Study on the Flower Biology of Camellia luteoflora—A Species with an Extremely Small Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Research Method
2.2.1. Flowering Phenology
2.2.2. Pollen Morphology
2.2.3. Pollen Viability
2.2.4. Estimation of Pollen/Ovule Ratio (P/O) and Outcrossing Index (OCI)
2.2.5. In Vitro Pollen Germination
2.2.6. Determination of Stigma Receptivity
2.2.7. Flower-Visiting Insects
2.2.8. Statistical Analysis
3. Results
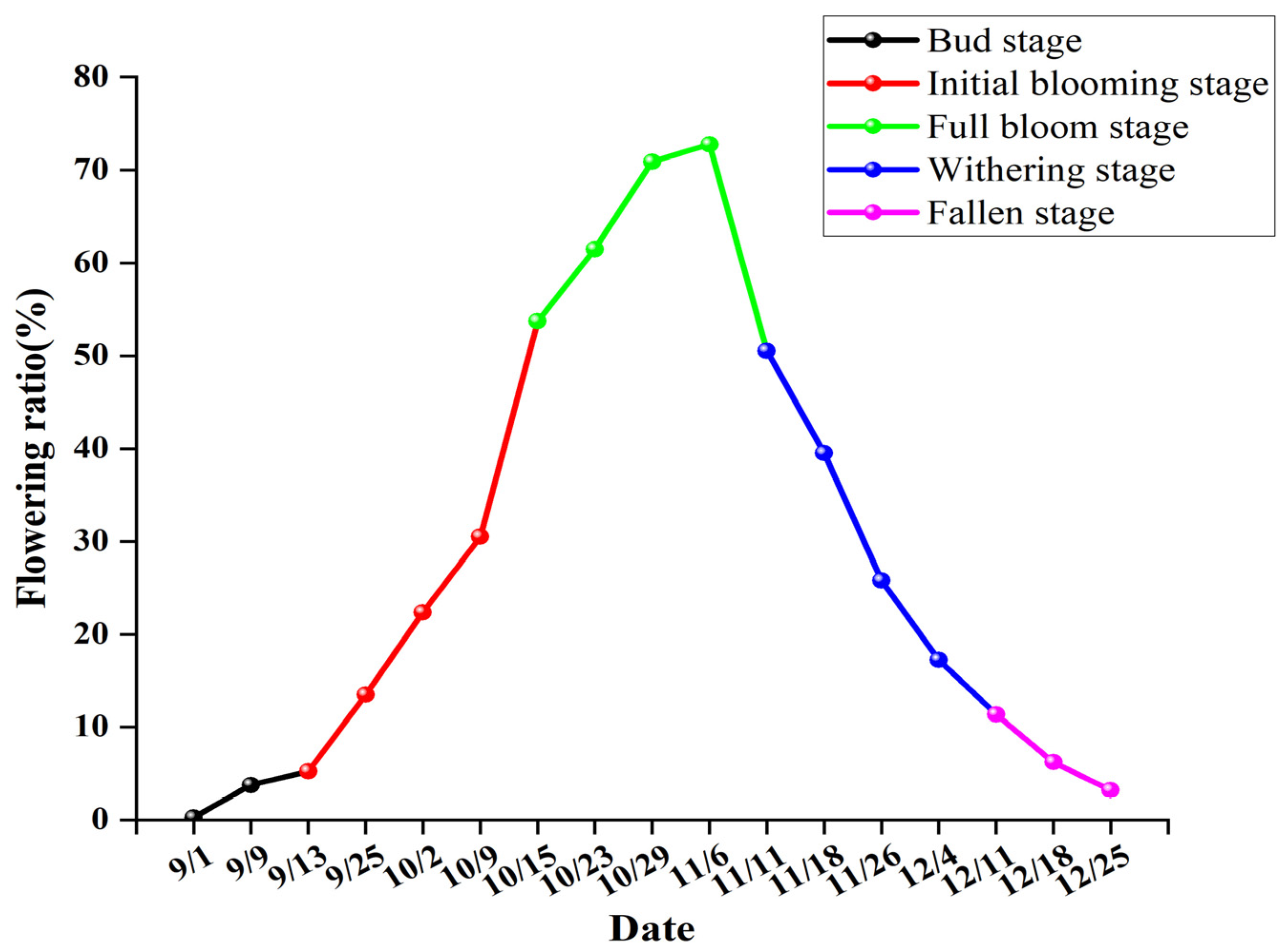
3.1. Flowering Phenology
- Bud stage: Typically lasting for 4–5 days, buds enlarge and become ellipsoidal, petals are pale yellow with a reddish tinge at the apex (Figure 2A).
