Endophyte Viability in Grass Seeds: Storage Conditions Affecting Survival and Control Methods
Abstract
1. Introduction
2. Materials and Methods
- (a)
- Variable temperature, average +23 ± 5 °C, variable humidity (56 ± 5%) (S_1);
- (b)
- Stable temperature +7 °C, stable humidity 55% (S_2);
- (c)
- Stable temperature −20 °C, stable humidity 10% (S_3).
3. Results
3.1. Effect of Seed Storage Conditions on Endophyte Viability
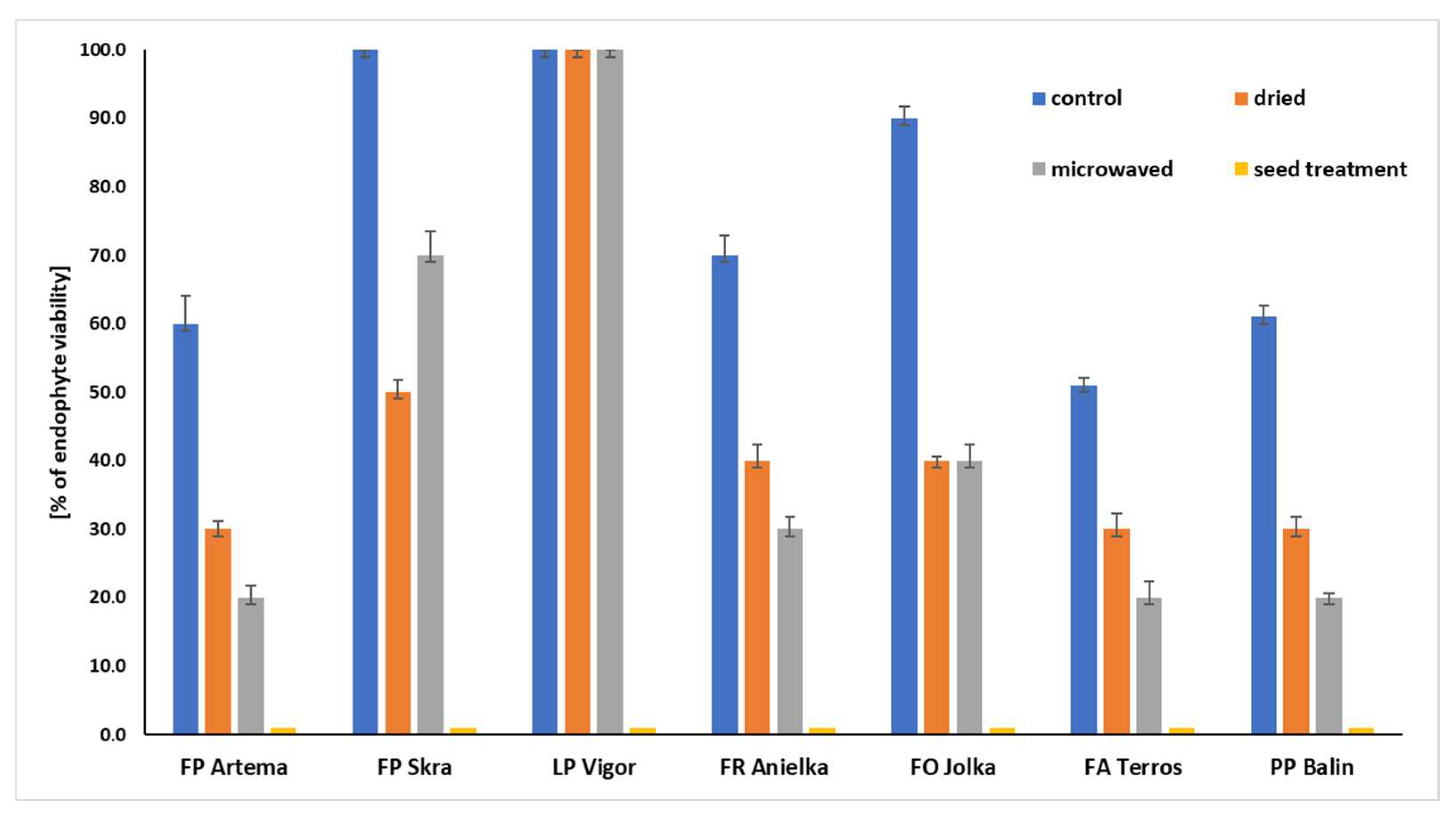
3.2. Effectiveness of Methods for Eliminating Epichloë from Seeds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rodriguez, R.J.; White, J.F.; Arnold, A.E.; Redman, R.S. Fungal endophytes: Diversity and functional roles. New Phytol. 2008, 178, 241–256. [Google Scholar] [CrossRef]
- Berg, G. Plant-microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef]
- Clay, K. Fungal endophytes of grasses: A defensive mutualism between plants and fungi. Ecology 1988, 69, 10–16. [Google Scholar] [CrossRef]
- Clay, K.; Schardl, C. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am. Nat. 2002, 160, S99–S127. [Google Scholar] [CrossRef]
- Bush, L.P.; Wilkinson, H.H.; Schardl, C.L. Bioprotective alkaloids of grass-endophyte associations. Plant Physiol. 1997, 114, 1–7. [Google Scholar] [CrossRef]
- White, J.F., Jr.; Cole, G.T. Endophyte—Host associations in forage grasses. IV. The endophyte of Festuca versusa. Mycologia 1986, 78, 102–107. [Google Scholar] [CrossRef]
- White, J.F., Jr. Widespread distribution of endophytes in the Poaceae. Plant Dis. 1987, 71, 340–342. [Google Scholar] [CrossRef]
- Ravel, C.; Charmet, G.; Balfourier, F. Influence of the fungal endophyte Acremonium lolii on agronomic traits of perennial ryegrass in France. Grass Forage Sci. 1995, 50, 75–80. [Google Scholar] [CrossRef]
- Saikkonen, K.; Faeth, S.H.; Helander, M.; Sullivan, T.J. Fungal endophytes: A continuum of interactions with host plants. Annu. Rev. Ecol. Syst. 1998, 29, 319–343. [Google Scholar] [CrossRef]
- Leyronas, C.; Raynal, G. Presence of Neotyphodium-like endophytes in European grasses. Ann. Appl. Biol. 2001, 139, 119–129. [Google Scholar] [CrossRef]
- Rolston, M.P.; Stewart, A.V.; Latch, G.C.M.; Hume, D.E. Endophytes in New Zealand grass seeds: Occurrence and implications for conservation of grass species. N. Z. J. Bot. 2002, 40, 365–372. [Google Scholar] [CrossRef]
- Wiewióra, B.; Prończuk, M. Porównanie metody mikroskopowej i immunologicznej do wykrywania grzybów endofitycznych w nasionach traw. Biul. IHAR 2006, 242, 277–284. [Google Scholar] [CrossRef]
