A Plant Growth-Promoting Bacterial Isolate, Bacillus velezensis 41S2, Enhances Seed Protein, Isoflavone Accumulation, and Stress Resilience in Soybean Under Salt–Alkaline Soil Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Carbon and Nitrogen Content Analysis
2.3. Determination of Protein Content
2.4. Determination of Isoflavone Content
2.5. Quantitative Analysis of Hydrogen Peroxide (H2O2)
2.6. RNA Extraction, Library Construction, and Transcriptome Sequencing
2.7. RNA Data Processing
2.8. Statistical Analysis
3. Results
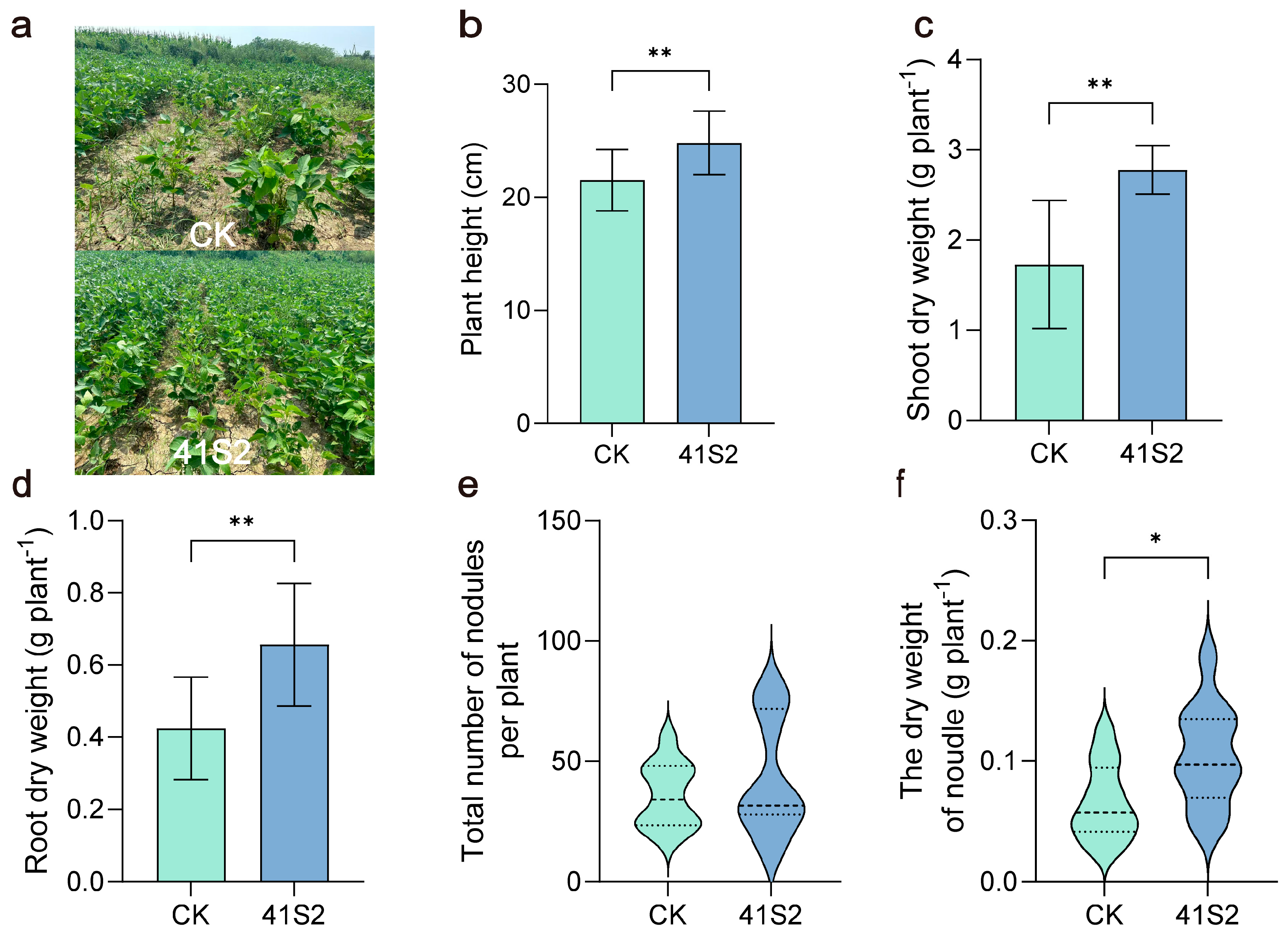
3.1. Effects of B. velezensis 41S2 on Soybean Growth Performance Under Salt–Alkaline Soil
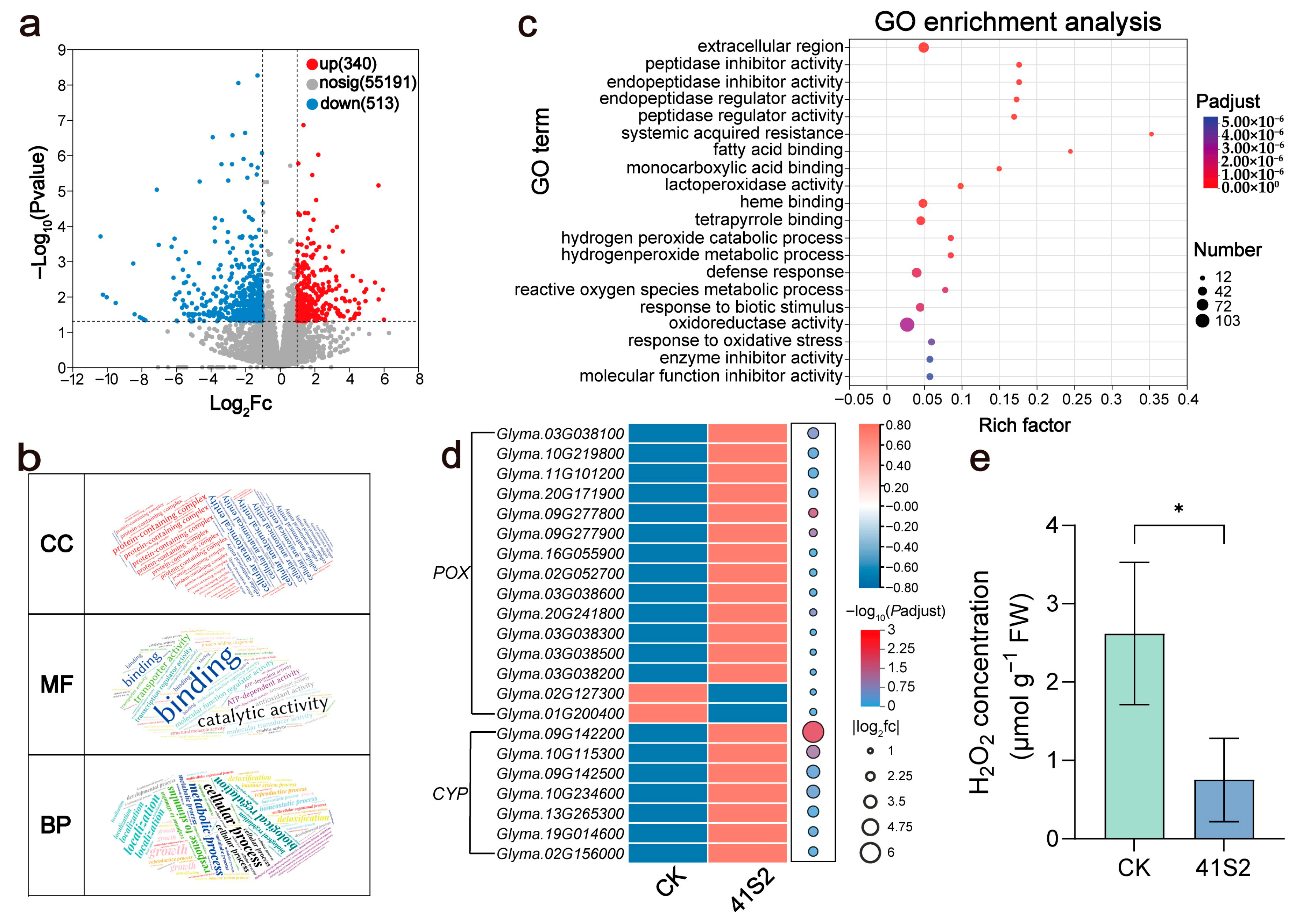
3.2. B. velezensis 41S2 Maintains ROS Homeostasis Induced by Salt–Alkaline Soil
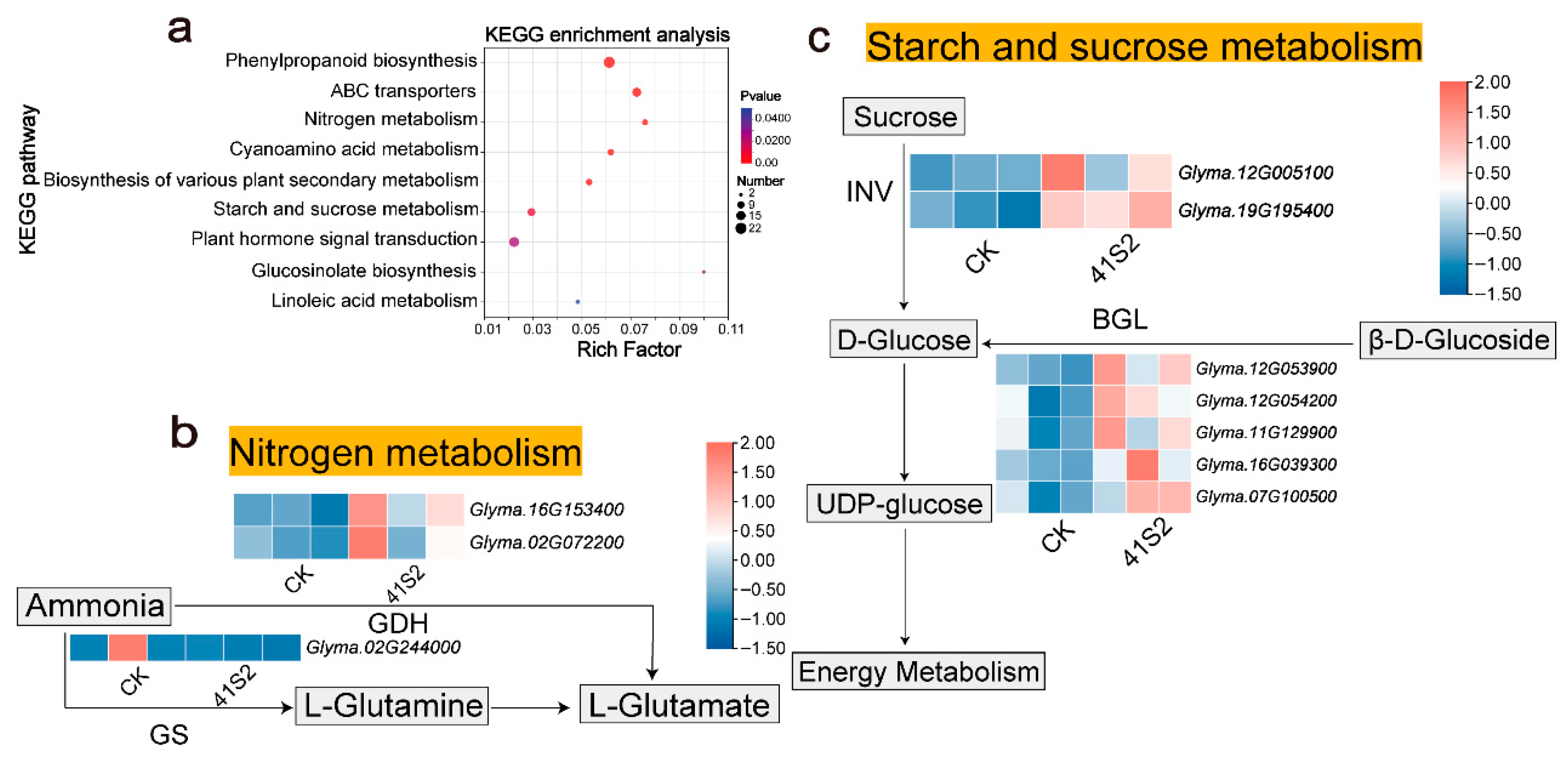
3.3. B. velezensis 41S2 Enhances Plant N and C Metabolism Under Salt–Alkaline Soil
3.4. B. velezensis 41S2 Enhances Soybean Seed Yield Under Salt–Alkaline Soil
3.5. B. velezensis 41S2 Improves Soybean Seed Quality Under Salt–Alkaline Soil
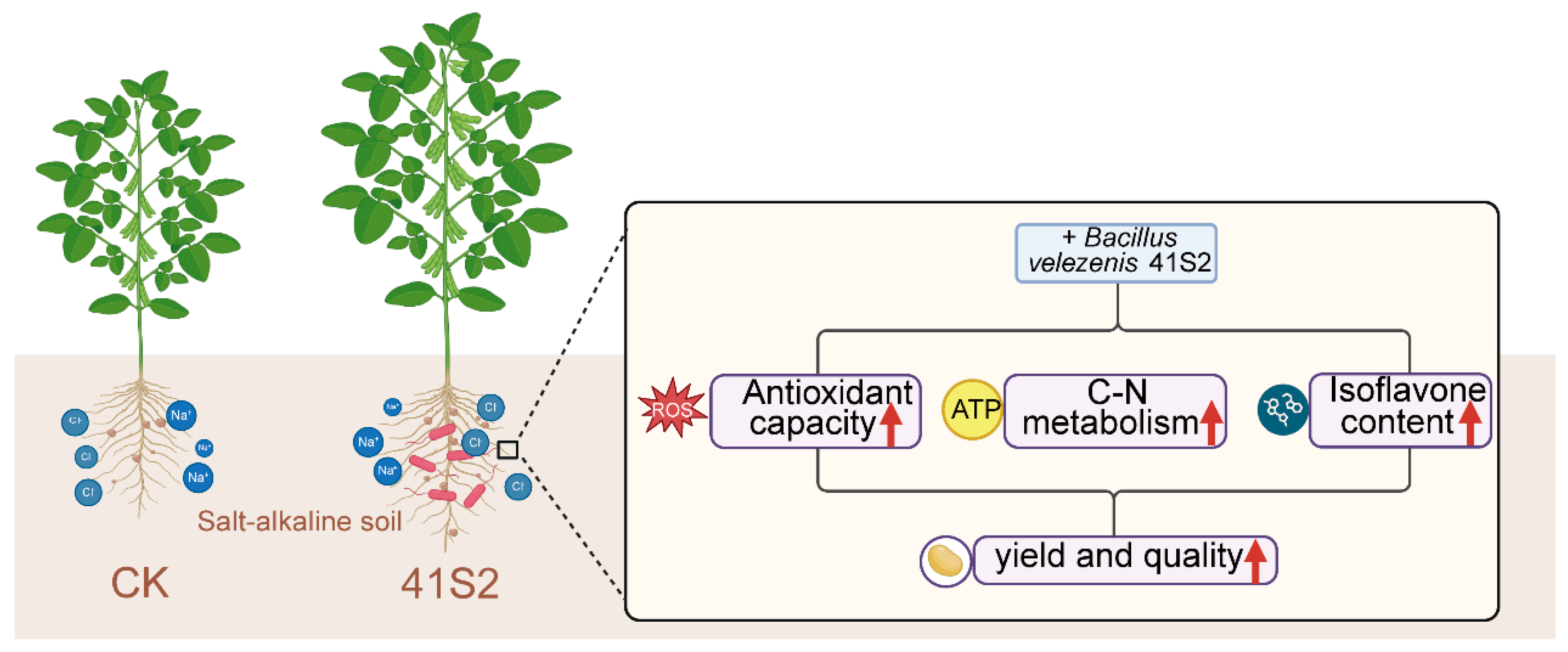
4. Discussion
4.1. B. velezensis 41S2 Enhances the Antioxidant Capacity of Soybean Under Salt–Alkaline Soil
4.2. Regulation of C and N Metabolic Homeostasis by B. velezensis 41S2 Under Salt–Alkaline Soil
4.3. B. velezensis 41S2 Enhances Soybean Yield and Quality Under Salt–Alkaline Soil
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- FAO. Global Status of Salt-Affected Soils; FAO: Rome, Italy, 2024; ISBN 978-92-5-139307-9. [Google Scholar]
- Mehrabi, S.S.; Sabokdast, M.; Bihamta, M.R.; Dedičová, B. The Coupling Effects of PGPR Inoculation and Foliar Spraying of Strigolactone in Mitigating the Negative Effect of Salt Stress in Wheat Plants: Insights from Phytochemical, Growth, and Yield Attributes. Agriculture 2024, 14, 732. [Google Scholar] [CrossRef]
- Xiao, F.; Zhou, H. Plant Salt Response: Perception, Signaling, and Tolerance. Front. Plant Sci. 2023, 13, 1053699. [Google Scholar] [CrossRef]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Hou, X.; Liang, X. Response Mechanisms of Plants Under Saline-Alkali Stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef]