- Initial blooming stage: The corolla unfolds into a campanulate shape, with an overall pale yellow color, stamens begin to emerge, and the anthers are yellow (Figure 2B).
- Full blooming stage: The corolla is fully expanded, petals are yellow, stamens are fully exserted, with yellow anthers (Figure 2C).
- Withering stage: The corolla exhibits irregular morphology (elliptical or campanulate), petals are yellow with localized red pigmentation, anthers are brownish-yellow (Figure 2D).
- Fallen stage: The corolla is umbellate, petals develop an overall red coloration, stamens are exserted, with brownish-yellow anthers (Figure 2E).
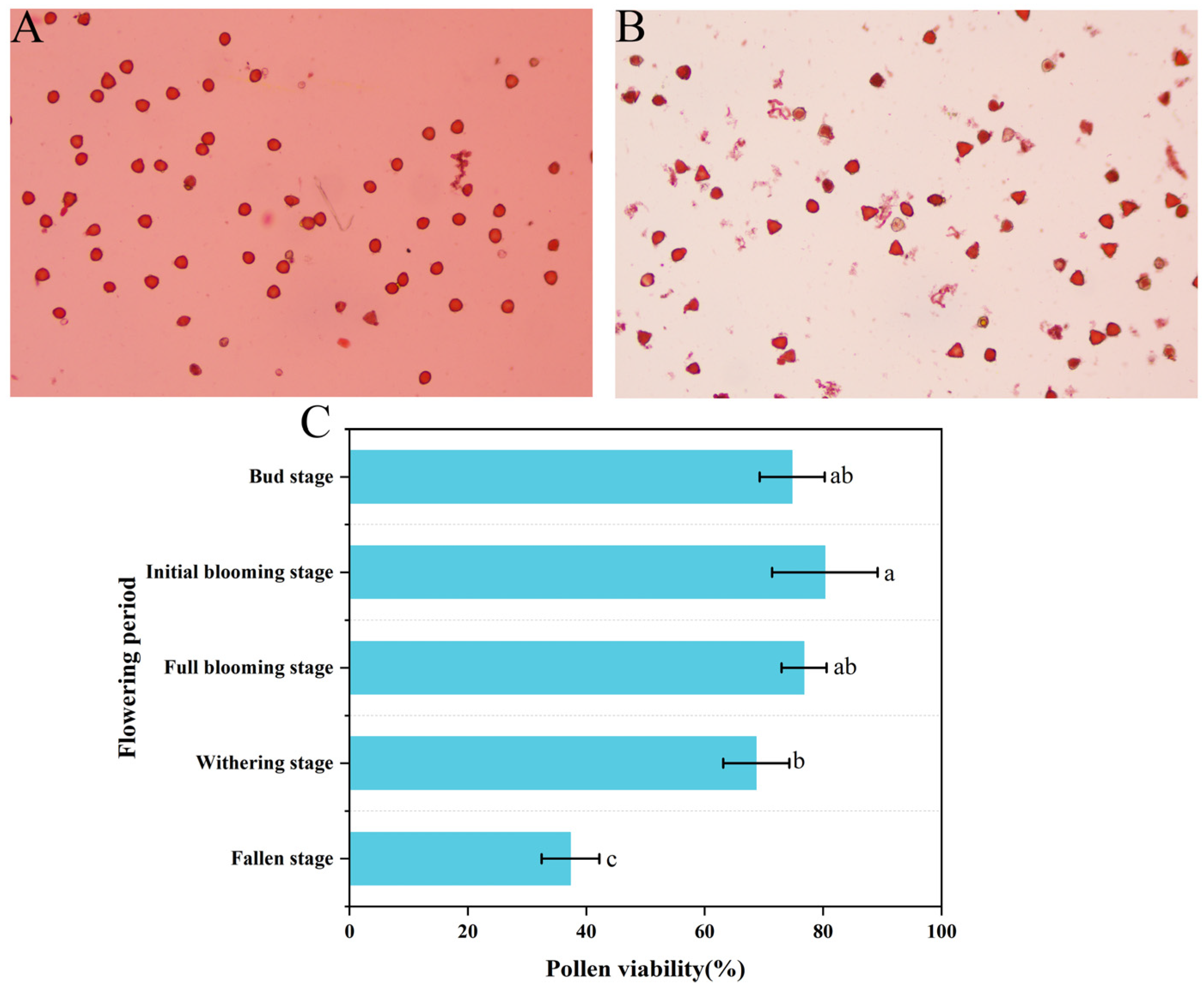
3.2. Pollen Morphology
3.3. Pollen Viability
3.4. Pollen Germinability
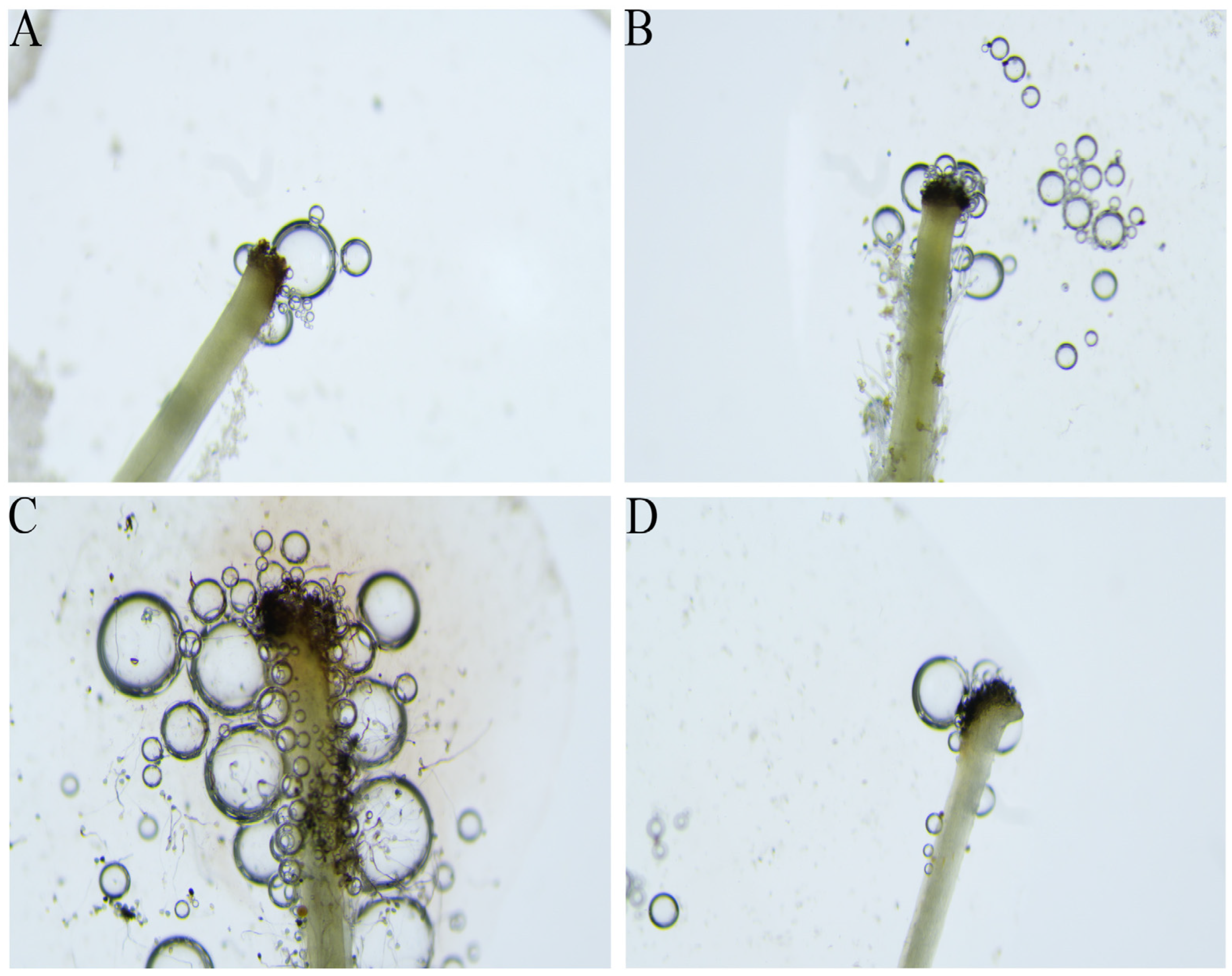
3.5. Stigma Receptivity
3.6. P/O Ratio and OCI Value
3.7. Flower Visitors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.Y.; Zong, X.H.; Wang, X.; Bai, X.J.; Liang, S.; Deng, H.-P. Population structure and living community characteristics of endangered Camellia luteoflora Li ex H. T. Chang. Plant Sci. J. 2016, 34, 539–546. [Google Scholar]
- Bai, X.J.; Shen, K.P.; Mu, J.; Weng, T.; Zang, L.P.; Ren, W.; Han, X.; Li, Q.; Tan, Q.; He, Y. Population structure and survival potentiality analysis of endangered Camellia luteoflora. J. Trop. Subtrop. Bot. 2022, 30, 718–726. [Google Scholar]
- Yang, W.; Liu, F.; Wu, G.; Liang, S.; Bai, X.; Liu, B.; Zhang, B.; Chen, H.; Yang, J. Widely targeted metabolomics analysis of the roots, stems, leaves, flowers, and fruits of Camellia luteoflora, a species with an extremely small population. Molecules 2024, 29, 4754. [Google Scholar] [CrossRef]
- Liu, Q.B.; Liu, B.Y.; Liang, S. Exploration of the causes of endangerment of Camellia luteoflora and countermeasures to deal with it. Environ. Prot. Technol. 2005, 11, 18–20. [Google Scholar]
- Wang, H. Study on Genetic Diversity of a Rare Andendangered Plant Camellia luteoflora Li ex H.T. Chang. Master’s Thesis, Southwest University, Chongqing, China, 2020. [Google Scholar]
