CRISPR/Cas9 Editing of the OsLOX3 Gene Enhances Rice Grain Weight and Seed Vigor
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Construction of CRISPR/Cas9 Editing Vector and Generation of Transgenic Plants
2.3. Detection of Transgenic-Positive Rice Lines and Screening of Mutant Plants
2.4. Detection of mRNA Expression Levels in Plants
2.5. Natural Aging Treatment and Seed Germination Experiment
2.6. Agronomic Trait Investigation of Mutant Plants
3. Results
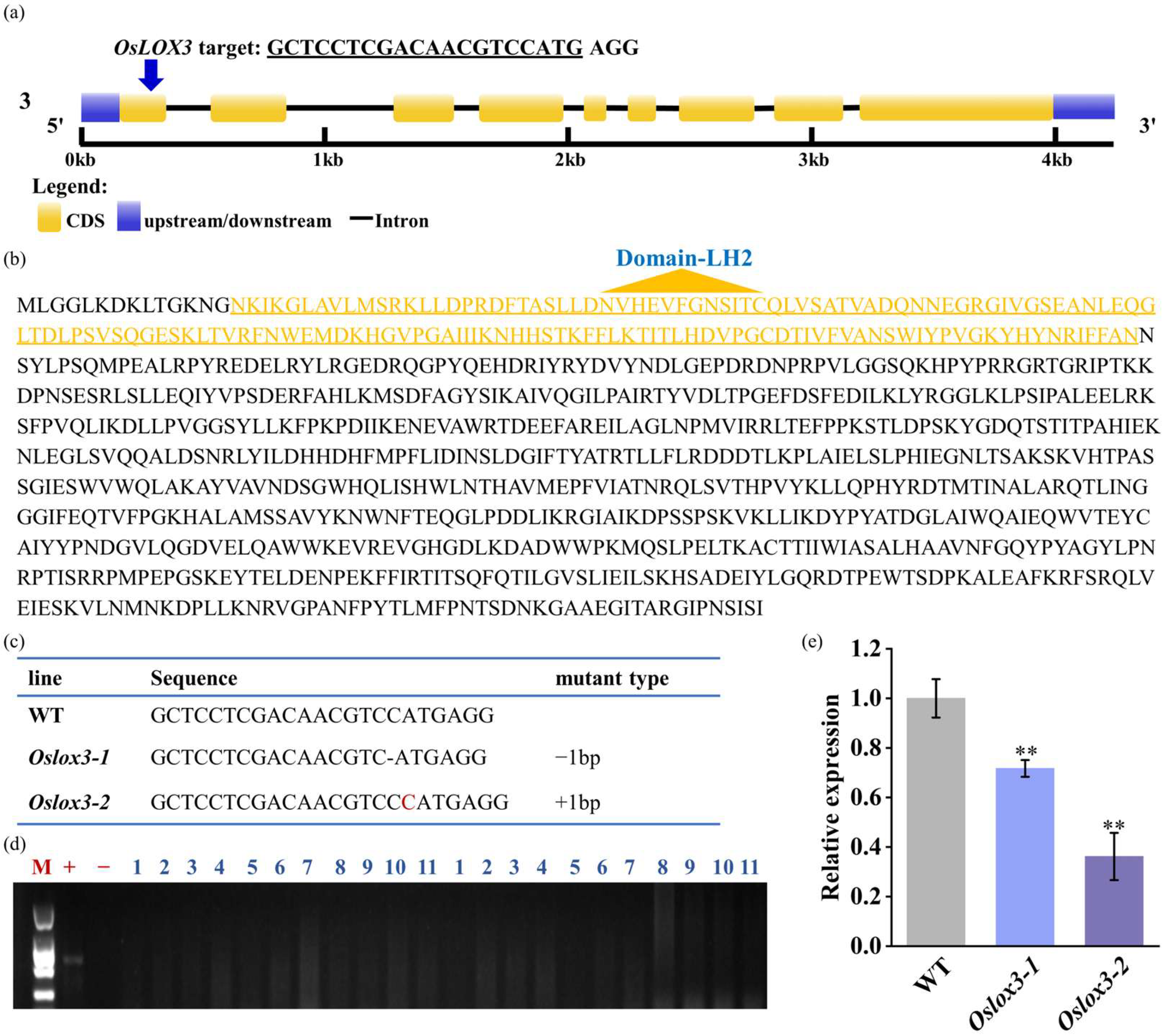
3.1. OsLOX3 Target Design and CRISPR/Cas9-LOX3 Vector Construction
3.2. Obtaining Mutant Plants with Two Different OsLOX3 Genetic Mutation Types
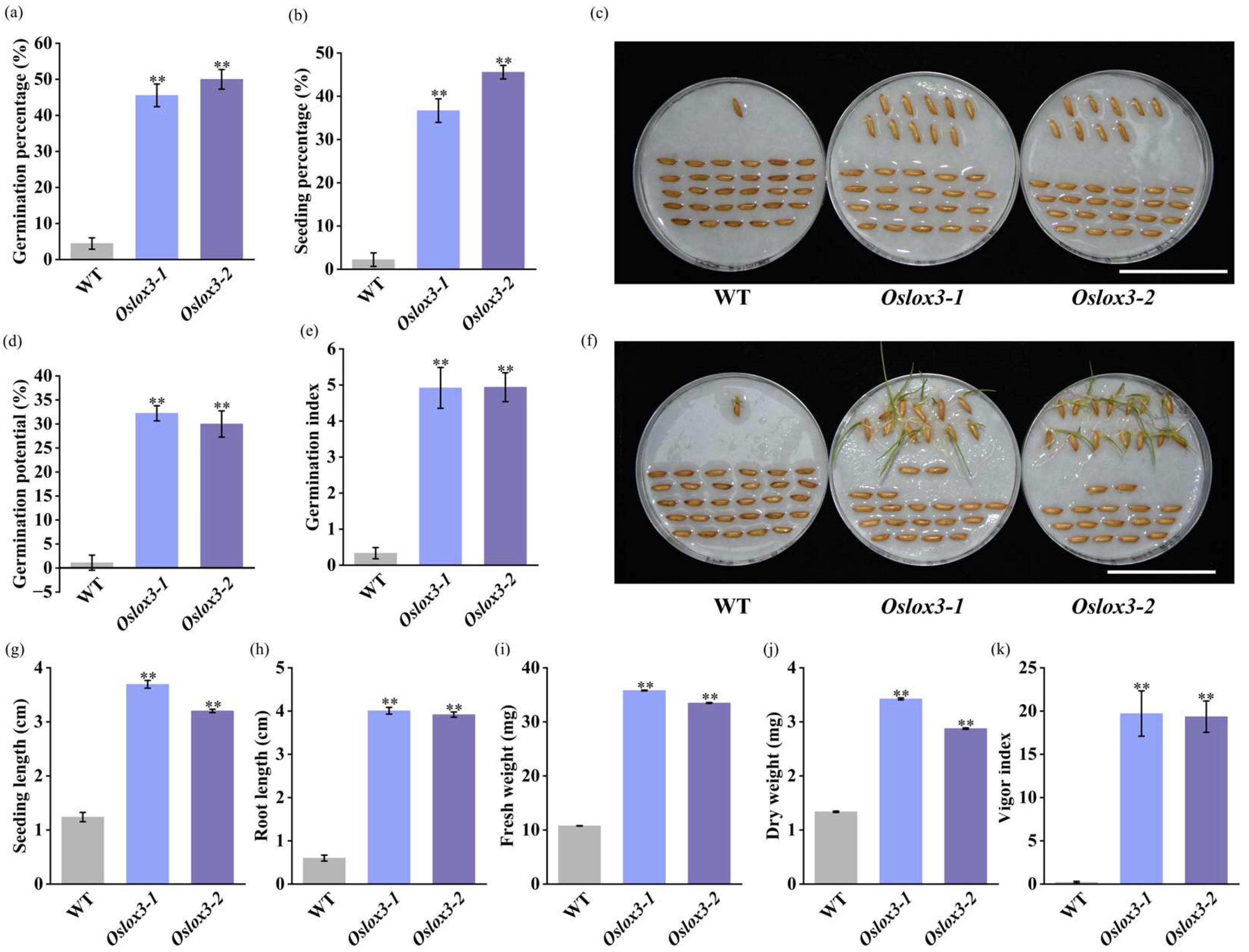
3.3. Seed Vigor of OsLOX3 Mutants Was Significantly Improved
3.4. The Grain Length and Grain Length-to-Width Ratio of OsLOX3 Mutants Were Significantly Increased
3.5. The Thousand-Grain Weight and Theoretical Yield of OsLOX3 Mutants Were Significantly Increased
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, C.J.; Yun, P.; Xia, J.F.; Zhou, K.N.; Wang, L.L.; Zhang, J.W.; Zhao, B.; Yin, D.K.; Fu, Z.; Wang, Y.L.; et al. CRISPR/Cas9-mediated editing of Wx and BADH2 genes created glutinous and aromatic two-line hybrid rice. Mol. Breed. 2023, 43, 24. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, M.; Song, J.T.; Seo, H.S. Seed size: A priority trait in cereal crops. Physiol. Plant 2013, 147, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xu, R.; Duan, P.; Li, Y. Control of grain size in rice. Plant Reprod. 2018, 31, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Jiang, L.; Zheng, J.; Wang, T.; Wang, H.; Huang, Y.; Hong, Z. Genetic bases of rice grain shape: So many genes, so little known. Trends Plant Sci. 2013, 18, 218–226. [Google Scholar] [CrossRef]
- Sakamoto, T.; Matsuoka, M. Identifying and exploiting grain yield genes in rice. Curr. Opin. Plant Biol. 2008, 11, 209–214. [Google Scholar] [CrossRef]
- Li, N.; Li, Y. Signaling pathways of seed size control in plants. Curr. Opin. Plant Biol. 2016, 33, 23–32. [Google Scholar] [CrossRef]
