Anatomical and Physiological Responses of Maize Nodal Roots to Shading Stress and Nitrogen Supply
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Field Experiments
2.2. Sampling and Measurement
2.2.1. Root Sampling and Measurement of Nodal Root Number and Diameter
2.2.2. Root Anatomical Traits
2.2.3. Collection of Root-Bleeding Sap
2.2.4. Measurement of Sucrose, Hormones, and Free Amino Acids in Root-Bleeding Sap
2.2.5. Amino Acid Extraction and Detection
2.2.6. Grain Yield and Yield Component
2.3. Data Processing and Statistical Analysis
3. Results
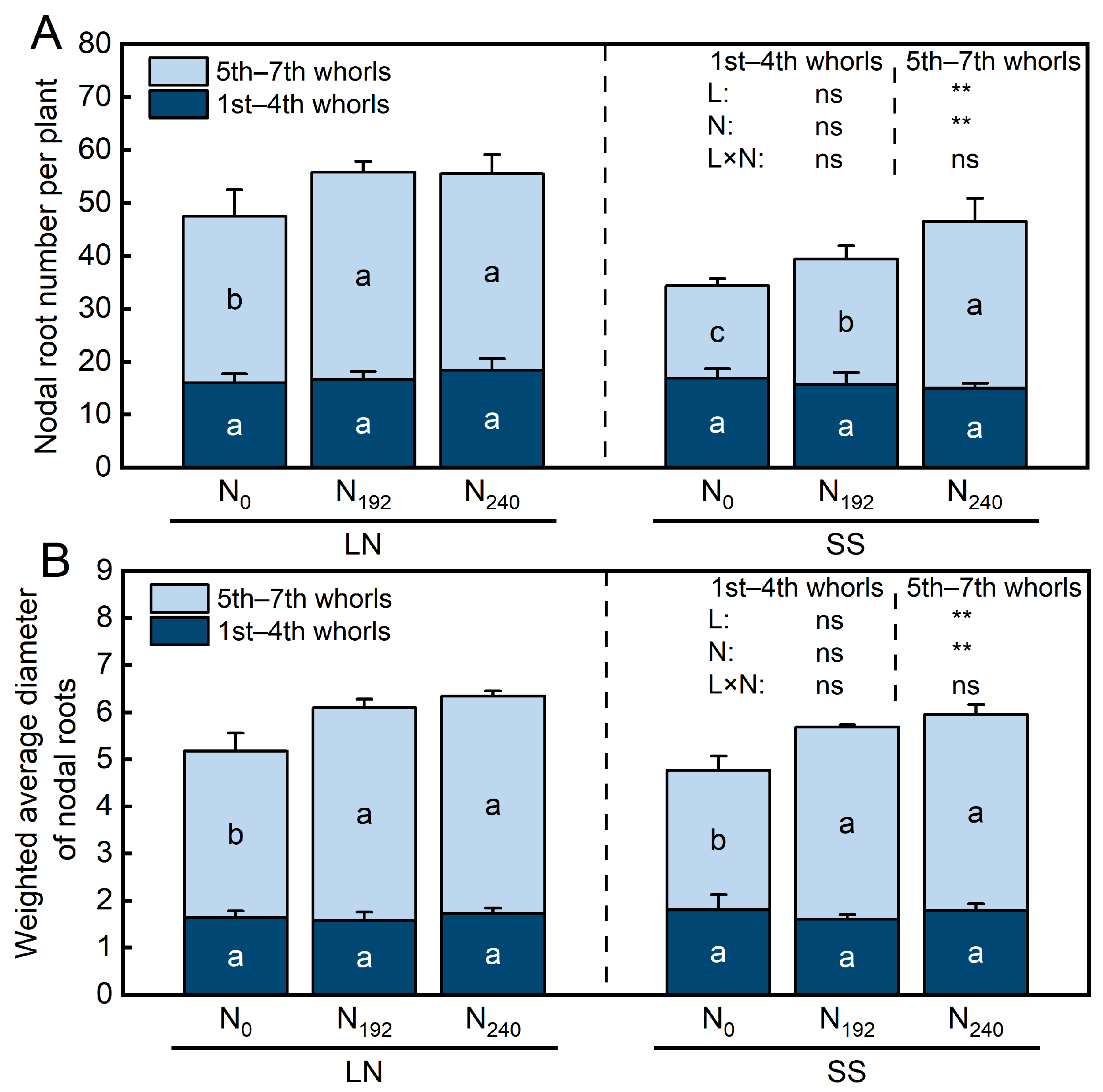
3.1. Nodal Root Number and Diameter
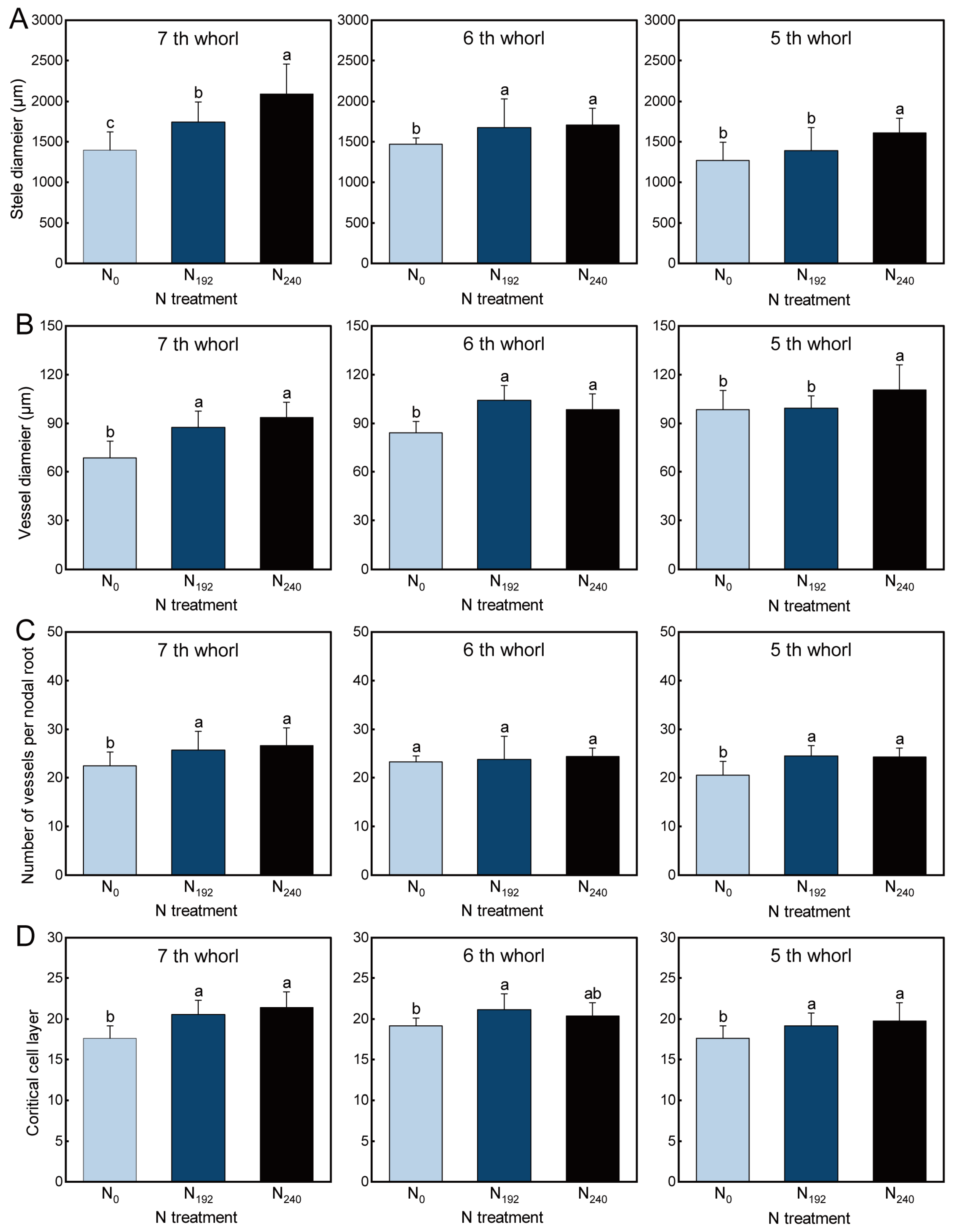
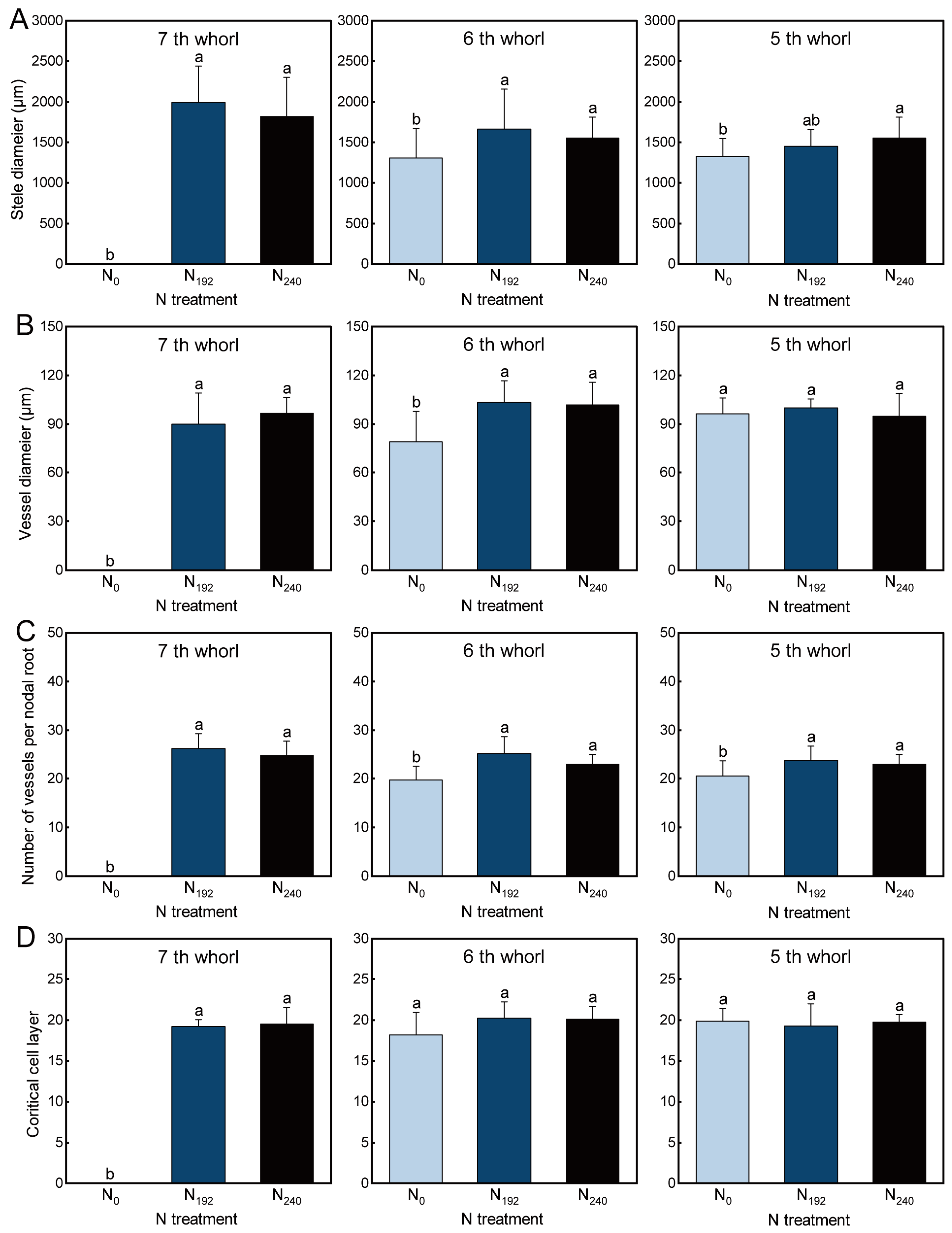
3.2. Root Anatomical Characteristics
3.2.1. Stele Diameter
3.2.2. Vessel Diameter
3.2.3. Number of Vessels per Nodal Root
3.2.4. Cortical Cell Layer Number
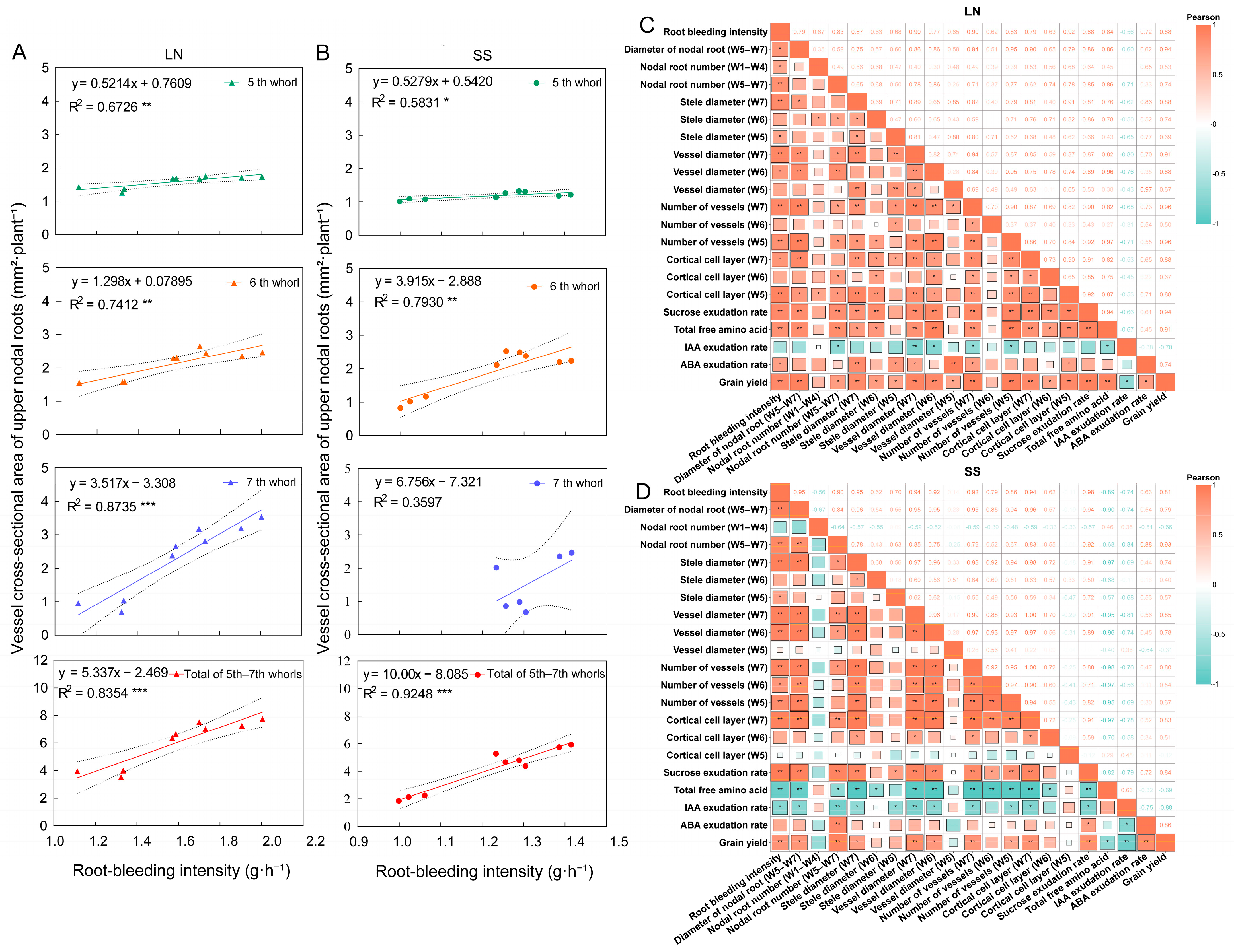
3.3. Root-Bleeding Intensity
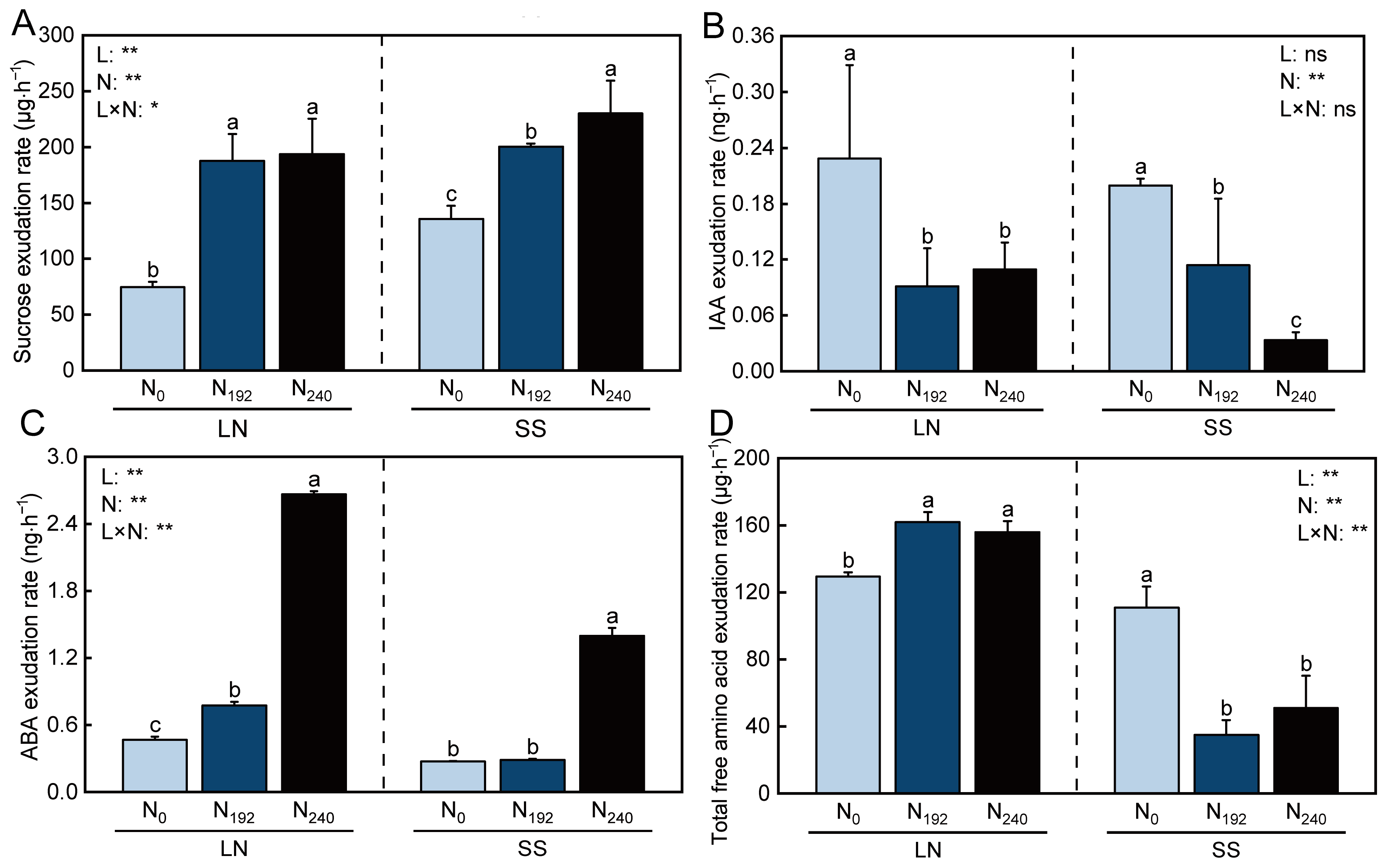
3.4. Sucrose Exudation Rate of Bleeding Sap
3.5. IAA and ABA Exudation Rates of Bleeding Sap
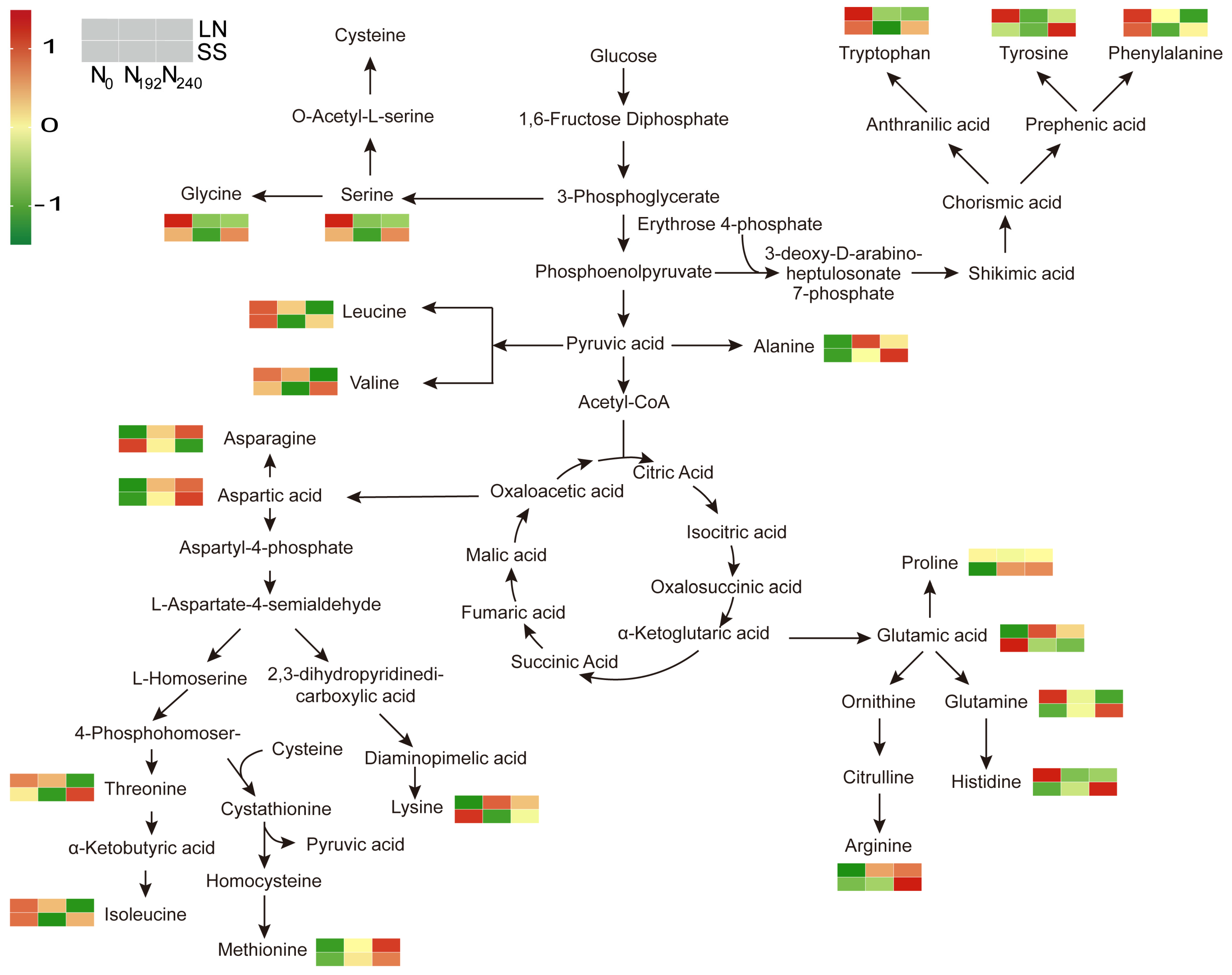
3.6. Amino Acid Content of Bleeding Sap
3.6.1. Total Free Amino Acid Exudation Rate
3.6.2. Amino Acid Exudation Rates
3.7. Grain Yield and Yield Components
3.8. Correlation Analysis
4. Discussion
4.1. Responses of Nodal Root Number, Diameter, and Anatomy to Nitrogen and Light
4.2. Responses of Root-Bleeding Intensity to Nitrogen and Light
4.3. Mechanisms Linking Nitrogen and Light Effects on Root-Bleeding Intensity Through Anatomical Changes
4.4. Responses of Sucrose and Plant Hormones (IAA and ABA) in Bleeding Sap to Nitrogen Supply and Shading
4.5. Responses of Free Amino Acids in Bleeding Sap to Nitrogen Supply and Shading
4.6. Responses of Grain Yield to Root Structural and Physiological Changes Under Nitrogen Supply and Shading
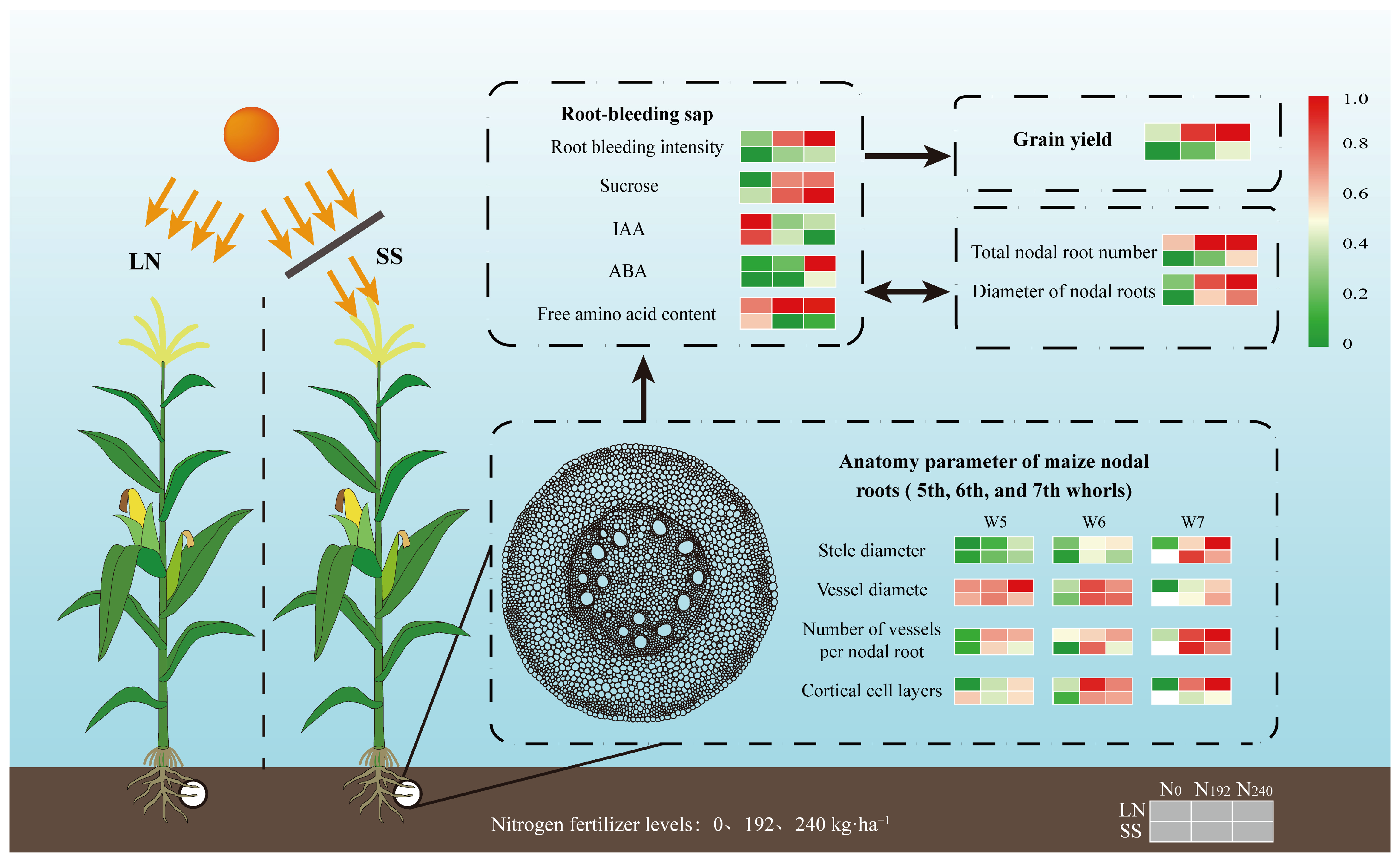
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | Abscisic Acid |
| IAA | Indole-3-Acetic Acid |
| GS-GOGAT | Glutamine Synthetase–Glutamate Synthase |
| LN | Normal Light |
| SS | Shading Stress |
References
- Sachan, D.S.; Khan, N.; Maurya, C.L.; Singh, B. Influence of different herbicides on the growth, growth attributes and yield of maize (Zea mays L.) under central plains zone of Uttar Pradesh. J. Exp. Agric. Int. 2024, 46, 9–19. [Google Scholar] [CrossRef]
- Palacios-Rojas, N.; McCulley, L.; Kaeppler, M.; Titcomb, T.J.; Gunaratna, N.S.; Lopez-Ridaura, S.; Tanumihardjo, S.A. Mining maize diversity and improving its nutritional aspects within agro-food systems. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1809–1834. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Yu, Y.; Qian, C.; Li, C.; Wei, S.; Li, C.; Gu, W. Nitrogen and chemical control management improve yield and quality in high-density planting of maize by promoting root-bleeding sap and nutrient absorption. Front. Plant Sci. 2022, 13, 754232. [Google Scholar] [CrossRef]
