Early Detection of Wheat Fusarium Head Blight During the Incubation Period Using FTIR-PAS
Abstract
1. Introduction
2. Materials and Methods
2.1. FHB Pathogen Culture and Wheat Infection
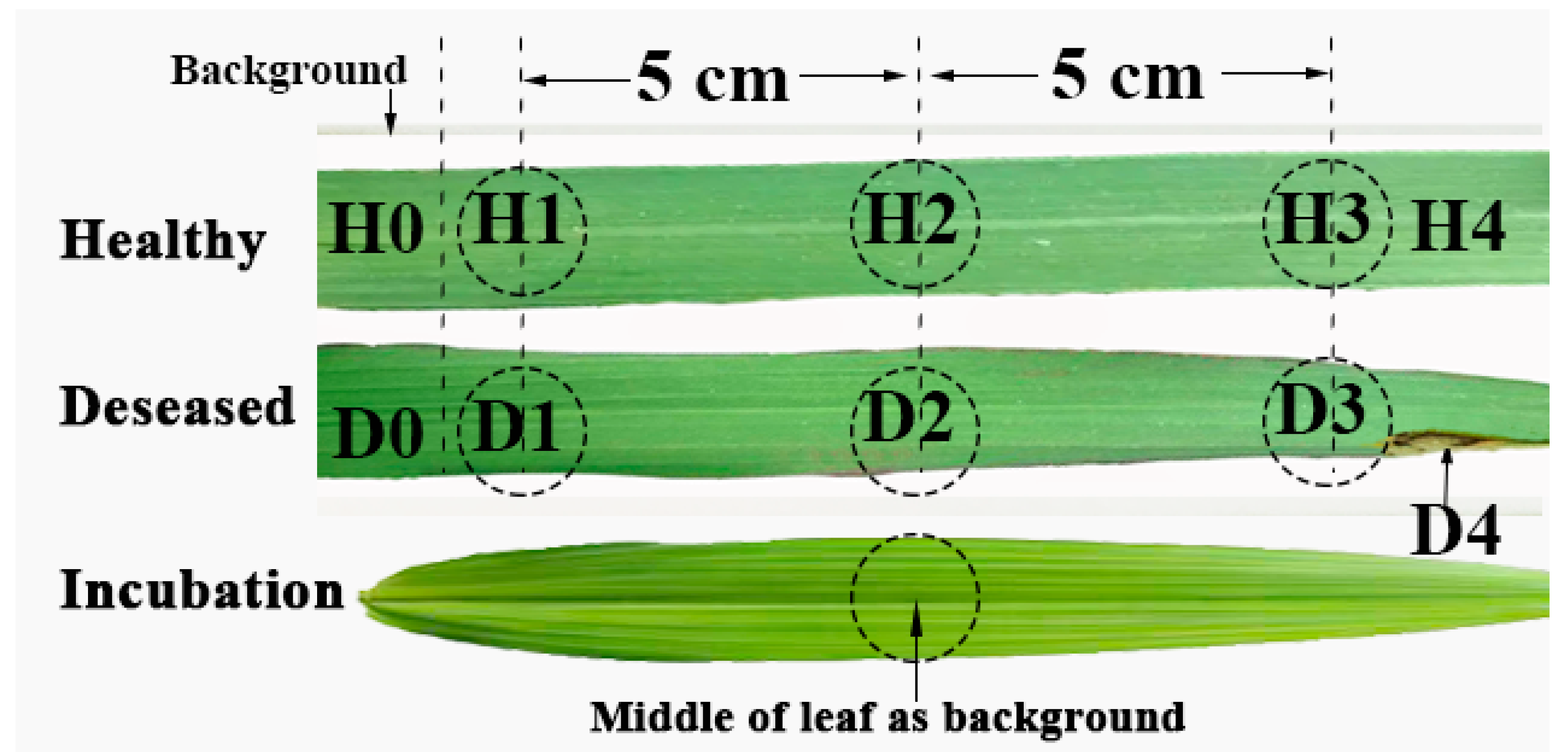
2.2. Leaf Sampling Point Division
2.3. Spectral Acquisition Parameters and Procedure
2.4. Spectral Data Preprocessing
2.5. Background Spectrum Removal
2.6. Data Similarity and Distance Analysis
2.7. Diagnostic Performance Evaluation Metrics
3. Results and Discussion
3.1. Analysis of Leaf Spectral Features and Functional Group Assignments at Different Scanning Depths
3.2. Spectral Feature Changes at Different Sampling Positions
3.3. Analysis of Leaf Spectral Features After Background Spectrum Subtraction
3.4. Synergistic Diagnosis of FHB Symptomatic Period Using Dual Bands at 1650/1050 cm−1
3.5. Synergistic Diagnosis of FHB Asymptomatic Period Using Dual Bands at 1650/1050 cm−1
3.6. Progressiveness Analysis of the Diagnostic Method
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NIRS | Near-Infrared Spectroscopy |
| MIRS | Mid-Infrared Spectroscopy |
| FTIR-PAS | Fourier Transform Infrared Photoacoustic Spectroscopy |
| FHB | Fusarium Head Blight |
| PLS | Partial Least Squares |
| PCA | Principal Component Analysis |
| PNN | Probabilistic Neural Network |
References
- Peng, Y.; Zhao, S.; Liu, J. Fused-deep-features based grape leaf disease diagnosis. Agronomy 2021, 11, 2234. [Google Scholar] [CrossRef]
- Zhao, S.; Peng, Y.; Liu, J.; Wu, S. Tomato leaf disease diagnosis based on improved convolution neural network by attention module. Agriculture 2021, 11, 651. [Google Scholar] [CrossRef]
- Wang, S.; Li, T.; Wang, Y.; Chen, L.; Jiang, F.; Zhang, X.; Wei, M.; Chen, S.; Xu, L.; Yang, N. MOS sensor array based on multi-modal data weighted composite membership optimization for rice blast detection in symptomless stage. Comput. Electron. Agric. 2025, 232, 110153. [Google Scholar] [CrossRef]
- Ba, W.; Jin, X.; Lu, J.; Rao, Y.; Zhang, T.; Zhang, X.; Zhou, J.; Li, S. Research on predicting early Fusarium head blight with asymptomatic wheat grains by micro-near infrared spectrometer. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 287, 122047. [Google Scholar] [CrossRef]
- Xiong, J.; Gu, S.; Rao, Y.; Zhang, X.; Wu, Y.; Lu, J.; Jin, X. An innovative fusion method with micro-vision and spectrum of wheat for detecting asymptomatic Fusarium head blight. J. Food Compos. Anal. 2025, 140, 107258. [Google Scholar] [CrossRef]
- Lv, G.; Du, C.; Ma, F.; Shen, Y.; Zhou, J. Responses of leaf cuticles to rice blast: Detection and identification using depth-profiling fourier transform mid-infrared photoacoustic Spectroscopy. Plant Dis. 2020, 104, 847–852. [Google Scholar]
- Gao, C.; Ji, X.; He, Q.; Gong, Z.; Sun, H.; Wen, T.; Guo, W. Monitoring of wheat fusarium head blight on spectral and textural analysis of UAV multispectral imagery. Agriculture 2023, 13, 293. [Google Scholar] [CrossRef]