- Fan, C.; Xing, Y.; Mao, H.; Lu, T.; Han, B.; Xu, C.; Li, X.; Zhang, Q. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 2006, 112, 1164–1171. [Google Scholar] [CrossRef]
- Huang, X.; Qian, Q.; Liu, Z.; Sun, H.; He, S.; Luo, D.; Xia, G.; Chu, C.; Li, J.; Fu, X. Natural variation at the DEP1 locus enhances grain yield in rice. Nat. Genet. 2009, 41, 494–497. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, J.; Li, Z.; Yi, C.; Liu, J.; Zhang, H.; Tang, S.; Gu, M.; Liang, G. Deletion in a quantitative trait gene qPE9-1 associated with panicle erectness improves plant architecture during rice domestication. Genetics 2009, 183, 315–324. [Google Scholar] [CrossRef]
- Duan, P.; Rao, Y.; Zeng, D.; Yang, Y.; Xu, R.; Zhang, B.; Dong, G.; Qian, Q.; Li, Y. SMALL GRAIN 1, which encodes a mitogen-activated protein kinase kinase 4, influences grain size in rice. Plant J. 2014, 77, 547–557. [Google Scholar] [CrossRef]
- Liu, S.; Hua, L.; Dong, S.; Chen, H.; Zhu, X.; Jiang, J.; Zhang, F.; Li, Y.; Fang, X.; Chen, F. OsMAPK6, a mitogen-activated protein kinase, influences rice grain size and biomass production. Plant J. 2015, 84, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.S.; Kim, Y.J.; Markkandan, K.; Koo, Y.J.; Song, J.T.; Seo, H.S. GW2 Functions as an E3 Ubiquitin Ligase for Rice Expansin-Like 1. Int. J. Mol. Sci. 2018, 19, 1904. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, X.; Li, Q.; Xiao, Y.; Zhang, G.; Yin, W.; Niu, M.; Meng, W.; Dong, N.; Liu, J.; et al. The U-box ubiquitin ligase TUD1 promotes brassinosteroid-induced GSK2 degradation in rice. Plant Commun. 2025, 6, 101255. [Google Scholar] [CrossRef]
- Song, X.J.; Huang, W.; Shi, M.; Zhu, M.Z.; Lin, H.X. A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat. Genet. 2007, 39, 623–630. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, W.; Liu, D.; Pan, D.; Yang, Y.; Chen, Z.; Ma, X.; Yin, W.; Niu, M.; Dong, N.; et al. Enhancing rice panicle branching and grain yield through tissue-specific brassinosteroid inhibition. Science 2024, 383, eadk8838. [Google Scholar] [CrossRef]