- Xu, L.Z.; Niu, J.F.; Li, C.J.; Zhang, F.S. Growth, nitrogen uptake and flow in maize plants affected by root growth restriction. J. Integr. Plant Biol. 2009, 51, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.F.; Gao, J.L.; Sun, J.Y.; Liu, J.; Su, Z.J.; Wang, Z.G. Effects of straw returning and potassium fertilizer application on root characteristics and yield of spring maize in China Inner Mongolia. Agron. J. 2021, 113, 4369–4385. [Google Scholar] [CrossRef]
- Guan, D.; Al-Kaisi, M.M.; Zhang, Y.; Duan, L.; Tan, W.; Zhang, M.; Li, Z. Tillage practices affect biomass and grain yield through regulating root growth, root-bleeding sap and nutrients uptake in summer maize. Field Crops Res. 2014, 157, 89–97. [Google Scholar] [CrossRef]
- Liu, K.; Li, T.; Chen, Y.; Huang, J.; Qiu, Y.; Li, S.; Wang, H.; Zhu, A.; Zhuo, X.; Yu, F.; et al. Effects of root morphology and physiology on the formation and regulation of large panicles in rice. Field Crops Res. 2020, 258, 107946. [Google Scholar] [CrossRef]
- Chen, P.P.; Gu, X.B.; Li, Y.N.; Qiao, L.R.; Li, Y.P.; Fang, H.; Yin, M.H.; Zhou, C.M. Effects of residual film on maize root distribution, yield and water use efficiency in Northwest China. Agric. Water Manag. 2022, 260, 107289. [Google Scholar] [CrossRef]
- Hodge, A. Plastic plants and patchy soils. J. Exp. Bot. 2006, 57, 401–411. [Google Scholar] [CrossRef]
- Hochholdinger, F.; Tuberosa, R. Genetic and genomic dissection of maize root development and architecture. Curr. Opin. Plant Biol. 2009, 12, 172–177. [Google Scholar] [CrossRef]
- Shao, H.; Xia, T.; Wu, D.; Chen, F.; Mi, G. Root growth and root system architecture of field-grown maize in response to high planting density. Plant Soil 2018, 430, 395–411. [Google Scholar] [CrossRef]
- Mi, G.; Chen, F.; Yuan, L.; Zhang, F. Ideotype root system architecture for maize to achieve high yield and resource use efficiency in intensive cropping systems. Adv. Agron. 2016, 139, 73–97. [Google Scholar] [CrossRef]
- Schneider, H.M.; Yang, J.T.; Brown, K.M.; Lynch, J.P. Nodal root diameter and node number in maize (Zea mays L.) interact to influence plant growth under nitrogen stress. Plant Direct 2020, 5, e00310. [Google Scholar] [CrossRef]
- York, L.M.; Lynch, J.P. Intensive field phenotyping of maize (Zea mays L.) root crowns identifies phenes and phene integration associated with plant growth and nitrogen acquisition. J. Exp. Bot. 2015, 66, 5493–5505. [Google Scholar] [CrossRef]
- Stamp, P.; Kiel, C. Root morphology of maize and its relationship to root lodging. J. Agron. Crop Sci. 2008, 168, 113–118. [Google Scholar] [CrossRef]
