Comprehensive Responses of Root System Architecture and Anatomy to Nitrogen Stress in Maize (Zea mays L.) Genotypes with Contrasting Nitrogen Efficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Site
2.3. Field Experiment Design
2.4. Phenotypic Sampling
2.4.1. Sampling Period
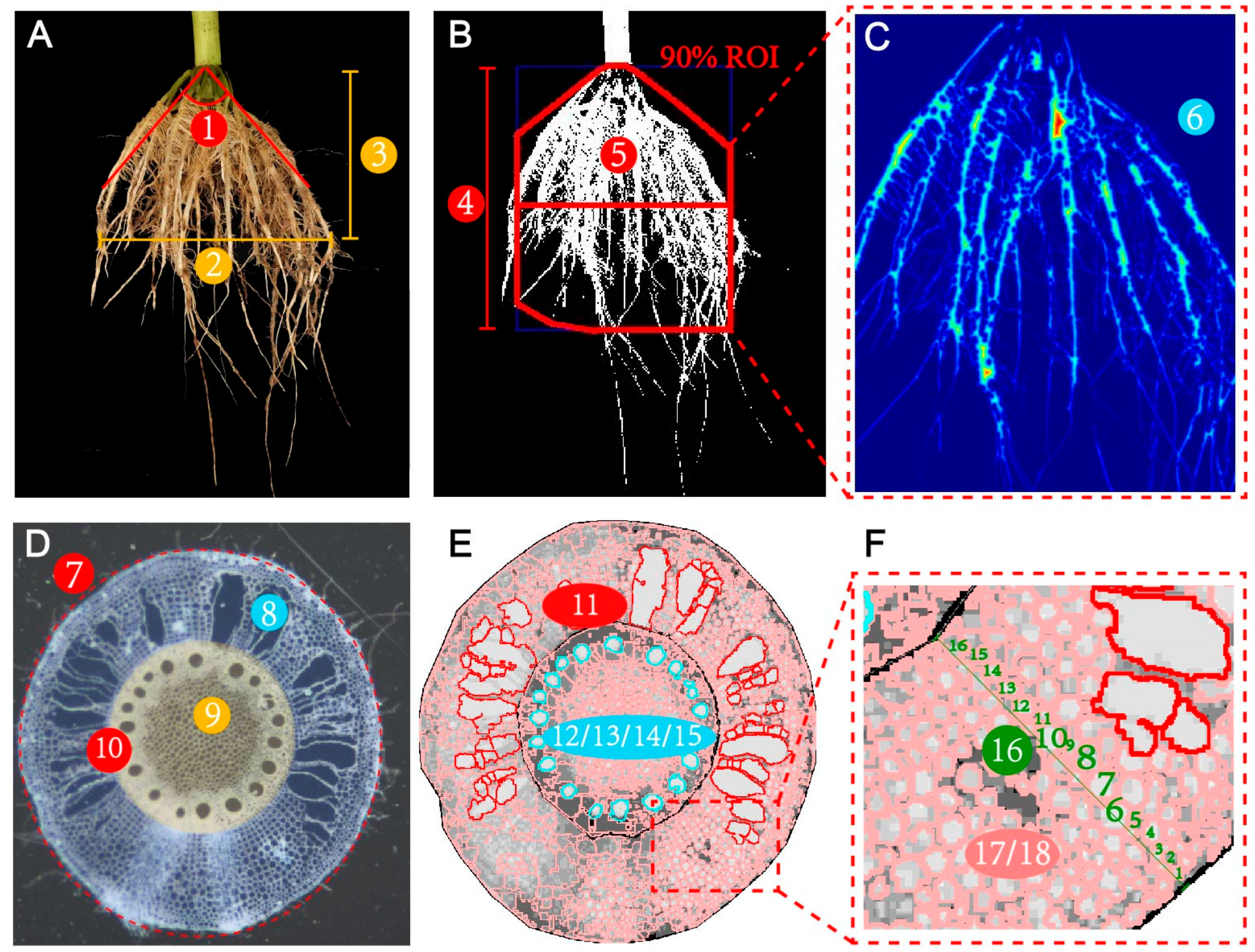
2.4.2. Root System Architecture Sampling and Measurement
2.4.3. Root Anatomy Sampling and Measurement
2.4.4. Grain-Yield-Related Traits
2.4.5. N-Efficiency Parameters Estimation
2.5. Transcriptome Analysis of Parental Inbred Lines
2.6. Statistical Analysis
3. Results
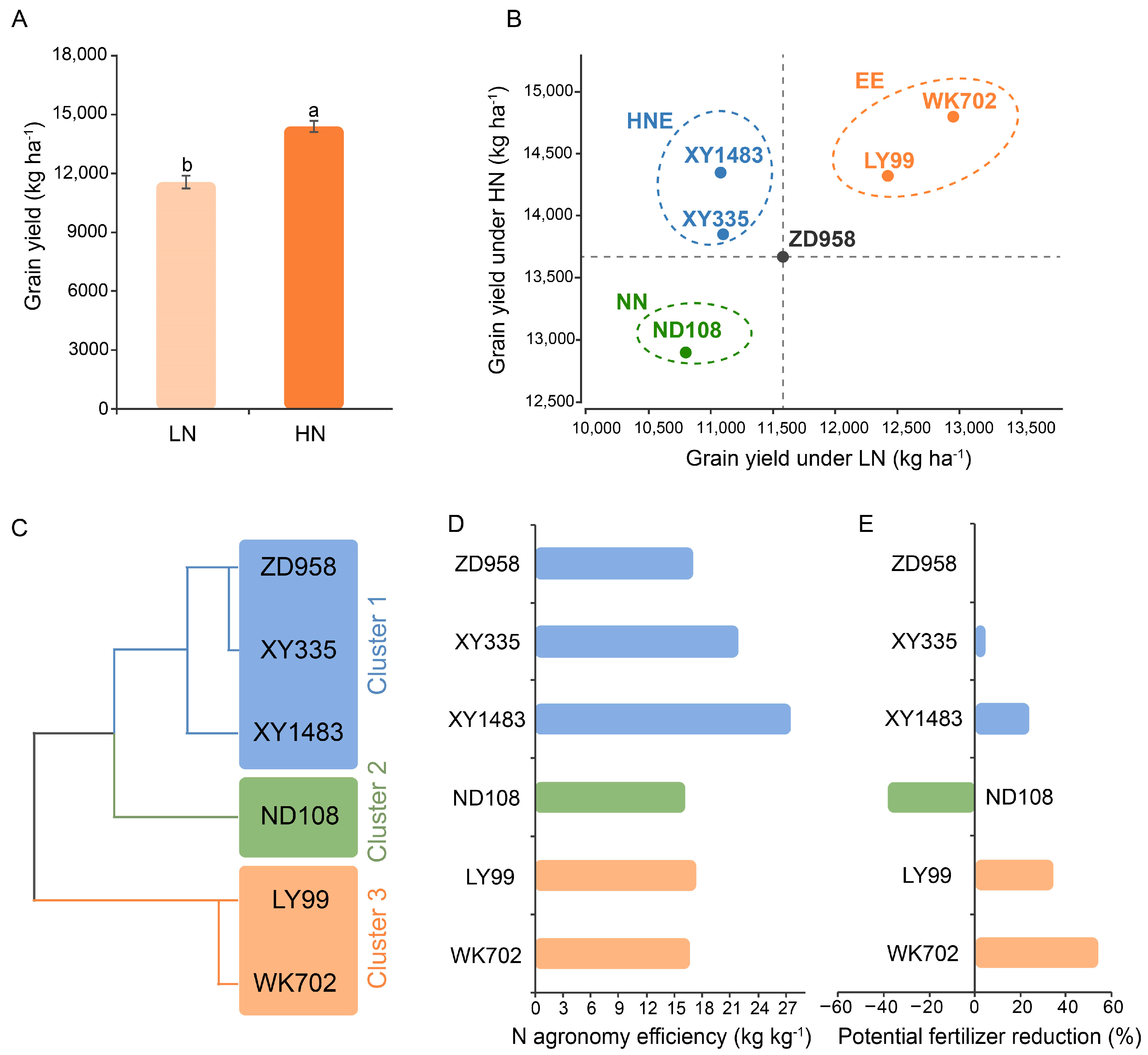
3.1. The Effects of N Levels and Hybrids on N-Efficiency Traits
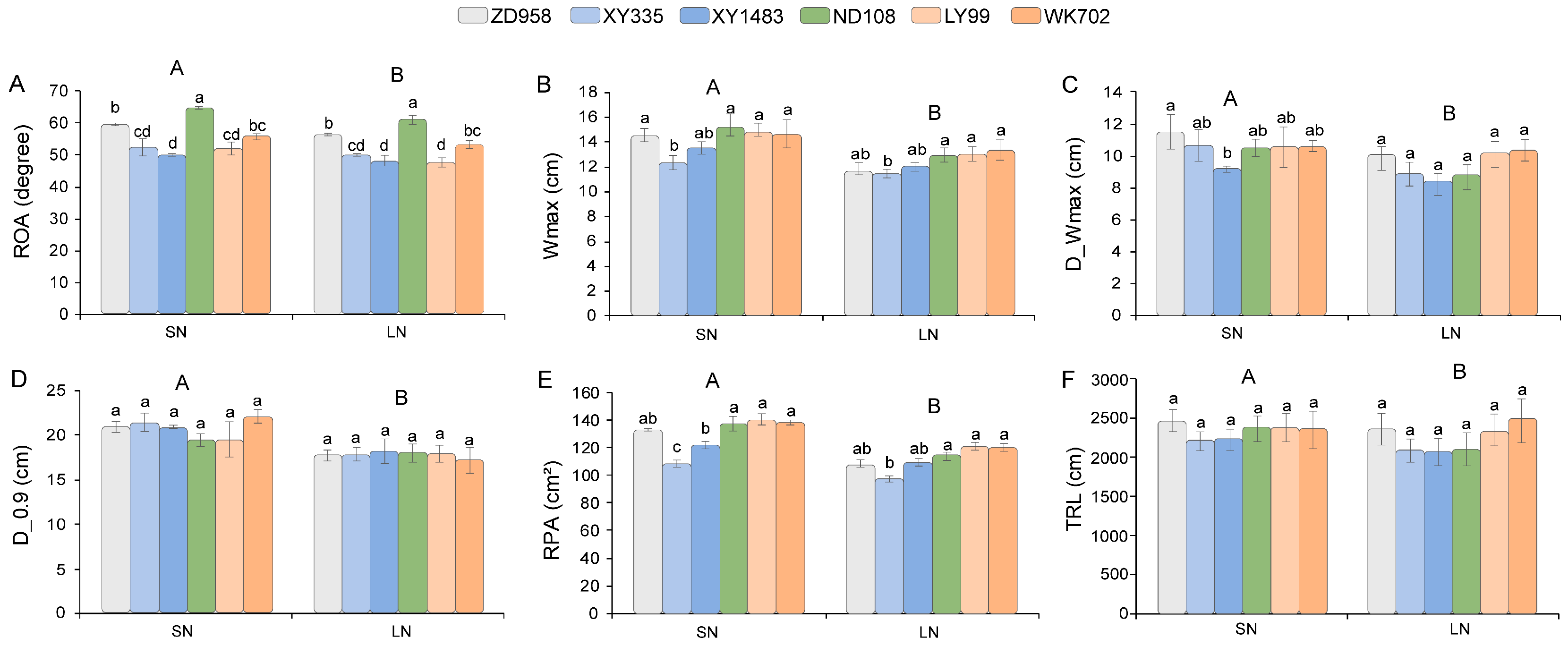
3.2. The Effects of N Levels and Hybrids on Root Architecture Traits
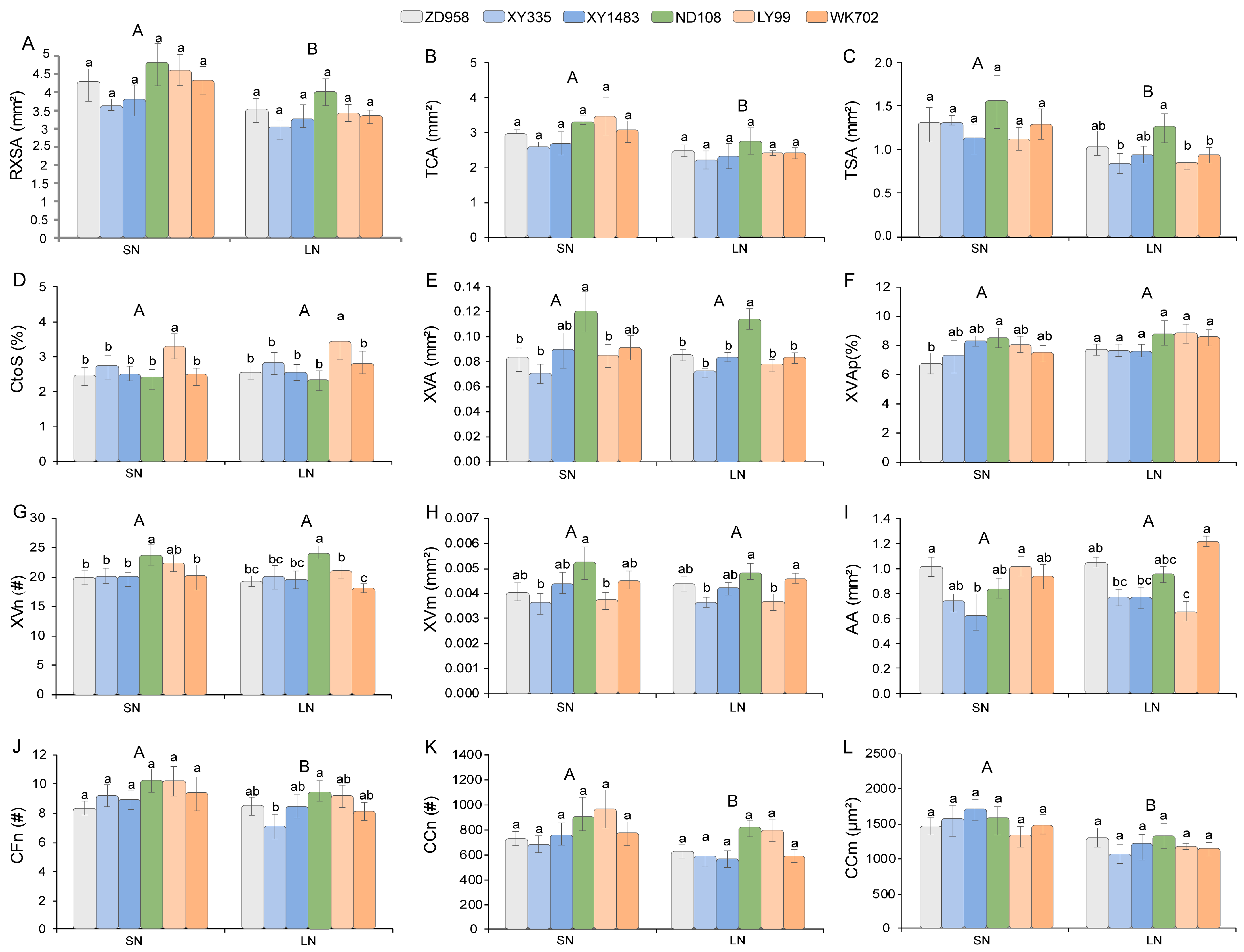
3.3. The Effects of N Levels and Hybrids on Root Anatomical Traits
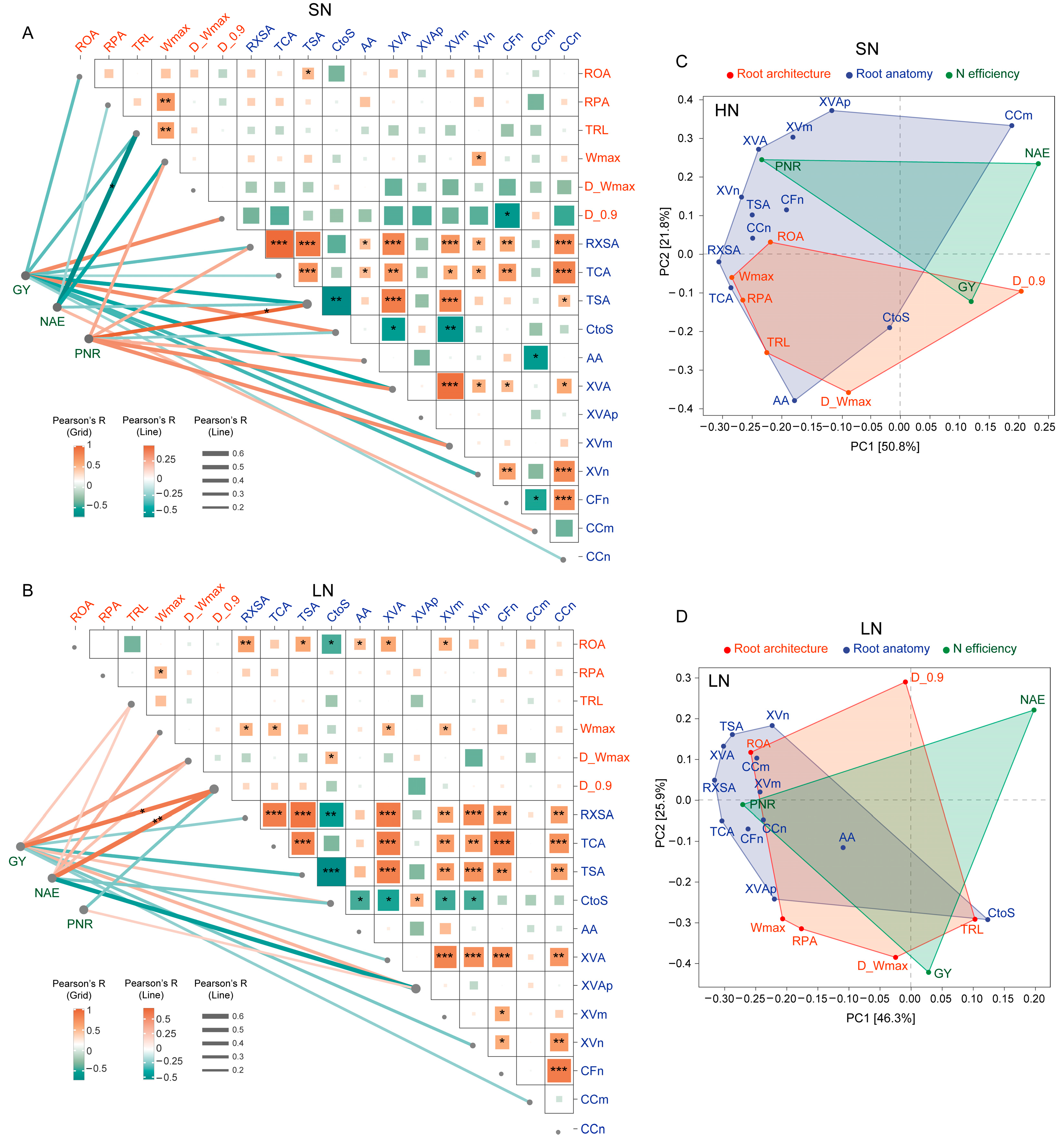
3.4. The Correlation Between Root and N-Efficiency Traits
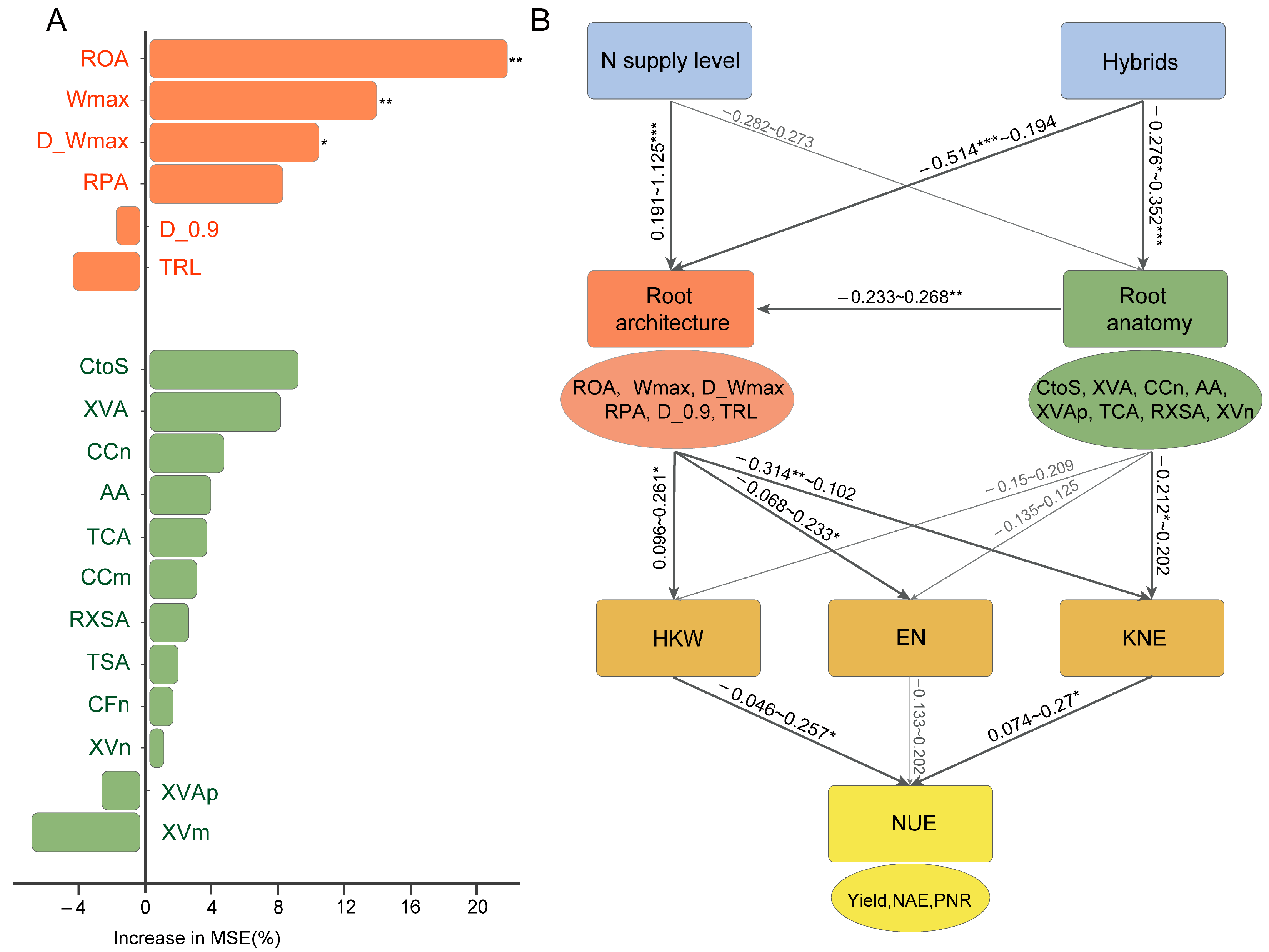
3.5. Random Forest and Structural Equation Modeling Analysis of the Root System and N Efficiency
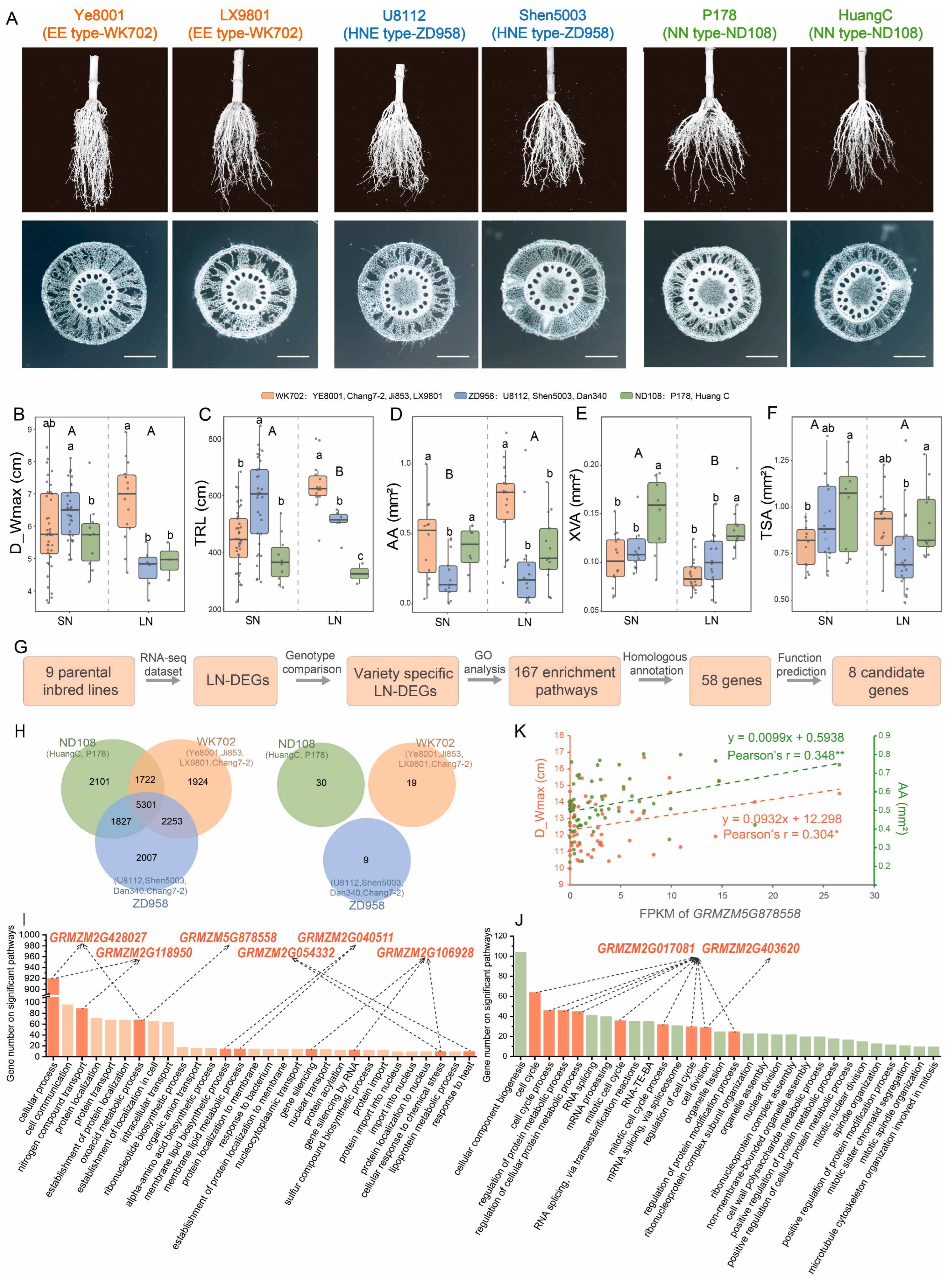
3.6. Transcriptome Analysis of Parental Inbred Lines for Root LN Response
4. Discussion
4.1. Integrated Root System Architecture Anatomy Responses to Low N and Key Traits for N Efficiency
4.2. Deciphering Root Anatomy–Architecture Coregulation Mechanisms in N-Efficient Varieties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Govindasamy, P.; Muthusamy, S.K.; Bagavathiannan, M.; Mowrer, J.; Jagannadham, P.T.K.; Maity, A.; Halli, H.M.; GK, S.; Vadivel, R.; TK, D.; et al. Nitrogen use efficiency—A key to enhance crop productivity under a changing climate. Front. Plant Sci. 2023, 14, 1121073. [Google Scholar] [CrossRef] [PubMed]
- Donovan, T.C.; Comas, L.H.; Schneekloth, J.; Schipanski, M. Nitrogen and water availability affect soil nitrogen mineralization and maize nitrogen uptake dynamics. Nutr. Cycl. Agroecosys. 2025, 130, 387–405. [Google Scholar] [CrossRef]
- Wang, C.; Shen, Y.; Fang, X.T.; Xiao, S.Q.; Liu, G.Y.; Wang, L.G.; Gu, B.J.; Zhou, F.; Chen, D.L.; Tian, H.Q.; et al. Reducing soil nitrogen losses from fertilizer use in global maize and wheat production. Nat. Geosci. 2024, 17, 1008. [Google Scholar] [CrossRef]
- Feyisa, D.S.; Jiao, X.Q.; Mojo, D. Wheat yield response to chemical nitrogen fertilizer application in Africa and China: A meta-analysis. J. Soil Sci. Plant Nutr. 2024, 24, 102–114. [Google Scholar] [CrossRef]
- Moll, R.H.; Kamprath, E.J.; Jackson, W.A. Analysis and Interpretation of Factors Which Contribute to Efficiency of Nitrogen. Agron. J. 1982, 74, 562–564. [Google Scholar] [CrossRef]
