Nutritional Value and Aerobic Stability of Safflower (Carthamus tinctorius L.) Silages Supplemented with Additives
Abstract
1. Introduction
2. Materials and Methods
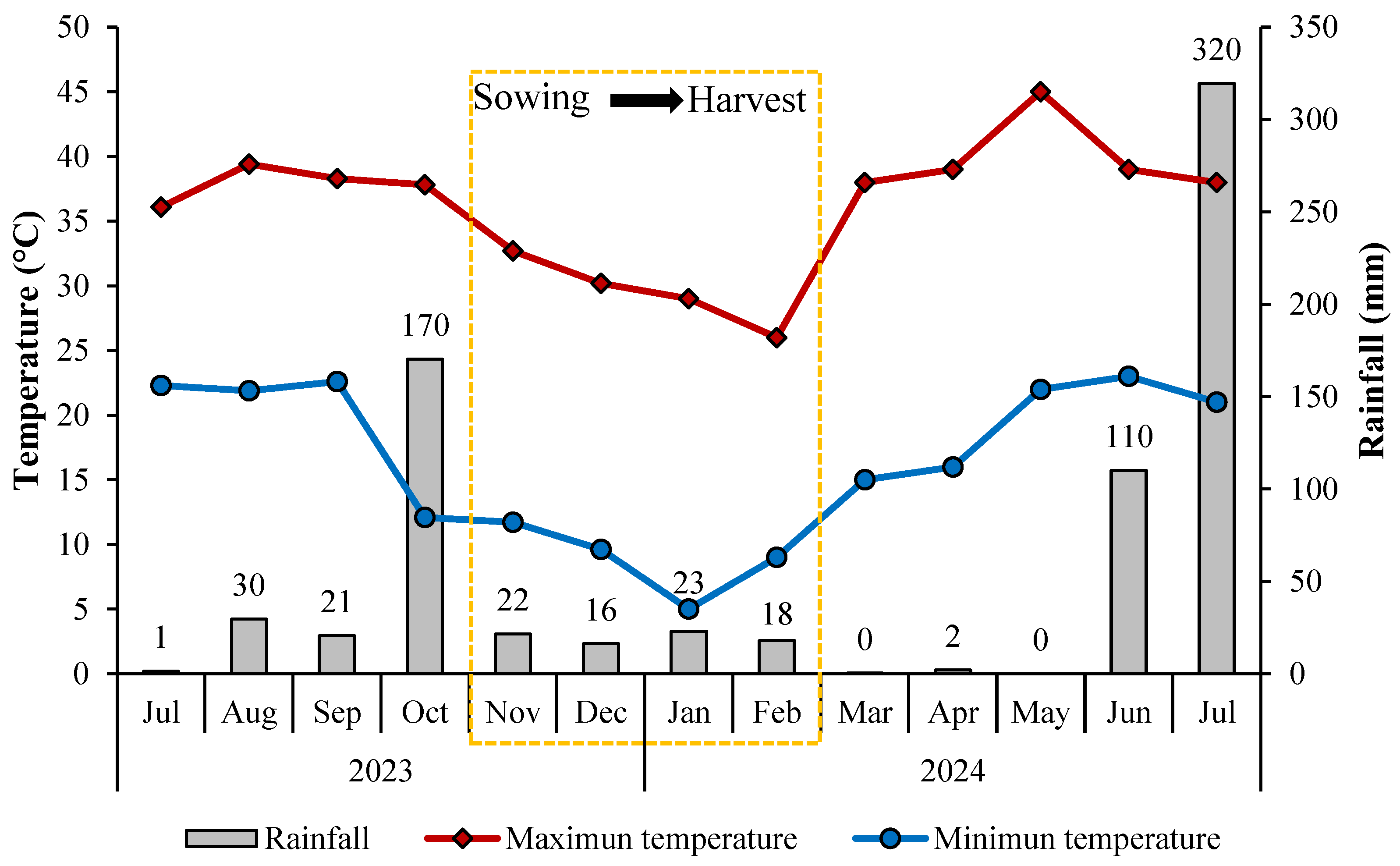
2.1. Location of the Experimental Site
2.2. Sowing and Characteristics of Safflower Forage Before Ensiling
2.3. Silage Preparation and Treatments Evaluated
2.4. Evaluation of Nutritive Value and Aerobic Stability
2.5. Statistical Analysis
3. Results and Discussion
3.1. Nutritional Value of Safflower Silage
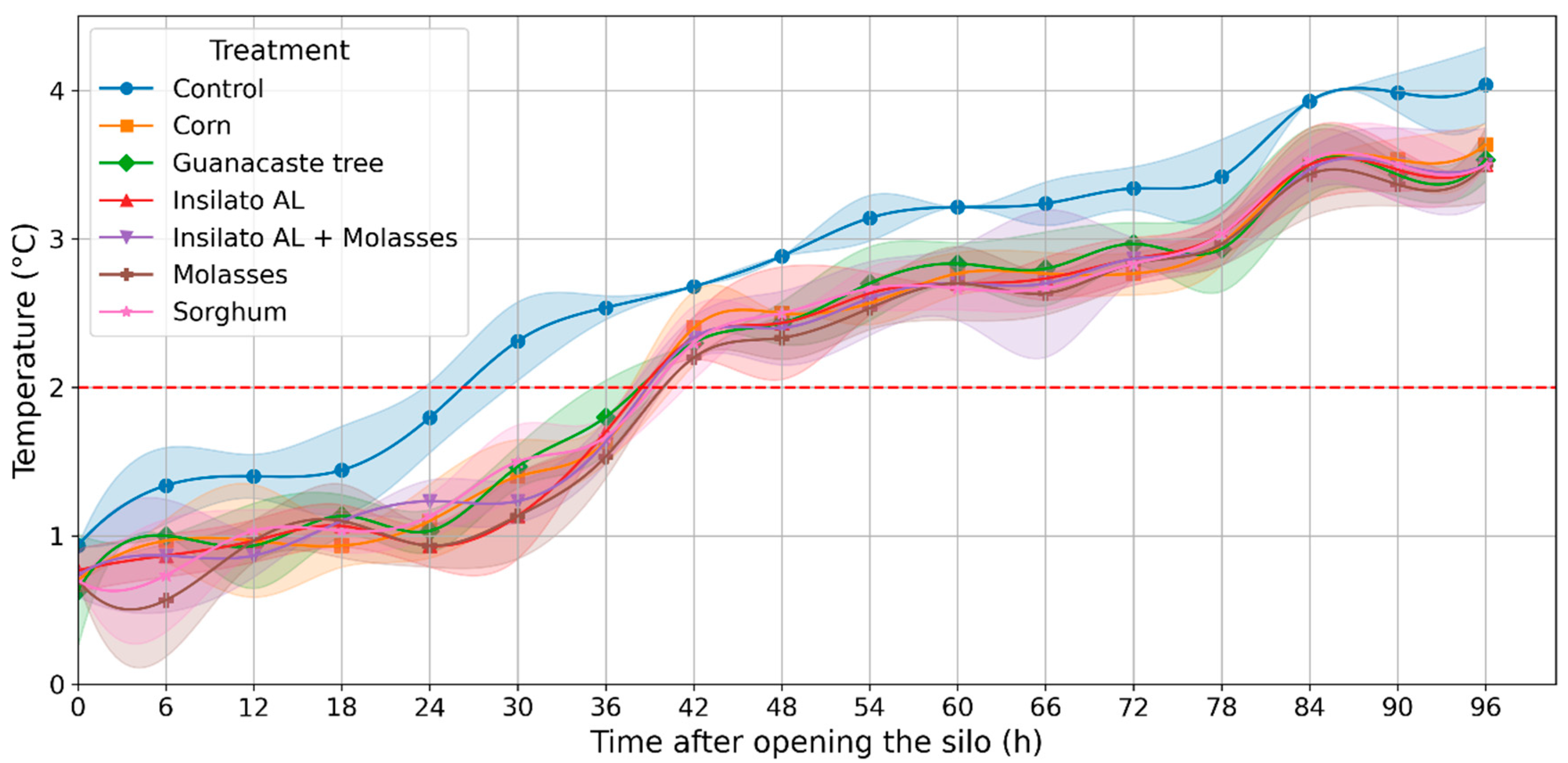
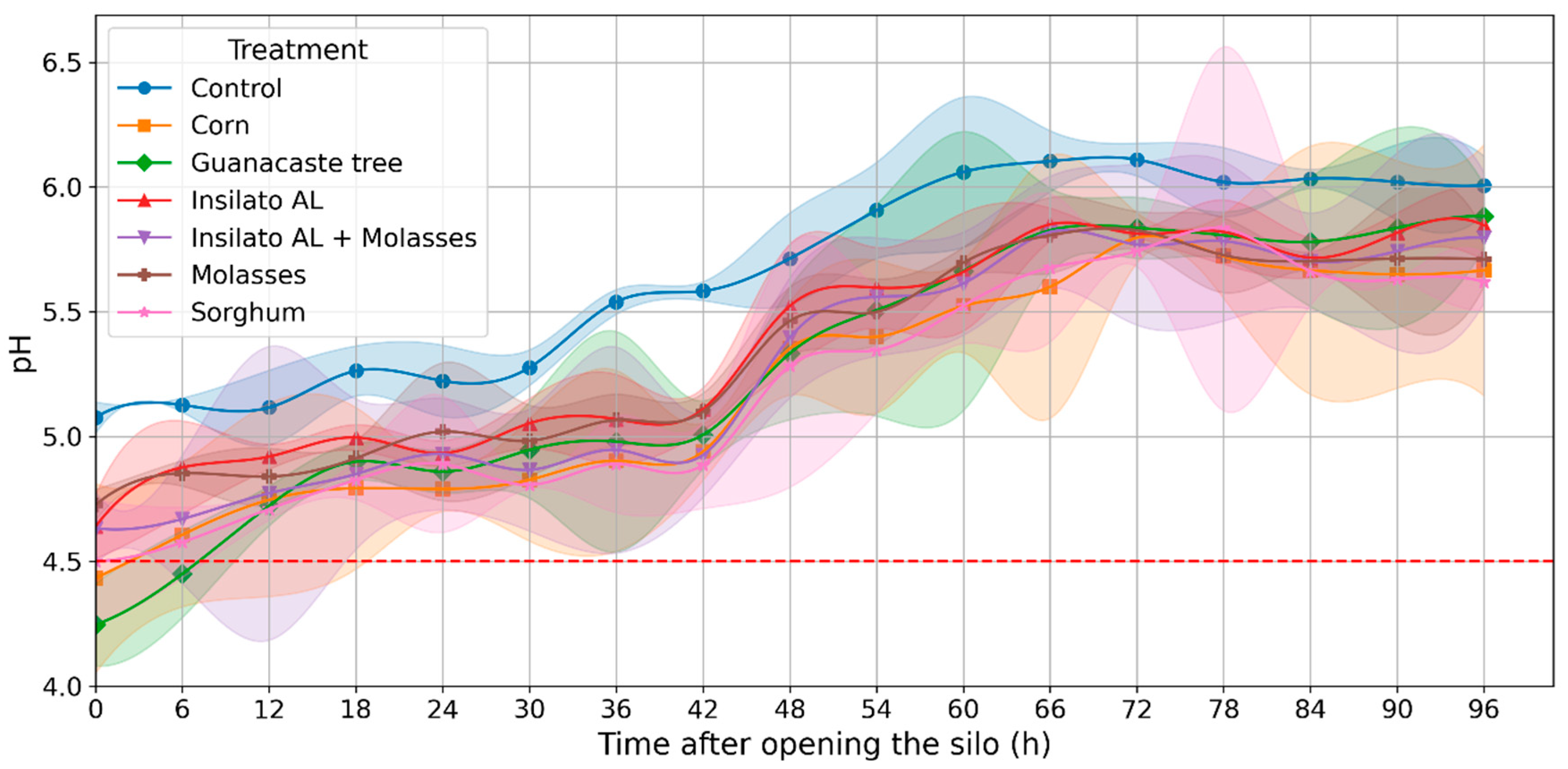
3.2. Evaluation of Aerobic Stability and pH
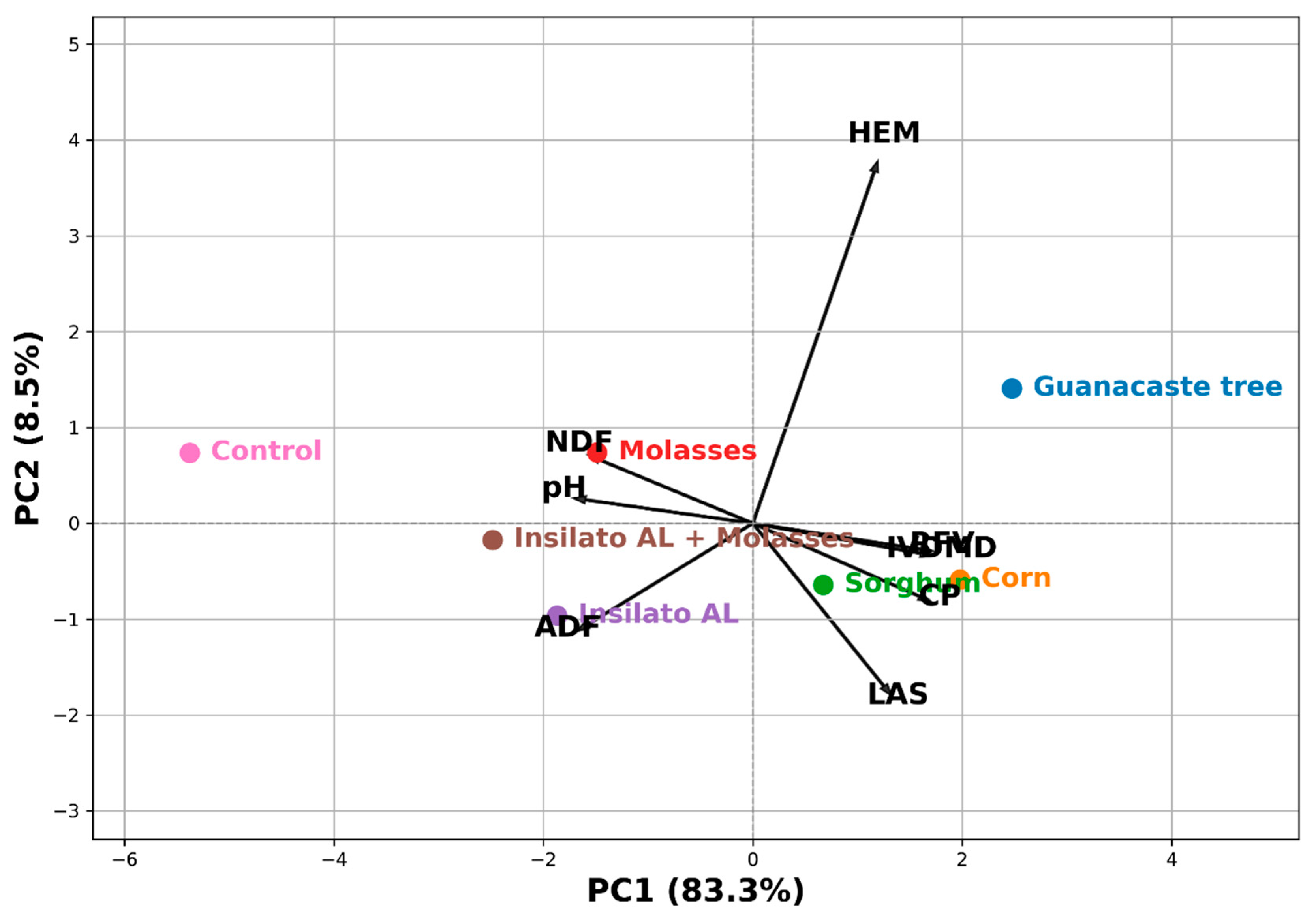
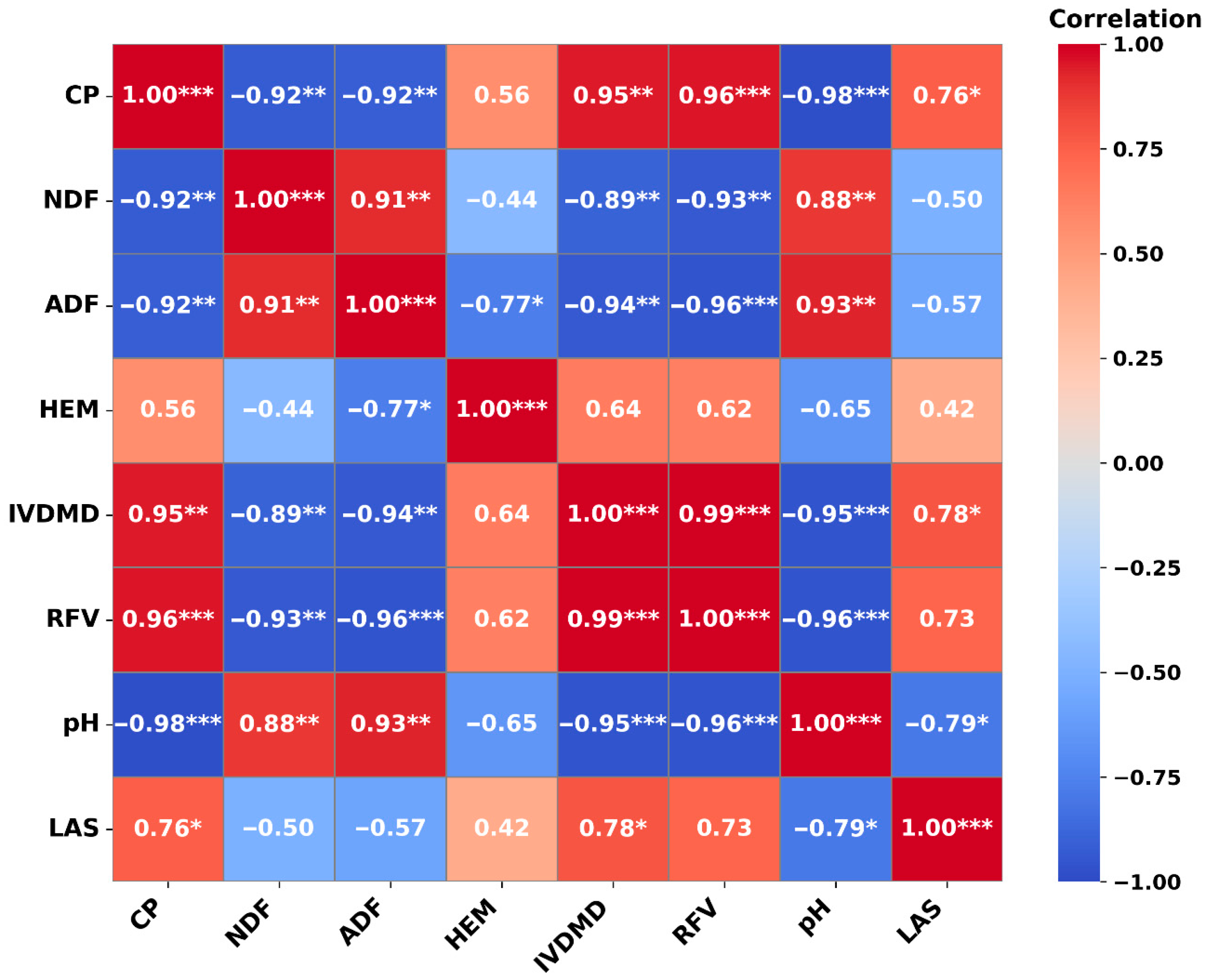
3.3. Principal Component Analysis and Pearson Correlation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SS | Safflower silage |
| DM | Dry matter |
| CP | Crude protein |
| NDF | Neutral detergent fiber |
| ADF | Acid detergent fiber |
| HEM | Hemicellulose |
| IVDMD | In vitro dry matter digestibility |
| RFV | Relative forage value |
| PCA | Principal component analysis |
References
- Lelis, D.L.; Morenz, M.J.F.; Paciullo, D.S.C.; Roseira, J.P.S.; Gomide, C.A.d.M.; Pereira, O.G.; Oliveira, J.S.e.; Lopes, F.C.F.; da Silva, V.P.; da Silveira, T.C.; et al. Effects of lactic acid bacteria on fermentation and nutritional value of BRS capiaçu elephant grass silage at two regrowth ages. Animals 2025, 15, 1150. [Google Scholar] [CrossRef]
- Silva, R.R.; Rodrigues, R.C.; Rodrigues, M.M.; Abdalla, A.L.; da Silva Cabral, L.; da Costa Araújo, D.L.; Olivera-Viciedo, D.; da Silva, E.C.; de Moura Zanine, A.; de Jesus Ferreira, D.; et al. Fermentation and nutritive value of pineapple stubble silage supplemented with leucaena hay. Agronomy 2024, 14, 2140. [Google Scholar] [CrossRef]
- Garay-Martínez, J.R.; Godina-Rodríguez, J.E.; Hernández-Rodríguez, B.; Maldonado-Torres, A.; López-Cantú, D.G.; Joaquín-Cancino, S. Caracterización de unidades de producción pecuaria en Aldama, Tamaulipas, México: Productores, hato y alimentación animal. Ecosist. Recur. Agropec. 2024, 11, e4091. [Google Scholar] [CrossRef]
