The Effect of Head Lettuce (Lactuca sativa var. capitata L.) Cultivation Under Glass with a Light Spectrum-Modifying Luminophore on Crop Traits
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Cultivation Conditions
2.3. Estimation of Morpho-Anatomical Parameters of Lettuce Plants
2.3.1. Lettuce Head Morphology
2.3.2. Lettuce Leaf Anatomy and Thickness
2.3.3. Lettuce Leaf Dry Weight Content
2.4. Biochemical Analysis
2.4.1. Phenolic Compound Content Determination
2.4.2. Cupric-Ion-Reducing Antioxidant Capacity Assay
2.4.3. L-Ascorbic Acid Content Determination
2.4.4. Sugar Content Determination
2.4.5. Free Amino Acid Content Determination
2.4.6. Determination of Nitrate Concentration (NO3−)
2.5. Statistical Analyses
3. Results
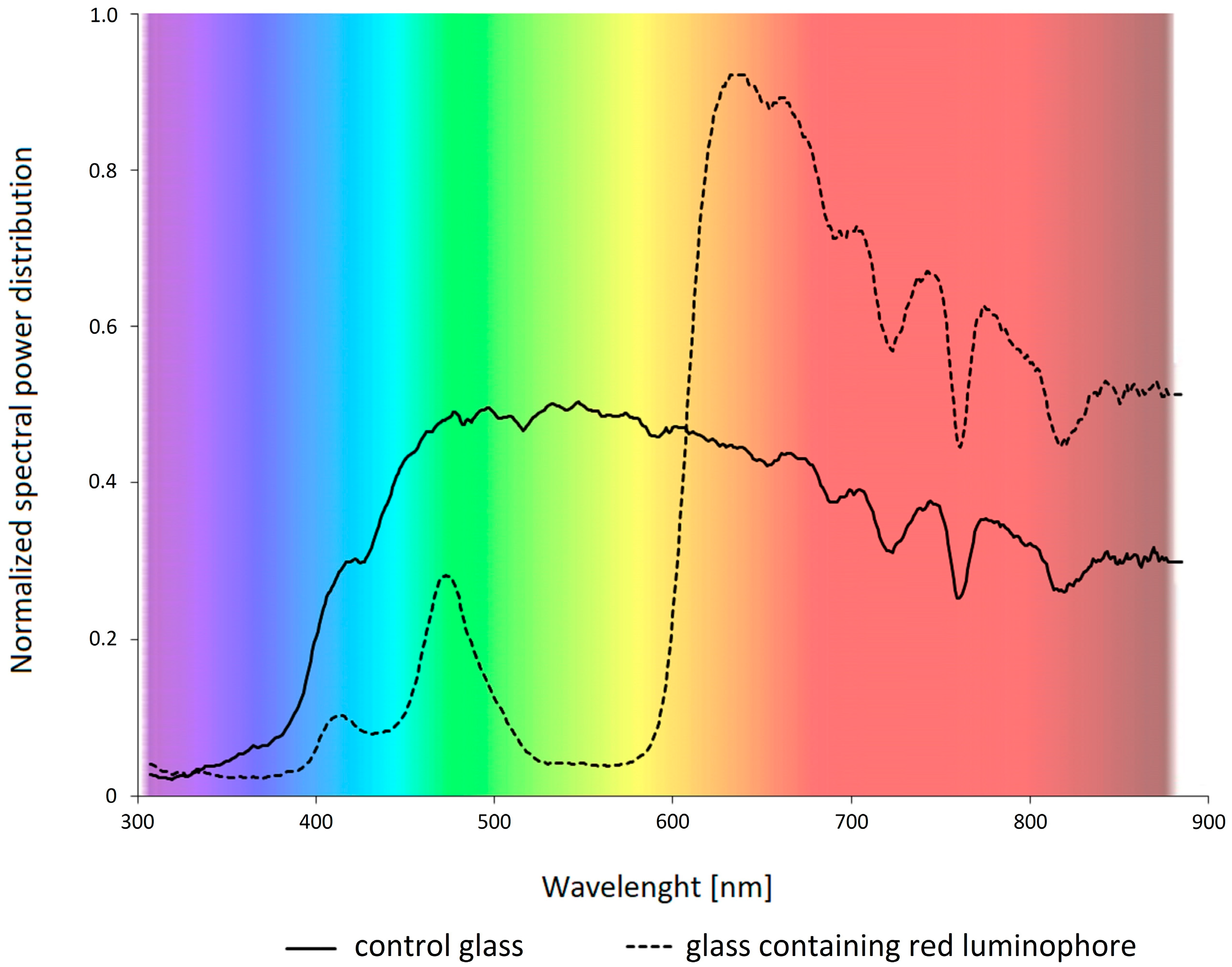
3.1. Cultivation Conditions
3.2. Morpho-Anatomical Traits
3.2.1. Morphology of Lettuce Plants
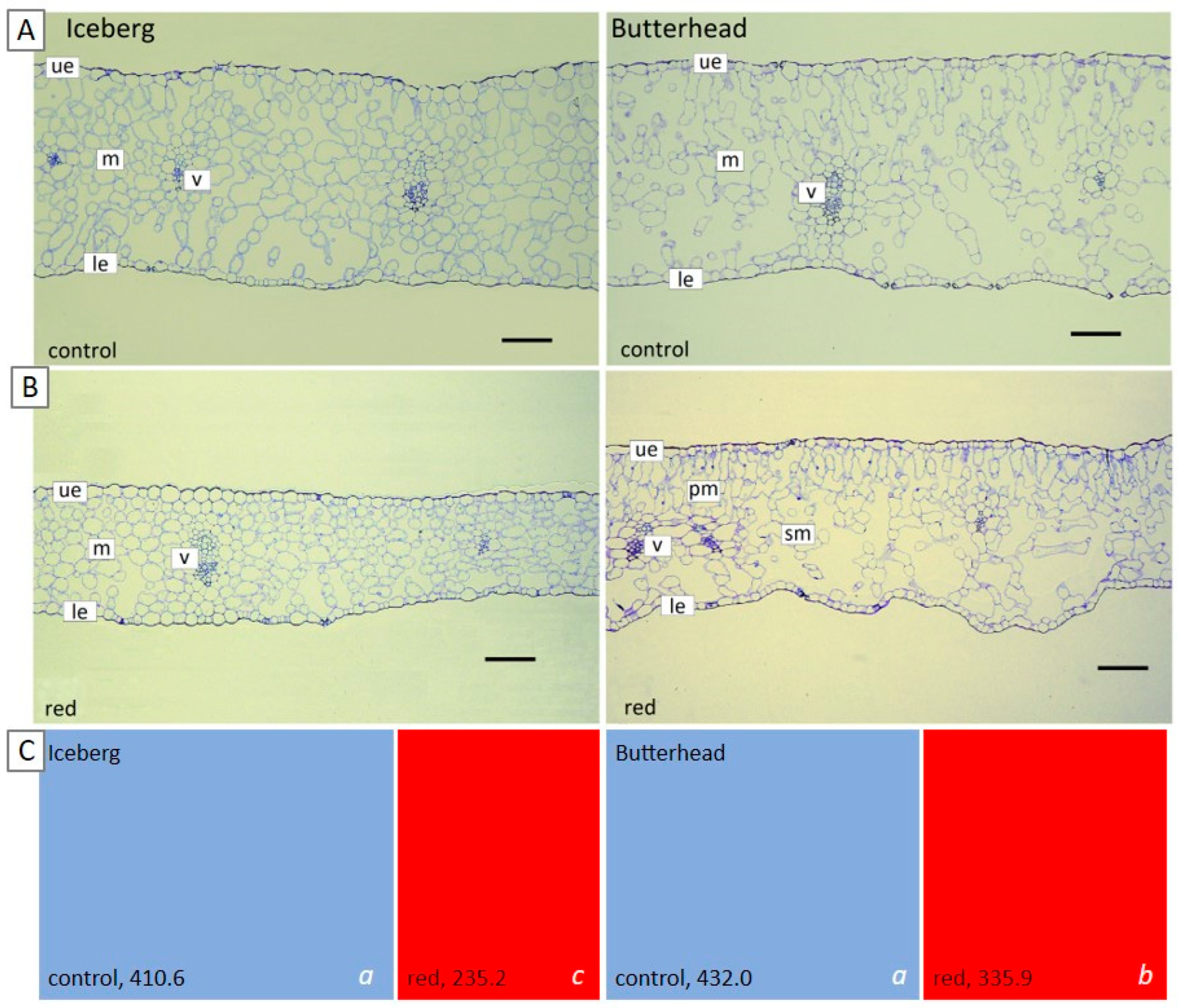
3.2.2. Anatomy of Lettuce Plants
3.2.3. Dry Weight Content
3.3. Antioxidant Properties of Lettuce Leaves
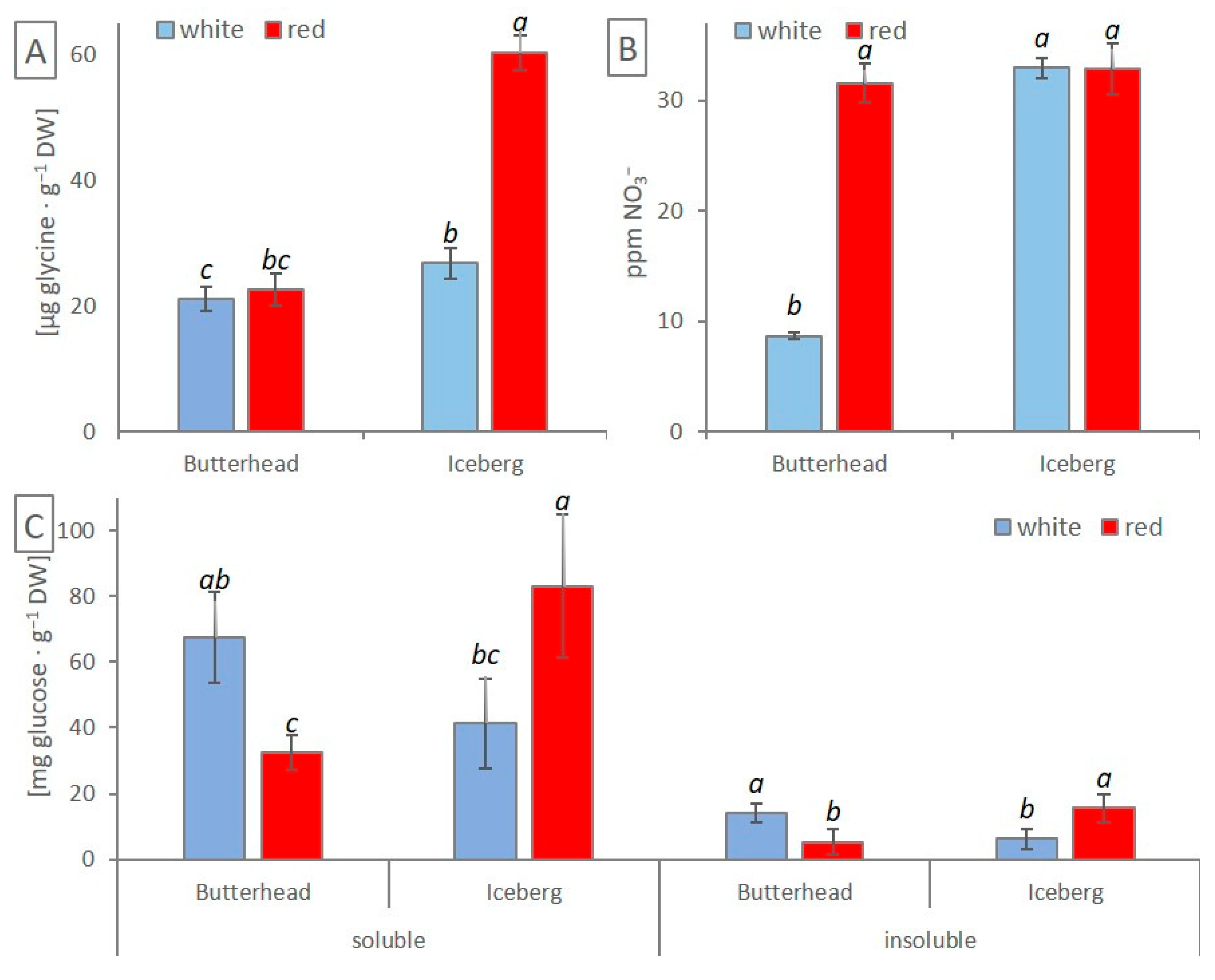
3.4. Amino Acids, NO3−, and Sugar Concentration in Lettuce Leaves
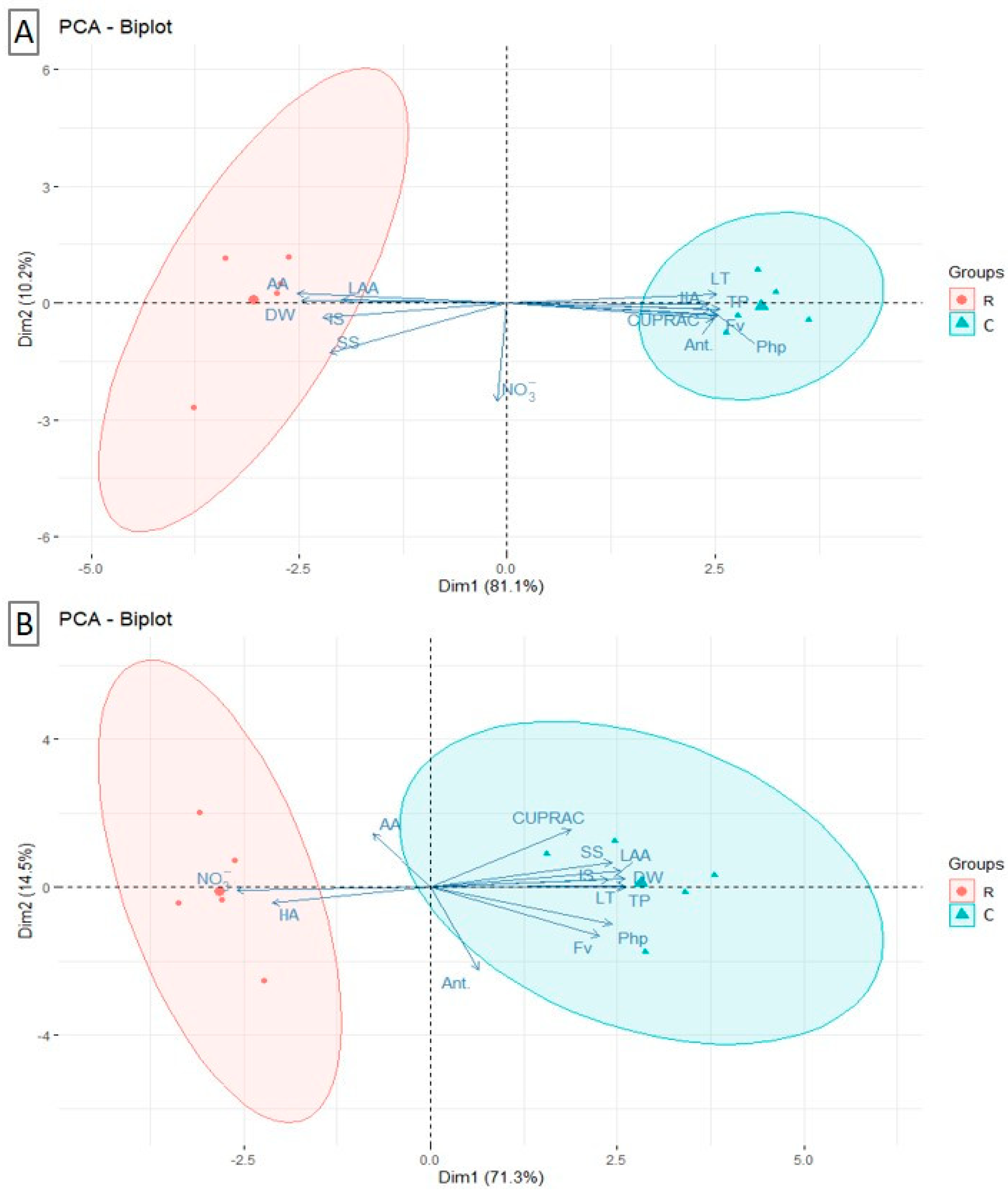