- Arroyo, M.T.K.; Tamburrino, Í.; Pliscoff, P.; Robles, V.; Colldecarrera, M.; Guerrero, P.C. Flowering phenology adjustment and flower longevity in a south american alpine species. Plants 2021, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Abe, T. Flowering phenology, display size, and fruit set in an understory dioecious shrub, Aucuba japonica (Cornaceae). Am. J. Bot. 2001, 88, 455–461. [Google Scholar] [CrossRef]
- Souza, E.H.; Souza, F.V.D.; Rossi, M.L.; Packer, R.M.; Cruz-Barros, M.A.V.; Martinelli, A.P. Pollen morphology and viability in Bromeliaceae. An. Da Acad. Bras. De Cienc. 2017, 89, 3067–3082. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, Y.; Iqbal, A.; Shi, P.; Li, J.; Yang, Y.; Lei, X. Optimization of culture medium and temperature for the in vitro germination of oil palm pollen. Sci. Hortic. 2017, 220, 134–138. [Google Scholar] [CrossRef]
- Fragallah, S.A.D.A.; Lig, S.; LI, N.; Ligate, E.J.; Chen, Y. Effects of sucrose, boric acid, pH, andincubation time on in vitro germination of pollen and tube growth of Chinese fir (Cunnighamial lanceolata L.). Forests 2019, 10, 102. [Google Scholar] [CrossRef]
- Wang, B.; Ma, G.; Lin, S.; He, X.; Chen, B.; Li, H.; Huang, L.; Yang, Y.; Wei, J. Floral Biology of Aquilaria sinensis (Lour.) Spreng. Horticulturae 2024, 10, 109. [Google Scholar] [CrossRef]
- Carpenedo, S.; Raseira, M.D.C.B.; Franzon, R.C.; Byrne, D.H.; Da Silva, J.B. Stigmatic receptivity of peach flowers submitted to heat stress. Acta Sci. Agron. 2020, 42, e42450. [Google Scholar] [CrossRef]
- He, J.-L.; Pei, L.-X.; Ji, B.-Y.; Wang, H.-B.; Zhong, H.; Dong, C.-M.; Chen, S.-Q.; Li, X.-Q.; Li, P.-P. Biological characteristics of flowers and examination of pollen viability at different developmental stages of Epimedium sagittatum (Sieb. et Zucc.) Maxim. Sci. Rep. 2024, 14, 18530. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Kaur, N.; Gill, P.P.S. Reproductive phenology of four pear cultivars grown under sub-tropics of Punjab. Plant Physiol. Rep. 2023, 28, 556–567. [Google Scholar] [CrossRef]
- Pauw, A.; Stofberg, J.; Waterman, R.J. Flies and flowers in Darwin’s race. Evolution 2009, 63, 268–279. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.Y.; Wang, X.Y.; Guo, W.; Yuan, T. Flowering Characteristics and Breeding System of Deutzia parviflora. J. Northwest For. Univ. 2025, 40, 290–300. [Google Scholar]