- Salmoral, G.; Ababio, B.; Holman, I.P. Drought impacts, coping responses and adaptation in the UK outdoor livestock sector: Insights to increase drought resilience. Land 2020, 9, 202. [Google Scholar] [CrossRef]
- Kahiu, N.; Anchang, J.; Alulu, V.; Fava, F.P.; Jensen, N.; Hanan, N.P. Leveraging browse and grazing forage estimates to optimize index-based livestock insurance. Sci. Rep. 2024, 14, 14834. [Google Scholar] [CrossRef]
- Ma, J.; Sun, G.; Shah, A.M.; Fan, X.; Li, S.; Yu, X. Silage additives improve fermentation quality, aerobic stability and rumen degradation in mixed silage composed of amaranth and corn straw. Front. Plant Sci. 2023, 14, 1189747. [Google Scholar] [CrossRef]
- Ochoa-Espinoza, X.M.; Montoya-Coronado, L.; Reta-Sanchez, D.G.; Borbón-Gracia, A.; Aguilera-Molina, N.A.; Ávila-Casillas, E.; Cota-Barreras, C.I.; Cano-Ríos, P. FORRCART 2020, nueva variedad de cártamo forrajero en México. Rev. Fitotec. Mex. 2021, 44, 275–277. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Hu, J.; Zhao, J.; Xu, G.; Dong, D.; Jia, Y.; Shao, T. Fermentation quality and aerobic stability evaluation of rice straw silage with different ensiling densities. Fermentation 2024, 10, 20. [Google Scholar] [CrossRef]
- Xu, G.; Yang, F.; Hu, J.; Wang, Y.; Dong, D.; Dong, Z.; Li, J.; Shao, T. Effect of ensiling density on fermentation characteristics and aerobic stability of Pennisetum giganteum silages. Agronomy 2024, 14, 1990. [Google Scholar] [CrossRef]
- Mosupiemang, M.; Emongor, V.E.; Malambane, G. A review of drought tolerance in safflower (Carthamus tinctorius L.). Int. J. Plant Sci. 2022, 34, 140–149. [Google Scholar] [CrossRef]
- Gültaş, H.T. Crop water stress index assessment of safflower (Carthamus tinctorius L.) under limited water conditions. Sci. Agric. 2025, 82, e20230285. [Google Scholar] [CrossRef]
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef]
