Biological Enzymatic Hydrolysis—Single Screw Co-Extrusion Treatment to Improve the Mechanical Properties of Biodegradable Straw Fiber Mulching Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
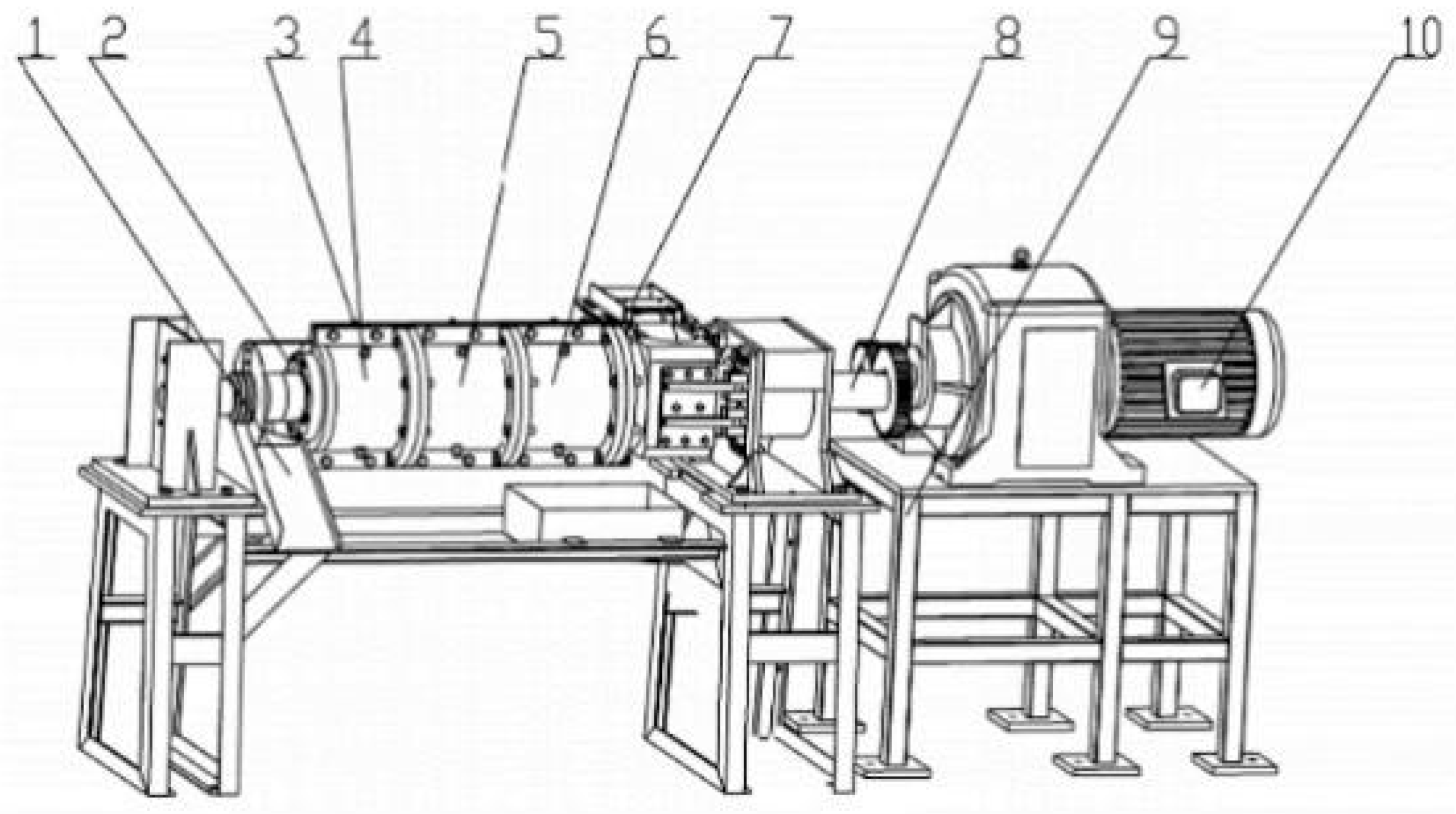
2.2. Instrumentation and Equipment
The Process of Making Straw Using Single Screw Extrusion Puffing
2.3. Pretreatment of Straw Using Biological Fermentation
2.3.1. Macroscopic Quality Loss During Fermentation
2.3.2. Physical Performance Testing
2.3.3. Test Methods for Changes in Chemical Properties
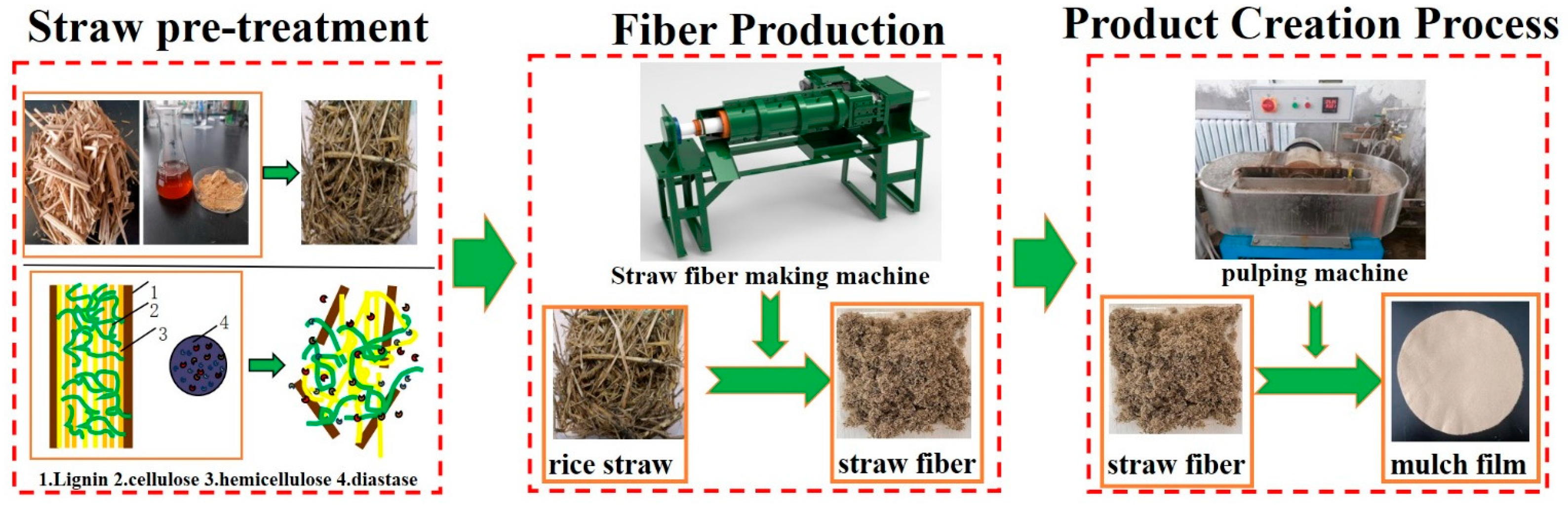
2.4. Preparation of Straw Fiber Mulch Film
2.4.1. Fiber Preparation Method for Mulch Film
2.4.2. Mechanical Pulping and Preparation Straw Fiber Mulch Film