- Siegel, M.R.; Latch, G.C.M.; Johnson, M.C. Acremonium fungal endophytes of Tall fescue and Perennial ryegrass: Significance and control. Plant Dis. 1985, 69, 179–183. [Google Scholar]
- Philipson, M.N.; Christey, M.C. The relationship of host and endophyte during flowering, seed formation and germination of Lolium perenne. N. Z. J. Bot. 1986, 2, 125–134. [Google Scholar] [CrossRef]
- Welty, R.E.; Azevedo, M.D.; Cook, K.L. Detecting viable Acremonium endophytes in leaf sheats and meristems of tall fescue and perennial ryegrass. Plant Dis. 1986, 70, 431–435. [Google Scholar] [CrossRef]
- Zhang, W.; Card, S.D.; Mace, W.J.; Christensen, M.J.; McGill, C.R.; Matthew, C. Defining the pathways of symbiotic Epichloë colonization in grass embryos with confocal microscopy. Mycologia 2017, 109, 153–161. [Google Scholar] [CrossRef]
- Latch, G.C.M.; Christensen, M.J. Ryegrass endophyte, incidence and control. N. Z. J. Agric. Res. 1982, 25, 443–448. [Google Scholar] [CrossRef]
- Siegel, M.R.; Varney, D.R.; Johnson, M.C.; Nesmith, W.C.; Buckner, R.C.; Bush, L.P.; Burrus, P.B.; Hardison, J.R. A fungal endophyte in tall fescue: Evaluation of control methods. Phytopathology 1984, 74, 937–941. [Google Scholar] [CrossRef]
- Williams, M.J.; Backman, E.M.; Clark, E.M.; White, J.F. Seed treatments for control of the tall fescue endophyte Acremonium coenophialum. Plant Dis. 1984, 68, 49–52. [Google Scholar] [CrossRef]
- Rolston, M.P.; Hare, M.D.; Moore, K.K.; Christensen, M.J. Viability of Lolium endophyte fungus in seed stored at different moisture contents and temperatures. N. Z. J. Exp. Agric. 1986, 14, 297–300. [Google Scholar] [CrossRef]
- Welty, R.E.; Azevedo, M.D.; Cooper, T.M. Influence of moisture content, temperature, and length of storage on seed germination and survival of endophytic fungi in seeds of tall fescue and perennial ryegrass. Phytopathology 1987, 77, 893–900. [Google Scholar] [CrossRef]
- Tian, P.; Le, T.-N.; Smith, K.F.; Forster, J.W.; Guthridge, K.M.; Spangenberg, G.C. Stability and viability of novel perennial ryegrass host–Neotyphodium endophyte associations. Crop Pasture Sci. 2013, 64, 39–50. [Google Scholar] [CrossRef]
- Hume, D.E.; Schmid, J.; Rolston, M.P.; Vijayan, P.; Hickey, M.J. Effect of climatic conditions on endophyte and seed viability in stored ryegrass seed. Seed Sci. Technol. 2011, 39, 481–489. [Google Scholar] [CrossRef]
- Caradus, J.R.; Chapman, D.F.; Tim Cookson, T.; Cotching, B.; Deighton, M.H.; Donnell, L.; Ferguson, J.; Finch, S.C.; Gard, S.; Hume, D.E.; et al. Epichloë endophytes—New perspectives on a key ingredient for resilient perennial grass pastures. Resilient Pastures-Grassl. Res. Pract. Ser. 2021, 17, 347–360. [Google Scholar] [CrossRef]