- Jabbari, H.; Golzardi, F.; Shariati, F.; Asadi, H. Effect of harvesting time on quantitative and qualitative characteristics of safflower cultivars forage in autumn planting. J. Crop Improv. 2023, 25, 65–81. [Google Scholar] [CrossRef]
- Khan, N.A.; Khan, N.; Tang, S.; Tan, Z. Optimizing corn silage quality during hot summer conditions of the tropics: Investigating the effect of additives on in-silo fermentation characteristics, nutrient profiles, digestibility and post-ensiling stability. Front. Plant Sci. 2023, 14, 1305999. [Google Scholar] [CrossRef]
- Wu, C.; Sun, W.; Huang, Y.; Dai, S.; Peng, C.; Zheng, Y.; Chen, C.; Hao, J. Effects of different additives on the bacterial community and fermentation mode of whole-plant paper mulberry silage. Front. Microbiol. 2022, 13, 904193. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Bao, J.; Li, W.; Sun, Z.; Gao, R.; Wu, Z.; Yu, Z. Effects of applying lactic acid bacteria and molasses on the fermentation quality, protein fractions and in vitro digestibility of baled alfalfa silage. Agronomy 2021, 11, 91. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, M.; Yang, R.; Sun, J.; Yu, Z.; Bai, C.; Xue, Y. Effect of regulation of whole-plant corn silage inoculated with LactoBacillus buchneri or Bacillus licheniformis regarding the dynamics of bacterial and fungal communities on aerobic stability. Plants 2024, 13, 1471. [Google Scholar] [CrossRef] [PubMed]
- Carbajal-Márquez, U.; Sánchez-Santillán, P.; Rojas-García, A.R.; Ayala-Monter, M.A.; Mendoza-Núñez, M.A.; Hernández-Valenzuela, D. Effect of parota (Enterolobium cyclocarpum) pod protein supplement on feed intake, digestibility and calf ruminal characteristics. Trop. Anim. Health Prod. 2021, 53, 323–331. [Google Scholar] [CrossRef]
- Rêgo, A.C.D.; Paiva, P.C.D.A.; Muniz, J.A.; Van Cleef, E.H.C.B.; Machado Neto, O.R.; Mata Junior, J.I.D. Mesquite pod meal in elephant grass silages. Acta Sci. Anim. Sci. 2013, 35, 251–258. [Google Scholar] [CrossRef]
- Vargas, T.V.; Hernández, R.M.E.; Gutiérrez, L.J.; Plácido, D.C.J.; Jiménez, C.A. Clasificación climática del estado de Tamaulipas, México. Ciencia UAT 2007, 2, 15–19. [Google Scholar]