3.5. Principal Component Analysis Biplots
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Food and Agriculture Organization. Production Quantities of Lettuce and Chicory. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 2 September 2022).
- Yang, X.; Gil, M.I.; Yang, Q.; Tomás-Barberán, F.A. Bioactive compounds in lettuce: Highlighting the benefits to human health and impacts of preharvest and postharvest practices. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4–45. [Google Scholar] [CrossRef] [PubMed]
- Aksakal, O.; Tabay, D.; Esringu, A.; Icoglu Aksakal, F.; Esim, N. Effect of proline on biochemical and molecular mechanisms in lettuce (Lactuca sativa L.) exposed to UV-B radiation. Photochem. Photobiol. Sci. 2017, 16, 246–254. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Křístková, E.; Doležalová, I.; Lebeda, A.; Vinter, V.; Novotná, A. Description of morphological characters of lettuce (Lactuca sativa L.) genetic resources. Hort. Sci. 2008, 35, 113–129. [Google Scholar] [CrossRef]
- Lebeda, A.; Ryder, E.; Grube, R.; Doležalová, I.; Křístková, E. Lettuce (Asteraceae; Lactuca spp.). In Genetic Resources, Chromosome Engineering, and Crop Improvement; Singh, R.J., Ed.; CRC Press: Boca Raton, FL, USA; Tailor and Francis Group: Boca Raton, FL, USA, 2007; Volume 3, pp. 377–472. [Google Scholar]