- Xiu, X.J.; He, X.Y.; Lin, J.X.; Jiang, C.N.; Huang, Y.F. Growth performance of 20 species of Michelia in Zhongshan arboretum from Guangdong. China. Subtrop. Plant Sci. 2013, 42, 342–344. [Google Scholar]
- Chen, H.D.; Yang, W.C.; Zhang, B.C.; Zhou, M.Q.; He, Q.Q.; Liang, S. The ultrastructure of antennal sensilla in Rhoptroceros cyatheae. J. Northeast For. Univ. 2024, 52, 154–160. [Google Scholar]
- Erdtman, G. Pollen Morphology and Plant Taxonomy. Angiosperms. An Introduction to Palynology; Almquist and Wiksel: Stockholm, Sweden, 1952. [Google Scholar]
- Aydin, S.; Vahideh, N.; Majid, S. Pollen viability and storage life in Leonurus cardiaca L. J. Appl. Res. Med. Aromat. Plants 2016, 3, 101–104. [Google Scholar]
- Zhang, H.; Wu, H.; Zhou, Q.; Zhao, R.; Sheng, Q.; Zhu, Z. Flowering characteristics and reproductive biology of Nymphaea hybrid, a precious water lily. Sci. Hortic. 2021, 287, 110268. [Google Scholar] [CrossRef]
- Cruden, R.W. Pollen-ovule ratios: A conservative indicator of breeding systems in flowering plants. Evolution 1977, 31, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Dafni, A. Pollination Ecology; Oxford Univ Press: New York, NY, USA, 1992. [Google Scholar]
- Huang, Z.W.; Yu, H.J.; Li, X.M.; Li, C.N.; Wang, H.Y.; Su, Q.; Zhou, Z.G.; Huang, C.Y.; Lu, S.Y. Effects of different culture conditions and storage methods on the pollen germination in Vitro of Camellia rostrata. Mol. Plant Breed. 2024, 22, 1992–1999. [Google Scholar]
- Zhang, X.N.; Ye, H.; Wu, F.Y.; Liu, H.L.; Ma, J.L. In vitro germination of Camellia osmantha pollen and its storage conditions research. Mol. Plant Breed. 2023, 1–17. [Google Scholar]
- Li, R.; Chang, H.; Liu, H.; Zhang, Y.; Liang, C.; Pu, G. The fertility research of “Huajin 6”, a new variety of honeysuckle. Sci. Rep. 2024, 14, 13729. [Google Scholar] [CrossRef]
- Ma, W.Z. Economic Insect Fauna of China (Coleoptera: Cetoniidae, Trichiidae, Valgidae); Science Press: Beijing, China, 1995. [Google Scholar]
- Erdtman, G.; Praglowski, J. Six notes on pollen morphology andpollen morphological techniques. Bot. Not. 1959, 112, 175–184. [Google Scholar]