- Garay-Martínez, J.R.; Maldonado-Moreno, N.; Ascencio-Luciano, A.; Joaquín-Cancino, S.; Bautista-Martínez, Y.; Granados-Rivera, L.D. Potencial forrajero de líneas experimentales de soya (Glycine max). Ecosist. Recur. Agropec. 2021, 8, e2932. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 21st ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Lucio-Ruiz, F.; Joaquín-Cancino, S.; Godina-Rodríguez, J.R.; Garay-Martínez, J.R. Yield and chemical composition of forage and silage of native maize under irrigated semi-arid conditions. Agrociencia 2023, 57, 722–732. [Google Scholar] [CrossRef]
- Tilley, J.M.A.; Terry, R.A. A two-stage technique for the in vitro digestion of forage crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Fortina, R.; Glorio Patrucco, S.; Barbera, S.; Tassone, S. Rumen Fluid from Slaughtered Animals: A Standardized Procedure for Sampling, Storage and Use in Digestibility Trials. Methods Protoc. 2022, 5, 59. [Google Scholar] [CrossRef] [PubMed]
- Mertens, D.R. Predicting intake and digestibility using mathematical models of ruminal function. J. Anim. Sci. 1987, 64, 1548–1558. [Google Scholar] [CrossRef]
- Undersander, D.; Moore, J.E.; Schneider, N. Relative forage quality. Focus Forage 2002, 4, 1–2. [Google Scholar]
- Ashbell, G.; Weinberg, Z.G.; Azrieli, A.; Hen, Y.; Horev, B. A simple system to study the aerobic determination of silages. Can. Agric. Eng. 1991, 33, 391–393. [Google Scholar]
- Aniceto, E.S.; Oliveira, T.S.; Meirelles, J.R.; Silva, I.N.; Mozelli Filho, E.J.L.; Gomes, R.S.; Arévalo, J.P.; Moraes, P.R. Evaluation of essential oils and their blends on the fermentative profile, microbial count, and aerobic stability of sorghum silage. Fermentation 2024, 10, 335. [Google Scholar] [CrossRef]
- SAS (Statistical Analysis System). SAS/STAT User’s Guide (Release 6.4); SAS Inst.: Cary, NC, USA, 2017. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 9th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Phil. Trans. R. Soc. Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Miche, V.; Thirion, B.; Grisel, O.; Blonndel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Waskom, M.L. Seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Chaudekar, S. An overview of Python for data analytics. Int. J. Res. Eng. Technol. 2022, 9, 461–468. [Google Scholar]