- Ahmed, H.A.; Tong, Y.-X.; Yang, Q.-C. Optimal control of environmental conditions affecting lettuce plant growth in a controlled environment with artificial lighting: A review. S. Afr. J. Bot. 2020, 130, 75–89. [Google Scholar] [CrossRef]
- Zhou, J.; Li, P.; Wang, J.; Fu, W. Growth, photosynthesis, and nutrient uptake at different light intensities and temperatures in lettuce. HortScience 2019, 54, 1925–1933. [Google Scholar] [CrossRef]
- Zhou, J.; Li, P.; Wang, J. Effects of Light Intensity and Temperature on the Photosynthesis Characteristics and Yield of Lettuce. Horticulturae 2022, 8, 178. [Google Scholar] [CrossRef]
- Boros, I.F.; Székely, G.; Balázs, L.; Csambalik, L.; Sipos, L. Effects of LED lighting environments on lettuce (Lactuca sativa L.) in PFAL systems—A review. Sci. Hortic. 2023, 321, 112351. [Google Scholar] [CrossRef]
- Zha, L.; Liu, W.; Yang, Q.; Zhang, Y.; Zhou, C.; Shao, M. Regulation of ascorbate accumulation and metabolism in lettuce by the red: Blue ratio of continuous light using LEDs. Front. Plant Sci. 2020, 11, 704. [Google Scholar] [CrossRef]