- Shao, W.H.; Diao, S.F.; Dong, R.X.; Jiang, J.M.; Yue, H.F. Study on geographic variation of morphology and economic character of fruit and seed of Sapindus mukorossi. For. Res. 2013, 26, 603–608. [Google Scholar]
- Chen, Y.J.; Li, H.; Guo, W.Z.; Xu, C.; Deng, L. The reproductive system and pollination biology of endangered Camellia huana. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2025, 49, 153–162. [Google Scholar]
- Liu, L.X.; Yao, X.H.; Zeng, H.T.; Teng, J.; Xu, H.; Chen, J.; Chang, J. Effects of flowering biology and pollination methods on fruit development of Camellia oleifera. J. Southwest Univ. (Nat. Sci. Ed.) 2025, 47, 93–103. [Google Scholar]
- Chai, S.F.; Chen, Z.Y.; Tang, J.M.; Wang, M.L.; Zou, R.; Wei, X. Breeding system and bird pollination of Camellia pubipetala, a narrowly endemic plant from karst regions of south China. Plant Species Biol. 2019, 34, 141–151. [Google Scholar] [CrossRef]
- Liu, Z.; Tao, J.; Ma, C.; Wen, M.; Xi, R.; Deng, X. Dynamic changes in endogenous substances in flowering organs of Camellia drupifera during the flowering stage. Forests 2024, 15, 1391. [Google Scholar] [CrossRef]
- Boavida, L.C.; Vieira, A.M.; Becker, J.D.; Feijo, J.A. Gametophyte interaction and sexual reproduction: How plants make a zygote. Int. J. Dev. Biol. 2005, 49, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Bedinger, P. The remarkable biology of pollen. Plant Cell 1992, 4, 879–887. [Google Scholar] [CrossRef]
- Qiu, J.; Gao, C.; Wei, H.; Wang, B.; Hu, Y.; Guo, Z.; Long, L.; Yang, L.; Li, H. Flowering biology of Rhododendron pulchrum. Horticulturae 2021, 7, 508. [Google Scholar] [CrossRef]
- Wan, X.Q.; Gao, C.; Song, Q.L.; Sun, D.C.; Lie, Y.M.; Wang, Q.M. Research on pollen morphology and germination characteristics of Camellia oleifera ‘Changlin’. Guihaia 2024, 1–13. [Google Scholar]
- Dafni, A.; Firmage, D. Pollen viability and longevity: Practical, ecological and evolutionary implications. Plant Syst. Evol. 2000, 222, 113–132. [Google Scholar] [CrossRef]