- Leyronas, C.; Meriaux, B.; Raynal, G. Chemical control of Neotyphodium spp. endophytes in perennial ryegrass and tall fescue seeds. Crop Sci. 2006, 46, 98–104. [Google Scholar] [CrossRef]
- Bouton, J.H.; Latch, G.C.M.; Hill, N.S.; Hoveland, C.S.; McCann, M.A.; Richard, H.; Watson, R.H.; Parish, J.A.; Hawkins, L.L.; Thompson, F.N. Reinfection of Tall Fescue Cultivars with Non-Ergot Alkaloid–Producing Endophytes. Agron. J. 2002, 94, 567–574. [Google Scholar]
- Ball, D.M.; Lacefield, G.D.; Agee, C.S.; Hoveland, C.S. Introduction and acceptance of novel endophytetall fescue in the USA. In New Zealand Grassland Association: Endophyte Symposium; New Zealand Grassland Association: Mosgiel, New Zealand, 2007; pp. 249–251. [Google Scholar]
- Caradus, J.R.; Johnson, L.J. Epichloë Fungal Endophytes-From a Biological Curiosity in Wild Grasses to an Essential Component of Resilient High Performing Ryegrass and Fescue Pastures. J. Fungi 2020, 6, 322. [Google Scholar] [CrossRef]
- Kirkby, K.A.; Hume, D.E.; Pratley, J.E.; Broster, J.C. Effect of temperature on endophyte and plant growth of annual ryegrass, perennial ryegrass and tall fescue. In Proceedings of the 17th Australasian Weeds Conference, Christchurch, New Zealand, 26–30 September 2010; pp. 56–59. [Google Scholar]
- International Seed Testing Association. International Rules for Seed Testing 2024; International Seed Testing Association: Bassersdorf, Switzerland, 2020; Volume 5, pp. 1–56. [Google Scholar]
- Saha, D.C.; Jackson, M.A.; Johnson-Cicalese, J.M. A rapid staining method for detection of endophyte fungi in turf and forage grasses. Am. Phytopathol. Soc. 1988, 2, 237–239. [Google Scholar] [CrossRef]
- Bouter, W.; Klooster, G. Practical endophyte—Work at the Barenbrug Grass Breeding Department. IOBC WPRS Bull. 1996, 19, 59–62. [Google Scholar]
- Welty, R.E.; Azevedo, M.D. Survival of endophyte hyphae in seeds of tall fescue stored one year. Phytopathology 1985, 75, 1331. [Google Scholar]
- Shelby, R.A.; Dalrymple, L.W.T. Incidence and distribution of the tall fescue endophyte in the United States. Plant Dis. 1987, 71, 783–786. [Google Scholar] [CrossRef]
- Wheatley, W.M.; Kemp, H.W.; Simpson, W.R.; Hume, D.E.; Nicol, H.I.; Kemp, D.R.; Launders, T.E. Viability of endemic endophyte (Neotyphodium lolii) and perennial ryegrass (Lolium perenne) seed at retail and wholesale outlets in south-eastern Australia. Seed Sci. Technol. 2007, 35, 360–370. [Google Scholar] [CrossRef]