- Sapkota, T.B.; Singh, B.; Takele, R. Improving nitrogen use efficiency and reducing nitrogen surplus through best fertilizer nitrogen management in cereal production: The case of India and China. Adv. Agron. 2023, 178, 233–294. [Google Scholar]
- Huang, Y.C.; Wang, H.H.; Zhu, Y.D.; Huang, X.; Li, S.; Wu, X.G.; Zhao, Y.; Bao, Z.G.; Qin, L.; Jin, Y.B.; et al. THP9 enhances seed protein content and nitrogen-use efficiency in maize. Nature 2022, 612, 292–300. [Google Scholar] [CrossRef]
- MacLaren, C.; Mead, A.; van Balen, D.; Claessens, L.; Etana, A.; de Hana, J.; Haagsma, W.; Jäck, O.; Keller, T.; Labuschangne, J.; et al. Long-term evidence for ecological intensification as a pathway to sustainable agriculture. Nat. Sustain. 2022, 5, 770–779. [Google Scholar] [CrossRef]
- George, T.S.; Bulgarelli, D.; Carminati, A.; Chen, Y.; Jones, D.; Kuzyakov, Y.; Schnepf, A.; Wissuwa, M.; Roose, T. Bottom-up perspective-The role of roots and rhizosphere in climate change adaptation and mitigation in agroecosystems. Plant Soil 2024, 500, 297–323. [Google Scholar] [CrossRef]
- Wang, R.F.; Zhong, Y.T.; Han, J.N.; Huang, L.L.; Wang, Y.Q.; Shi, X.G.; Li, M.F.; Zhuang, Y.; Ren, W.; Liu, X.T.; et al. NIN-LIKE PROTEIN3.2 inhibits repressor Aux/IAA14 expression and enhances root biomass in maize seedlings under low nitrogen. Plant Cell 2024, 36, 4388–4403. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, Z.; Amtmann, A. Food for thought: How nutrients regulate root system architecture. Curr. Opin. Plant Bio. 2017, 39, 80–87. [Google Scholar] [CrossRef]
- Liu, X.J.; Huang, K.; Chu, C.C. The genetic basis of nitrogen-dependent root system architecture in plants. Front. Agr. Sci. Eng. 2025, 12, 3–15. [Google Scholar]
- Morris, E.C.; Griffiths, M.; Golebiowska, A.; Mairhofer, S.; Burr-Hersy, J.; Goh, T.; von Wangenheim, D.; Atkinson, B.; Sturrock, C.J.; Lynch, J.P.; et al. Shaping 3D root system architecture. Curr. Biol. 2017, 27, 919–930. [Google Scholar] [CrossRef]
- Ren, W.; Zhao, L.F.; Liang, J.X.; Wang, L.F.; Chen, L.M.; Li, P.C.; Liu, Z.G.; Li, X.J.; Zhang, Z.H.; Li, J.P.; et al. Genome-wide dissection of changes in maize root system architecture during modern breeding. Nat. Plants 2022, 8, 1408–1422. [Google Scholar] [CrossRef]
- Lynch, J.P. Root phenotypes for improved nutrient capture: An underexploited opportunity for global agriculture. New Phytol. 2019, 223, 548–564. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, W.; Yi, X.; Li, Q.; Cai, H.G.; Ali, F.; Yuan, L.X.; Mi, G.H.; Pan, Q.C.; Chen, F.J. Local nitrogen application increases maize post-silking nitrogen uptake of responsive genotypes via enhanced deep root growth. J. Integr. Agr. 2023, 22, 235–250. [Google Scholar] [CrossRef]
