google Scholar] [CrossRef]
- Vaculik, M.; Lukacova, Z.; Bokor, B.; Martinka, M.; Tripathi, D.K.; Lux, A. Alleviation mechanisms of metal(loid) stress in plants by silicon: A review. J. Exp. Bot. 2020, 71, 6744–6757. [Google Scholar] [CrossRef]
- Rogalla, H.; Römheld, V. Role of leaf apoplast in silicon-mediated manganese tolerance of Cucumis sativus L. Plant Cell Environ. 2002, 25, 549–555. [Google Scholar] [CrossRef]
- Gu, H.H.; Qiu, H.; Tian, T.; Zhan, S.S.; Deng, T.H.; Chaney, R.L.; Wang, S.Z.; Tang, Y.T.; Morel, J.L.; Qiu, R.L. Mitigation ef-fects of silicon rich amendments on heavy metal accumulation in rice (Oryza sativa L.) Planted on multi-metal contami-nated acidic soil. Chemosphere 2011, 83, 1234–1240. [Google Scholar] [CrossRef]
- Ma, J.; Cai, H.; He, C.; Zhang, W.; Wang, L. A hemicellulose-bound form of silicon inhibits cadmium ion uptake in rice (Oryza sativa) cells. New Phytol. 2015, 206, 1063–1074. [Google Scholar] [CrossRef]
- Greger, M.; Kabir, A.H.; Landberg, T.; Maity, P.J.; Lindberg, S. Silicate reduces cadmium uptake into cells of wheat. Environ. Pollut. 2016, 211, 90–97. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, W.; Ye, D.; Liu, X.; Peng, C.; Tang, Y.; Su, L.; Cheng, S.; Cao, K.; Lei, Q.; et al. Mitigation mechanism of silicon and iron co-application to cadmium toxicity in tomato seedlings by integrated transcriptomic and physio-logical correlation analysis. Front. Plant Sci. 2025, 16, 1555618. [Google Scholar] [CrossRef]
- Panda, A.; Fatnani, D.; Parida, A.K. Uptake, impact, adaptive mechanisms, and phytoremediation of heavy metals by plants: Role of transporters in heavy metal sequestration. Plant Physiol. Biochem. 2025, 221, 109578. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Wu, L.; Wang, Q.; Wang, Y.; Luo, H.; Song, J.; Yang, M.; Yao, H.; Chen, S. Physiological and molecular mechanisms of medicinal plants in response to cadmium stress: Current status and future perspective. J. Hazard. Mater. 2023, 450, 131008. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Adamakis, I.S.; Dobrikova, A.; Apostolova, E.; Hanc, A.; Moustakas, M. Excess zinc supply reduces cadmium uptake and mitigates cadmium toxicity effects on chloroplast structure, oxidative stress, and photosystem II photochem-ical efficiency in Salvia sclarea plants. Toxics 2022, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Abd, A.E.; Alqarawi, A.A.; Egamberdieva, D. Bioremediation of adverse impact of cadmium toxicity on cassia italica mill by arbuscular mycorrhizal fungi. Saudi J. Biol. Sci. 2016, 23, 39–47. [Google Scholar] [CrossRef]
- Batool, T.; Javied, S.; Ashraf, K.; Sultan, K.; Zaman, Q.U.; Haider, F.U. Alleviation of cadmium stress by silicon supplemen-tation in peas by the modulation of morpho-physio-biochemical variables and health risk assessment. Life 2022, 12, 1479. [Google Scholar] [CrossRef] [PubMed]
- Tamma, A.A.; Lejcuś, K.; Fiałkiewicz, W.; Marczak, D. Advancing phytoremediation: A review of soil amendments for heavy metal contamination management. Sustainability 2025, 17, 5688. [Google Scholar] [CrossRef]
- Rodríguez-Serrano, M.; Romero-Puertas, M.C.; Pazmiño, D.M.; Testillano, P.S.; Risueño, M.C.; Del Río, L.A.; Sandalio, L.M. Cellular response of pea plants to cadmium toxicity: Cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol. 2009, 150, 229–243. [Google Scholar] [CrossRef]
- Sardar, R.; Ahmed, S.; Shah, A.A.; Yasin, N.A. Selenium nanoparticles reduced cadmium uptake, regulated nutritional ho-meostasis and antioxidative system in coriandrum sativum grown in cadmium toxic conditions. Chemosphere 2022, 287, 132332. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.; Jha, A.B.; Dubey, R.S.; Sharma, P. Mitigating cadmium accumulation and toxicity in plants: The promising role of nanoparticles. Sci. Total Environ. 2024, 912, 168826. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, X.; Yan, J.; Fan, W.; Li, T.; Wang, S.; Liu, P. Silicon reduces cadmium accumulation and toxicity by regulating transcriptional and physiological pathways, and promotes the early growth of tomato seedlings. Ind. Crops Prod. 2023, 206, 117720. [Google Scholar] [CrossRef]
- Song, J.; Sun, Z.; Saud, S.; Fahad, S.; Nawaz, T. Exploring the deleterious effects of heavy metal cadmium on antioxidant defense and photosynthetic pathways in higher plants. Plant Stress 2025, 15, 100716. [Google Scholar] [CrossRef]
- Guo, L.; Yao, H.; Chen, W.; Wang, X.; Ye, P.; Xu, Z.; Zhang, S.; Wu, H. Natural products of medicinal plants: Biosynthesis and bioengineering in post-genomic era. Hortic. Res. 2022, 9, uhac223. [Google Scholar] [CrossRef]
- Kazemi, E.M.; Kolahi, M.; Yazdi, M.; Goldson-Barnaby, A. Anatomic features, tolerance index, secondary metabolites and protein content of chickpea (Cicer arietinum) seedlings under cadmium induction and identification of PCS and FC genes. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2020, 26, 1551–1568. [Google Scholar] [CrossRef]
- Golubkina, N.; Moldovan, A.; Fedotov, M.; Kekina, H.; Kharchenko, V.; Folmanis, G.; Alpatov, A.; Caruso, G. Iodine and selenium biofortification of chervil plants treated with silicon nanoparticles. Plants 2021, 10, 2528. [Google Scholar] [CrossRef] [PubMed]
- Walczak-Skierska, J.; Krakowska-Sieprawska, A.; Monedeiro, F.; Zloch, M.; Pomastowski, P.; Cichorek, M.; Olszewski, J.; Glowacka, K.; Guzewska, G.; Szultka-Mlynska, M. Silicon’s influence on polyphenol and flavonoid profiles in pea (Pisum sativum L.) Under cadmium exposure in hydroponics: A study of metabolomics, extraction efficacy, and antimicrobial properties of extracts. ACS Omega 2024, 9, 14899–14910. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).