- Liu, L.; Dong, Y.; Huang, W.; Du, X.; Ma, H. Monitoring wheat fusarium head blight using unmanned aerial vehicle hyperspectral imagery. Remote Sens. 2020, 12, 3811. [Google Scholar] [CrossRef]
- Bao, W.; Huang, C.; Hu, G.; Su, B.; Yang, X. Detection of Fusarium head blight in wheat using UAV remote sensing based on parallel channel space attention. Comput. Electron. Agric. 2024, 217, 108630. [Google Scholar] [CrossRef]
- Sheng, Q.; Ma, H.; Zhang, J.; Gui, Z.; Huang, W.; Chen, D.; Wang, B. Coupling Multi-Source Satellite Remote Sensing and Meteorological Data to Discriminate Yellow Rust and Fusarium Head Blight in Winter Wheat. Phyton 2025, 94, 421. [Google Scholar] [CrossRef]
- Peiris, K.; Pumphrey, M.; Dong, Y.; Maghirang, E.; Berzonsky, W.; Dowell, F. Near-infrared spectroscopic method for identification of fusarium head blight damage and prediction of deoxynivalenol in single wheat kernels. Cereal Chem. 2010, 87, 511–517. [Google Scholar] [CrossRef]
- Matengu, T.T.; Bullock, P.R.; Mkhabela, M.S.; Zvomuya, F.; Henriquez, M.A.; Ojo, E.R.; Fernando, W.D. Weather-based models for forecasting Fusarium head blight risks in wheat and barley: A review. Plant Pathol. 2024, 73, 492–505. [Google Scholar] [CrossRef]
- Terentev, A.; Dolzhenko, V.; Fedotov, A.; Eremenko, D. Current state of hyperspectral remote sensing for early plant disease detection: A review. Sensors 2022, 22, 757. [Google Scholar] [CrossRef]
- Khaled, A.Y.; Abd Aziz, S.; Bejo, S.K.; Nawi, N.M.; Seman, I.A.; Onwude, D.I. Early detection of diseases in plant tissue using spectroscopy–applications and limitations. Appl. Spectrosc. Rev. 2018, 53, 36–64. [Google Scholar] [CrossRef]
- Lv, G.; Du, C.; Ma, F.; Shen, Y.; Zhou, J. Rapid and nondestructive detection of pesticide residues by depth-profiling Fourier transform infrared photoacoustic spectroscopy. ACS Omega 2018, 3, 3548–3553. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Du, C.; Ma, F.; Shen, Y.; Zhou, J. In situ detection of rice leaf cuticle responses to nitrogen supplies by depth-profiling Fourier transform photoacoustic spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117759. [Google Scholar] [CrossRef]
- Huang, J.; Glæsner, N.; Triolo, J.M.; Bekiaris, G.; Bruun, S.; Liu, F. Application of Fourier transform mid-infrared photoacoustic spectroscopy for rapid assessment of phosphorus availability in digestates and digestate-amended soils. Sci. Total Environ. 2022, 832, 155040. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Ma, F.; Du, C. Application of FTIR-PAS in rapid assessment of rice quality under climate change conditions. Foods 2021, 10, 159. [Google Scholar] [CrossRef]
- Suresh, R.; Álvarez, Á.; Sandoval, C.; Ramírez, E.; Santander, P.; Mangalaraja, R.; Yáñez, J. Fe2O3/NiO nanocomposites: Synthesis, characterization and roxarsone sensing by Fourier transform infrared photoacoustic spectroscopy. New J. Chem. 2023, 47, 12806–12815. [Google Scholar] [CrossRef]