- Saengwilai, P.; Tian, X.L.; Lynch, J.P. Low crown root number enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiol. 2024, 166, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Lankhost, J.A.; Stomph, T.J.; Schneider, H.M.; Chen, Y.; Mi, G.H.; Yuan, L.; Evers, J.B. Root plasticity improves maize nitrogen use when nitrogen is limiting: An analysis using 3D plant modelling. J. Exp. Bot. 2024, 75, 5989–6005. [Google Scholar] [CrossRef]
- Du, P.Z.; Lynch, J.P.; Sun, Z.; Sun, Z.L.; Li, F.M. Does root respiration and root anatomical traits affect crop yield under stress? A meta-analysis and experimental study. Plant Soil 2025, 509, 763–777. [Google Scholar] [CrossRef]
- Schneider, H.M.; Postma, J.A.; Wojciechowski, T.; Kuppe, C.; Lynch, J.P. Root cortical senescence improves growth under suboptimal availability of N, P, and K. Plant Physiol. 2017, 174, 2333–2347. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wu, G.; Strock, C.; Li, L.; Dong, S.; Zhang, J.; Zhao, B.; Lynch, J.P.; Liu, P. Root anatomical phenotypes related to growth under low nitrogen availability in maize (Zea mays L.) hybrids. Plant Soil 2022, 474, 265–276. [Google Scholar] [CrossRef]
- Lynch, J.P.; Strock, C.F.; Schneider, H.M.; Sidhu, J.S.; Ajmera, I.; Galindo-Castañedaet, T.; Klein, S.P.; Hanlon, M.T. Root anatomy and soil resource capture. Plant Soil 2021, 466, 21–63. [Google Scholar] [CrossRef]
- Postma, J.A.; Lynch, J.P. Root cortical aerenchyma enhances the growth of maize on soils with suboptimal availability of nitrogen, phosphorus, and potassium. Plant Physiol. 2011, 156, 1190–1201. [Google Scholar] [CrossRef]
- Saengwilai, P.; Nord, E.A.; Chimungu, J.G.; Brown, K.M.; Lynch, J.P. Root cortical aerenchyma enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiol. 2014, 166, 726–735. [Google Scholar] [CrossRef]
- Gao, K.; Chen, F.J.; Yuan, L.X.; Zhang, F.S.; Mi, G.H. A comprehensive analysis of root morphological changes and nitrogen allocation in maize in response to low nitrogen stress. Plant Cell Environ. 2015, 38, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Kumi, F.; Obour, P.B.; Arthur, E.; Moore, S.E.; Asare, P.A.; Asiedu, J.; Angnuureng, D.B.; Atiah, K.; Amoah, K.K.; Amponsah, S.K.; et al. Quantifying root-induced soil strength, measured as soil penetration resistance, from different crop plants and soil types. Soil Tillage Res. 2023, 233, 105811. [Google Scholar] [CrossRef]
- Yang, J.T.; Schneider, H.M.; Brown, K.M.; Lynch, J.P. Genotypic variation and nitrogen stress effects on root anatomy in maize are node specific. J. Exp. Bot. 2019, 70, 5311–5325. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Lynch, J.P.; Brown, K.M. Responses of root architectural and anatomical traits to low nitrogen stress in rice. bioRxiv 2024. [Google Scholar] [CrossRef]