2.5. Fiber Morphology Analysis
2.6. SEM Ultrastructural Characterization
2.7. Determination of Fiber Polymerization
2.8. Fiber Crystallinity Determination
3. Results
3.1. Results of Biological Fermentation Pretreatment Test
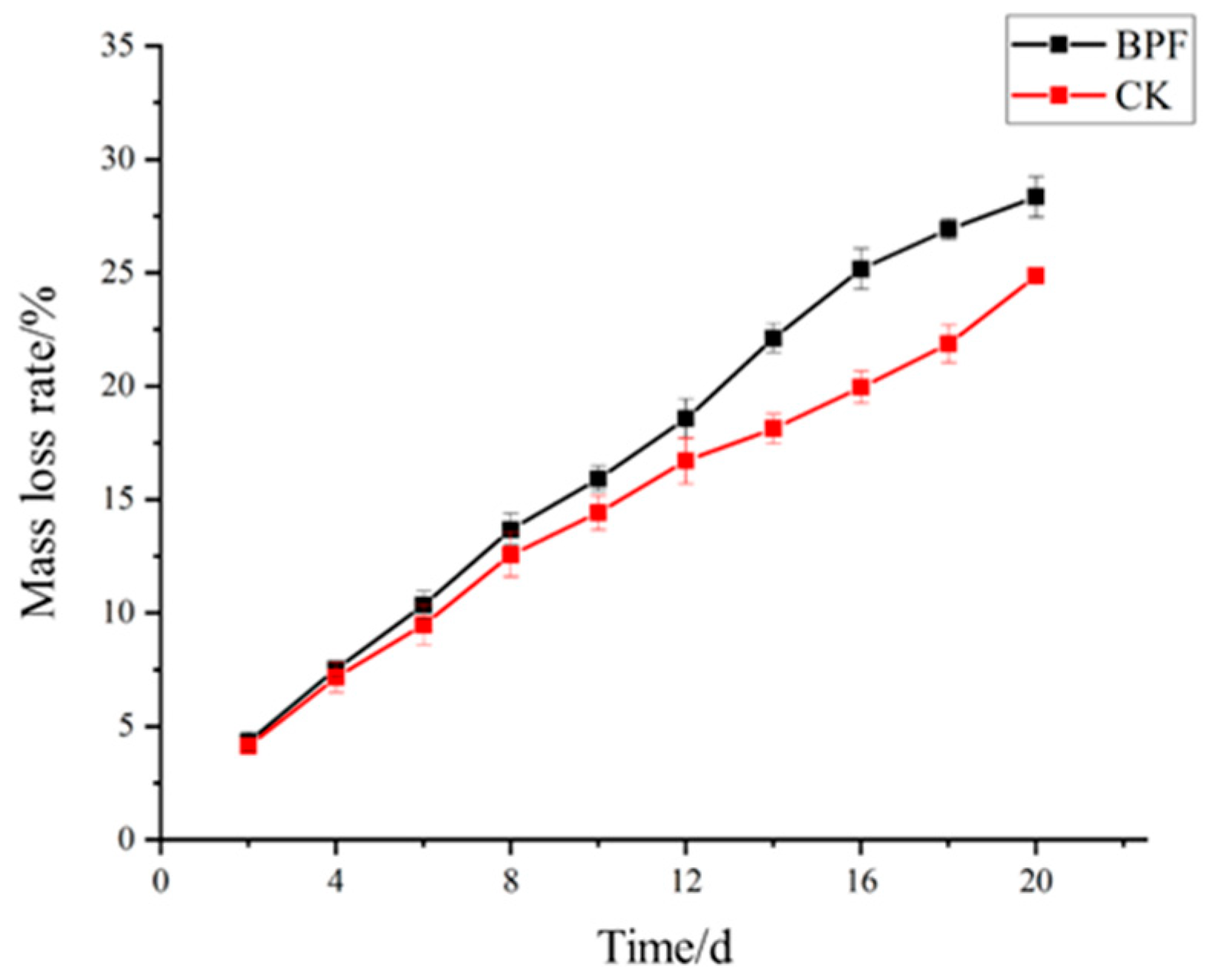
3.1.1. Mass Loss Rate
3.1.2. Changes in Mechanical Properties During Fermentation
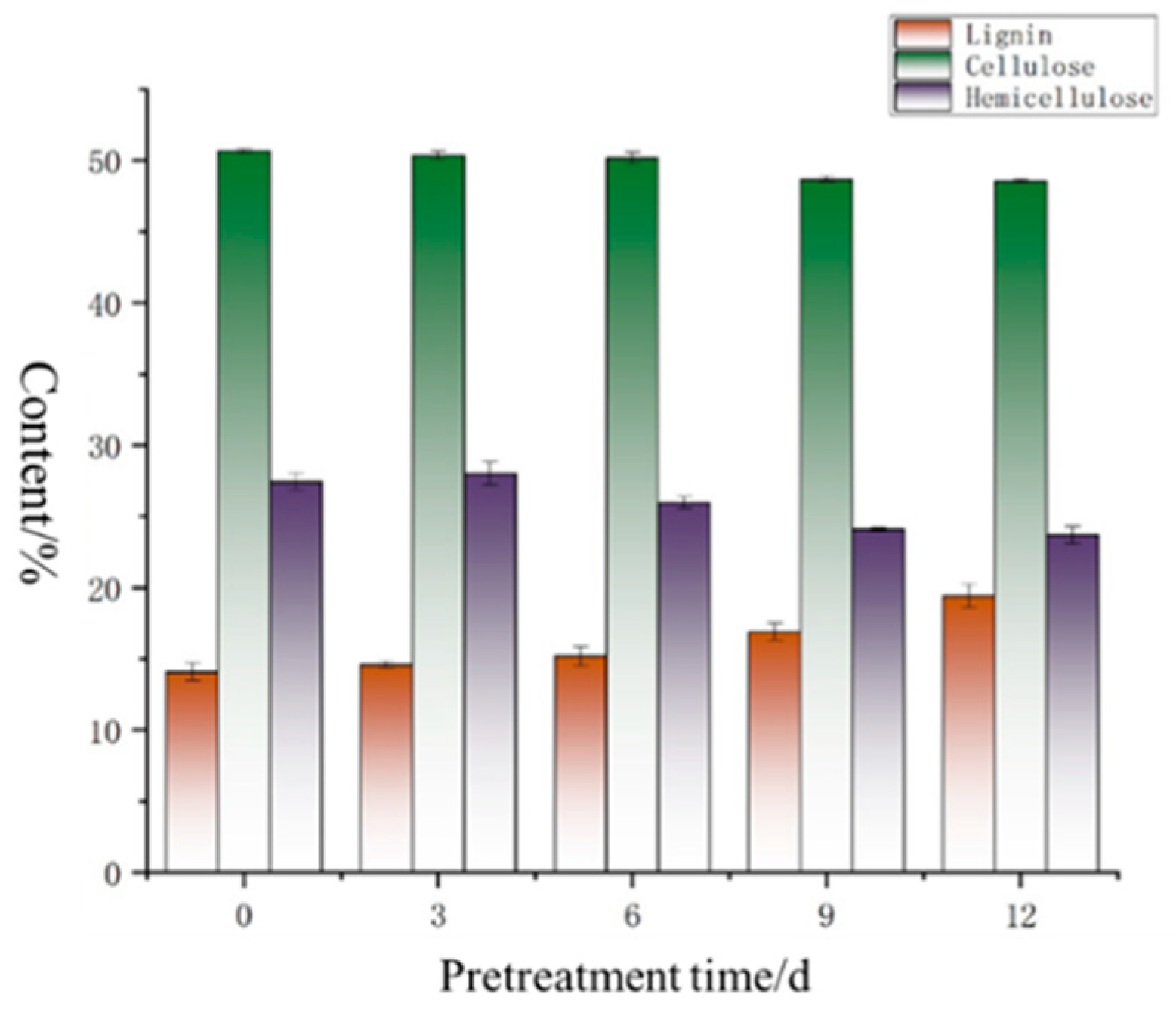
3.1.3. Changes in Chemical Composition
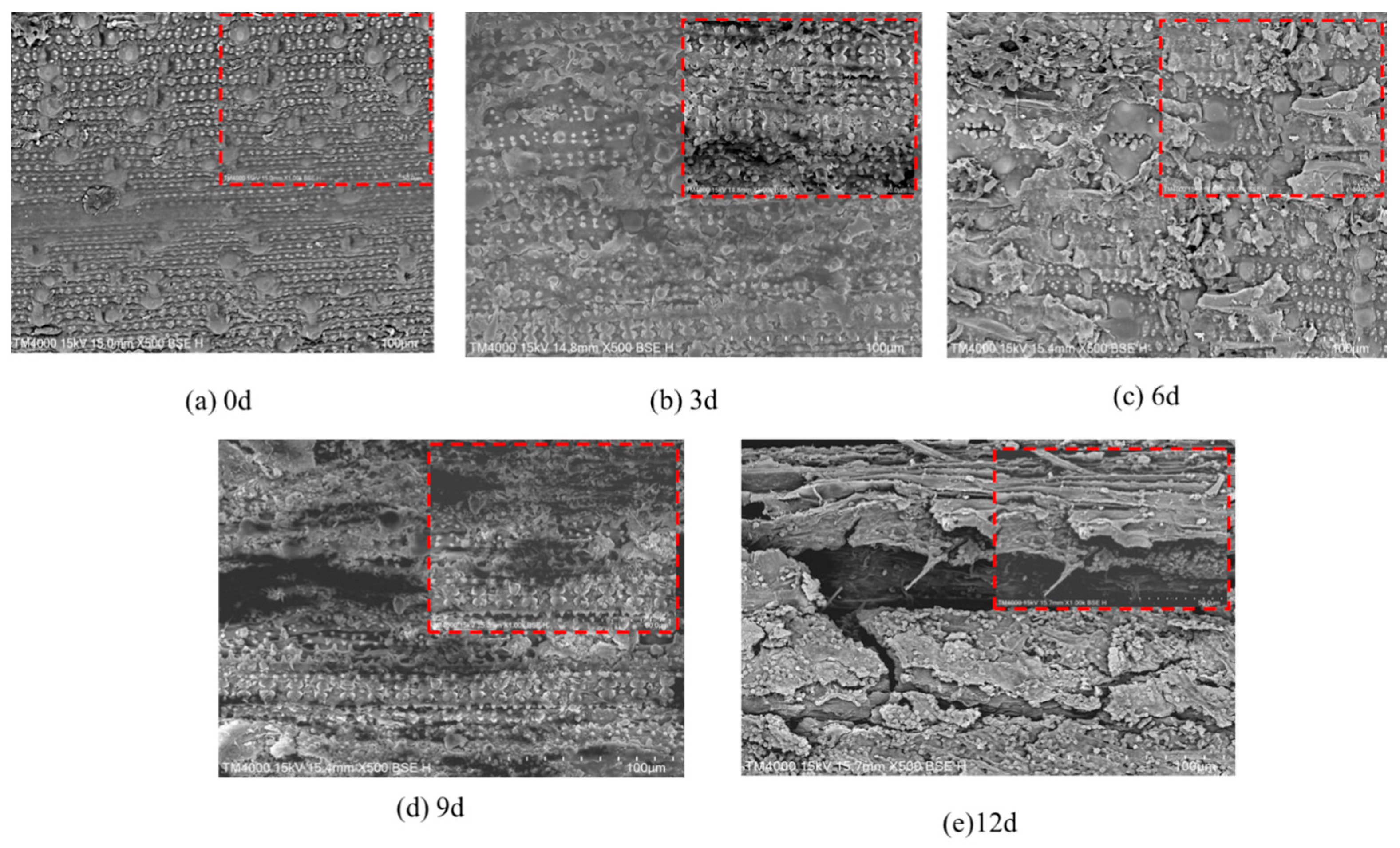
3.2. Results of SEM Ultrastructural Characterization
3.3. Results of Fiber Morphology Analysis
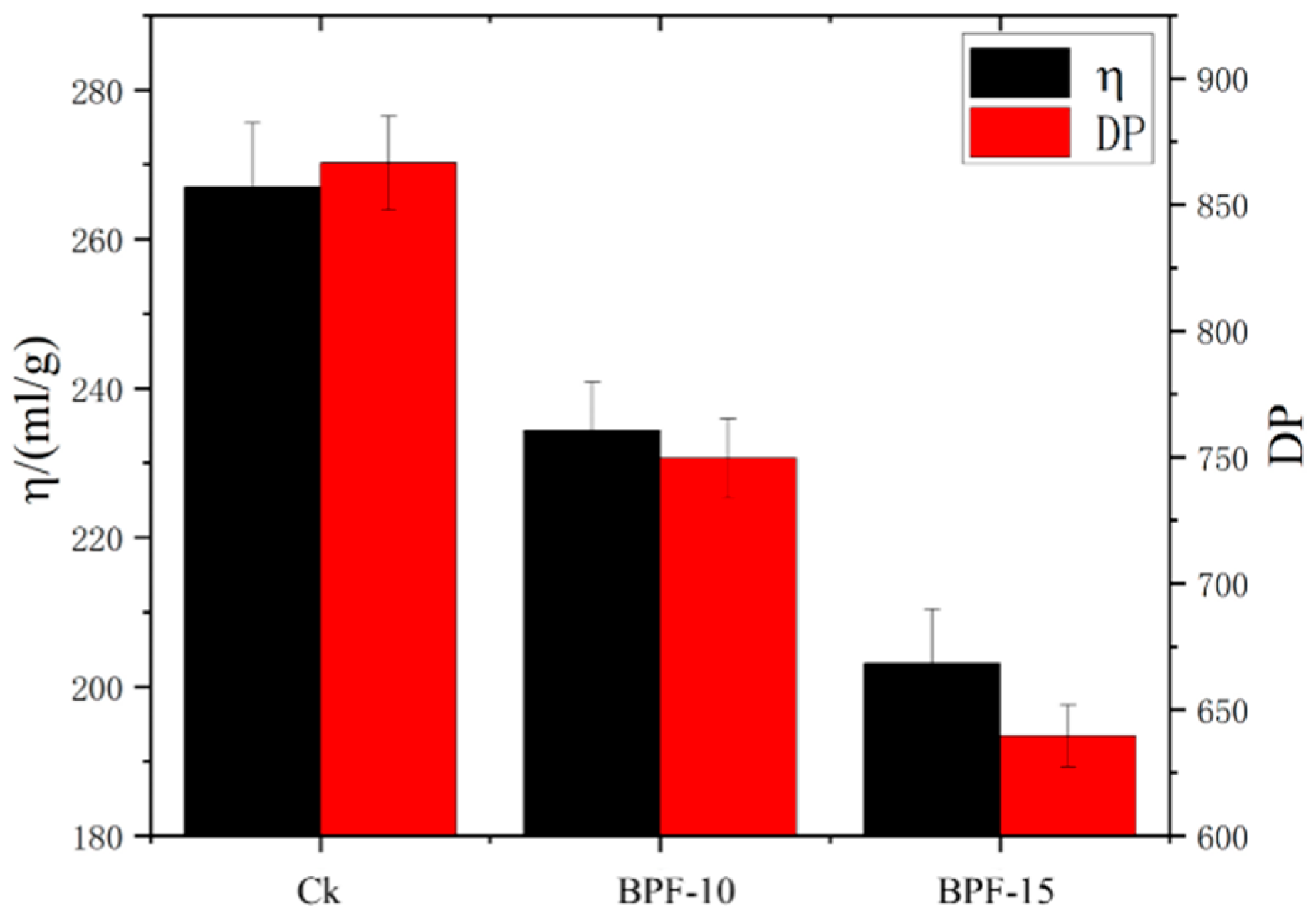
3.4. Results of Cellulose Degree of Polymerization (DP) Analysis
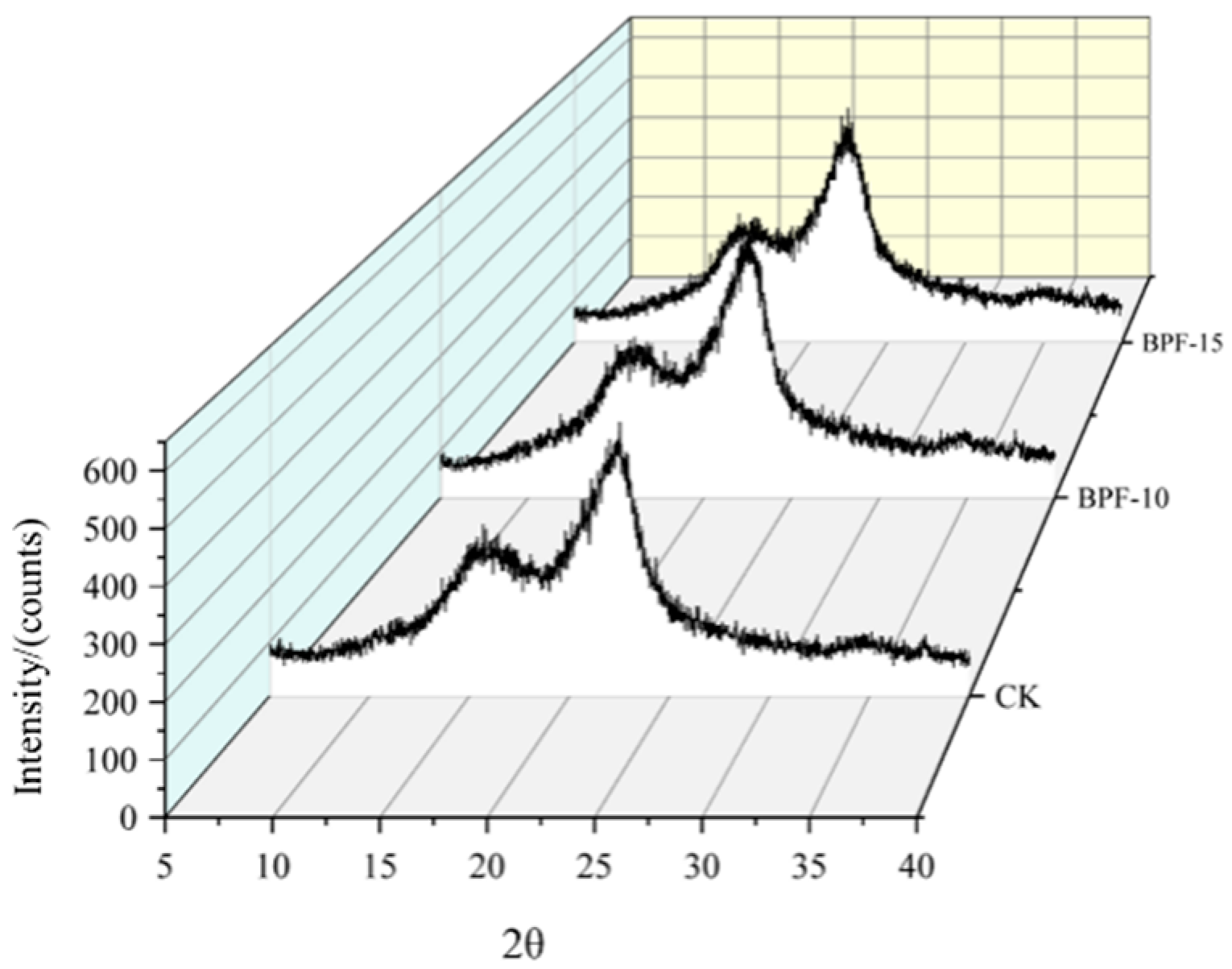
3.5. Results of the Crystallinity Test