- Ennos, A.R.; Crook, M.J.; Grimshaw, C.G. The anchorage mechanics of maize, Zea mays. J. Exp. Bot. 1993, 44, 147–153. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, Y.; Turner, D.; Duan, Y.; Wang, D.; Shen, Q. Root physiological and morphological characteristics of two rice cultivars with different nitrogen-use efficiency. Pedosphere 2010, 20, 446–455. [Google Scholar] [CrossRef]
- Yang, J.T.; Schneider, H.M.; Brown, K.M.; Lynch, J.P. Genotypic variation and nitrogen stress effects on root anatomy in maize are node specific. J. Exp. Bot. 2019, 70, 5311–5325. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, F.; Yuan, L.; Mi, G. The physiological mechanism underlying root elongation in response to nitrogen deficiency in crop plants. Planta 2020, 251, 84. [Google Scholar] [CrossRef]
- Gao, K.; Chen, F.; Yuan, L.; Zhang, F.; Mi, G. A comprehensive analysis of root morphological changes and nitrogen allocation in maize in response to low nitrogen stress. Plant Cell Environ. 2015, 38, 740–750. [Google Scholar] [CrossRef]
- Jia, X.; Wu, G.; Strock, C.F.; Li, L.; Dong, S.; Zhang, J.; Zhao, B.; Lynch, J.P.; Liu, P. Root anatomical phenotypes related to growth under low nitrogen availability in maize (Zea mays L.) hybrids. Plant Soil 2022, 474, 265–276. [Google Scholar] [CrossRef]
- Gheith, E.M.S.; El-Badry, O.Z.; Lamlom, S.F.; Ali, H.M.; Siddiqui, M.H.; Ghareeb, R.Y.; El-Sheikh, M.H.; Jebril, J.; Abdelsalam, N.R.; Kandil, E.E. Maize (Zea mays L.) productivity and nitrogen use efficiency in response to nitrogen application levels and time. Front. Plant Sci. 2022, 13, 941343. [Google Scholar] [CrossRef]
- Su, W.; Ahmad, S.; Ahmad, I.; Han, Q. Nitrogen fertilization affects maize grain yield through regulating nitrogen uptake, radiation and water use efficiency, photosynthesis and root distribution. PeerJ 2020, 8, e10291. [Google Scholar] [CrossRef]
- Kang, S.; Shi, W.; Zhang, J. An improved water-use efficiency for maize grown under regulated deficit irrigation. Field Crops Res. 2000, 67, 207–214. [Google Scholar] [CrossRef]
- Wang, P.; Yu, A.; Wang, F.; Wang, Y.; Lyu, H.; Shang, Y.; Yang, X.; Liu, Y.; Yin, B.; Zhang, D.; et al. Nitrogen reduction by 20% with green manure retention reduces soil evaporation, promotes maize transpiration and improves water productivity in arid areas. Field Crops Res. 2024, 315, 109488. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Malhi, S.S.; Li, S.; Gao, Y.; Tian, X. Chapter 7: Nutrient and water management effects on crop production, and nutrient and water use efficiency in dryland areas of China. Adv. Agron. 2009, 102, 223–265. [Google Scholar]
- Sakaigaichi, T.; Morita, S.; Abe, J.; Yamaguchi, T. Diurnal and phenological changes in the rate of nitrogen transportation monitored by bleeding in field-grown rice plants (Oryza sativa L.). Plant Prod. Sci. 2007, 10, 270–276. [Google Scholar] [CrossRef]
- Tang, Y.H.; Shi, W.B.; Xia, X.; Zhao, D.Q.; Wu, Y.Q.; Tao, J. Morphological, microstructural and lignin-related responses of herbaceous peony stem to shading. Sci. Hortic. 2022, 293, 110734. [Google Scholar] [CrossRef]