- Galindo-Castañeda, T.; Lynch, J.P.; Six, J.; Hartmann, M. Improving soil resource uptake by plants through capitalizing on synergies between root architecture and anatomy and root-associated microorganisms. Front. Plant Sci. 2022, 13, 827369. [Google Scholar] [CrossRef]
- Yang, Y.; Bao, W.K.; Hu, H.; Wu, N.; Li, F.L.; Wang, Z.L.; Hu, B.; Yang, T.H.; Li, X.J. Environmental factors drive latitudinal patterns of fine-root architectures of 96 xerophytic species in the dry valleys of Southwest China. Sci. Total Environ. 2024, 950, 175352. [Google Scholar] [CrossRef]
- Fujii, K. Plant strategy of root system architecture and exudates for acquiring soil nutrients. Ecol. Res. 2024, 39, 623–633. [Google Scholar] [CrossRef]
- Heymans, A.; Couvreur, V.; LaRue, T.; Paez-Garcia, A.; Lobet, G. GRANAR, a computational tool to better understand the functional importance of monocotyledon root anatomy. Plant Physiol. 2020, 182, 707–720. [Google Scholar] [CrossRef]
- Galindo-Castañeda, T.; Brown, K.M.; Kuldau, G.A.; Roth, G.W.; Wenner, N.G.; Ray, S.; Schneider, H.; Lynch, J.P. Root cortical anatomy is associated with differential pathogenic and symbiotic fungal colonization in maize. Plant Cell Environ. 2019, 42, 2999–3014. [Google Scholar] [CrossRef]
- Lynch, J.P.; Galindo-Castañeda, T.; Schneider, H.M.; Sidhu, J.S.; Rangarajan, H.; York, L.M. Root phenotypes for improved nitrogen capture. Plant Soil 2024, 502, 31–85. [Google Scholar] [CrossRef]
- York, L.M.; Galindo-Castaneda, T.; Schussler, J.R.; Lynch, J.P. Evolution of US maize (Zea mays L.) root architectural and anatomical phenes over the past 100 years corresponds to increased tolerance of nitrogen stress. J. Exp. Bot. 2015, 66, 2347–2358. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Y.Y.; Sheng, D.C.; Zhang, S.; Guo, S.C.; Yan, Y.; Zhao, F.C.; Wang, P.; Huang, S.B. Optimizing root system architecture to improve root anchorage strength and nitrogen absorption capacity under high plant density in maize. Field Crops Res. 2023, 303, 109109. [Google Scholar] [CrossRef]
- Lopez-Valdivia, I.; Yang, X.; Lynch, J.P. Large root cortical cells and reduced cortical cell files improve growth under suboptimal nitrogen regimes in silico. Plant Physiol. 2023, 192, 2261–2275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Y.; Xu, G.; Li, J.S.; Yan, J.B.; Li, H.H.; Yang, X.H. Patterns of genomic variation in Chinese maize inbred lines and implications for genetic improvement. Theor. Appl. Genet. 2018, 131, 1207–1221. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.P.; Zhao, H.N.; Ren, L.H.; Song, W.B.; Zeng, B.; Guo, J.J.; Wang, B.B.; Liu, Z.P.; Chen, J.; Li, W.; et al. Genome-wide genetic changes during modern breeding of maize. Nat. Genet. 2012, 44, 812–815. [Google Scholar] [CrossRef]