3.6. Results of Mechanical Property Determination
4. Discussion
4.1. Discussion on Results of Biological Fermentation Pretreatment Experiments
4.1.1. Mass Loss Rate
4.1.2. Changes in Mechanical Properties During Fermentation
4.1.3. Changes in Chemical Composition
4.2. Discussion on SEM Ultrastructural Characterization
4.3. Discussion on Fiber Morphology Analysis
4.4. Analysis of Cellulose Degree of Polymerization
4.5. Analysis of Crystallinity Test Results
4.6. Determination of Mechanical Properties
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Liu, Y.; Zhou, Z.; Liu, Y.; Ge, S.; Shen, C.; Liu, Z.; Wang, J. Impacts of microplastics on agroecosystem multifunctionality: From plant production to soil microbial diversity and functions. Environ. Chem. Ecotoxicol. 2025, 7, 1634–1647. [Google Scholar] [CrossRef]
- Graf, M.; Reay, M.K.; Florent, P.J.; Brown, R.W.; Chadwick, D.R.; Jones, D.L. Differential effects of field-aged versus new LDPE and PLA/PBAT plastic film fragments on soil quality and crop productivity. J. Hazard. Mater. 2025, 496, 139398. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-D.; Park, M.-H.; Byun, H.-S. Mechanical and water barrier properties of starch/PVA composite films by adding nano-sized poly(methyl methacrylate-co-acrylamide) particles. Carbohydr. Polym. 2012, 87, 676–686. [Google Scholar] [CrossRef]
- Moreno, M.M.; González-Mora, S.; Villena, J.; Campos, J.A.; Moreno, C. Deterioration pattern of six biodegradable, potentially low-environmental impact mulches in field conditions. J. Environ. Manag. 2017, 200, 490–501. [Google Scholar] [CrossRef]