- Casal, J.J. Shade avoidance. Arabidopsis Book 2012, 10, e0157. [Google Scholar] [CrossRef]
- Ma, J.; Li, X.; He, M.; Li, Y.; Lu, W.; Li, M.; Sun, B.; Zheng, Y. A joint transcriptomic and metabolomic analysis reveals the regulation of shading on lignin biosynthesis in asparagus. Int. J. Mol. Sci. 2023, 24, 1539. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, B.; Dong, S.T.; Liu, P.; Ren, B.Z.; Zhang, J.W. Response of summer maize photosynthate accumulation and distribution to shading stress assessed by using 13CO2 stable isotope tracer in the field. Front. Plant Sci. 2017, 8, 1821. [Google Scholar] [CrossRef]
- Huang, D.; Li, W.; Chen, J.R.; Dong, L. Morphological plasticity, photosynthesis and chlorophyll fluorescence of Athyrium pachyphlebium at different shade levels. Photosynthetica 2011, 49, 611–618. [Google Scholar] [CrossRef]
- Yamazaki, J.; Shinomiya, Y. Effect of partial shading on the photosynthetic apparatus and photosystem stoichiometry in sunflower leaves. Photosynthetica 2013, 51, 3–12. [Google Scholar] [CrossRef]
- Wang, J.; Yao, R.; Sun, Z.; Wang, M.; Jiang, C.; Zhao, X.; Liu, X.; Zhong, C.; Zhang, H.; Zhao, S.; et al. Effects of shading on morphology, photosynthesis characteristics, and yield of different shade-tolerant peanut varieties at the flowering stage. Front. Plant Sci. 2024, 15, 1429800. [Google Scholar] [CrossRef]
- Urban, A.; Rogowski, P.; Wasilewska-Dębowska, W.; Romanowska, E. Understanding maize response to nitrogen limitation in different light conditions for the improvement of photosynthesis. Plants 2021, 10, 1932. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, Z.; Zhao, B.; Dong, S.; Liu, P.; Zhang, J. Shade stress decreased maize grain yield, dry matter, and nitrogen accumulation. Agron. J. 2020, 112, 2768–2776. [Google Scholar] [CrossRef]
- Yamasaki, A. Root-pressure driven xylem sap flow in greenhouse melon (Cucumis melo L.): Diurnal change and the effects of shading, growth stage, rootstock and fruit number. Plant Soil 2003, 255, 409–412. [Google Scholar] [CrossRef]
- Nardini, A.; Grego, F.; Trifilò, P.; Salleo, S. Changes of xylem sap ionic content and stem hydraulics in response to irradiance in Laurus nobilis. Tree Physiol. 2010, 30, 628–635. [Google Scholar] [CrossRef]
- Schurr, U. Xylem sap sampling—New approaches to an old topic. Trends Plant Sci. 1998, 3, 293–298. [Google Scholar] [CrossRef]
- Wang, H.; Xu, R.; Li, Y.; Yang, L.; Shi, W.; Liu, Y.; Chang, S.; Hou, F.; Jia, Q. Enhance root-bleeding sap flow and root lodging resistance of maize under a combination of nitrogen strategies and farming practices. Agric. Water Manag. 2019, 224, 105742. [Google Scholar] [CrossRef]