- D’Andrea, K.; Otegui, M.E.; Cirilo, A.G. Kernel number determination differs among maize hybrids in response to nitrogen. Field Crop. Res. 2008, 105, 228–239. [Google Scholar] [CrossRef]
- Shao, H.; Shi, D.F.; Shi, W.J.; Ban, X.B.; Chen, Y.C.; Ren, W.; Chen, F.J.; Mi, G.H. Genotypic difference in the plasticity of root system architecture of field-grown maize in response to plant density. Plant Soil 2019, 439, 201–217. [Google Scholar] [CrossRef]
- Trachsel, S.; Kaeppler, S.M.; Brown, K.M.; Lynch, J.P. Shovelomics: High throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant Soil. 2011, 341, 75–87. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, J.L.; Li, D.D.; Li, P.C.; He, K.H.; Ali, F.; Mi, G.H.; Chen, F.J.; Yuan, L.X.; Pan, Q.C. Plasticity of root anatomy during domestication of a maize-teosinte derived population. J. Exp. Bot. 2022, 73, 139–153. [Google Scholar] [CrossRef]
- Burton, A.L.; Williams, M.; Lynch, J.P.; Brown, K.M. RootScan: Software for high-throughput analysis of root anatomical traits. Plant Soil. 2012, 357, 189–203. [Google Scholar] [CrossRef]
- Liu, Z.G.; Zhao, Y.; Guo, S.; Cheng, S.; Guan, Y.J.; Cai, H.G.; Mi, G.H.; Yuan, L.X.; Chen, F.J. Enhanced crown root number and length confers potential for yield improvement and fertilizer reduction in nitrogen-efficient maize cultivars. Field Crop. Res. 2019, 241, 107562. [Google Scholar] [CrossRef]
- Chen, X.C.; Chen, F.J.; Chen, Y.; Gao, Q.; Yang, X.L.; Yuan, L.X.; Zhang, F.S.; Mi, G.H. Modern maize hybrids in Northeast China tolerate exhibit increased yield potential and resource use efficiency despite the adverse climate change. Global Change Biol. 2013, 19, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Jevtic, S.; Drapek, C.; Gaillochet, C.; Brockman, A.; Cadman, J.; Flesh, T.; Kral, N. A scalable method for modulating plant gene expression using a multispecies genomic model and protoplast-based massively parallel reporter assay. bioRxiv 2024. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Jiao, S.; Chen, W.; Wang, J.; Du, N.; Li, Q.; Wei, G. Soil microbiomes with distinct assemblies through vertical soil profiles drive the cycling of multiple nutrients in reforested ecosystems. Microbiome 2018, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Hair, J.F.; Hult, G.T.M.; Ringle, C.M.; Sarstedt, M. A Primer on Partial Least Squares Structural Equation Modeling (PLS-SEM), 2nd ed.; Sage: Thousand Oaks, CA, USA, 2017. [Google Scholar]
- Li, P.C.; Zhang, Z.H.; Xiao, G.; Zhao, Z.; He, K.H.; Yang, X.H.; Pan, Q.C.; Mi, G.H.; Jia, Z.T.; Yan, J.B.; et al. Genomic basis determining root system architecture in maize. Theor. Appl. Genet. 2024, 137, 102. [Google Scholar] [CrossRef]