- Liao, M.L.; Sun, L.N.; Meng, F.; Du, L.; Lin, M.; Huang, L.Y. Pollen viability measurement and storage conditions of three Camellia plants. Guangxi For. Sci. 2021, 50, 539–543. [Google Scholar]
- Okusaka, K.; Hiratsuka, S. Fructose inhibits pear pollen germination on agar medium without loss of viability. Sci. Hortic. 2009, 122, 51–55. [Google Scholar] [CrossRef]
- Su, M.; Wang, D.; Li, Z.-D.; Hao, J.-H.; Dong, S.; Yuan, X.; Li, X.; Gao, L.; Yang, G.; Chu, X.; et al. Establishment of in vitro pollen germination system in C4 model plant Foxtail Millet. Plant Cell Tissue Organ Cult. 2024, 156, 98. [Google Scholar] [CrossRef]
- Li, Z.Q.; Geng, F.; Nie, R.M.; Hu, Y.C.; Yang, Z.Y.; Wang, Z.L.; Chen, L.Q. Pollen germination and viability preservation of Camellia reticulata under different storage conditions. Mol. Plant Breed. 2025, 23, 1195–1201. [Google Scholar]
- Liu, X.; Xiao, Y.; Wang, Y.; Chen, F.; Huang, R.; Jiang, Y. The in vitro germination and storage characteristics of Keteleeria fortunei var. cyclolepis pollen provide a reference for cross breeding. Protoplasma 2020, 257, 1221–1230. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, L.; Wu, X.; Li, Y.; Lin, J. Boron influences pollen germination and pollen tube growth in Picea meyeri. Tree Physiol. 2003, 23, 345–351. [Google Scholar] [CrossRef]
- Wani, M.S.; Hamid, M.; Tantray, Y.R.; Gupta, R.C.; Munshi, A.; Singh, V. In vitro pollen germination of Betula utilis, a typical tree line species in Himalayas. S. Afr. J. Bot. 2020, 131, 214–221. [Google Scholar] [CrossRef]
- Cui, M.; Pham, M.D.; Lee, H.; Lee, B.; Myung, J.; Hwang, H.; Chun, C. Ultrastructural changes in developmental stages of anther and pollen grains as affected by short-term exposure to low temperatures in strawberry. Environ. Exp. Bot. 2023, 205, 105135. [Google Scholar] [CrossRef]
- Masoomi-Aladizgeh, F.; Najeeb, U.; Hamzelou, S.; Pascovici, D.; Amirkhani, A.; Tan, D.K.Y.; Mirzaei, M.; Haynes, P.A.; Atwell, B.J. Pollen development in cotton (Gossypium hirsutum) is highly sensitive to heat exposure during the tetrad stage. Plant Cell Environ. 2021, 44, 2150–2166. [Google Scholar] [CrossRef] [PubMed]