- Bilo, F.; Pandini, S.; Sartore, L.; Depero, L.E.; Gargiulo, G.; Bonassi, A.; Federici, S.; Bontempi, E. A sustainable bioplastic obtained from rice straw. J. Clean. Prod. 2018, 200, 357–368. [Google Scholar] [CrossRef]
- Beniwal, P.; Toor, A.P. Advancement in tensile properties of polylactic acid composites reinforced with rice straw fibers. Ind. Crop. Prod. 2023, 192, 116098. [Google Scholar] [CrossRef]
- Bajpai, P.; Mishra, S.P.; Mishra, O.P.; Kumar, S.; Bajpai, P.K.; Singh, S. Biochemical Pulping of Bagasse. Biotechnol. Prog. 2004, 20, 1270–1272. [Google Scholar] [CrossRef]
- Sun, E.; Zhang, Y.; Yong, C.; Qu, P.; Huang, H.; Xu, Y. Biological fermentation pretreatment accelerated the depolymerization of straw fiber and its mechanical properties as raw material for mulch film. J. Clean. Prod. 2021, 284, 124688. [Google Scholar] [CrossRef]
- Banvillet, G.; Gatt, E.; Belgacem, N.; Bras, J. Cellulose fibers deconstruction by twin-screw extrusion with in situ enzymatic hydrolysis via bioextrusion. Bioresour. Technol. 2021, 327, 124819. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Longhai, L.; Gao, C.; Fan, S.; Xu, X.; Jiang, T.; Liu, S.; Li, R.; Chen, H. Optimization of the parameters for the preparation of straw fiber raw material by biological pretreatment and synergistic expansion blasting. Trans. Chin. Soc. Agric. Eng. 2024, 40, 183–190. [Google Scholar] [CrossRef]
- Chen, H.-r.; Chen, H.-t.; Liu, S.; Dun, G.-q.; Zhang, Y. Effect of Plasticizers on Properties of Rice Straw Fiber Film. J. Northeast. Agric. Univ. (Engl. Ed.) 2014, 21, 67–72. [Google Scholar] [CrossRef]
- ISO 5270:2012; Pulps–Laboratory Sheets–Determination of Physical Properties. The International Organization for Standardization: Geneva, Switzerland, 2012.
- Hallac, B.B.; Ragauskas, A.J. Analyzing cellulose degree of polymerization and its relevancy to cellulosic ethanol. Biofuels Bioprod. Biorefining 2011, 5, 215–225. [Google Scholar] [CrossRef]
- BS 6306-1; Methods for Determination of Limiting Viscosity Number of Cellulose in Dilute Solutions Cupri-Ethylene-Diamine (CED) Method. British Standard Institution: London, UK, 1982.