- Clement, S.L.; Youssef, N.N.; Bruehl, G.W.; Kaiser, W.J.; Elberson, L.R.; Bradley, V. Effects of different storage temperatures on grass seed germination and Neotyphodium survival. In Proceedings of the 5th International Symposium on Neotyphodi-um/Grass Interactions, Fayetteville, AR, USA, 23–26 May 2004; Kallenbach, R., Rosenkrans, C., Jr., Lock, T.R., Eds.; Volume 511, pp. 163–165. [Google Scholar]
- Cheplick, G.P. Persistence of endophytic fungi in cultivars of Lolium perenne grown from seeds stored for 22 years. Am. J. Bot. 2017, 104, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Hume, D.E.; Card, S.D.; Rolston, P.M. Effects of storage conditions on endophyte and seed viability in pasture grasses. In Proceedings of the 22nd International Grassland Congress, Sydney, Australia, 15–19 September 2013; pp. 405–408. [Google Scholar]
- Titei, V. The quality of forage from perennial ryegrass (Lolium perenne) and tall fescue (Festuca arundinacea) under the conditions of Moldova. Sci. Pap.-Ser. D-Anim. Sci. 2023, 66, 183–190. [Google Scholar]
- Oliveira, J.A.; Castro, M.J. Incidence and viability of Acremonium endophytes in tall fescue accessions from North Spain. Genet. Resour. Crop Evol. 1997, 44, 519–522. [Google Scholar] [CrossRef]
- Latch, G.C.M.; Hunt, W.F.; Musgrave, D.R. Endophytic fungi affect growth of perennial ryegrass. N. Z. J. Agric. Res. 1985, 2, 165–168. [Google Scholar] [CrossRef]
- Schardl, C.L.; Young, C.A.; Hesse, U.; Amyotte, S.G.; Andreeva, K.; Calie, P.J.; Fleetwood, D.J.; Haws, D.C.; Moore, N.; Oeser, B.; et al. Plant-symbiotic fungi as chemical engineers: Multi-genome analysis of the clavicipitaceae reveals dynamics of alkaloid loci. PLoS Genet. 2013, 9, e1003323. [Google Scholar] [CrossRef]
- Stach, A.J. Grzyby endofityczne traw—Nasi wrogowie czy sprzymierzeńcy? Kosm.-Probl. Nauk. Biol. 2016, 65, 257–266. [Google Scholar]
- Rolston, M.P.; Archie, W.J.; Simpson, W.R. Tolerance of AR1 Neotyphodium endophyte to fungicides used in perennial ryegrass seed production. N. Z. Plant Prot. 2002, 55, 322–326. [Google Scholar] [CrossRef]
- Harvey, I.C.; Fletcher, L.R.; Emms, L.M. Effects of several fungicides on the Lolium endophyte in ryegrass plants, seeds and in culture. N. Z. J. Agric. Res. 1982, 25, 601–606. [Google Scholar] [CrossRef]
- Fletcher, L.R.; Easton, H.S. Advances in endophyte research. Progress and priorities in temperate areas. In Proceedings of the XIX International Grassland Congress, São Pedro, Brazil, 11–21 February 2001; pp. 595–603. [Google Scholar]
- Tyagi, A.; Lama Tamang, T.; Kashtoh, H.; Mir, R.A.; Mir, Z.A.; Manzoor, S.; Manzar, N.; Gani, G.; Vishwakarma, S.K.; Almalki, M.A.; et al. A Review on Biocontrol Agents as Sustainable Approach for Crop Disease Management: Applications, Production, and Future Perspectives. Horticulturae 2024, 10, 805. [Google Scholar] [CrossRef]