- Li, T.F.; Lin, T.; Xu, B.Y.; Liu, Z.C.; Luo, L.J. Study on pollen germinating ability and storage tolerance of Camellia azalea. Biotechnol. Bull. 2008, S1, 239–243. [Google Scholar]
- Xiong, H.; Zou, F.; Yuan, D.; Zhang, X.; Tan, X. Orthogonal test design for optimising the culture medium for in vitro pollen germination of feijoa (Acca sellowiana cv. Unique). New Zealand J. Crop. Hortic. Sci. 2016, 44, 192–202. [Google Scholar] [CrossRef]
- Chen, J.Y.; Deng, L.X.; Huang, L.; Xu, C.R.; Liu, X.H. Study on viability determination, culture medium selection and storage conditions of Camellia Liberofilamenta pollen. Guizhou For. Sci. Technol. 2022, 50, 1–5. [Google Scholar]
- Zhao, R.; Hu, X.; Yuan, D.; Masabni, J.; Xiong, H.; Zou, F. Orthogonal test design for optimizing culture medium for in vitro pollen germination of interspecific oil tea hybrids. An. Da Acad. Bras. De Cienc. 2021, 93, e20190431. [Google Scholar] [CrossRef]
- Zhang, D.Y. The Evolution of Plant Life History and Reproductive Ecology; SciPress: Beijing, China, 2004. [Google Scholar]
- Lv, Z.J. Study on Breeding System and Fruit Characters of Camellia Osmantha; Guangxi University: Nanning, China, 2023. [Google Scholar]


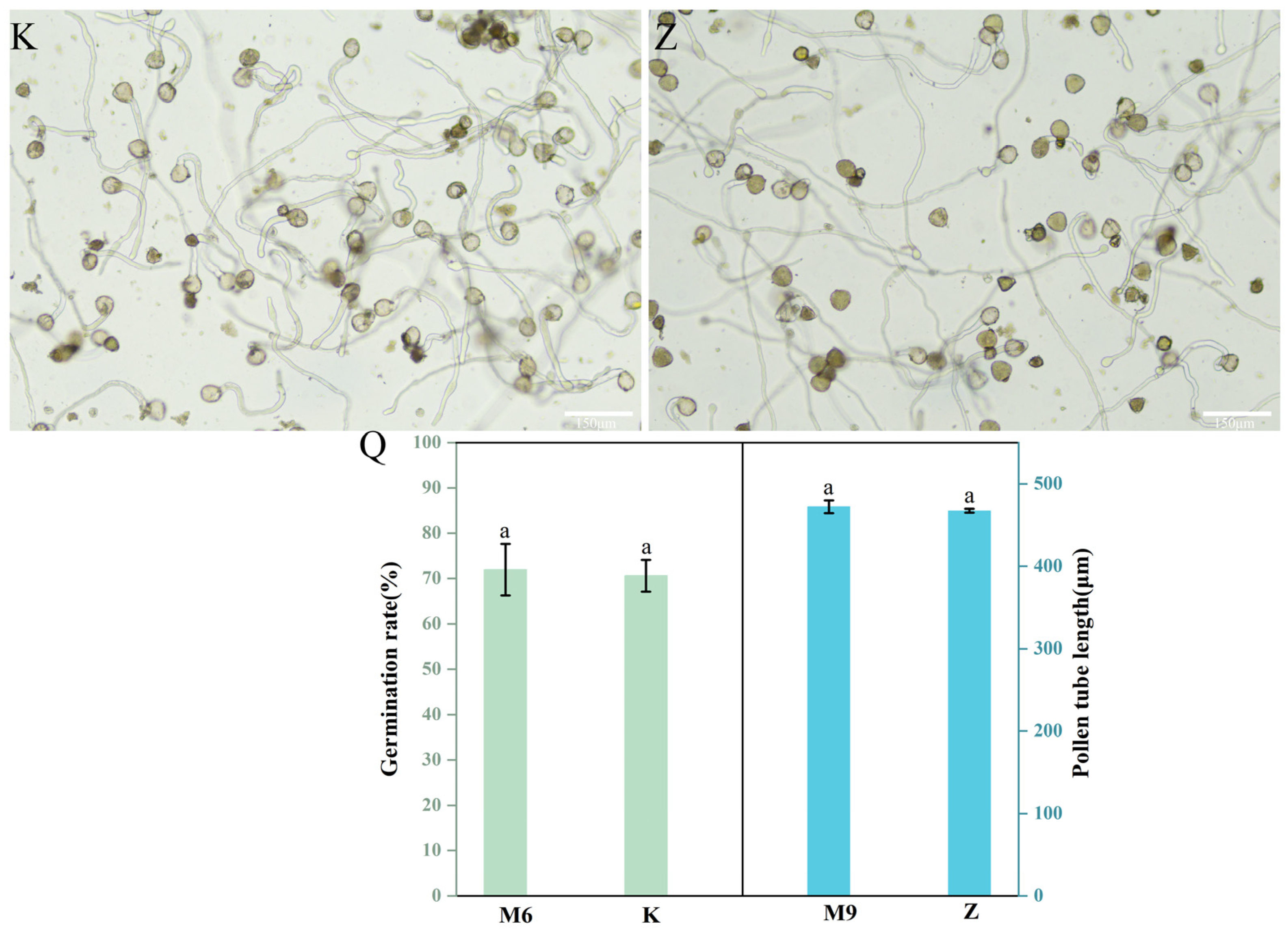


| No. | Sucrose (g/L) | H3BO3 (g/L) | CaCl2/(g/L) | Temperature (°C) | Germination Rate (%) | Pollen Tube Length (μm) |
|---|---|---|---|---|---|---|
| M1 | 50 | 0.1 | 0.1 | 18 | 40.81 ± 3.42 cd | 151.29 ± 23.42 g |
| M2 | 50 | 0.15 | 0.2 | 24 | 61.59 ± 4.20 b | 365.94 ± 16.41 c |
| M3 | 50 | 0.2 | 0.3 | 30 | 20.75 ± 1.07 gh | 206.80 ± 2.44 ef |
| M4 | 50 | 0.25 | 0.4 | 36 | 0.96 ± 0.24 j | 60.84 ± 12.09 i |
| M5 | 75 | 0.1 | 0.2 | 36 | 6.10 ± 1.97 j | 70.98 ± 12.49 hi |
| M6 | 75 | 0.15 | 0.1 | 30 | 71.96 ± 5.68 a | 422.55 ± 46.53 b |
| M7 | 75 | 0.2 | 0.4 | 24 | 62.33 ± 6.71 b | 357.86 ± 28.23 c |
| M8 | 75 | 0.25 | 0.3 | 18 | 31.68 ± 3.23 ef | 81.95 ± 2.22 hi |
| M9 | 100 | 0.1 | 0.3 | 24 | 42.29 ± 0.44 cd | 472.15 ± 7.86 a |
| M10 | 100 | 0.15 | 0.4 | 18 | 26.70 ± 4.64 dfg | 170.84 ± 14.92 fg |
| M11 | 100 | 0.2 | 0.1 | 36 | 2.20 ± 1.17 j | 55.12 ± 2.99 i |
| M12 | 100 | 0.25 | 0.2 | 30 | 56.42 ± 7.23 b | 337.83 ± 62.40 c |
| M13 | 125 | 0.1 | 0.4 | 30 | 18.09 ± 5.53 hi | 273.37 ± 13.41 d |