- Fu, H.; Yang, Y. How Plants Tolerate Salt Stress. Curr. Issues Mol. Biol. 2023, 45, 5914–5934. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity Induced Physiological and Biochemical Changes in Plants: An Omic Approach towards Salt Stress Tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic Adjustment and Energy Limitations to Plant Growth in Saline Soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, H.; Yang, Y.; Feng, X.; Chen, X.; Xiao, F.; Lin, H.; Guo, Y. Insights into Plant Salt Stress Signaling and Tolerance. J. Genet. Genom. 2024, 51, 16–34. [Google Scholar] [CrossRef]
- Yang, S.; Xu, Y.; Tang, Z.; Jin, S.; Yang, S. The Impact of Alkaline Stress on Plant Growth and Its Alkaline Resistance Mechanisms. Int. J. Mol. Sci. 2024, 25, 13719. [Google Scholar] [CrossRef]
- Ali, A.; Petrov, V.; Yun, D.-J.; Gechev, T. Revisiting Plant Salt Tolerance: Novel Components of the SOS Pathway. Trends Plant Sci. 2023, 28, 1060–1069. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Sui, N. Sensitivity and Responses of Chloroplasts to Salt Stress in Plants. Front. Plant Sci. 2024, 15, 1374086. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Goh, H.; Azodi, C.B.; Krishnamoorthi, S.; Liu, M.-J.; Urano, D. Evolutionarily Conserved Hierarchical Gene Regulatory Networks for Plant Salt Stress Response. Nat. Plants 2021, 7, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of Plant Responses and Adaptation to Soil Salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Mangu, V.R.; Bedre, R.; Sanchez, L.; Pilcher, W.; Zandkarimi, H.; Baisakh, N. Salt Adaptation Mechanisms of Halophytes: Improvement of Salt Tolerance in Crop Plants. In Elucidation of Abiotic Stress Signaling in Plants: Functional Genomics Perspectives; Pandey, G.K., Ed.; Springer: New York, NY, USA, 2015; Volume 2, pp. 243–279. ISBN 978-1-4939-2540-7. [Google Scholar]
- Kotula, L.; Caparros, P.G.; Zörb, C.; Colmer, T.D.; Flowers, T.J. Improving Crop Salt Tolerance Using Transgenic Approaches: An Update and Physiological Analysis. Plant Cell Environ. 2020, 43, 2932–2956. [Google Scholar] [CrossRef]
- Qin, H.; Li, Y.; Huang, R. Advances and Challenges in the Breeding of Salt-Tolerant Rice. Int. J. Mol. Sci. 2020, 21, 8385. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-Tolerant Plant Growth Promoting Rhizobacteria for Enhancing Crop Productivity of Saline Soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, G.; Potestio, S.; Visioli, G. The Contribution of PGPR in Salt Stress Tolerance in Crops: Unravelling the Molecular Mechanisms of Cross-Talk between Plant and Bacteria. Plants 2023, 12, 2197. [Google Scholar] [CrossRef]