- Li, C.; Du, C.; Zeng, Y.; Ma, F.; Shen, Y.; Xing, Z.; Zhou, J. Two-dimensional visualization of nitrogen distribution in leaves of Chinese cabbage (Brassica rapa subsp. chinensis) by the Fourier transform infrared photoacoustic spectroscopy technique. J. Agric. Food Chem. 2016, 64, 7696–7701. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Du, C.; Ma, F.; Shen, Y.; Liang, D.; Zhou, J. Rapid diagnosis of nitrogen status in rice based on Fourier transform infrared photoacoustic spectroscopy (FTIR-PAS). Plant Methods 2019, 15, 94. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liu, R.; Wang, S.; Yang, J.; Tao, N.; Wang, X.; Zhou, Q.; Xu, C. Direct identification and quantitation of protein peptide powders based on multi-molecular infrared spectroscopy and multivariate data fusion. J. Agric. Food Chem. 2023, 71, 10819–10829. [Google Scholar] [CrossRef]
- Cutshaw, G.; Uthaman, S.; Hassan, N.; Kothadiya, S.; Wen, X.; Bardhan, R. The emerging role of Raman spectroscopy as an omics approach for metabolic profiling and biomarker detection toward precision medicine. Chem. Rev. 2023, 123, 8297–8346. [Google Scholar] [CrossRef]
- Kheiri, A.; Moosawi Jorf, S.A.; Malihipour, A. Infection process and wheat response to Fusarium head blight caused by Fusarium graminearum. Eur. J. Plant Pathol. 2019, 153, 489–502. [Google Scholar] [CrossRef]
- Du, C.; Zhou, J.; Liu, J. Identification of Chinese medicinal fungus Cordyceps sinensis by depth-profiling mid-infrared photoacoustic spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Sun, J.; Tian, Y.; Wu, X.; Dai, C.; Li, B. Spectral classification of lettuce cadmium stress based on information fusion and VISSA-GOA-SVM algorithm. J. Food Process Eng. 2019, 42, e13085. [Google Scholar] [CrossRef]
- Li, Y.; Pan, T.; Li, H.; Chen, S. Non-invasive quality analysis of thawed tuna using near infrared spectroscopy with baseline correction. J. Food Process Eng. 2020, 43, e13445. [Google Scholar] [CrossRef]
- Liu, J.; Abbas, I.; Noor, R.S. Development of deep learning-based variable rate agrochemical spraying system for targeted weeds control in strawberry crop. Agronomy 2021, 11, 1480. [Google Scholar] [CrossRef]
- Tao, T.; Wei, X. STBNA-YOLOv5: An Improved YOLOv5 Network for Weed Detection in Rapeseed Field. Agriculture 2024, 15, 22. [Google Scholar] [CrossRef]
- Chang, Z.; Zhao, M.; Qin, B.; Hong, L. Polar-localized OsLTPG22 regulates rice leaf cuticle deposition and drought response. Plant Stress 2024, 14, 100586. [Google Scholar] [CrossRef]
- Farber, C.; Li, J.; Hager, E.; Chemelewski, R.; Mullet, J.; Rogachev, A.Y.; Kurouski, D. Complementarity of raman and infrared spectroscopy for structural characterization of plant epicuticular waxes. Acs Omega 2019, 4, 3700–3707. [Google Scholar] [CrossRef]
