Continuous Cropping Duration Alters Green Pepper Root Exudate Composition and Triggers Rhizosphere Feedback Inhibition
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location and Plant Cultivation
2.2. Different Years of Continuous Cropping Experiment
2.3. Exogenous Addition of Root Exudate Experiment
2.3.1. Experimental Design
2.3.2. Sampling and Analysis
2.3.3. Extraction, Sequencing, Quantitative PCR, and Bioinformatics Analysis of Soil Total DNA
2.4. Data Processing and Statistical Analysis
3. Results
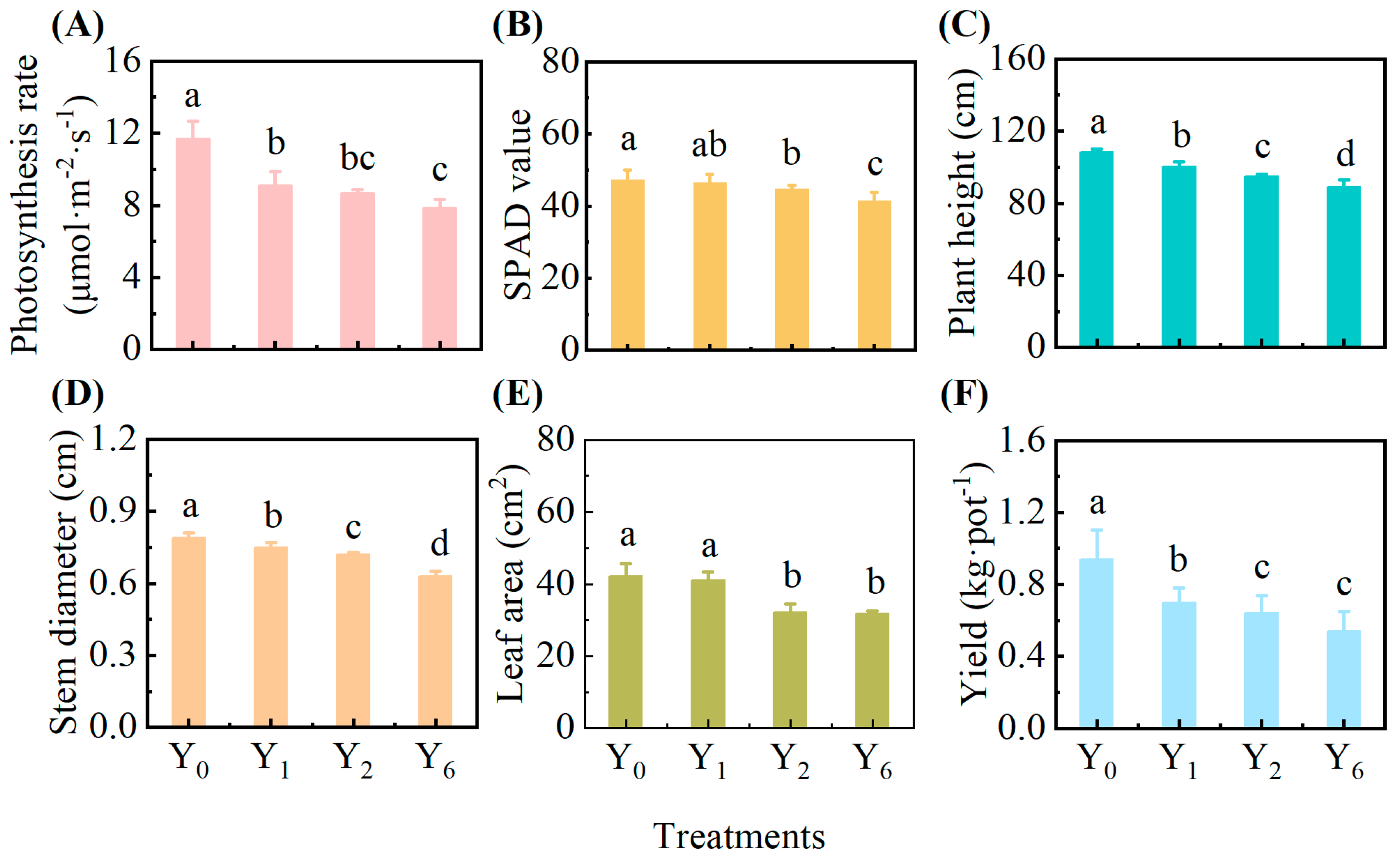
3.1. Effect of Continuous Cropping on Growth of Pepper Plants
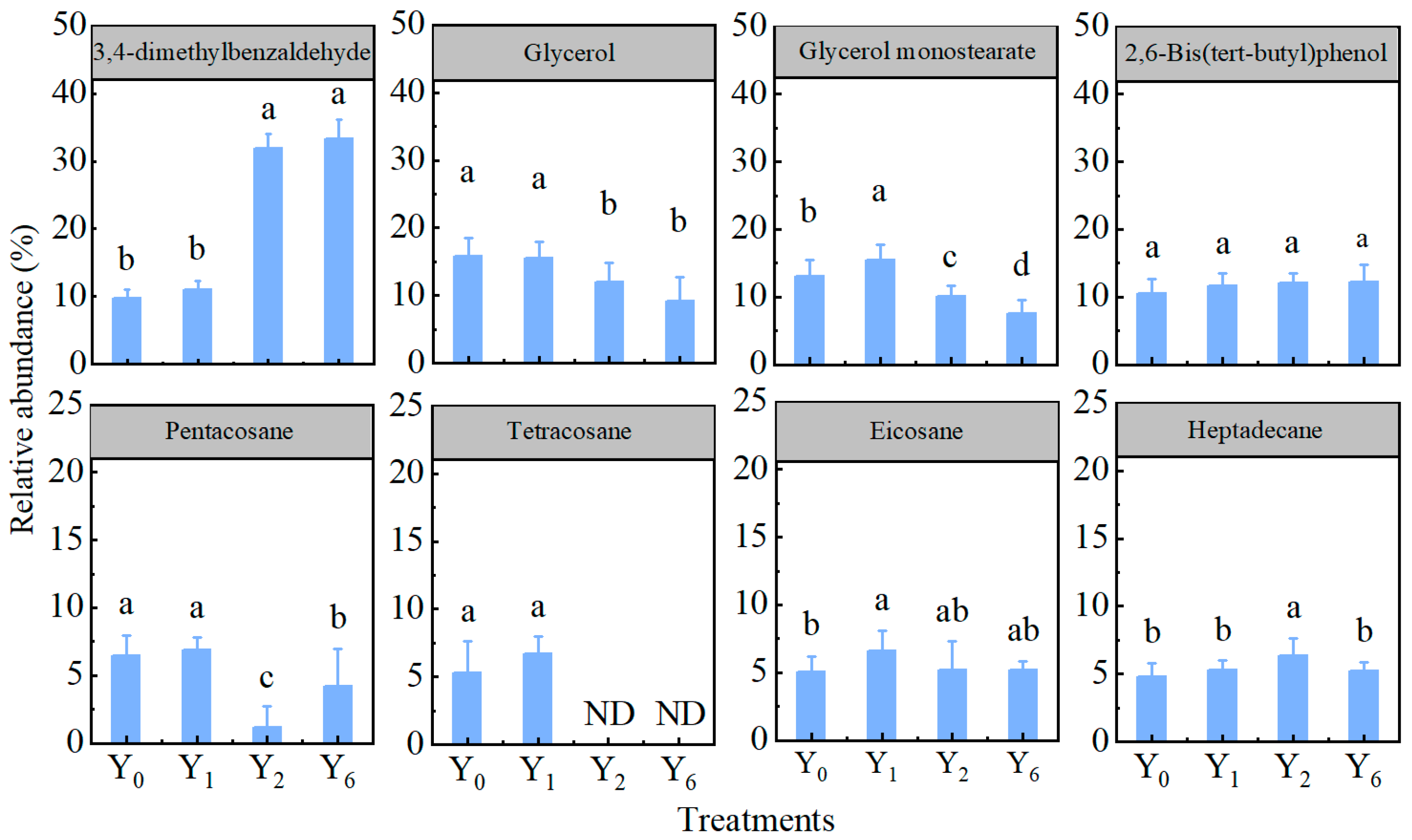
3.2. Effect of Continuous Cropping on Root Exudates of Pepper
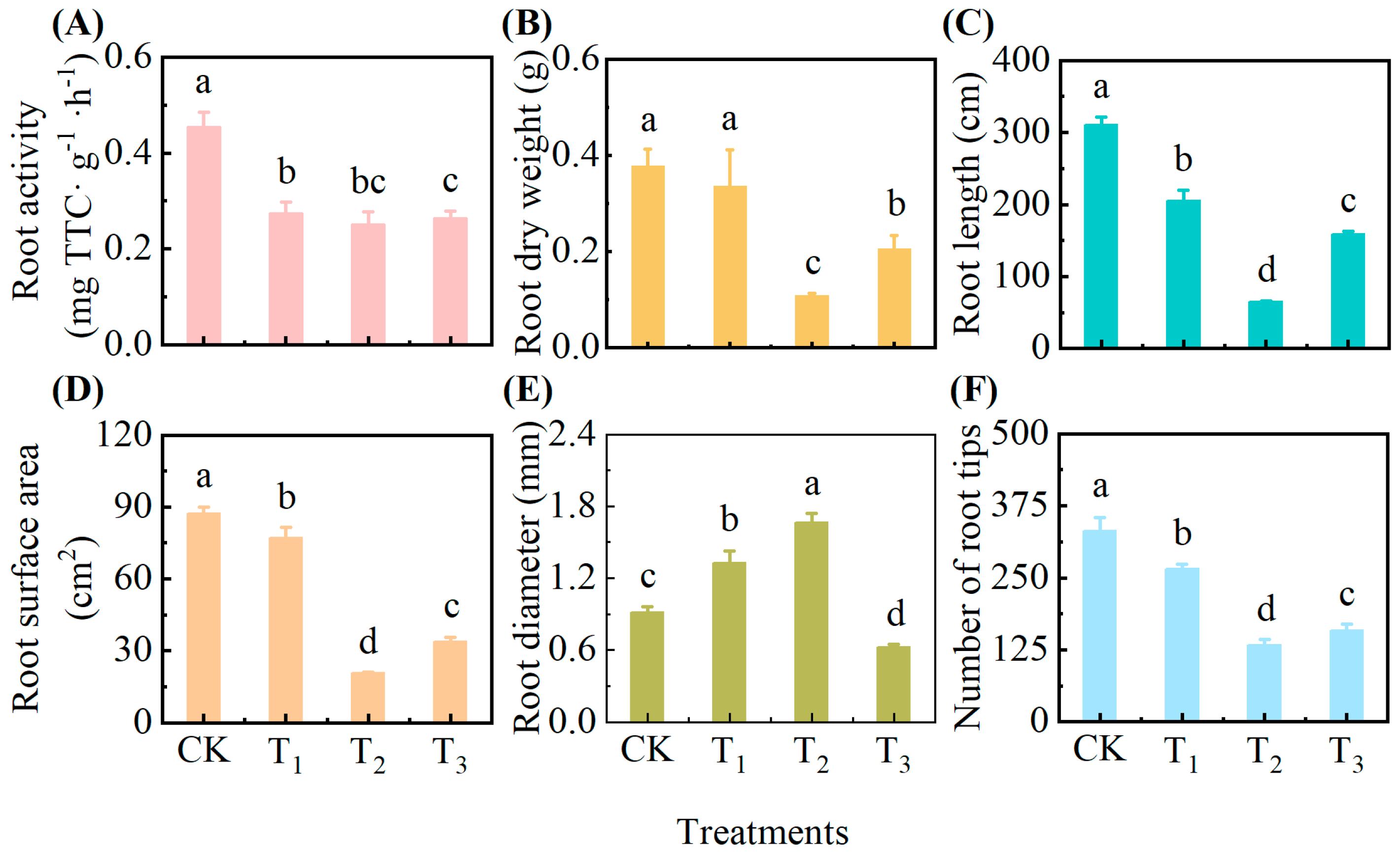
3.3. Effect of Exogenous Addition of Root Exudates on Pepper Root Traits
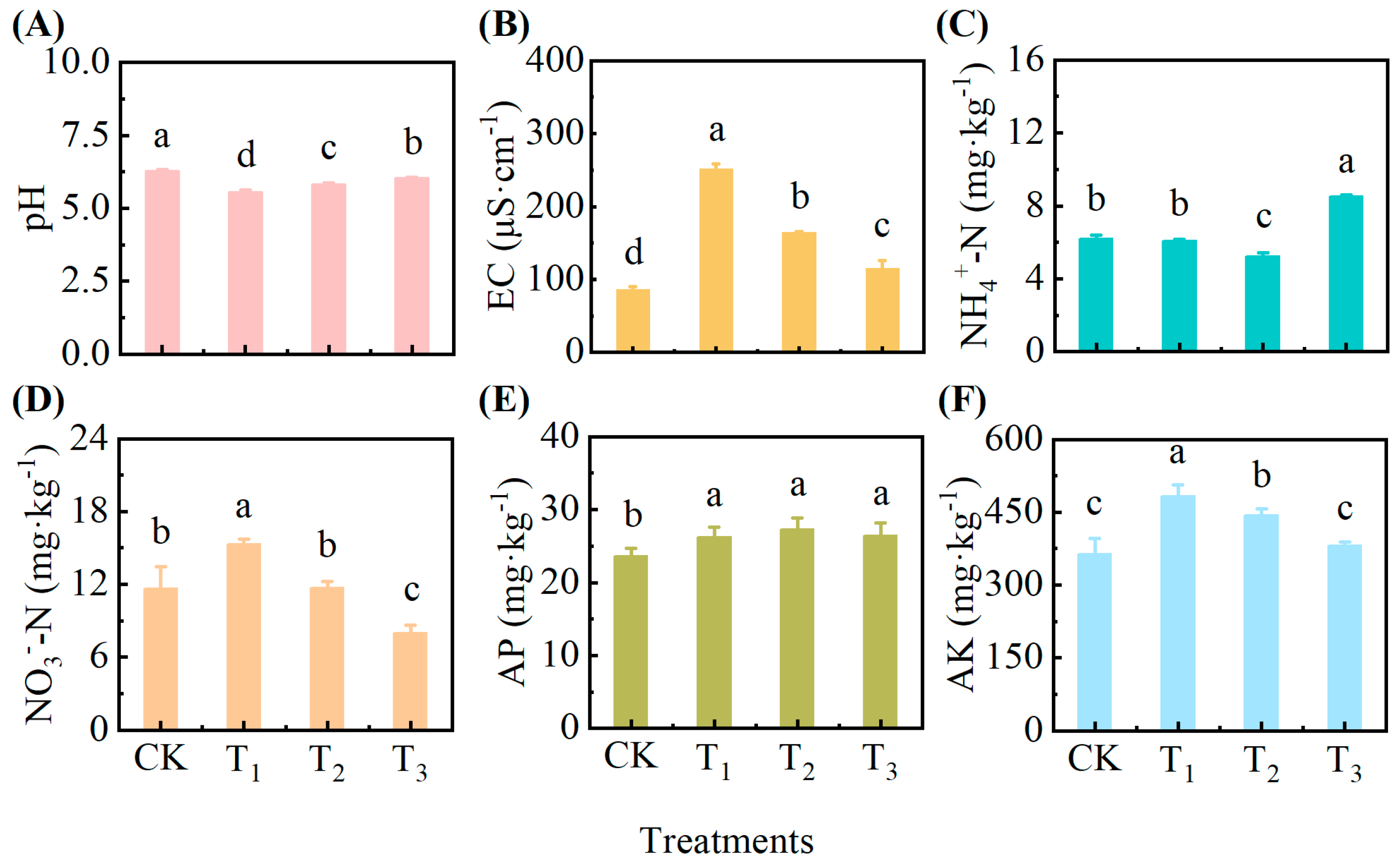
3.4. Effect of Exogenous Addition of Root Exudates on Soil Physicochemical Properties
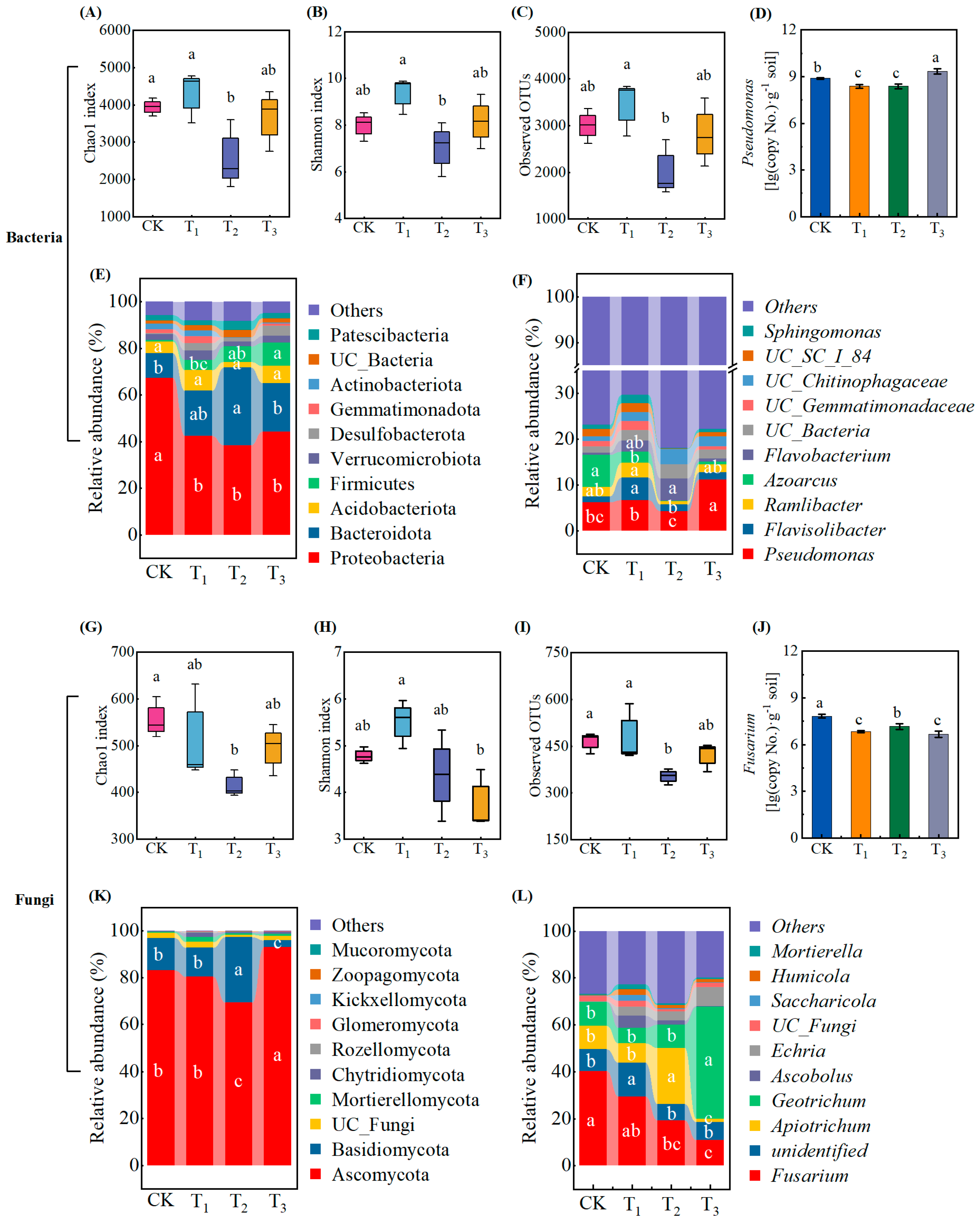
3.5. Effect of Exogenous Addition of Root Exudates on Soil Microbial Diversity and Composition
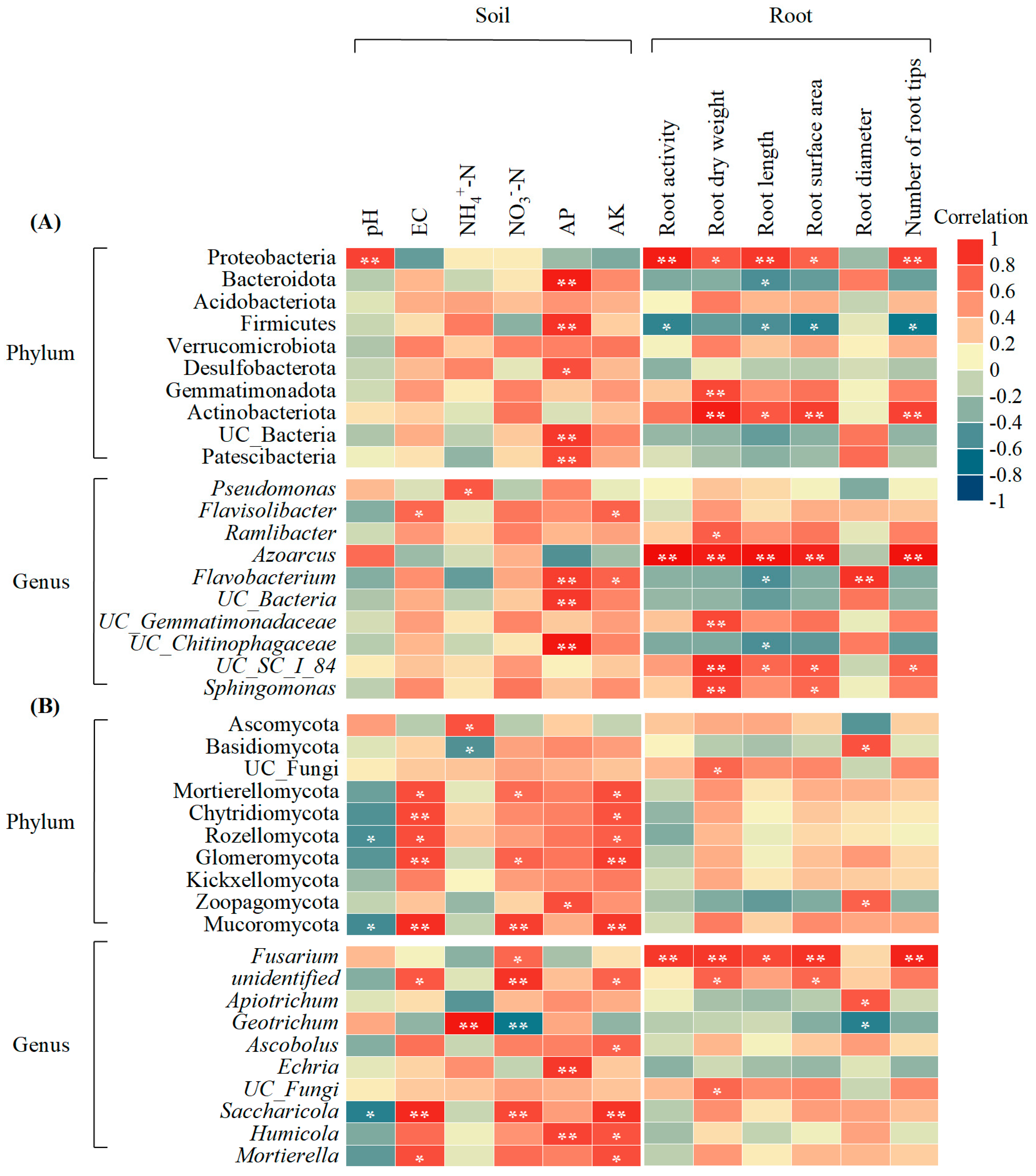
3.6. Correlation Analysis of Soil, Microbial Communities, and Root Traits
4. Discussion
4.1. Effects of Continuous Cropping on Green Pepper Plant Growth and Root Exudate Composition
4.2. The Exogenous Addition of Root Exudates Disrupts the Equilibrium of the Plant–Soil Ecosystem
4.3. Limitations and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, J.; Pei, H.; Xie, H. Synergistic effects of organic fertilizer and corn straw on microorganisms of pepper continuous cropping soil in China. Bioengineered 2020, 11, 1258–1268. [Google Scholar] [CrossRef]
- Zou, X.; Ma, Y.; Dai, X.; Li, X.; Yang, S. Spread and industry development of pepper in china. Acta Hortic. Sin. 2020, 47, 1715–1726. (In Chinese) [Google Scholar]
- Xiong, W.; Li, Z.; Liu, H.; Xue, C.; Zhang, R.; Wu, H.; Li, R.; Shen, Q. The effect of long-term continuous cropping of black pepper on soil bacterial communities as determined by 454 pyrosequencing. PLoS ONE 2015, 10, e0136946. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Q.; Liu, S.Z.; Liu, X.; Zhang, S.R.; Li, L.; Zhao, H.T.; Zhao, J.; Zhang, J.B.; Cai, Z.C. Plant pathological condition is associated with fungal community succession triggered by root exudates in the plant-soil system. Soil Biol. Biochem. 2020, 151, 108046. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, Z.; Li, Z.W.; Jiang, Y.H.; Weng, B.Q.; Lin, W.X. Variations of rhizosphere bacterial communities in tea (Camellia sinensis L.) continuous cropping soil by high-throughput pyrosequencing approach. J. Appl. Microbiol. 2016, 121, 787–799. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, C.; Su, Y.; Peng, W.; Lu, R.; Liu, Y.; Huang, H.; He, X.; Yang, M.; Zhu, S. Soil acidification caused by excessive application of nitrogen fertilizer aggravates soil-borne diseases: Evidence from literature review and field trials. Agric. Ecosyst. Environ. 2022, 340, 108176. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, Y.; Tian, M.; Shah Jahan, M.; Shu, S.; Sun, J.; Li, P.; Ahammed, G.J.; Guo, S. Mixing of biochar, vinegar and mushroom residues regulates soil microbial community and increases cucumber yield under continuous cropping regime. Appl. Soil Ecol. 2021, 161, 103883. [Google Scholar] [CrossRef]
- Cui, X.; Yuan, J.; Yang, X.; Wei, C.; Bi, Y.; Sun, Q.; Meng, J.; Han, X. Biochar application alters soil metabolites and nitrogen cycle-related microorganisms in a soybean continuous cropping system. Sci. Total Environ. 2024, 917, 170522. [Google Scholar] [CrossRef]
- Liao, J.; Xia, P. Continuous cropping obstacles of medicinal plants: Focus on the plant-soil-microbe interaction system in the rhizosphere. Sci. Hortic. 2024, 328, 112927. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, W.; Guan, H.; Wang, K.; Xiang, P.; Wei, F.; Yang, S.; Miao, C.; Ma, L.Q. Biochar increases Panax notoginseng’s survival under continuous cropping by improving soil properties and microbial diversity. Sci. Total Environ. 2022, 850, 157990. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, P.; Zhang, W.; Yu, C.; Luo, Z. Continuous cropping of potato changed the metabolic pathway of root exudates to drive rhizosphere microflora. Front. Microbiol. 2023, 14, 1318586. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhou, Y.; Wang, G.; Dai, K.; Li, J.; Song, X.; Xu, Y.; Cui, Y.; Yang, X. Biochar-based organic fertilizer application promotes the alleviation of tobacco (Nicotiana tabacum L.) continuous cropping obstacles by improving soil chemical properties and microbial community structure. BMC Plant Biol. 2025, 25, 271. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wu, L.; Zhu, Q.; Wang, J.; Qin, X.; Xu, J.; Kong, L.; Chen, J.; Lin, S.; Umar Khan, M.; et al. The role of organic acids on microbial deterioration in the Radix pseudostellariae rhizosphere under continuous monoculture regimes. Sci. Rep. 2017, 7, 3497. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.N.; Schachtman, D.P. Root exudates impact plant performance under abiotic stress. Trends Plant Sci. 2021, 27, 80–91. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, Q.; Zhang, Z.; Li, Y.; Xu, N.; Qu, Q.; Lu, T.; Pan, X.; Qian, H. Enantioselective effects of imazethapyr on Arabidopsis thaliana root exudates and rhizosphere microbes. Sci. Total Environ. 2020, 716, 137121. [Google Scholar] [CrossRef]
- Ma, J.Q.; Xie, Y.; Yang, Y.S.; Jing, C.L.; You, X.W.; Yang, J.; Sun, C.Y.; Qin, S.F.; Chen, J.H.; Cao, K.X.; et al. Amf colonization affects allelopathic effects of zea mays l. Root exudates and community structure of rhizosphere bacteria. Front. Plant Sci. 2022, 13, 1050104. [Google Scholar] [CrossRef]
- Huang, W.; Sun, D.; Wang, R.; An, Y. Integration of transcriptomics and metabolomics reveals the responses of sugar beet to continuous cropping obstacle. Front. Plant Sci. 2021, 12, 711333. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, H.; Tang, C.; Zhang, Y.; Lin, H.; Shen, Y.; Wang, J.; Wan, S. Effects of continuous cropping on root exudates of different resistance peanut (Arachis hypogaea L.) varieties and allelochemicals content in soil. Chin. J. Oil Crop Sci. 2015, 37, 467–474. (In Chinese) [Google Scholar]
- Cheng, F.; Ali, M.; Liu, C.; Deng, R.; Cheng, Z.H. Garlic allelochemical diallyl disulfide alleviates autotoxicity in the root exudates caused by long-term continuous cropping of tomato. J. Agric. Food Chem. 2020, 68, 11684–11693. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wang, H.; Tan, F.; Wu, B.; Liu, L.; Qin, H.; Yang, Z.; He, M. Rhizosphere metabolic cross-talk from plant-soil-microbe tapping into agricultural sustainability: Current advance and perspectives. Plant Physiol. Biochem. 2024, 210, 108619. [Google Scholar] [CrossRef]
- Lian, J.; Li, G.; Zhang, J.; Massart, S. Nitrogen fertilization affected microbial carbon use efficiency and microbial resource limitations via root exudates. Sci. Total Environ. 2024, 950, 174933. [Google Scholar] [CrossRef]
- Li, J.; Li, W.L.; Xu, X.L. Root exudates induce rhizosphere effect benefits for plant N use efficiency and fitness of relatives for Glycine max. Plant Soil. 2021, 469, 234–258. [Google Scholar] [CrossRef]