- Gao, Y.; Zou, H.; Wang, B.; Yuan, F. Progress and Applications of Plant Growth-Promoting Bacteria in Salt Tolerance of Crops. Int. J. Mol. Sci. 2022, 23, 7036. [Google Scholar] [CrossRef]
- Ha-Tran, D.M.; Nguyen, T.T.M.; Hung, S.-H.; Huang, E.; Huang, C.-C. Roles of Plant Growth-Promoting Rhizobacteria (PGPR) in Stimulating Salinity Stress Defense in Plants: A Review. Int. J. Mol. Sci. 2021, 22, 3154. [Google Scholar] [CrossRef]
- Chinachanta, K.; Shutsrirung, A.; Santasup, C.; Pathom-Aree, W.; Luu, D.T.; Herrmann, L.; Lesueur, D.; Prom-u-thai, C. Rhizoactinobacteria Enhance Growth and Antioxidant Activity in Thai Jasmine Rice (Oryza sativa) KDML105 Seedlings under Salt Stress. Plants 2023, 12, 3441. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, S.; Mukherjee, A.; Rastogi, R.P.; Verma, J.P. Salt-Tolerant Plant Growth-Promoting Bacillus pumilus Strain JPVS11 to Enhance Plant Growth Attributes of Rice and Improve Soil Health under Salinity Stress. Microbiol. Res. 2021, 242, 126616. [Google Scholar] [CrossRef]
- Chebotar, V.K.; Zaplatkin, A.N.; Chizhevskaya, E.P.; Gancheva, M.S.; Voshol, G.P.; Malfanova, N.V.; Baganova, M.E.; Khomyakov, Y.V.; Pishchik, V.N. Phytohormone Production by the Endophyte Bacillus safensis TS3 Increases Plant Yield and Alleviates Salt Stress. Plants 2023, 13, 75. [Google Scholar] [CrossRef]
- Cheng, Y.; Cheng, X.; Wei, K.; Wang, Y. Comparative Transcriptome Analysis of Salt-Tolerant and -Sensitive Soybean Cultivars under Salt Stress. Int. J. Mol. Sci. 2024, 25, 9818. [Google Scholar] [CrossRef]
- Lu, P.; Dai, S.-Y.; Yong, L.-T.; Zhou, B.-H.; Wang, N.; Dong, Y.-Y.; Liu, W.-C.; Wang, F.-W.; Yang, H.-Y.; Li, X.-W. A Soybean Sucrose Non-Fermenting Protein Kinase 1 Gene, GmSNF1, Positively Regulates Plant Response to Salt and Salt–Alkali Stress in Transgenic Plants. Int. J. Mol. Sci. 2023, 24, 12482. [Google Scholar] [CrossRef]
- Kim, I.-S. Current Perspectives on the Beneficial Effects of Soybean Isoflavones and Their Metabolites on Plants. Food Sci. Biotechnol. 2022, 31, 515–526. [Google Scholar] [CrossRef]