- Ahmad, N.; Jiang, Z.; Zhang, L.; Hussain, I.; Yang, X. Insights on phytohormonal crosstalk in plant response to nitrogen stress: A focus on plant root growth and development. Int. J. Mol. Sci. 2023, 24, 3631. [Google Scholar] [CrossRef]
- Li, G.H.; Zhao, B.; Dong, S.T.; Zhang, J.W.; Liu, P.; Ren, B.Z.; Lu, D.L.; Lu, W.P. Morphological and physiological characteristics of maize roots in response to controlled-release urea under different soil moisture conditions. Agron. J. 2019, 111, 1849–1864. [Google Scholar] [CrossRef]
- Fang, H.; Qin, W.; Wang, L.; Zhang, M.; Yang, X. Solar brightening/dimming over China’s Mainland: Effects of atmospheric aerosols, anthropogenic emissions, and meteorological conditions. Remote Sens. 2021, 13, 88. [Google Scholar] [CrossRef]
- Cui, Z.; Chen, X.; Miao, Y.; Zhang, F.; Sun, Q.; Schroder, J.; Zhang, H.; Li, J.; Shi, L.; Xu, J.; et al. On-farm evaluation of the improved soil Nmin–based nitrogen management for summer maize in North China Plain. Agron. J. 2008, 100, 517–525. [Google Scholar] [CrossRef]
- Yang, H.; Dong, B.; Wang, Y.; Qiao, Y.; Shi, C.; Jin, L.; Liu, M. Photosynthetic base of reduced grain yield by shading stress during the early reproductive stage of two wheat cultivars. Sci. Rep. 2020, 10, 14353. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, N.; Chen, L. Paraffin section observation of flower bud differentiation of Chimonanthus praecox in Kunming and comparison of the differentiation processes in different regions, China. Hortic. Plant J. 2022, 8, 221–229. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, K.L.; Dong, S.T.; Liu, P.; Zhao, B.; Zhang, J.W. Effects of integrated agronomic practices management on root growth and development of summer maize. Eur. J. Agron. 2017, 84, 140–151. [Google Scholar] [CrossRef]
- Aguirrezabal, L.; Pellerin, S.; Tardieu, F. Carbon nutrition, root branching and elongation: Can the present state of knowledge allow a predictive approach at a whole-plant level? Environ. Exp. Bot. 1993, 33, 121–130. [Google Scholar] [CrossRef]
- Yu, Y.; Qian, C.; Gu, W.; Li, C. Responses of root characteristic parameters and plant dry matter accumulation, distribution and transportation to nitrogen levels for spring maize in Northeast China. Agriculture 2021, 11, 308. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Yang, X.; Wang, B.; Gu, W.; Wang, Y. Effects of carbon-based fertilizer on maize root morphology, root bleeding rate and components in Northeast China. Agronomy 2023, 13, 814. [Google Scholar] [CrossRef]
- Noguchi, A.; Kageyama, M.; Shinmachi, F.; Schmidhalter, U.; Hasegawa, I. Potential for using plant xylem sap to evaluate inorganic nutrient availability in soil. Soil Sci. Plant Nutr. 2005, 51, 333–341. [Google Scholar] [CrossRef]
- Morita, S.; Okamoto, M.; Abe, J.; Yamagishi, J. Bleeding rate of field-grown maize with reference to root system development. Jpn. J. Crop Sci. 2000, 69, 80–85. [Google Scholar] [CrossRef]