- Kirfman, G.W.; Brandenburg, R.L.; Garner, G.B. Relationship between insect abundance and endophyte infestation level in tall fescue in Missouri. J. Kans. Entomol. Soc. 1986, 59, 552–554. [Google Scholar]
- Cappelli, C.; Buonaurio, R. Occurrence of endophytic fungi in grass seeds and plants in Italy. In Proceedings of the 4th International Neotyphodium/Grass Interactions Symposium, Soest, Germany, 27–29 September 2001; Paul, V.H., Dapprich, P.D., Eds.; Universitat-Gesamthochschule Paderborn, Abtailung Soest: Soest, Germany, 2001; pp. 131–137. [Google Scholar]
- Bayle, L.; Doussinault, G.; Reynaud, G. Management of Neotyphodium endophytes in France: Current situation and perspectives. In Proceedings of the 5th International Symposium on Fungal Endophytes of Grasses, Christchurch, New Zealand, 22–26 February 2003; pp. 107–110. [Google Scholar]


| Sources of Variation | Results of Analysis | ||
|---|---|---|---|
| MS | F-Calc. | p | |
| cultivar (n = 7) | 6484.46 | 505.7 | 0.000 |
| storage condition (n = 4) | 3069.00 | 239.4 | 0.000 |
| interaction (n = 28) | 215.42 | 16.8 | 0.000 |
| error | 12.82 | ||
| Sources of Variation | Results of Analysis | ||
|---|---|---|---|
| MS | F-Calc. | p | |
| cultivar (n = 7) | 3991.96 | 469.6 | 0.000 |
| elimination method (n = 4) | 20,521.14 | 2414.3 | 0.000 |
| interaction (n = 28) | 670.06 | 78.8 | 0.000 |
| error | 8.5 | ||
| Endophytes Exposed to | Conditions/Treatments Applied | Correlation Coefficients with Initial (Control) Viability |
|---|---|---|
| seed storage | +23 °C | 0.80 *** |
| +7 °C | 0.89 *** | |
| −20 °C | 0.76 *** | |
| elimination | dryer | 0.71 *** |
| microwave | 0.87 *** | |
| Raxil Gel | nd. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiewióra, B.; Żurek, G. Endophyte Viability in Grass Seeds: Storage Conditions Affecting Survival and Control Methods. Agronomy 2025, 15, 1977. https://doi.org/10.3390/agronomy15081977
Wiewióra B, Żurek G. Endophyte Viability in Grass Seeds: Storage Conditions Affecting Survival and Control Methods. Agronomy. 2025; 15(8):1977. https://doi.org/10.3390/agronomy15081977
Chicago/Turabian StyleWiewióra, Barbara, and Grzegorz Żurek. 2025. "Endophyte Viability in Grass Seeds: Storage Conditions Affecting Survival and Control Methods" Agronomy 15, no. 8: 1977. https://doi.org/10.3390/agronomy15081977
APA StyleWiewióra, B., & Żurek, G. (2025). Endophyte Viability in Grass Seeds: Storage Conditions Affecting Survival and Control Methods. Agronomy, 15(8), 1977. https://doi.org/10.3390/agronomy15081977