- Soliman, M.H.; Abdulmajeed, A.M.; Alhaithloul, H.; Alharbi, B.M.; El-Esawi, M.A.; Hasanuzzaman, M.; Elkelish, A. Saponin Biopriming Positively Stimulates Antioxidants Defense, Osmolytes Metabolism and Ionic Status to Confer Salt Stress Tolerance in Soybean. Acta Physiol. Plant. 2020, 42, 114. [Google Scholar] [CrossRef]
- Nagrale, D.T.; Chaurasia, A.; Kumar, S.; Gawande, S.P.; Hiremani, N.S.; Shankar, R.; Gokte-Narkhedkar, N.; Renu; Prasad, Y.G. PGPR: The Treasure of Multifarious Beneficial Microorganisms for Nutrient Mobilization, Pest Biocontrol and Plant Growth Promotion in Field Crops. World J. Microbiol. Biotechnol. 2023, 39, 100. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, D.; Li, Z.; Yang, Y.; Peng, Z.; Meng, C.; Ma, S.; Xu, K.; Li, Y.; Ding, G.; et al. Kaempferol Drives Genotype-Specific Microbiota Bacillaceae to Enhance Nitrogen Acquisition in Rapeseed. J. Adv. Res. 2025, in press. [CrossRef]
- Sharma, A.; Dev, K.; Sourirajan, A.; Choudhary, M. Isolation and Characterization of Salt-Tolerant Bacteria with Plant Growth-Promoting Activities from Saline Agricultural Fields of Haryana, India. J. Genet. Eng. Biotechnol. 2021, 19, 99. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Zhao, D.; Li, Z.; Sui, X.; Zhang, H.; Liu, J.; Li, Y.; Zhang, C.-S.; Zheng, Y. Ensifer Sp. GMS14 Enhances Soybean Salt Tolerance for Potential Application in Saline Soil Reclamation. J. Environ. Manag. 2024, 349, 119488. [Google Scholar] [CrossRef]
- Taliercio, E.; Gillenwater, J.; Woodruff, L.; Fallen, B. Glycine Soja, PI424025, Is a Valuable Genetic Resource to Improve Soybean Seed-Protein Content and Composition. PLoS ONE 2024, 19, e0310544. [Google Scholar] [CrossRef]
- Jin, W.; Zhou, H.; Zhao, H.; Pei, Y.; Su, F.; Li, Y.; Luo, T. Isolation, In Vitro Antioxidant Capacity, Hypoglycemic Activity and Immunoactivity Evaluation of Polysaccharides from Coriandrum sativum L. Antioxidants 2025, 14, 149. [Google Scholar] [CrossRef]
- Hsia, S.-Y.; Hsiao, Y.-H.; Li, W.-T.; Hsieh, J.-F. Aggregation of Soy Protein-Isoflavone Complexes and Gel Formation Induced by Glucono-δ-Lactone in Soymilk. Sci. Rep. 2016, 6, 35718. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-Based Genome Alignment and Genotyping with HISAT2 and HISAT-Genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie Enables Improved Reconstruction of a Transcriptome from RNA-Seq Reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate Transcript Quantification from RNA-Seq Data with or without a Reference Genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Klopfenstein, D.V.; Zhang, L.; Pedersen, B.S.; Ramírez, F.; Warwick Vesztrocy, A.; Naldi, A.; Mungall, C.J.; Yunes, J.M.; Botvinnik, O.; Weigel, M.; et al. GOATOOLS: A Python Library for Gene Ontology Analyses. Sci. Rep. 2018, 8, 10872. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive Oxygen Species Signalling in Plant Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Feng, L.; Li, Q.; Zhou, D.; Jia, M.; Liu, Z.; Hou, Z.; Ren, Q.; Ji, S.; Sang, S.; Lu, S.; et al. B. subtilis CNBG-PGPR-1 Induces Methionine to Regulate Ethylene Pathway and ROS Scavenging for Improving Salt Tolerance of Tomato. Plant J. 2023, 117, 193–211. [Google Scholar] [CrossRef]
- Singh, A.; Panwar, R.; Mittal, P.; Hassan, M.I.; Singh, I.K. Plant Cytochrome P450s: Role in Stress Tolerance and Potential Applications for Human Welfare. Int. J. Biol. Macromol. 2021, 184, 874–886. [Google Scholar] [CrossRef]
- Kidwai, M.; Ahmad, I.Z.; Chakrabarty, D. Class III Peroxidase: An Indispensable Enzyme for Biotic/Abiotic Stress Tolerance and a Potent Candidate for Crop Improvement. Plant Cell Rep. 2020, 39, 1381–1393. [Google Scholar] [CrossRef]
- Wang, P.; Liu, W.-C.; Han, C.; Wang, S.; Bai, M.-Y.; Song, C.-P. Reactive Oxygen Species: Multidimensional Regulators of Plant Adaptation to Abiotic Stress and Development. J. Integr. Plant Biol. 2024, 66, 330–367. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Yan, J.; Li, W.; Wang, L.; Zhao, J.; Ma, X.; Li, A.; Wang, H.; Kong, L. A Member of Wheat Class III Peroxidase Gene Family, TaPRX-2A, Enhanced the Tolerance of Salt Stress. BMC Plant Biol. 2020, 20, 392. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Fujita, M. Plant Responses and Tolerance to Salt Stress: Physiological and Molecular Interventions. Int. J. Mol. Sci. 2022, 23, 4810. [Google Scholar] [CrossRef]
- Liang, M.; Hu, F.; Xie, D.; Chen, Z.; Zheng, Q.; Xie, Q.; Zheng, F.; Liu, D.; Jian, S.; Chen, H.; et al. Physiological Measurements and Transcriptome Survey Reveal How Semi-Mangrove Clerodendrum Inerme Tolerates Saline Adversity. Front Plant Sci. 2022, 13, 882884. [Google Scholar] [CrossRef]