- Wang, H.W.; Ma, C.Y.; Xu, F.J.; Lu, F.; Zhang, W.; Dai, C.C. Root endophyte-enhanced peanut-rhizobia interaction is associated with regulation of root exudates. Microbiol. Res. 2021, 250, 126765. [Google Scholar] [CrossRef] [PubMed]
- Afridi, M.S.; Kumar, A.; Javed, M.A.; Dubey, A.; de Medeiros, F.H.V.; Santoyo, G. Harnessing root exudates for plant microbiome engineering and stress resistance in plants. Microbiol. Res. 2024, 279, 127564. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Geng, H.-Y.; Li, W.-X.; Li, Y.-Y.; Lu, Y.-S.; Xie, K.-Z.; Sun, L.L.; Zhang, J.-X.; Peng, H.-L.; Shi, C.-H.; et al. Innate root exudates contributed to contrasting coping strategies in response to ralstonia solanacearum in resistant and susceptible tomato cultivars. J. Agric. Food Chem. 2023, 71, 20092–20104. [Google Scholar] [CrossRef]
- Vora, S.M.; Ankati, S.; Patole, C.; Podile, A.R.; Archana, G. Alterations of primary metabolites in root exudates of intercropped Cajanus Cajan-zea mays modulate the adaptation and proteome of Ensifer (Sinorhizobium) fredii NGR234. Microb. Ecol. 2022, 83, 1008–1025. [Google Scholar] [CrossRef]
- Zhu, S.; Zhao, W.; Sun, S.; Yang, X.; Mao, H.; Sheng, L.; Chen, Z. Community metagenomics reveals the processes of cadmium resistance regulated by microbial functions in soils with Oryza sativa root exudate input. Sci. Total Environ. 2024, 949, 175015. [Google Scholar] [CrossRef]
- Bertilsson, S.; Eisenhauer, N.; Scheu, S.; Jousset, A. Bacterial diversity stabilizes community productivity. PLoS ONE 2012, 7, e34517. [Google Scholar]
- Liu, L.; Wu, J.; Liu, M.; Wang, M.; Huo, Y.; Wei, F.; Wu, M. Microbial communities in continuous Panax notoginseng cropping soil. Agronomy 2025, 15, 486. [Google Scholar] [CrossRef]
- Zhou, N.; Mei, C.-M.; Zhu, X.-Y.; Zhao, J.-J.; Ma, M.-G.; Li, W.-D. Research progress of rhizosphere microorganisms in fritillaria l. Medicinal plants. Front. Bioeng. Biotech. 2022, 10, 1054757. [Google Scholar] [CrossRef]
- Peng, S.; Zhou, P.; Wu, K.; Xing, D.; He, J. Research progress on the response and regulatory measures of chili peppers to continuous cropping obstacles in China. J. Anhui Agric. Sci. 2023, 51, 15–17. [Google Scholar]
- Zhang, F.; Chen, Y.; Wu, C.; Wang, X.; Fan, S.; Wang, J. Investigation and analysis on current situation of continuous cropping obstacle of greenhouse pepper in jiangxi province. N. Hortic. 2018, 17, 75–81. (In Chinese) [Google Scholar]