| M14 | 125 | 0.15 | 0.3 | 36 | 0 j | 0 j |
| M15 | 125 | 0.2 | 0.2 | 18 | 37.74 ± 1.96 de | 231.29 ± 26.09 e |
| M16 | 125 | 0.25 | 0.1 | 24 | 45.96 ± 1.85 c | 267.84 ± 16.81 d |
| CK1 | 0 | 0 | 0 | 18 | 42.94 ± 2.24 cd | 101.39 ± 9.13 h |
| CK2 | 0 | 0 | 0 | 24 | 34.08 ± 4.34 e | 164.57 ± 8.20 g |
| CK3 | 0 | 0 | 0 | 30 | 13.20 ± 2.12 i | 218.72 ± 3.60 e |
| CK4 | 0 | 0 | 0 | 36 | 0 j | 0 i |
| Factor | Sucrose | H3BO3 | CaCl2 | Temperature | |
|---|---|---|---|---|---|
| Germination rate (%) | K1 | 31.03 | 26.82 | 40.23 | 34.24 |
| K2 | 43.02 | 40.06 | 40.46 | 53.04 | |
| K3 | 31.90 | 30.76 | 23.68 | 41.81 | |
| K4 | 25.45 | 33.75 | 27.02 | 2.31 | |
| Range | 17.57 | 13.24 | 16.78 | 50.73 | |
| Pollen tube length (μm) | Z1 | 196.22 | 241.95 | 224.20 | 158.84 |
| Z2 | 233.33 | 239.83 | 251.51 | 365.95 | |
| Z3 | 381.94 | 212.77 | 190.23 | 310.14 | |
| Z4 | 193.12 | 260.92 | 215.73 | 46.74 | |
| Range | 188.82 | 48.15 | 61.28 | 319.21 |
| Stage | Stigma Receptivity |
|---|---|
| Bud stage | ++ |
| Initial blooming stage | +++ |
| Full blooming stage | +++++ |
| Withering stage | ++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Liu, B.; Yang, W.; Song, Y.; Liang, S.; Chen, H.; Zhou, M.; Wu, G. Study on the Flower Biology of Camellia luteoflora—A Species with an Extremely Small Population. Agronomy 2025, 15, 2098. https://doi.org/10.3390/agronomy15092098
Liu F, Liu B, Yang W, Song Y, Liang S, Chen H, Zhou M, Wu G. Study on the Flower Biology of Camellia luteoflora—A Species with an Extremely Small Population. Agronomy. 2025; 15(9):2098. https://doi.org/10.3390/agronomy15092098
Chicago/Turabian StyleLiu, Fen, Bangyou Liu, Weicheng Yang, YueHua Song, Sheng Liang, Hangdan Chen, Mengqing Zhou, and Gaoyin Wu. 2025. "Study on the Flower Biology of Camellia luteoflora—A Species with an Extremely Small Population" Agronomy 15, no. 9: 2098. https://doi.org/10.3390/agronomy15092098
APA StyleLiu, F., Liu, B., Yang, W., Song, Y., Liang, S., Chen, H., Zhou, M., & Wu, G. (2025). Study on the Flower Biology of Camellia luteoflora—A Species with an Extremely Small Population. Agronomy, 15(9), 2098. https://doi.org/10.3390/agronomy15092098