- Gao, J.; Shi, J.; Dong, S.; Liu, P.; Zhao, B.; Zhang, J. Grain yield and root characteristics of summer maize (Zea mays L.) under shade stress conditions. J. Agron. Crop Sci. 2017, 203, 562–573. [Google Scholar] [CrossRef]
- Jia, Q.; Chen, K.; Chen, Y.; Ali, S.; Manzoor, S.; Sohail, A.; Fahad, S. Mulch covered ridges affect grain yield of maize through regulating root growth and root-bleeding sap under simulated rainfall conditions. Soil Tillage Res. 2018, 175, 101–111. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef]
- Gruber, B.D.; Giehl, R.F.H.; Friedel, S.; von Wirén, N. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol. 2013, 163, 161–179. [Google Scholar] [CrossRef]
- Giehl, R.F.H.; von Wirén, N. Root nutrient foraging. Plant Physiol. 2014, 166, 509–517. [Google Scholar] [CrossRef]
- Chen, L.Q.; Qu, X.Q.; Hou, B.H.; Sosso, D.; Osorio, S.; Fernie, A.R.; Frommer, W.B. Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security. J. Exp. Bot. 2012, 63, 5635–5650. [Google Scholar] [CrossRef]
- Alonso, A.P.; Raymond, P.; Hernould, M.; Rondeau-Mouro, C.; de Graaf, A.; Chourey, P.S.; Lahaye, M.; Shachar-Hill, Y.; Rolin, D.; Dieuaide-Noubhani, M. A metabolic flux analysis to study the role of sucrose synthase in the regulation of the carbon partitioning in central metabolism in maize root tips. Metab. Eng. 2007, 9, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Bush, D.R. Carbohydrate export from the leaf: A highly regulated process and target to enhance photosynthesis and productivity. Plant Physiol. 2011, 155, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Chen, F.; Liu, J.; Zhang, F.; Mi, G. Inhibition of maize root growth by high nitrate supply is correlated with reduced IAA levels in roots. J. Plant Physiol. 2008, 165, 942–951. [Google Scholar] [CrossRef]
- Qin, S.; Zhang, Z.; Ning, T.; Ren, S.; Su, L.; Li, Z. Abscisic acid and aldehyde oxidase activity in maize ear leaf and grain relative to post-flowering photosynthetic capacity and grain filling rate under different water/nitrogen treatments. Plant Physiol. Biochem. 2013, 70, 69–80. [Google Scholar] [CrossRef]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot. 2011, 62, 1399–1409. [Google Scholar] [CrossRef]
- Ma, W.; Li, J.; Qu, B.; He, X.; Zhao, X.; Li, B.; Fu, X.; Tong, Y. Auxin biosynthetic gene TAR2 is involved in low nitrogen-mediated reprogramming of root architecture in Arabidopsis. Plant J. 2014, 78, 70–79. [Google Scholar] [CrossRef]
- Walch-Liu, P.; Ivanov, I.I.; Filleur, S.; Gan, Y.; Remans, T.; Forde, B.G. Nitrogen regulation of root branching. Ann. Bot. 2006, 97, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Rook, F.; Corke, F.; Card, R.; Munz, G.; Smith, C.; Bevan, M.W. Impaired sucrose-induction mutants reveal the modulation of sugar-induced starch biosynthetic gene expression by abscisic acid signaling. Plant J. 2001, 26, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Song, H.X.; Li, S.X. Effects of water and N supply on maize bleeding sap and its nutrient contents. Plant Nutr. Fertil. Sci. 2004, 10, 574–578. [Google Scholar] [CrossRef]