- Silva, C.d.S.; Araújo, G.G.L.d.; Santos, E.M.; Oliveira, J.S.d.; Silva, T.G.F.d.; Araújo, C.d.A.; Novaes, J.J.d.S.; de Macedo, A.; de Araújo, J.S.; Lima, D.O.; et al. Fermentative characteristics, nutritional aspects, aerobic stability, and microbial populations of total mixed ration silages based on relocated sorghum silage and cactus pear for sheep diets. Agronomy 2025, 15, 506. [Google Scholar] [CrossRef]
- Grote, A.J.; Nieman, C.C.; Thomas, I.R., Jr.; Coffey, K.P.; Muir, J.P.; Klotz, J.L. Effect of quebracho tannin (Schinopsis quebracho-colorado (Schltdl.) F.A. Barkley and T. Meyer) on silage nutritive value, ergovaline concentration, and fermentation parameters of tall fescue (Schedonorus arundinaceus (Shreb.) Dumort) with two dry-matter levels. Agronomy 2023, 13, 694. [Google Scholar] [CrossRef]
- Ji, Z.; Shi, Y.; Jiang, L.; Wang, X.; Zhu, G.; Zhou, G. Double-cropping systems based on maize, sorghum, and alfalfa: Impact on nutritive value and silage fermentation quality. Agronomy 2025, 15, 630. [Google Scholar] [CrossRef]
- Ekanem, N.J.; Inyang, U.A.; Ikwunze, K. Chemical composition, secondary metabolites and nutritive value of elephant ear tree (Enterolobium cyclocarpum (Jacq.) Griseb): A review. Niger. J. Anim. Prod. 2022, 49, 277–286. [Google Scholar] [CrossRef]
- Sandoval-González, L.; Miranda-Romero, L.A.; Lara-Bueno, A.; Huerta-Bravo, M.; Uribe-Gómez, M.; Martínez-Martínez, M. In vitro fermentation and the correlation of the nutritional content of leucaena associated with star grass. Rev. Mex. Cienc. Agrícolas 2016, 7, 3185–3196. [Google Scholar]