- Chen, W.; Guo, X.; Guo, Q.; Tan, X.; Wang, Z. Long-term chili monoculture alters environmental variables affecting the dominant microbial community in rhizosphere soil. Front. Microbiol. 2021, 12, 681953. [Google Scholar] [CrossRef] [PubMed]
- Ankati, S.; Podile, A.R. Metabolites in the root exudates of groundnut change during interaction with plant growth promoting rhizobacteria in a strain-specific manner. J. Plant Physiol. 2019, 243, 153057. [Google Scholar] [CrossRef]
- Xu, L.; Ma, L.; Wei, R.; Ma, Y.; Ma, T.; Dang, J.; Chen, Z.; Li, S.; Ma, S.; Chen, G. Effect of continuous cropping on growth and lobetyolin synthesis of the medicinal plant Codonopsis pilosula (Franch.) Nannf. Based on the integrated analysis of plant-metabolite-soil factors. J. Agric. Food Chem. 2024, 72, 19604–19617. [Google Scholar] [CrossRef]
- Wu, H.; Qin, X.; Wang, J.; Wu, L.; Chen, J.; Fan, J.; Zheng, L.; Tangtai, H.; Arafat, Y.; Lin, W.; et al. Rhizosphere responses to environmental conditions in Radix pseudostellariae under continuous monoculture regimes. Agric. Ecosyst. Environ. 2019, 270–271, 19–31. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A.; Aly, I.N.; Abdel-Satar, M.A.; Khalil, M.S.; Verreet, J.A. PCR identification of fusarium genus based on nuclear ribosomal-DNA sequence data. Afric. J. Biotechnol. 2003, 2, 82–85. [Google Scholar]
- Tissier, M.L.; Kletty, F.; Handrich, Y.; Habold, C. Monocultural sowing in mesocosms decreases the species richness of weeds and invertebrates and critically reduces the fitness of the endangered European hamster. Oecologia 2018, 186, 589–599. [Google Scholar] [CrossRef]
- Guo, X.-X.; Tian, L.; Song, B.-Q.; Li, Y.-H.; Huang, C.-Y.; Li, Z.; Zhang, P.; Jian, C.-Y.; Han, K.; Xue, C.-L.; et al. Effects of continuous cropping and application of bio-organic fertilizer on photosynthetic performance, dry matter accumulation and distribution of sugar beet. Sci. Rep. 2025, 25, 2512. [Google Scholar] [CrossRef]
- Jiao, R.; Xu, X.; Yang, H.; Feng, H.; Zhang, S.; Zhang, J.; Li, C.; Li, J. Study on the effect and its mechanism of continuous cropping on potato growth and soil health. Agric. Res. Arid Areas 2018, 36, 94–100. (In Chinese) [Google Scholar]