- Qiao, J.; Jiang, H.; Lin, Y.; Shang, L.; Wang, M.; Li, D.; Fu, X.; Geisler, M.; Qi, Y.; Gao, Z.; et al. A novel miR167a-OsARF6-OsAUX3 module regulates grain length and weight in rice. Mol. Plant 2021, 14, 1683–1698. [Google Scholar] [CrossRef]
- Qin, Q.; Li, G.; Jin, L.; Huang, Y.; Wang, Y.; Wei, C.; Xu, Z.; Yang, Z.; Wang, H.; Li, Y. Auxin response factors (ARFs) differentially regulate rice antiviral immune response against rice dwarf virus. PLoS Pathog. 2020, 16, e1009118. [Google Scholar] [CrossRef]
- Rong, C.Y.; Liu, Y.X.; Chang, Z.Y.; Liu, Z.Y.; Ding, Y.F.; Ding, C.Q. Cytokinin oxidase/dehydrogenase family genes exhibit functional divergence and overlap in rice growth and development, especially in control of tillering. J. Exp. Bot. 2022, 73, 3552–3568. [Google Scholar] [CrossRef]
- Zürcher, E.; Müller, B. Cytokinin Synthesis, Signaling, and Function—Advances and New Insights. Int. Rev. Cell Mol. Biol. 2016, 324, 1–38. [Google Scholar]
- Suzuki, Y.; Higo, K.; Hagiwara, K.; Ohtsubo, K.; Kobayashi, A.; Nozu, Y. Production and Use of Monoclonal Antibodies against Rice Embryo Lipoxygenase-3. Biosci. Biotechnol. Biochem. 1992, 56, 678–679. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, S.F.; Liu, X.; Chen, L.M.; Liu, S.J.; Zhai, H.Q.; Wan, J.M. Storability of high quality rice variety W017. J. Nanjing Agric. Univ. 2007, 30, 133–135. [Google Scholar]
- Su, S.; Tang, P.; Zuo, R.; Chen, H.; Zhao, T.; Yang, S.; Yang, J. Exogenous Jasmonic Acid Alleviates Blast Resistance Reduction Caused by LOX3 Knockout in Rice. Biomolecules 2023, 13, 1197. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Miao, Z.; Kong, D.; Zhang, A.; Wang, F.; Liu, G.; Yu, X.; Luo, L.; Liu, Y. Application of CRISPR/Cas9 Technology in Rice Germplasm Innovation and Genetic Improvement. Genes 2024, 15, 1492. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, S.; Han, B.; Li, J. The integrated genomics of crop domestication and breeding. Cell 2022, 185, 2828–2839. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.B.; Tan, Y.N.; Sun, Z.Z.; Yu, D.; Wang, X.F.; Yuan, G.L.; Yuan, D.Y.; Duan, M.J. Using CRISPR/Cas9-Mediated Targeted Mutagenesis of qSH1 Reduces the Seed Shattering in Rice. Sci. Agric. Sin. 2018, 51, 2631–2641. [Google Scholar]