- Friero, I.; Larriba, E.; Melgarejo, P.A.M.; Clemente, M.S.J.; Alarcon, M.V.; Albacete, A.; Salguero, J.; Pérez, J.M. Transcriptomic and hormonal analysis of the roots of maize seedlings grown hydroponically at low temperature. Plant Sci. 2022, 326, 111525. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.R.; Liu, Z.; Guo, J.; Jia, Z.T.; Shi, Y.D.; Kang, K.; Peng, W.S.; Wang, Z.K.; Chen, L.M.; Neuhäuser, B.; et al. ZmNRT1.1B (ZmNPF6.6) determines nitrogen use efficiency via regulation of nitrate transport and signaling in maize. Plant Biotechnol. J. 2024, 22, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xu, J.Y.; Zhao, Y.D.; Wang, Y.C.; Ling, Z.; Ning, L.H.; Shabala, S.; Zhao, H. Transcription factor ZmEREB97 regulates nitrate uptake in maize (Zea mays) root. Plant Physiol. 2024, 196, 535–550. [Google Scholar] [CrossRef]
- Pal, G.; Saxena, S.; Kumar, K.; Verma, A.; Kumar, D.; Shukla, P.; Pandey, A.; White, J.; Verma, S.K. Seed endophytic bacterium Lysinibacillus sp. (ZM1) from maize (Zea mays L.) shapes its root architecture through modulation of auxin biosynthesis and nitrogen metabolism. Plant Physiol. Bioch. 2024, 212, 108731. [Google Scholar] [CrossRef]
- Martín-Roldán, M.; Würsig, H.; Tarkka, M.T.; Hartwig, R.P.; Wimmer, M.A.; Blagodatskaya, E. Maize roots modulate microbial functional traits in the rhizosphere to mitigate drought stress. Soil Biol. Biochem. 2025, 207, 109837. [Google Scholar] [CrossRef]
- Ma, N.N.; Dong, L.N.; Lv, W.; Lv, J.L.; Meng, Q.W.; Liu, P. Transcriptome analysis of maize seedling roots in response to nitrogen-, phosphorus-, and potassium deficiency. Plant Soil. 2020, 447, 637–658. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Li, Y.Y.; Pan, L.Y.; Lambers, H.; Wang, X.R. Intercropping promotes maize growth by enhancing accumulation of specific metabolites in the rhizosphere and synergistic interaction between arbuscular mycorrhizal fungi and Bacillus. Plant Soil. 2025; published online. [Google Scholar] [CrossRef]
- Adhikari, A.; Roy, D.; Adhikari, S.; Saha, S.; Ghosh, P.K.; Shaw, A.K.; Hossain, Z. microRNAomic profiling of maize root reveals multifaceted mechanisms to cope with Cr (VI) stress. Plant Physiol. Biochem. 2023, 198, 107693. [Google Scholar] [CrossRef]
- Yu, P.; White, P.J.; Hochholdinger, F.; Li, C.J. Phenotypic plasticity of the maize root system in response to heterogeneous nitrogen availability. Planta 2014, 240, 667–678. [Google Scholar] [CrossRef]
- Ding, Y.; Huang, X.; Li, Y.; Liu, H.Y.; Zhang, Q.C.; Liu, X.M.; Xu, J.M.; Di, H.J. Nitrate leaching losses mitigated with intercropping of deep-rooted and shallow-rooted plants. J. Soil Sediments. 2021, 21, 364–375. [Google Scholar] [CrossRef]