- Farber, C.; Wang, R.; Chemelewski, R.; Mullet, J.; Kurouski, D. Nanoscale structural organization of plant epicuticular wax probed by atomic force microscope infrared spectroscopy. Anal. Chem. 2019, 91, 2472–2479. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Shi, J.; Zhang, F.; Zou, X.; Holmes, M.; Zhang, W.; Huang, X.; Cui, X.; Xue, J. Determination of retrogradation degree in starch by mid-infrared and Raman spectroscopy during storage. Food Anal. Methods 2017, 10, 3694–3705. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, J.; Wu, B.; Sun, J.; Dai, C. Identification of tea varieties by mid-infrared diffuse reflectance spectroscopy coupled with a possibilistic fuzzy c-means clustering with a fuzzy covariance matrix. J. Food Process Eng. 2019, 42, e13298. [Google Scholar] [CrossRef]
- Lara, I.; Belge, B.; Goulao, L.F. A focus on the biosynthesis and composition of cuticle in fruits. J. Agric. Food Chem. 2015, 63, 4005–4019. [Google Scholar] [CrossRef]
- Ge, S.; Qin, K.; Ding, S.; Yang, J.; Jiang, L.; Qin, Y.; Wang, R. Gas chromatography–mass spectrometry metabolite analysis combined with transcriptomic and proteomic provide new insights into revealing cuticle formation during pepper development. J. Agric. Food Chem. 2022, 70, 12383–12397. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Li, Y.-L.; Fu, Z.-L.; Huang, Q.; Yue, X.-G.; Wang, Y.; Zhu, K.-M.; Wang, Z.; Ge, Y.-S.; Wang, Z.-H. Transcriptome analysis of Brassica napus wax-deficient mutant revealed the dynamic regulation of leaf wax biosynthesis is associated with Basic pentacysteine 6. Int. J. Agric. Biol. 2019, 21, 1228–1234. [Google Scholar]
- Liu, H.; Han, X.; Fadiji, T.; Li, Z.; Ni, J. Prediction of the cracking susceptibility of tomato pericarp: Three-point bending simulation using an extended finite element method. Postharvest Biol. Technol. 2022, 187, 111876. [Google Scholar] [CrossRef]
- Huang, H.; He, X.; Sun, Q.; Liu, G.; Tang, Y.; Sun, J. Differential changes in cuticular wax affect the susceptibility to fruit decay in pitaya after harvest: A cultivar comparative study. Postharvest Biol. Technol. 2024, 210, 112751. [Google Scholar] [CrossRef]
- Reshma, T.; Balan, S.; Dileep, C. First report of rice grain discolouration and leaf blight caused by Pantoea ananatis in the Kuttanad agro-ecosystem, Kerala, India. Can. J. Plant Pathol. 2023, 45, 30–34. [Google Scholar] [CrossRef]
- Munzert, K.S.; Engelsdorf, T. Plant cell wall structure and dynamics in plant–pathogen interactions and pathogen defence. J. Exp. Bot. 2025, 76, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Feng, Z.; Dai, S.; Zhang, P.; Wei, X. Using UAV multispectral remote sensing with appropriate spatial resolution and machine learning to monitor wheat scab. Agriculture 2022, 12, 1785. [Google Scholar] [CrossRef]