- Li, Y.; Du, S.; Sun, L.; Cheng, Q.; Hao, J.; Lu, Q.; Ge, G.; Wang, Z.; Jia, Y. Effects of lactic acid bacteria and molasses additives on dynamic fermentation quality and microbial community of native grass silage. Front. Microbiol. 2022, 13, 830121. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Weinberg, Z.G.; Ogunade, I.M.; Cervantes, A.A.P.; Arriola, K.G.; Jiang, Y.; Kim, D.; Li, X.; Gonçalves, M.C.M.; Vyas, D.; et al. Meta-analysis of effects of inoculation with homofermentative and facultative heterofermentative lactic acid bacteria on silage fermentation, aerobic stability, and the performance of dairy cows. J. Dairy Sci. 2017, 100, 4587–4603. [Google Scholar] [CrossRef]
- Neumann, M.; Baldissera, E.; Alessi Ienke, L.; Martins de Souza, A.; Piemontez de Oliveira, P.E.; Harry Bumbieris Junior, V. Nutritional Value Evaluation of Corn Silage from Different Mesoregions of Southern Brazil. Agriculture 2024, 14, 1055. [Google Scholar] [CrossRef]
- Sallam, S.M.; Rady, A.M.S.; Attia, M.F.A.; Elazab, M.A.; Vargas-Bello-Pérez, E.; Kholif, A.E. Different maize silage cultivars with or without urea as a feed for ruminant: Chemical composition and in vitro fermentation and nutrient degradability. Chil. J. Agric. Anim. Sci. 2024, 40, 137–149. [Google Scholar] [CrossRef]
- Ren, X.; Tian, H.; Zhao, K.; Li, D.; Xiao, Z.; Yu, Y.; Liu, F. Research on pH value detection method during maize silage secondary fermentation based on computer vision. Agriculture 2022, 12, 1623. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E. The relationship of silage temperature with the microbiological status of the face of corn silage bunkers. J. Dairy Sci. 2010, 93, 2620–2629. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Davies, D.R. The aerobic stability of silage: Key findings and recent developments. Grass Forage Sci. 2013, 68, 1–19. [Google Scholar] [CrossRef]
- Ávila, C.L.S.; Carvalho, B.F. Silage fermentation-updates focusing on the performance of micro-organisms. J. Appl. Microbiol. 2020, 128, 966–984. [Google Scholar] [CrossRef]
- Pang, H.; Zhou, P.; Yue, Z.; Wang, Z.; Qin, G.; Wang, Y.; Tan, Z.; Cai, Y. Fermentation characteristics, chemical composition, and aerobic stability in whole crop corn silage treated with lactic acid bacteria or Artemisia argyi. Agriculture 2024, 14, 1015. [Google Scholar] [CrossRef]