- Hu, R.; Ding, Z.J.; Tian, Y.B.; Cao, Y.X.; Hou, J.; Wang, X.X. Localized nitrogen supply facilitates rice yield and nitrogen use efficiency by enabling root-zone nitrogen distribution and root growth. Front. Sustain. Food Syst. 2024, 8, 1326311. [Google Scholar] [CrossRef]
- Kong, D.L.; Wang, J.J.; Wu, H.F.; Valcerde-Barrantes, O.J.; Wang, R.L.; Zeng, H.; Kardol, P.; Zhang, H.Y.; Feng, Y.L. Nonlinearity of root trait relationships and the root economics spectrum. Nat. Commun. 2019, 10, 2203. [Google Scholar] [CrossRef]
- Voxeur, A.; Wang, Y.; Sibout, R. Lignification: Different mechanisms for a versatile polymer. Curr. Opin. Plant Biol. 2015, 23, 83–90. [Google Scholar] [CrossRef]
- Ertiro, B.T.; Das, B.; Kosgei, T.; Tesfaye, A.T.; Labuschagne, M.T.; Worku, M.; Olsen, M.S.; Chaikam, V.; Gowda, M. Relationship between grain yield and quality traits under optimum and low-nitrogen stress environments in tropical maize. Agronomy 2022, 12, 438. [Google Scholar] [CrossRef]
- Fry, E.L.; Evans, A.L.; Sturrock, C.J.; Bullock, J.M.; Bardgett, R.D. Root architecture governs plasticity in response to drought. Plant Soil. 2018, 433, 189–200. [Google Scholar] [CrossRef]
- Silva, J.R.; Yule, T.; Ribas, A.C.A.; Scremin-Dias, E. Do root secondary xylem functional traits differ between growth forms in Fabaceae species in a seasonally dry Neotropical environment? Ann. Bot. 2023, 132, 401–412. [Google Scholar] [CrossRef]
- Yu, P.; Li, C.H.; Li, M.; He, X.M.; Wang, D.N.; Li, H.J.; Marcon, C.; Li, Y.; Perez-Limón, S.; Chen, X.P.; et al. Seedling root system adaptation to water availability during maize domestication and global expansion. Nat. Genet. 2024, 56, 1245–1256. [Google Scholar] [CrossRef]
- Hochholdinger, F.; Marcon, C.; Baldauf, J.; Yu, P.; Frey, F.P. Proteomics of maize root development. Front. Plant Sci. 2018, 9, 143. [Google Scholar] [CrossRef]
- Smith, S.J.; Trevisan, W.; McCunn, A.; Huffman, W.E. Global dependence on corn belt dent maize germplasm: Challenges and opportunities. Crop Sci. 2022, 62, 2039–2066. [Google Scholar] [CrossRef]
| Hybrids | SN | LN | ||||||
|---|---|---|---|---|---|---|---|---|
| GY (kg ha−1) | EN (104 ha−1) | KNE (ear−1) | HKW (g) | GY (kg ha−1) | EN (104 ha−1) | KNE (ear−1) | HKW (g) | |
| ZD958 | 13,650.0 ± 445.0 ab | 5.7 ± 0.2 ab | 556.8 ± 22.7 b | 24.9 ± 1.5 a | 11,529.6 ± 444.5 ab | 5.7 ± 0.1 a | 522.0 ± 33.7 b | 25.5 ± 1.2 a |
| XY335 | 13,772.6 ± 199.3 ab | 5.8 ± 0.1 a | 561.7 ± 28.1 b | 26.56 ± 0.4 a | 11,111.5 ± 455.7 ab | 5.6 ± 0.1 ab | 560.5 ± 24.0 b | 23.7 ± 0.5 a |
| XY1483 | 14,453.8 ± 592.1 a | 5.5 ± 0.1 b | 617.7 ± 54.0 a | 25.4 ± 1.4 a | 11,110.5 ± 427.3 ab | 5.5 ± 0.1 abc | 553.8 ± 43.3 b | 23.0 ± 1.2 a |