- Almoujahed, M.B.; Rangarajan, A.K.; Whetton, R.L.; Vincke, D.; Eylenbosch, D.; Vermeulen, P.; Mouazen, A.M. Non-destructive detection of fusarium head blight in wheat kernels and flour using visible near-infrared and mid-infrared spectroscopy. Chemom. Intell. Lab. Syst. 2024, 245, 105050. [Google Scholar] [CrossRef]
- Hong, Q.; Jiang, L.; Zhang, Z.; Ji, S.; Gu, C.; Mao, W.; Li, W.; Liu, T.; Li, B.; Tan, C. A lightweight model for wheat ear fusarium head blight detection based on RGB images. Remote Sens. 2022, 14, 3481. [Google Scholar] [CrossRef]





| Wavenumber (cm−1) | Diffusion Length (µm) with Moving Mirror Velocities (cm·s−1) | ||
|---|---|---|---|
| 0.3 | 0.4 | 0.6 | |
| 2000 | 7.3 | 6.3 | 5.2 |
| 1650 | 8.0 | 6.9 | 5.7 |
| 1050 | 10.0 | 8.7 | 7.1 |
| 800 | 10.9 | 9.5 | 7.8 |
| Wavenumber (cm−1) | Cellular Layer | Cuticle Layer | ||
|---|---|---|---|---|
| Vibration | Attribution | Vibration | Attribution | |
| 1650 | Amide I | Proteins, Nucleosides | — | — |
| 1550 | Amide II | Proteins, Nucleosides | ν(C=C) | Unsaturated aliphatics |
| 1050 | ν(C-O) | Saccharides, Aliphatics | ν(C-O) | Alcohol |
| δ(N-H) | Proteins, Nucleosides | — | — | |
| Evaluation Indicators | Sample Size | Misdiagnosis Details | ||
|---|---|---|---|---|
| 0.4 | 0.5 | 0.6 | ||
| Healthy set | 50 | 16 | 10 | 8 |
| Diseased set | 80 | 10 | 8 | 23 |
| Total amount | 130 | 26 | 18 | 30 |
| Proportion | — | 0.20 | 0.14 | 0.24 |
| F1-Score | — | 0.84 | 0.89 | 0.78 |
| Methods | Information Interpretation | Onset Stage | Accuracy |
|---|---|---|---|
| MIR spectroscopy | LDA | Symptomatic | 93% |
| Multi-spectral | kNN, SVM, XGBoost | Symptomatic | 84.64–85.02% |
| RGB image | RGB-YOLOv4 | Symptomatic | 93.69% |
| NIR spectroscopy | SVM | Asymptomatic (organ) | 73.33% |
| Micro-vision and spectrum | Multiple feature fusion and modeling | Asymptomatic (organ) | 75.11–90.95% |
| FTIR-PAS (proposed method) | Ratio of absorption intensity | Symptomatic | 86% |
| Asymptomatic | 82% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, G.; Li, J.; Shan, D.; Liu, F.; Mao, H.; Sun, W. Early Detection of Wheat Fusarium Head Blight During the Incubation Period Using FTIR-PAS. Agronomy 2025, 15, 2100. https://doi.org/10.3390/agronomy15092100
Lv G, Li J, Shan D, Liu F, Mao H, Sun W. Early Detection of Wheat Fusarium Head Blight During the Incubation Period Using FTIR-PAS. Agronomy. 2025; 15(9):2100. https://doi.org/10.3390/agronomy15092100
Chicago/Turabian StyleLv, Gaoqiang, Jiaqi Li, Didi Shan, Fei Liu, Hanping Mao, and Weihong Sun. 2025. "Early Detection of Wheat Fusarium Head Blight During the Incubation Period Using FTIR-PAS" Agronomy 15, no. 9: 2100. https://doi.org/10.3390/agronomy15092100
APA StyleLv, G., Li, J., Shan, D., Liu, F., Mao, H., & Sun, W. (2025). Early Detection of Wheat Fusarium Head Blight During the Incubation Period Using FTIR-PAS. Agronomy, 15(9), 2100. https://doi.org/10.3390/agronomy15092100