- Gonda, M.; Rufo, C.; Gonzalez-Andujar, J.L.; Vero, S. Mitigating aflatoxin B1 in high-moisture sorghum silage: Aspergillus flavus growth and aflatoxin B1 prediction. Front. Microbiol. 2024, 15, 1360343. [Google Scholar] [CrossRef]
- Chaves, A.R.D.; Moraes, L.G.; Montaño, A.S.; da Cunha, F.F.; Theodoro, G.d.F. Analysis of principal components for the assessment of silage corn hybrid performance under water deficit. Agriculture 2023, 13, 1335. [Google Scholar] [CrossRef]
- Contreras-Govea, F.E.; Muck, R.E.; Broderick, G.A.; Weimer, P.J. Lactobacillus plantarum effects on silage fermentation and in vitro microbial yield. Anim. Feed. Sci. Technol. 2013, 179, 61–68. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Widodo, S.; Shiddieqy, M.I.; Wahyono, T.; Widiawati, Y.; Muttaqin, Z. Analysis of correlation between nutrient content, digestibility, and gas production of forages in Indonesia. Adv. Anim. Vet. 2023, 11, 1770–1778. [Google Scholar] [CrossRef]
| PH (cm) | TFF | TFDM | Leaf | Stem | Inflorescence | Leaf | Stem | Inflorescence |
|---|---|---|---|---|---|---|---|---|
| t/ha | % | |||||||
| 91 ± 5 | 8.75 ± 0.58 | 2.64 ± 0.34 | 0.55 ± 0.04 | 1.18 ± 0.16 | 0.91 ± 0.15 | 22 ± 1 | 44 ± 1 | 34 ± 1 |
| CP | NDF | ADF | HEM | IVDMD | RFV | |||
| g/kg | ||||||||
| 129 ± 3.6 | 532 ± 5.2 | 241 ± 6.6 | 291 ± 7.2 | 524 ± 5.8 | 92 ± 4.2 | |||
| Treatment | Description |
|---|---|
| Control | Safflower silage without the addition of additives |
| Guanacaste tree * | Safflower silage supplemented with Guanacaste pod meal [Enterolobium cyclocarpum (Jacq.) Griseb.] |
| Corn * | Safflower silage supplemented with white corn meal |
| Sorghum * | Safflower silage supplemented with red sorghum meal |
| Molasses ¥ | Safflower silage supplemented with molasses diluted with water (66% molasses and 34% water) |
| Insilato AL ¥ | Safflower silage supplemented with homofermentative inoculant dissolved in water (1.25 mL/L of water) |
| Insilato AL + molasses ¥ | Safflower silage supplemented with Insilato AL + molasses treatments |