- Wong, C.E.; Teo, Z.W.N.; Shen, L.; Yu, H. Seeing the lights for leafy greens in indoor vertical farming. Trends Food Sci. Technol. 2020, 106, 48–63. [Google Scholar] [CrossRef]
- Nozue, H.; Gomi, M. Usefulness of broad-spectrum white LEDs to envision future plant factory. In Smart Plant Factory: The Next Generation Indoor Vertical Farms; Kozai, T., Ed.; Springer: Singapore, 2018; pp. 197–210. [Google Scholar]
- Riahi, J.; Vergura, S.; Mezghani, D.; Mami, A. Smart and renewable energy system to power a temperature-controlled greenhouse. Energies 2021, 14, 5499. [Google Scholar] [CrossRef]
- Majeed, Y.; Khan, M.U.; Waseem, M.; Zahid, U.; Mahmood, F.; Majeed, F.; Sultan, M.; Raza, A. Renewable energy as an alternative source for energy management in agriculture. Energy Rep. 2023, 10, 344–359. [Google Scholar] [CrossRef]
- Olvera-Gonzalez, E.; Escalante-Garcia, N.; Myers, D.; Ampim, P.; Obeng, E.; Alaniz-Lumbreras, D.; Castaño, V. Pulsed LED-Lighting as an Alternative Energy Savings Technique for Vertical Farms and Plant Factories. Energies 2021, 14, 1603. [Google Scholar] [CrossRef]