- Huang, X.; Yang, S.; Gong, J.; Zhao, Q.; Feng, Q.; Zhan, Q.; Zhao, Y.; Li, W.; Cheng, B.; Xia, J.; et al. Genomic architecture of heterosis for yield traits in rice. Nature 2016, 537, 629–633. [Google Scholar] [CrossRef]
- Guo, T.; Yu, H.; Qiu, J.; Li, J.Y.; Han, B.; Lin, H.X. Advances in rice genetics and breeding by molecular design in China. Sci. Sin. Vitae 2019, 49, 1185–1212. [Google Scholar]
- Viswanath, K.K.; Varakumar, P.; Pamuru, R.R.; Basha, S.J.; Mehta, S.; Rao, A.D. Plant Lipoxygenases and Their Role in Plant Physiology. J. Plant Biol. 2020, 63, 83–95. [Google Scholar] [CrossRef]
- Wang, R.; Shen, W.B.; Jiang, L.; Liu, L.L.; Zhai, H.Q.; Wan, J.M. Prokaryotic Expression Purification and Characterization of a Novel Rice Seed Lipoxygenase Gene OsLOX1. Chin. J. Rice Sci. 2008, 22, 118–124. [Google Scholar] [CrossRef]
- Wu, Y.J.; Wu, X.S.; Shen, Z.H.; Zhang, Y.; Wu, J.D.; Lu, Y.X.; Yu, Z.L. Innovation of Rice Germplasm for Storage Tolerance and Related Technology Research. Grain Storage 2005, 34, 17–20. [Google Scholar]
- Mou, C.; Chen, Y.; Zhang, P.; Tong, Q.; Zhu, Z.; Ma, T.; Wang, P.; Fu, K.; Chen, C.; Huang, Y.; et al. Prolongation of seed viability and grain quality in rice by editing OsLOX1 using CRISPR/Cas9. Mol. Breed. 2024, 44, 72. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Wang, Y.; Wang, K.; Wu, F.; Wei, Y.; Jiang, J.; Zhu, Y.; Wang, F.; Xie, H.; Xiao, Y.; et al. OsLOX1 positively regulates seed vigor and drought tolerance in rice. Plant Mol. Biol. 2025, 115, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, H.; Zhang, L.; Shi, Y.; Song, Y.; Wang, X.; Cai, Q.; He, W.; Xie, H.; Zhang, J. The lipoxygenase OsLOX10 affects seed longevity and resistance to saline-alkaline stress during rice seedlings. Plant Mol. Biol. 2023, 111, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Marla, S.S.; Singh, V.K. LOX genes in blast fungus (Magnaporthe grisea) resistance in rice. Funct. Integr. Genom. 2012, 12, 265–275. [Google Scholar] [CrossRef]