- Patel, M.K.; Kumar, M.; Li, W.; Luo, Y.; Burritt, D.J.; Alkan, N.; Tran, L.-S.P. Enhancing Salt Tolerance of Plants: From Metabolic Reprogramming to Exogenous Chemical Treatments and Molecular Approaches. Cells 2020, 9, 2492. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, E.; Zhang, X.; Wang, Q. Silicon Alleviates Salinity Stress in Licorice (Glycyrrhiza uralensis) by Regulating Carbon and Nitrogen Metabolism. Sci. Rep. 2021, 11, 1115. [Google Scholar] [CrossRef]
- Zhang, X.; He, P.; Guo, R.; Huang, K.; Huang, X. Effects of Salt Stress on Root Morphology, Carbon and Nitrogen Metabolism, and Yield of Tartary Buckwheat. Sci. Rep. 2023, 13, 12483. [Google Scholar] [CrossRef]
- AbuQamar, S.F.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.-S.M.; Elrys, A.S.; El-Mageed, T.A.A.; Semida, W.M.; Abdelkhalik, A.; Mosa, W.F.A.; Al Kafaas, S.S.; et al. Halotolerant Plant Growth-Promoting Rhizobacteria Improve Soil Fertility and Plant Salinity Tolerance for Sustainable Agriculture—A Review. Plant Stress 2024, 12, 100482. [Google Scholar] [CrossRef]
- Alishahi, F.; Alikhani, H.A.; Khoshkholgh-Sima, N.A.; Etesami, H. Mining the Roots of Various Species of the Halophyte Suaeda for Halotolerant Nitrogen-Fixing Endophytic Bacteria with the Potential for Promoting Plant Growth. Int. Microbiol. 2020, 23, 415–427. [Google Scholar] [CrossRef] [PubMed]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How Does Proline Treatment Promote Salt Stress Tolerance During Crop Plant Development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Lu, M.; Riaz, M.; Tong, K.; Yu, H.; Gao, G.; Niu, Y. Exogenous Proline Enhances Salt Acclimation in Soybean Seedlings: Modifying Physicochemical Properties and Controlling Proline Metabolism through the Ornithine-Glutamate Dual Pathway. Ecotoxicol. Environ. Saf. 2025, 294, 118012. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Li, L.; Liu, X.; Chen, M.; Wan, S.; Li, G. Salt Stress Inhibits Photosynthesis and Destroys Chloroplast Structure by Downregulating Chloroplast Development–Related Genes in Robinia Pseudoacacia Seedlings. Plants 2023, 12, 1283. [Google Scholar] [CrossRef]
- Zahra, N.; Al Hinai, M.S.; Hafeez, M.B.; Rehman, A.; Wahid, A.; Siddique, K.H.M.; Farooq, M. Regulation of Photosynthesis under Salt Stress and Associated Tolerance Mechanisms. Plant Physiol. Biochem. 2022, 178, 55–69. [Google Scholar] [CrossRef]