- Jeremiasz, O.; Sobik, P.; Sala, A.; Pluta, A.; Szendera, F. Photoluminescent Dye, in Particular for Photovoltaic Modules and Method of Producing a Photoluminescent Dye, in Particular for Photovoltaic Module. PL Patent 240248 B1, 29 July 2019. [Google Scholar]
- Tokarz, K.M.; Makowski, W.; Tokarz, B.; Muszyńska, E.; Gajewski, Z.; Mazur, S.; Kunicki, E.; Jeremiasz, O.; Sobik, P.; Nowak, P.; et al. Performance of the Photosynthetic Apparatus under Glass with a Luminophore Modifying Red-To-Far-Red-Light Ratio—A Case Study. Cells 2023, 12, 1552. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.Q.; Harbick, K.; Eylands, N.J.; Kortshagen, U.R.; Ferry, V.E. Design guidelines for luminescent solar concentrator greenhouses in the United States. Adv. Sust. Sys. 2025, 9, 2400749. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006, First Update 2007; World Soil Resources Reports No. 103; FAO: Rome, Italy, 2006. [Google Scholar]
- The Joint Research Centre: EU Science Hub. Photovoltaic Geographical Information System (PVGIS). Available online: https://joint-research-centre.ec.europa.eu/photovoltaic-geographical-information-system-pvgis_en (accessed on 11 August 2025).