- Wang, Y.; Zhou, Y.; Ye, J.; Jin, C.; Hu, Y. Continuous cropping inhibits photosynthesis of Polygonatum odoratum. Plants 2023, 12, 3374. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Du, J.; Li, Y.; Tang, H.; Yin, Z.; Yang, L.; Ding, X. Evolutions and managements of soil microbial community structure drove by continuous cropping. Front. Microbiol. 2022, 13, 839494. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Liu, B.; Teng, A.; Zhang, L.; Wang, H.; Yang, Z.; Li, J.; Xia, Y.; Wang, J. The impact of continuous cropping on phenolic acids in muskmelon soil and the colonization of Trichoderma viride. Agronomy 2024, 14, 1344. [Google Scholar] [CrossRef]
- Song, H.; Gao, X.; Wang, X.; Xing, L.; Feng, B. Effects of exogenous phthalic and cinnamic acids on enzyme activities and microbial community structures in rhizosphere soil of adzuki bean. Agric. Sci. Technol. 2017, 18, 1935–1940+1954. [Google Scholar]
- Li, T.; Ge, L.; Zhao, R.; Peng, C.; Zhou, X.; Li, P.; Liu, Z.; Song, H.; Tang, J.; Zhang, C.; et al. Phenolic compounds weaken the impact of drought on soil enzyme activity in global wetlands. Front. Microbiol. 2024, 15, 1372866. [Google Scholar] [CrossRef]
- Aubert, S.; Gout, E.; Bligny, R.; Douce, R. Multiple effects of glycerol on plant cell metabolism. Phosphorus-31 nuclear magnetic resonance studies. J. Biol. Chem. 1994, 269, 21420–21427. [Google Scholar] [CrossRef]
- Oubohssaine, M.; Hnini, M.; Rabeh, K. Exploring lipid signaling in plant physiology: From cellular membranes to environmental adaptation. J. Plant Physiol. 2024, 300, 154295. [Google Scholar] [CrossRef]
- Wang, Z.H.; Fang, H.; Chen, M. Effects of root exudates of woody species on the soil anti-erodibility in the rhizosphere in a karst region, China. PeerJ 2017, 5, e3029. [Google Scholar] [CrossRef]
- Bouhaouel, I.; Richard, G.; Fauconnier, M.L.; Ongena, M.; Franzil, L.; Gfeller, A.; Amara, H.S.; du Jardin, P. Identification of barley (Hordeum vulgare L. subsp. vulgare) root exudates allelochemicals, their autoallelopathic activity and against Bromus diandrus Roth. Germination. Agronomy 2019, 9, 345. [Google Scholar] [CrossRef]
- Feng, F.J.; Yang, C.Y.; Li, M.J.; Zhan, S.Y.; Liu, H.Y.; Chen, A.G.; Wang, J.M.; Zhang, Z.Y.; Gu, L. Key molecular events involved in root exudates-mediated replanted disease of Rehmannia glutinosa. Plant Physiol. Biochem. 2022, 172, 136–150. [Google Scholar] [CrossRef]
- Zhang, W.; Qiu, H.; Zhang, C.; Hai, L. Identification of chemicals in potato root exudates under different years of continuous cropping and their biologic effects. Chin. J. Eco-Agric. 2018, 26, 1811–1818. (In Chinese) [Google Scholar]