- Liu, N.N.; Jiang, L.; Zhang, W.W.; Liu, L.L.; Zhai, H.Q.; Wan, J.M. Role of LOX3 Gene in Alleviating Adverse Effects of Drought and Pathogens in Rice. Rice Sci. 2008, 15, 276–282. [Google Scholar] [CrossRef]
- Montillet, J.L.; Chamnongpol, S.; Rustérucci, C.; Dat, J.; van de Cotte, B.; Agnel, J.P.; Battesti, C.; Inzé, D.; Van Breusegem, F.; Triantaphylidès, C. Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves. Plant Physiol. 2005, 138, 1516–1526. [Google Scholar] [CrossRef]


| Usage | Primer Name | Primer Sequence (5′-3′) | |
|---|---|---|---|
| Forward | Reverse | ||
| Vector constructs | OsLOX3-cas9 | cagGCTCCTCGACAACGTCCATG | aacCATGGACGTTGTCGAGGAGC |
| Hygromycin detection | hpt-t | GATGTTGGCGACCTCGTATTGG | CGTGCTTTCAGCTTCGATGTAGGAG |
| Cas9 detection | Cas9 | CATCCAGAAAGCCCAGGTGT | GTTCCTGGTCCACGTACATA |
| Target sequencing | OsLOX3 T0 | AGTTCTTCCCCATCCATTG | CCTTGATTCTTTCTACATAGCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, P.; Gao, J.; Jia, J.; Meng, D.; Dai, Z.; Zhong, M.; Liu, J.; Tian, X. CRISPR/Cas9 Editing of the OsLOX3 Gene Enhances Rice Grain Weight and Seed Vigor. Agronomy 2025, 15, 2112. https://doi.org/10.3390/agronomy15092112
Yu P, Gao J, Jia J, Meng D, Dai Z, Zhong M, Liu J, Tian X. CRISPR/Cas9 Editing of the OsLOX3 Gene Enhances Rice Grain Weight and Seed Vigor. Agronomy. 2025; 15(9):2112. https://doi.org/10.3390/agronomy15092112
Chicago/Turabian StyleYu, Ping, Jiadong Gao, Junting Jia, Deyao Meng, Zhangyan Dai, Mingsheng Zhong, Jun Liu, and Xiangrong Tian. 2025. "CRISPR/Cas9 Editing of the OsLOX3 Gene Enhances Rice Grain Weight and Seed Vigor" Agronomy 15, no. 9: 2112. https://doi.org/10.3390/agronomy15092112
APA StyleYu, P., Gao, J., Jia, J., Meng, D., Dai, Z., Zhong, M., Liu, J., & Tian, X. (2025). CRISPR/Cas9 Editing of the OsLOX3 Gene Enhances Rice Grain Weight and Seed Vigor. Agronomy, 15(9), 2112. https://doi.org/10.3390/agronomy15092112