- Kiełkowska, A.; Dziurka, M. Changes in polyamine pattern mediates sex differentiation and unisexual flower development in monoecious cucumber (Cucumis sativus L.). Physiol. Plant. 2021, 171, 48–65. [Google Scholar] [CrossRef]
- Makowski, W.; Tokarz, K.M.; Tokarz, B.; Banasiuk, R.; Witek, K.; Królicka, A. Elicitation-Based Method for Increasing the Production of Antioxidant and Bactericidal Phenolic Compounds in Dionaea muscipula J. Ellis Tissue. Molecules 2020, 25, 1794. [Google Scholar] [CrossRef] [PubMed]
- Swain, T.; Hillis, W.E. Phenolic constituents of Prunus domestica. I. Quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Tokarz, K.; Makowski, W.; Banasiuk, R.; Królicka, A.; Piwowarczyk, B. Response of Dionaea muscipula J. Ellis to light stress in in vitro: Physiological study. Plant Cell Tiss. Organ Cult. 2018, 134, 65–77. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Makowski, W.; Królicka, A.; Nowicka, A.; Zwyrtková, J.; Tokarz, B.; Pecinka, A.; Banasiuk, R.; Tokarz, K.M. Transformed tissue of Dionaea muscipula J. Ellis as a source of biologically active phenolic compounds with bactericidal properties. Appl. Microbiol. Biotechnol. 2021, 105, 1215–1226. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Özyurt, D. Comparative Evaluation of Various Total Antioxidant Capacity Assays Applied to Phenolic Compounds with the CUPRAC Assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef]
- Makowski, W.; Królicka, A.; Tokarz, B.; Szopa, A.; Ekiert, H.; Tokarz, K.M. Temporary immersion bioreactors as a useful tool for obtaining high productivity of phenolic compounds with strong antioxidant properties from Pontechium maculatum. Plant Cell Tiss. Organ Cult. 2023, 153, 525–537. [Google Scholar] [CrossRef]
- Krełowska-Kułas, M. Badanie Jakości Produktów Spożywczych (Food Quality Testing); PWE: Warsaw, Poland, 1993. (In Polish) [Google Scholar]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Miernicka, K.; Tokarz, B.; Makowski, W.; Mazur, S.; Banasiuk, R.; Tokarz, K.M. The Adjustment Strategy of Venus Flytrap Photosynthetic Apparatus to UV-A Radiation. Cells 2022, 11, 3030. [Google Scholar] [CrossRef]
- Kamińska, I.; Lukasiewicz, A.; Klimek-Chodacka, M.; Długosz-Grochowska, O.; Rutkowska, J.; Szymonik, K.; Baranski, R. Antioxidative and osmoprotecting mechanisms in carrot plants tolerant to soil salinity. Sci. Rep. 2022, 12, 7266. [Google Scholar] [CrossRef]
- Wojciechowska, R.; Kolton, A.; Dlugosz-Grochowska, O.; Knop, E. Nitrate content in Valerianella locusta L. plants is affected by supplemental LED lighting. Sci. Hortic. 2016, 211, 179–186. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org (accessed on 5 September 2024).
- Azizi, S.; Aliniaeifard, S.; Zarbakhsh, S.; Esmaeili, S.; Baghalian, K.; Gruda, N.S. Photobiology, photosynthesis, and plant responses under artificial lighting in controlled environment agriculture. Sci. Hortic. 2025, 349, 114248. [Google Scholar] [CrossRef]
- Trouwborst, G.; Hogewoning, S.W.; van Kooten, O.; Harbinson, J.; van Ieperen, W. Plasticity of photosynthesis after the ‘red light syndrome’ in cucumber. Environ. Exp. Bot. 2016, 121, 75–82. [Google Scholar] [CrossRef]
- Dougher, T.A.; Bugbee, B. Differences in the Response of Wheat, Soybean and Lettuce to Reduced Blue Radiation. Photochem. Photobiol. 2001, 73, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Izzo, L.G.; Mickens, M.A.; Aronne, G.; Gómez, C. Spectral effects of blue and red light on growth, anatomy, and physiology of lettuce. Physiol. Plant. 2021, 172, 2191–2202. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Kjaer, K.H.; Adnan, M.; Naznin, M.T.; Lim, J.D.; Sung, I.J.; Park, C.H.; Lim, Y.S. The Evaluation of Growth Performance, Photosynthetic Capacity, and Primary and Secondary Metabolite Content of Leaf Lettuce Grown under Limited Irradiation of Blue and Red LED Light in an Urban Plant Factory. Agriculture 2020, 10, 28. [Google Scholar] [CrossRef]