- Wankhade, A.; Wilkinson, E.; Britt, D.W.; Kaundal, A. A review of plant–microbe interactions in the rhizosphere and the role of root exudates in microbiome engineering. Appl. Sci. 2025, 13, 7127. [Google Scholar] [CrossRef]
- Shen, F.; Wu, W.; Li, Y.; Liu, D.; Teng, Y. Study on microecological effects of root exudates of garden plants with different concentrations. Ecol. Environ. Sci. 2021, 30, 313–319. (In Chinese) [Google Scholar]
- Ma, Z.; Wang, Q.; Wang, X.; Chen, X.; Wang, Y.; Mao, Z. Effects of biochar on replant disease by amendment soil environment. Commun. Soil Sci. Plant Anal. 2021, 52, 673–685. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, J.; Dawuda, M.M.; Xie, J.; Yu, J.; Li, J.; Zhang, X.; Tang, C.; Wang, C.; Gan, Y. Appropriate ammonium-nitrate ratio improves nutrient accumulation and fruit quality in pepper (Capsicum annuum L.). Agronomy 2019, 9, 683. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, L.; Yang, J.; Zhang, Z.; Awasthi, M.K.; Li, H. Insight to bacteria community response of organic management in apple orchard-bagasse fertilizer combined with biochar. Chemosphere 2022, 286, 131693. [Google Scholar] [CrossRef]
- Yang, S.; Huang, T.; Zhang, H.; Guo, H.; Xu, J.; Cheng, Y. Pollutants reduction via artificial mixing in a drinking water reservoir: Insights into bacterial metabolic activity, biodiversity, interactions and co-existence of core genera. Sci. Total Environ. 2023, 898, 165473. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, G.; Cheng, Y.; Shi, P.; Yang, C.; Yang, H.; Xu, Z. Soil acidification in continuously cropped tobacco alters bacterial community structure and diversity via the accumulation of phenolic acids. Sci. Rep. 2019, 9, 12499. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Liang, J.; Qin, Z.; Ning, T.; Wei, X.; Yang, B.; Wang, Q.; Xu, Y.; Shen, F. Unveiling the sustained effects of plant root exudates on soil microbiome and resistome and the related functional traits. J. Environ. Manag. 2025, 376, 124485. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhao, J.; Yuan, J.; Hale, L.; Wen, T.; Huang, Q.; Vivanco, J.M.; Zhou, J.; Kowalchuk, G.A.; Shen, Q. Root exudates drive soil-microbe-nutrient feedbacks in response to plant growth. Plant Cell Environ. 2020, 44, 613–628. [Google Scholar] [CrossRef]
- Zhang, W.-J.; Xu, Z.-R.; Zhao, S.-H.; Sun, J.-Y.; Yang, X. Development of a microbial fermentation process for detoxification of gossypol in cottonseed meal. Anim. Feed Sci. Tech. 2007, 135, 176–186. [Google Scholar] [CrossRef]