- Shahid, M.; Al-Khattaf, F.S.; Danish, M.; Zeyad, M.T.; Atef Hatamleh, A.; Mohamed, A.; Ali, S. PGPR Kosakonia Radicincitans KR-17 Increases the Salt Tolerance of Radish by Regulating Ion-Homeostasis, Photosynthetic Molecules, Redox Potential, and Stressor Metabolites. Front Plant Sci. 2022, 13, 919696. [Google Scholar] [CrossRef]
- Sevgi, B.; Leblebici, S. Exogenous Sucrose Alleviates Salt Stress in Sunflower (Helianthus annuus L.) and Canola (Brassica napus L.) by Modulating Osmotic Adjustment and Antioxidant Defense System. Physiol. Mol. Biol. Plants 2025, 31, 405–418. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Mohammed, D.M.; Fahmy, M.A.; Elesawi, I.E.; Ahmed, A.E.; Algopishi, U.B.; Elrys, A.S.; Desoky, E.-S.M.; Mosa, W.F.A.; et al. Drought-Tolerant Plant Growth-Promoting Rhizobacteria Alleviate Drought Stress and Enhance Soil Health for Sustainable Agriculture: A Comprehensive Review. Plant Stress 2024, 14, 100632. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Singh, K.; Chandra, R. Recent Advances of Plant Growth Promoting Rhizobacteria (PGPR) for Eco-Restoration of Polluted Soil. Clean. Eng. Technol. 2024, 23, 100845. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, X. Symbiotic Nitrogen Fixation: The Role of Rhizobia in Enhancing Legume Growth and Soil Fertility. Mol. Microbiol. Res. 2024, 14, 109–118. [Google Scholar] [CrossRef]
- Ni, H.; Hou, X.; Tian, S.; Liu, C.; Zhang, G.; Peng, Y.; Chen, L.; Wang, J.; Chen, Q.; Xin, D. Insights into the Early Steps of the Symbiotic Interaction between Soybean (Glycine max) and Sinorhizobium fredii Symbiosis Using Transcriptome, Small RNA, and Degradome Sequencing. J. Agric. Food Chem. 2024, 72, 17084–17098. [Google Scholar] [CrossRef]
- Han, Q.; Zhu, G.; Qiu, H.; Li, M.; Zhang, J.; Wu, X.; Xiao, R.; Zhang, Y.; Yang, W.; Tian, B.; et al. Quality Traits Drive the Enrichment of Massilia in the Rhizosphere to Improve Soybean Oil Content. Microbiome 2024, 12, 224. [Google Scholar] [CrossRef]