- Ohtake, N.; Ishikura, M.; Suzuki, H.; Yamori, W.; Goto, E. Continuous irradiation with alternating red and blue light enhances plant growth while keeping nutritional quality in lettuce. HortScience 2018, 53, 1804–1809. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Son, K.H.; Oh, M.M. Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. HortScience 2013, 48, 988–995. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Far-red radiation interacts with relative and absolute blue and red photon flux densities to regulate growth, morphology, and pigmentation of lettuce and basil seedlings. Sci. Hortic. 2019, 255, 269–280. [Google Scholar] [CrossRef]
- Chen, X.L.; Xue, X.Z.; Guo, W.Z.; Wang, L.C.; Qiao, X.J. Growth and nutritional properties of lettuce affected by mixed irradiation of white and supplemental light provided by light-emitting diode. Sci. Hortic. 2016, 200, 111–118. [Google Scholar] [CrossRef]
- Possart, A.; Fleck, C.; Hiltbrunner, A. Shedding (far-red) light on phytochrome mechanisms and responses in land plants. Plant Sci. 2014, 217, 36–46. [Google Scholar] [CrossRef]
- Amitrano, C.; Junker, A.; D’Agostino, N.; De Pascale, S.; De Micco, V. Integration of high-throughput phenotyping with anatomical traits of leaves to help understanding lettuce acclimation to a changing environment. Planta 2022, 256, 68. [Google Scholar] [CrossRef] [PubMed]
- Almeida, P.H.; Mógor, Á.F.; Ribeiro, A.Z.; Heinrichs, J.; Amano, E. Increase in lettuce (Lactuca sativa L.) production by foliar calcium application. Aust. J. Basic Appl. Sci. 2016, 10, 161–167. [Google Scholar]
- Lee, J.-S.; Chandra, D.; Son, J. Growth, Physicochemical, Nutritional, and Postharvest Qualities of Leaf Lettuce (Lactuca sativa L.) as Affected by Cultivar and Amount of Applied Nutrient Solution. Horticulturae 2022, 8, 436. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, Y.; Zhang, Y.; Bian, Z.; Fanourakis, D.; Yang, Q.; Li, T. Morphological and physiological properties of indoor cultivated lettuce in response to additional far-red light. Sci. Hortic. 2019, 257, 108725. [Google Scholar] [CrossRef]
- Chen, X.L.; Li, Y.L.; Wang, L.C.; Guo, W.Z. Red and blue wavelengths affect the morphology, energy use efficiency and nutritional content of lettuce (Lactuca sativa L.). Sci. Rep. 2021, 11, 8374. [Google Scholar] [CrossRef]
- Samuolienė, G.; Viršilė, A.; Miliauskienė, J.; Haimi, P.J.; Laužikė, K.; Brazaitytė, A.; Duchovskis, P. The physiological response of lettuce to red and blue light dynamics over different photoperiods. Front. Plant Sci. 2021, 11, 610174. [Google Scholar] [CrossRef]
- Tokarz, K.; Piwowarczyk, B.; Wysocka, A.; Wójtowicz, T.; Makowski, W.; Golemiec, E. Response of grass pea (Lathyrus sativus L.) photosynthetic apparatus to short-term intensive UV-A: Red radiation. Acta Physiol. Plant. 2019, 41, 1–7. [Google Scholar] [CrossRef]
- Li, L.; Tong, Y.X.; Lu, J.L.; Li, Y.M.; Yang, Q.C. Lettuce growth, nutritional quality, and energy use efficiency as affected by red–blue light combined with different monochromatic wavelengths. HortScience 2020, 55, 613–620. [Google Scholar] [CrossRef]
- Chen, X.L.; Wang, L.C.; Li, T.; Yang, Q.C.; Guo, W.Z. Sugar accumulation and growth of let-tuce exposed to different lighting modes of red and blue LED light. Sci. Rep. 2019, 9, 6926. [Google Scholar]
- Borbély, P.; Gasperl, A.; Pálmai, T.; Ahres, M.; Asghar, M.A.; Galiba, G.; Müller, M.; Kocsy, G. Light Intensity- and Spectrum-Dependent Redox Regulation of Plant Metabolism. Antioxidants 2022, 11, 1311. [Google Scholar] [CrossRef] [PubMed]
- Viršilė, A.; Brazaitytė, A.; Vaštakaitė-Kairienė, V.; Miliauskienė, J.; Jankauskienė, J.; Novičkovas, A.; Laužikė, K.; Samuolienė, G. The distinct impact of multi-color LED light on nitrate, amino acid, soluble sugar and organic acid contents in red and green leaf lettuce cultivated in controlled environment. Food Chem. 2020, 310, 125799. [Google Scholar] [CrossRef]