| ND108 | 12,886.4 ± 439.2 b | 5.6 ± 0.1 ab | 528.4 ± 31.8 b | 27.05 ± 1.6 a | 10,893.5 ± 528.1 b | 5.4 ± 0.1 c | 547.4 ± 26.3 b | 23.5 ± 0.9 a |
| LY99 | 14,380.5 ± 499.8 a | 5.6 ± 0.1 ab | 574.4 ± 19.9 b | 23.91 ± 0.9 a | 12,255.9 ± 340.6 ab | 5.6 ± 0.1 ab | 563.3 ± 28.2 b | 24.2 ± 0.9 a |
| WK702 | 14,766.4 ± 423.0 a | 5.8 ± 0.1 a | 545.1 ± 46.9 b | 27.41 ± 0.5 a | 12,717.7 ± 791.6 a | 5.5 ± 0.1 bc | 611.8 ± 35.7 a | 26.1 ± 1 a |
| Gene ID | Gene Names | Source | GO Enrichment Pathways | Reference |
|---|---|---|---|---|
| GRMZM2G403620 | rs2—rough sheath2 | ND108 | cell cycle process (CCP) | [52] |
| GRMZM2G017081 | cyc9—cyclin9 | ND108 | mitotic cell cycle process (MCCP), cell cycle process, mitotic cell cycle, regulation of cell cycle, regulation of protein modification process, cell division | [53] |
| GRMZM2G428027 | nnr5—nitrate reductase5 | WK702 | cellular process (CP) | [54,55] |
| GRMZM2G118950 | amt3—ammonium transporter3 | WK702 | nitrogen compound transport (NCT), cellular process | [56] |
| GRMZM2G054332 | abcg1—ABC transporter G family member 1 | WK702 | cellular process, response to heat (RH) | [57] |
| GRMZM5G878558 | nnr2—nitrate reductase2 | WK702 | oxoacid metabolic process (OMP), cellular process | [10,58] |
| GRMZM2G040511 | pco091925 | WK702 | membrane lipid biosynthetic process, membrane lipid metabolic process (MLMP) | [59] |
| GRMZM2G106928 | sod14—superoxide dismutase14 | WK702 | gene silencing by RNA, gene silencing, cellular response to chemical stress (CRCS) | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Hou, Y.; Yan, J.; Cheng, S.; Wang, Y.; Feng, G.; Cai, H. Comprehensive Responses of Root System Architecture and Anatomy to Nitrogen Stress in Maize (Zea mays L.) Genotypes with Contrasting Nitrogen Efficiency. Agronomy 2025, 15, 2083. https://doi.org/10.3390/agronomy15092083
Chen Z, Hou Y, Yan J, Cheng S, Wang Y, Feng G, Cai H. Comprehensive Responses of Root System Architecture and Anatomy to Nitrogen Stress in Maize (Zea mays L.) Genotypes with Contrasting Nitrogen Efficiency. Agronomy. 2025; 15(9):2083. https://doi.org/10.3390/agronomy15092083
Chicago/Turabian StyleChen, Zhe, Yuzhuo Hou, Jianxin Yan, Song Cheng, Yin Wang, Guozhong Feng, and Hongguang Cai. 2025. "Comprehensive Responses of Root System Architecture and Anatomy to Nitrogen Stress in Maize (Zea mays L.) Genotypes with Contrasting Nitrogen Efficiency" Agronomy 15, no. 9: 2083. https://doi.org/10.3390/agronomy15092083
APA StyleChen, Z., Hou, Y., Yan, J., Cheng, S., Wang, Y., Feng, G., & Cai, H. (2025). Comprehensive Responses of Root System Architecture and Anatomy to Nitrogen Stress in Maize (Zea mays L.) Genotypes with Contrasting Nitrogen Efficiency. Agronomy, 15(9), 2083. https://doi.org/10.3390/agronomy15092083