- Puiatti, M.; Sodek, L. Waterlogging affects nitrogen transport in the xylem of soybean. Plant Physiol. Biochem. 1999, 37, 767–773. [Google Scholar] [CrossRef]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Konishi, N.; Ishiyama, K.; Matsuoka, K.; Maru, I.; Hayakawa, T.; Yamaya, T.; Kojima, S. NADH-dependent glutamate synthase plays a crucial role in assimilating ammonium in the Arabidopsis root. Physiol. Plant. 2014, 152, 138–151. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Zhao, Y.; Zhang, Y.; Zhang, J.; Ma, H.; Han, Y. Transcriptome analysis reveals nitrogen deficiency induced alterations in leaf and root of three cultivars of potato (Solanum tuberosum L.). PLoS ONE 2020, 15, e0240662. [Google Scholar] [CrossRef]
- Prinsi, B.; Espen, L. Mineral nitrogen sources differently affect root glutamine synthetase isoforms and amino acid balance among organs in maize. BMC Plant Biol. 2015, 15, 96. [Google Scholar] [CrossRef]
- Han, M.; Zhang, C.; Suglo, P.; Sun, S.; Wang, M.; Su, T. L-Aspartate: An essential metabolite for plant growth and stress acclimation. Molecules 2021, 26, 1887. [Google Scholar] [CrossRef]
- Murchie, E.H.; Ferrario-Méry, S.; Valadier, M.H.; Foyer, C.H. Short-term nitrogen-induced modulation of phosphoenolpyruvate carboxylase in tobacco and maize leaves. J. Exp. Bot. 2000, 51, 1349–1356. [Google Scholar] [CrossRef]
- Antunes, A.P.; Hibberd, J.M.; Muench, D.G. Nitrogen stress and the expression of asparagine synthetase in roots and nodules of soybean (Glycine max). Physiol. Plant. 2008, 133, 736–743. [Google Scholar] [CrossRef]
- Good, A.G.; Johnson, S.J.; De Pauw, M.; Carroll, R.T.; Savidov, N.; Vidmar, J.; Lu, Z.; Taylor, G.; Stroeher, V. Engineering nitrogen use efficiency with alanine aminotransferase. Can. J. Bot. 2007, 85, 252–262. [Google Scholar] [CrossRef]
- Won, C.; Shen, X.; Mashiguchi, K.; Zheng, Z.; Dai, X.; Cheng, Y.; Kasahara, H.; Kamiya, Y.; Chory, J.; Zhao, Y. Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCA proteins. Proc. Natl. Acad. Sci. USA 2011, 108, 18518–18523. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.K.; Devi, S.; Buckseth, T.; Ali, N.; Singh, R.K.; Zinta, R.; Dua, V.K.; Chakrabarti, S.K. Precision phenotyping of contrasting potato (Solanum tuberosum L.) varieties in a novel aeroponics system for improving nitrogen use efficiency: In search of key traits and genes. J. Integr. Agric. 2020, 19, 51–61. [Google Scholar] [CrossRef]
- Li, M.; Guo, R.; Jiao, Y.; Jin, X.; Zhang, H.; Shi, L. Comparison of salt tolerance in Soja based on metabolomics of seedling roots. Front. Plant Sci. 2017, 8, 1101. [Google Scholar] [CrossRef]