- Akhtar, K.; ul Ain, N.; Prasad, P.V.V.; Naz, M.; Aslam, M.M.; Djalovic, I.; Riaz, M.; Ahmad, S.; Varshney, R.K.; He, B.; et al. Physiological, Molecular, and Environmental Insights into Plant Nitrogen Uptake, and Metabolism under Abiotic Stresses. Plant Genome 2024, 17, e20461. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Feng, N.; Zheng, D.; Liu, M.; Zhang, R.; Huang, X.; Huang, A.; Chen, Z. Exogenous Hemin Alleviates NaCl Stress by Promoting Photosynthesis and Carbon Metabolism in Rice Seedlings. Sci. Rep. 2023, 13, 3497. [Google Scholar] [CrossRef]
- Trush, K.; Pal’ove-Balang, P. Biosynthesis and Role of Isoflavonoids in Legumes under Different Environmental Conditions. Plant Stress 2023, 8, 100153. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; Li, W.; Wang, C.; Dai, H.; Xu, R.; Zhang, Y.; Zhang, L. Integrative Analysis of Metabolome and Transcriptome Reveals Regulatory Mechanisms of Flavonoid Biosynthesis in Soybean under Salt Stress. Front. Plant Sci. 2024, 15, 1415867. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Hua, S.; Li, Z.; Wang, Z.; Zhao, D.; Jing, C.; Li, Y.; Zhang, C.; Zheng, Y.; Wang, Y.; et al. A Plant Growth-Promoting Bacterial Isolate, Bacillus velezensis 41S2, Enhances Seed Protein, Isoflavone Accumulation, and Stress Resilience in Soybean Under Salt–Alkaline Soil Conditions. Agronomy 2025, 15, 2103. https://doi.org/10.3390/agronomy15092103
Zheng H, Hua S, Li Z, Wang Z, Zhao D, Jing C, Li Y, Zhang C, Zheng Y, Wang Y, et al. A Plant Growth-Promoting Bacterial Isolate, Bacillus velezensis 41S2, Enhances Seed Protein, Isoflavone Accumulation, and Stress Resilience in Soybean Under Salt–Alkaline Soil Conditions. Agronomy. 2025; 15(9):2103. https://doi.org/10.3390/agronomy15092103
Chicago/Turabian StyleZheng, Han, Shutian Hua, Zhe Li, Ziyan Wang, Donglin Zhao, Changliang Jing, Yiqiang Li, Chengsheng Zhang, Yanfen Zheng, Youqiang Wang, and et al. 2025. "A Plant Growth-Promoting Bacterial Isolate, Bacillus velezensis 41S2, Enhances Seed Protein, Isoflavone Accumulation, and Stress Resilience in Soybean Under Salt–Alkaline Soil Conditions" Agronomy 15, no. 9: 2103. https://doi.org/10.3390/agronomy15092103
APA StyleZheng, H., Hua, S., Li, Z., Wang, Z., Zhao, D., Jing, C., Li, Y., Zhang, C., Zheng, Y., Wang, Y., & Jiang, M. (2025). A Plant Growth-Promoting Bacterial Isolate, Bacillus velezensis 41S2, Enhances Seed Protein, Isoflavone Accumulation, and Stress Resilience in Soybean Under Salt–Alkaline Soil Conditions. Agronomy, 15(9), 2103. https://doi.org/10.3390/agronomy15092103