| Treatments | CP | NDF | ADF | HEM | IVDMD | RFV | pH |
|---|---|---|---|---|---|---|---|
| g/kg | |||||||
| Guanacaste tree | 142 a | 490 b | 195 d | 296 a | 618 a | 117 a | 4.25 e |
| Corn | 140 a | 488 b | 205 cd | 283 ab | 612 a | 116 ab | 4.43 d |
| Sorghum | 138 a | 494 b | 212 bc | 282 ab | 605 a | 113 b | 4.50 cd |
| Molasses | 128 c | 509 a | 221 a | 288 b | 584 b | 107 c | 4.73 b |
| Insilato AL | 131 bc | 504 a | 226 a | 278 b | 576 b | 106 c | 4.64 bc |
| Insilato AL + molasses | 133 b | 506 a | 223 ab | 283 ab | 572 b | 105 c | 4.63 bc |
| Control | 120 d | 511 a | 234 a | 278 b | 535 c | 97 d | 5.07 a |
| p-Value | <0.0001 | <0.0001 | <0.0001 | 0.0297 | <0.0001 | <0.0001 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garay-Martínez, J.R.; Lucio-Ruíz, F.; Godina-Rodríguez, J.E.; Ochoa-Espinoza, X.M.; Joaquín-Cancino, S.; Orzuna-Orzuna, J.F. Nutritional Value and Aerobic Stability of Safflower (Carthamus tinctorius L.) Silages Supplemented with Additives. Agronomy 2025, 15, 2071. https://doi.org/10.3390/agronomy15092071
Garay-Martínez JR, Lucio-Ruíz F, Godina-Rodríguez JE, Ochoa-Espinoza XM, Joaquín-Cancino S, Orzuna-Orzuna JF. Nutritional Value and Aerobic Stability of Safflower (Carthamus tinctorius L.) Silages Supplemented with Additives. Agronomy. 2025; 15(9):2071. https://doi.org/10.3390/agronomy15092071
Chicago/Turabian StyleGaray-Martínez, Jonathan Raúl, Fernando Lucio-Ruíz, Juan Eduardo Godina-Rodríguez, Xochilt Militza Ochoa-Espinoza, Santiago Joaquín-Cancino, and José Felipe Orzuna-Orzuna. 2025. "Nutritional Value and Aerobic Stability of Safflower (Carthamus tinctorius L.) Silages Supplemented with Additives" Agronomy 15, no. 9: 2071. https://doi.org/10.3390/agronomy15092071
APA StyleGaray-Martínez, J. R., Lucio-Ruíz, F., Godina-Rodríguez, J. E., Ochoa-Espinoza, X. M., Joaquín-Cancino, S., & Orzuna-Orzuna, J. F. (2025). Nutritional Value and Aerobic Stability of Safflower (Carthamus tinctorius L.) Silages Supplemented with Additives. Agronomy, 15(9), 2071. https://doi.org/10.3390/agronomy15092071