- Sajeena, A.; Nair, D.S.; Sreepavan, K. Non-pathogenic Fusarium oxysporum as a biocontrol agent. Indian Phytopathol. 2020, 73, 177–183. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, L.; Zhang, J.; Wu, M.; Chen, W.; Jiang, D.; Li, G. Production of anti-fungal volatiles by non-pathogenic Fusarium oxysporum and its efficacy in suppression of Verticillium wilt of cotton. Plant Soil 2015, 392, 101–114. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2023, 4, 226–239. [Google Scholar] [CrossRef]
- Tang, Z.; Tan, W.; Li, R.; Weng, L.; Chen, X.; Xi, B.; Lv, D. Advances in rhizosphere microbiome and rhizosphere immunity effect: A review. J. Agric. Food Chem. 2025, 24, 14707–14721. [Google Scholar] [CrossRef]
- Nansahwang, A.; Leksungnoen, P.; Armatmontree, C.; Aramrak, S.; Kongsil, P.; Wisawapipat, W. Phosphate mineral solubility controls on cassava root exudates, rhizosphere nutrient availability, and plant nutrient accumulation. Rhizosphere 2022, 23, 100575. [Google Scholar] [CrossRef]
- Fan, X.; Ge, A.-H.; Qi, S.; Guan, Y.; Wang, R.; Yu, N.; Wang, E. Root exudates and microbial metabolites: Signals and nutrients in plant-microbe interactions. Sci. China Life Sci. 2025, 68, 2290–2302. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Lian, D.; Zhang, S.; Yao, Y.; Lin, B.; Hong, J.; Wu, S.; Li, H. Continuous Cropping Duration Alters Green Pepper Root Exudate Composition and Triggers Rhizosphere Feedback Inhibition. Agronomy 2025, 15, 2010. https://doi.org/10.3390/agronomy15082010
Li Z, Lian D, Zhang S, Yao Y, Lin B, Hong J, Wu S, Li H. Continuous Cropping Duration Alters Green Pepper Root Exudate Composition and Triggers Rhizosphere Feedback Inhibition. Agronomy. 2025; 15(8):2010. https://doi.org/10.3390/agronomy15082010
Chicago/Turabian StyleLi, Zhou, Dongmei Lian, Shaoping Zhang, Yunfa Yao, Bizhen Lin, Jianji Hong, Songhai Wu, and Honghong Li. 2025. "Continuous Cropping Duration Alters Green Pepper Root Exudate Composition and Triggers Rhizosphere Feedback Inhibition" Agronomy 15, no. 8: 2010. https://doi.org/10.3390/agronomy15082010
APA StyleLi, Z., Lian, D., Zhang, S., Yao, Y., Lin, B., Hong, J., Wu, S., & Li, H. (2025). Continuous Cropping Duration Alters Green Pepper Root Exudate Composition and Triggers Rhizosphere Feedback Inhibition. Agronomy, 15(8), 2010. https://doi.org/10.3390/agronomy15082010