- Zimmermann, S.E.; Benstein, R.M.; Flores-Tornero, M.; Blau, S.; Anoman, A.D.; Rosa-Téllez, S.; Gerlich, S.C.; Salem, M.A.; Alseekh, S.; Kopriva, S.; et al. The phosphorylated pathway of serine biosynthesis links plant growth with nitrogen metabolism. Plant Physiol. 2021, 186, 1487–1506. [Google Scholar] [CrossRef]
- Maqbool, S.; Hassan, M.A.; Xia, X.; York, L.M.; Rasheed, A.; He, Z. Root system architecture in cereals: Progress, challenges, and perspective. Plant J. 2022, 110, 23–48. [Google Scholar] [CrossRef] [PubMed]
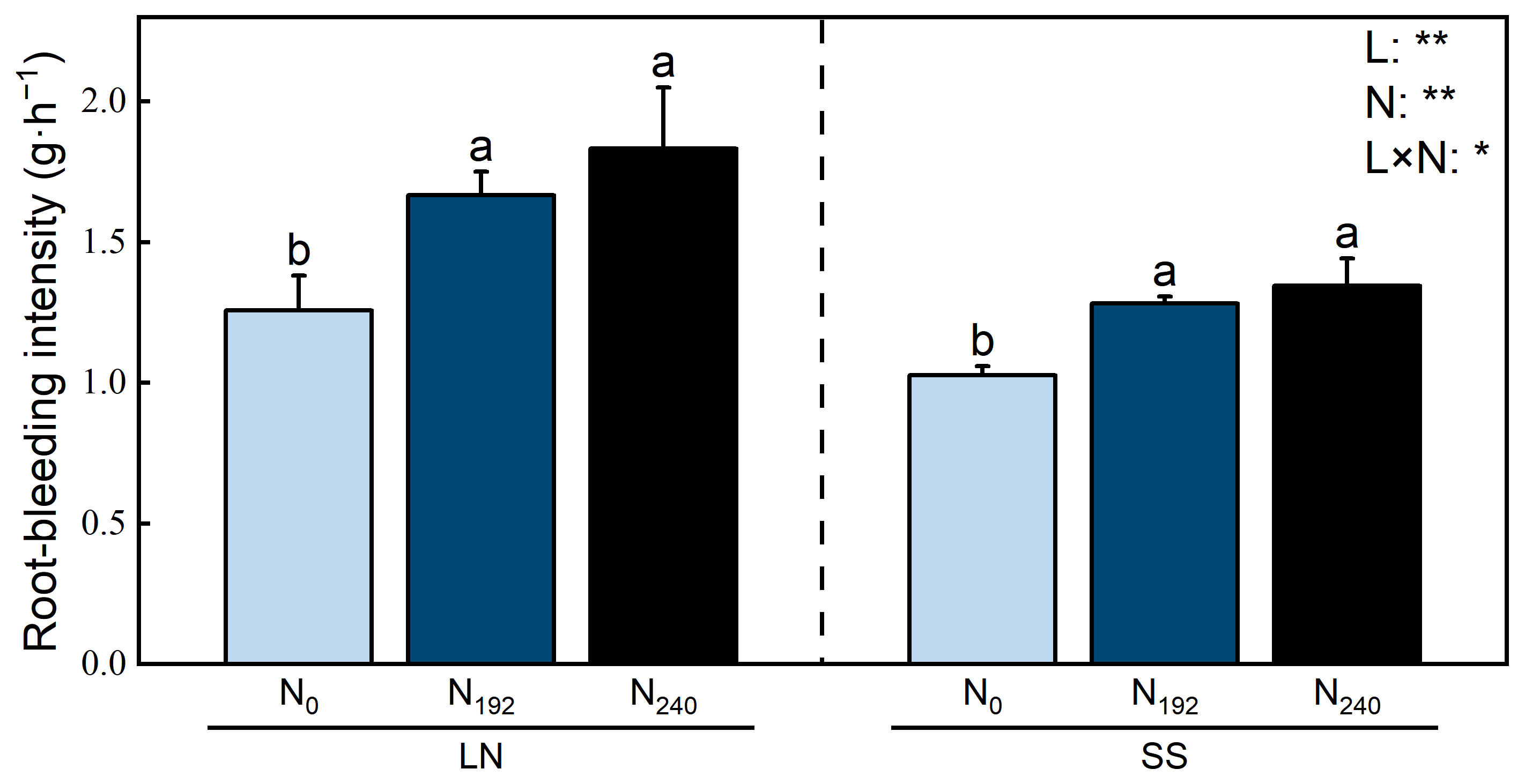
| Treatment | Light Condition | Nitrogen Level |
|---|---|---|
| LN-N0 | Normal light | 0 kg·ha−1 |
| LN-N192 | Normal light | 192 kg·ha−1 |
| LN-N240 | Normal light | 240 kg·ha−1 |
| SS-N0 | 50% Shading | 0 kg·ha−1 |
| SS-N192 | 50% Shading | 192 kg·ha−1 |
| SS-N240 | 50% Shading | 240 kg·ha−1 |
| Treatment | Ear Length (cm) | Ear Diameter (cm) | Kernel Rows per Ear | Kernels per Row | 100-Grain Weight (g) | Grain Yield (g·plant−1) | |
|---|---|---|---|---|---|---|---|
| LN | N0 | 19.82 ± 0.34 b | 4.78 ± 0.08 c | 16.40 ± 1.59 a | 38.40 ± 1.61 b | 31.09 ± 1.79 b | 206.88 ± 6.87 c |
| N192 | 21.34 ± 0.49 a | 5.00 ± 0.04 b | 16.10 ± 1.74 a | 42.67 ± 0.49 a | 41.18 ± 0.75 a | 287.17 ± 1.64 b | |
| N240 | 21.73 ± 0.88 a | 5.15 ± 0.10 a | 16.97 ± 1.01 a | 43.07 ± 0.97 a | 40.36 ± 1.40 a | 306.25 ± 5.12 a | |
| SS | N0 | 14.27 ± 0.84 c | 4.70 ± 0.08 b | 15.87 ± 0.08 a | 25.43 ± 0.87 c | 30.02 ± 1.10 b | 133.05 ± 3.65 c |
| N192 | 15.60 ± 0.26 b | 4.95 ± 0.11 a | 16.67 ± 0.11 a | 30.70 ± 1.68 b | 38.44 ± 2.87 a | 169.99 ± 4.59 b | |
| N240 | 18.07 ± 0.55 a | 4.89 ± 0.04 a | 16.50 ± 0.04 a | 36.63 ± 3.71 a | 36.14 ± 2.21 a | 212.54 ± 11.75 a | |
| Source of variation | |||||||
| Light | ** | ** | ns | ** | ** | ** | |
| Nitrogen | ** | ** | ns | ** | ** | ** | |
| Light × Nitrogen | * | ns | ns | * | ns | ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Dai, M.; Liu, S.; Ma, Y.; Qin, Z.; Liu, C.; Wang, R. Anatomical and Physiological Responses of Maize Nodal Roots to Shading Stress and Nitrogen Supply. Agronomy 2025, 15, 1949. https://doi.org/10.3390/agronomy15081949
Liu J, Dai M, Liu S, Ma Y, Qin Z, Liu C, Wang R. Anatomical and Physiological Responses of Maize Nodal Roots to Shading Stress and Nitrogen Supply. Agronomy. 2025; 15(8):1949. https://doi.org/10.3390/agronomy15081949
Chicago/Turabian StyleLiu, Junren, Mingmei Dai, Shengqun Liu, Yue Ma, Zhanxiang Qin, Chang Liu, and Rui Wang. 2025. "Anatomical and Physiological Responses of Maize Nodal Roots to Shading Stress and Nitrogen Supply" Agronomy 15, no. 8: 1949. https://doi.org/10.3390/agronomy15081949
APA StyleLiu, J., Dai, M., Liu, S., Ma, Y., Qin, Z., Liu, C., & Wang, R. (2025). Anatomical and Physiological Responses of Maize Nodal Roots to Shading Stress and Nitrogen Supply. Agronomy, 15(8), 1949. https://doi.org/10.3390/agronomy15081949