- Miyagi, A.; Uchimiya, H.; Kawai-Yamada, M. Synergistic effects of light quality, carbon dioxide and nutrients on metabolite compositions of head lettuce under artificial growth conditions mimicking a plant factory. Food Chem. 2017, 218, 561–568. [Google Scholar] [CrossRef]
- Signore, A.; Bell, L.; Santamaria, P.; Wagstaff, C.; Van Labeke, M.C. Red light is effective in reducing nitrate concentration in rocket by increasing nitrate reductase activity, and contributes to increased total glucosinolates content. Front. Plant Sci. 2020, 11, 604. [Google Scholar] [CrossRef] [PubMed]
- Brazaitytė, A.; Vaštakaitė-Kairienė, V.; Sutulienė, R.; Rasiukevičiūtė, N.; Viršilė, A.; Miliauskienė, J.; Laužikė, K.; Valiuškaitė, A.; Dėnė, L.; Chrapačienė, S.; et al. Phenolic Compounds Content Evaluation of Lettuce Grown under Short-Term Preharvest Daytime or Nighttime Supplemental LEDs. Plants 2022, 11, 1123. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.J.; Rihan, H.Z.; Aljafer, N.; Fuller, M.P. The Impact of Light Spectrum and Intensity on the Growth, Physiology, and Antioxidant Activity of Lettuce (Lactuca sativa L.). Plants 2021, 10, 2162. [Google Scholar] [CrossRef]
- Chadwick, M.; Gawthrop, F.; Michelmore, R.W.; Wagstaff, C.; Methven, L. Perception of bitterness, sweetness and liking of different genotypes of lettuce. Food Chem. 2016, 197, 66–74. [Google Scholar] [CrossRef]
- Chadwick, M.; Swann, J.R.; Gawthrop, F.; Michelmore, R.; Scaglione, D.; Jose-Truco, M.; Wagstaff, C. Mapping taste and flavour traits to genetic markers in lettuce Lactuca sativa. Food Chem. Mol. Sci. 2024, 9, 100215. [Google Scholar] [CrossRef] [PubMed]
- Galvão, V.C.; Fankhauser, C. Sensing the light environment in plants: Photoreceptors and early signaling steps. Curr. Opin. Neurobiol. 2015, 34, 46–53. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nesi, A.N.; Araújo, W.L.; Braun, H.P. Amino acid catabolism in plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef]
- Trovato, M.; Funck, D.; Forlani, G.; Okumoto, S.; Amir, R. Amino acids in plants: Regulation and functions in development and stress defense. Front. Plant Sci. 2021, 12, 772810. [Google Scholar] [CrossRef]


| Lettuce Type | Greenhouse | Dry Weight Content [% ±SD] |
|---|---|---|
| Iceberg | Control | 4.1 ± 0.02 d |
| Red | 4.4 ± 0.02 c | |
| Butterhead | Control | 5.7 ± 0.05 a |
| Red | 4.7 ± 0.03 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokarz, B.; Gajewski, Z.; Makowski, W.; Mazur, S.; Kiełkowska, A.; Kunicki, E.; Jeremiasz, O.; Szendera, W.; Wesołowski, W.; Tokarz, K.M. The Effect of Head Lettuce (Lactuca sativa var. capitata L.) Cultivation Under Glass with a Light Spectrum-Modifying Luminophore on Crop Traits. Agronomy 2025, 15, 2090. https://doi.org/10.3390/agronomy15092090
Tokarz B, Gajewski Z, Makowski W, Mazur S, Kiełkowska A, Kunicki E, Jeremiasz O, Szendera W, Wesołowski W, Tokarz KM. The Effect of Head Lettuce (Lactuca sativa var. capitata L.) Cultivation Under Glass with a Light Spectrum-Modifying Luminophore on Crop Traits. Agronomy. 2025; 15(9):2090. https://doi.org/10.3390/agronomy15092090
Chicago/Turabian StyleTokarz, Barbara, Zbigniew Gajewski, Wojciech Makowski, Stanisław Mazur, Agnieszka Kiełkowska, Edward Kunicki, Olgierd Jeremiasz, Waldemar Szendera, Wojciech Wesołowski, and Krzysztof M. Tokarz. 2025. "The Effect of Head Lettuce (Lactuca sativa var. capitata L.) Cultivation Under Glass with a Light Spectrum-Modifying Luminophore on Crop Traits" Agronomy 15, no. 9: 2090. https://doi.org/10.3390/agronomy15092090
APA StyleTokarz, B., Gajewski, Z., Makowski, W., Mazur, S., Kiełkowska, A., Kunicki, E., Jeremiasz, O., Szendera, W., Wesołowski, W., & Tokarz, K. M. (2025). The Effect of Head Lettuce (Lactuca sativa var. capitata L.) Cultivation Under Glass with a Light Spectrum-Modifying Luminophore on Crop Traits. Agronomy, 15(9), 2090. https://doi.org/10.3390/agronomy15092090