- Evans, R.; Wallis, A.F.A. Cellulose molecular weights determined by viscometry. J. Appl. Polym. Sci. 1989, 37, 2331–2340. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, X.; Li, G.; Li, J.; Li, C.; Che, L.; Zhang, L. Enhanced bioenergy production in rural areas: Synthetic urine as a pre-treatment for dry anaerobic fermentation of wheat straw. J. Clean. Prod. 2020, 260, 121164. [Google Scholar] [CrossRef]
- Mattonai, M.; Pawcenis, D.; del Seppia, S.; Łojewska, J.; Ribechini, E. Effect of ball-milling on crystallinity index, degree of polymerization and thermal stability of cellulose. Bioresour. Technol. 2018, 270, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, A.; Oddershede, J.; Lilholt, H.; Thomsen, A.B.; Ståhl, K. On the determination of crystallinity and cellulose content in plant fibres. Cellulose 2005, 12, 563–576. [Google Scholar] [CrossRef]
- Seth, R.S. The measurement and significance of fines. In Proceedings of the Annual Meeting-Pulp and Paper Technical Association of Canada, Montreal, QC, Canada, 25–29 January 1999; Pulp and Paper Technical Association of Canada: Brossard, QC, Canada, 2002; Volume 88, pp. C97–C102. [Google Scholar]
- Truong, Y.B.; Shen, W.; Parker, I. Effect of primary fines and surface charge of hardwood pulps on AKD sizing. Appita J. 2003, 56, 30–34. [Google Scholar]
- Chandra, R.P.; Wu, J.; Saddler, J.N. The Application of Fiber Quality Analysis (FQA) and Cellulose Accessibility Measurements To Better Elucidate the Impact of Fiber Curls and Kinks on the Enzymatic Hydrolysis of Fibers. ACS Sustain. Chem. Eng. 2019, 7, 8827–8833. [Google Scholar] [CrossRef]
- Wang, S.; Gao, W.; Chen, K.; Xiang, Z.; Zeng, J.; Wang, B.; Xu, J. Deconstruction of cellulosic fibers to fibrils based on enzymatic pretreatment. Bioresour. Technol. 2018, 267, 426–430. [Google Scholar] [CrossRef]
- Lin, X.; Wu, Z.; Zhang, C.; Liu, S.; Nie, S. Enzymatic pulping of lignocellulosic biomass. Ind. Crop. Prod. 2018, 120, 16–24. [Google Scholar] [CrossRef]
- Gupta, C.; Jain, P.; Kumar, D.; Dixit, A.K. Production of cellulase enzyme from isolated fungus and its application as efficient refining aid for production of security paper. Int. J. Appl. Microbiol. Biotechnol. Res. 2015, 3, 11–19. [Google Scholar]
| Pre-Treatment Time/d | Tensile Strength/Mpa |
|---|---|
| 0 | 2.87 ± 0.18 |
| 3 | 2.68 ± 0.21 |
| 6 | 2.5 ± 0.2 |
| 9 | 2.2 ± 0.15 |
| 12 | 1.89 ± 0.37 |
| Time (d) | Fine Content (%) | Average Fiber Length (μm) |
|---|---|---|
| 0 | 59.6 | 0.489 ± 0.007 |
| 10 | 64.8 | 0.437 ± 0.009 |
| 15 | 69.8 | 0.431 ± 0.005 |
| Samples | [] (mL/g) | DP |
|---|---|---|
| Ck | 267 | 866.51 |
| BPF-10 | 234.35 | 749.60 |
| BPF-15 | 203.17 | 639.64 |
| Sample | Crystallinity Index (wt.%) |
|---|---|
| Ck | 53.33 |
| BPF-10 | 60.78 |
| BPF-15 | 52.14 |
| Sample | Tensile Strength /N·m·g−1 | Tear Strength /mN·m2·g−1 | Burst Strength /kPa·m2·g−1 | Initial Pulping Degree /°SR | Pulping Time /min |
|---|---|---|---|---|---|
| Ck | 6.15 ± 0.21 b | 0.74 ± 0.07 b | 1.03 ± 0.05 b | 16 ± 2 b | 101 ± 3 a |
| BPF-10 | 9.57 ± 0.16 a | 0.86 ± 0.03 a | 1.15 ± 0.06 a | 21 ± 1 a | 81 ± 2 b |
| BPF-15 | 5.32 ± 0.17 c | 0.68 ± 0.05 b | 0.97 ± 0.07 b | 23 ± 1 a | 70 ± 3 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, T.; Wang, X.; Yang, H.; Gao, C.; Hongyang, M.; Xu, X.; Cong, S.; Sun, Y.; Pei, T.; Wang, B.; et al. Biological Enzymatic Hydrolysis—Single Screw Co-Extrusion Treatment to Improve the Mechanical Properties of Biodegradable Straw Fiber Mulching Films. Agronomy 2025, 15, 1923. https://doi.org/10.3390/agronomy15081923
Jiang T, Wang X, Yang H, Gao C, Hongyang M, Xu X, Cong S, Sun Y, Pei T, Wang B, et al. Biological Enzymatic Hydrolysis—Single Screw Co-Extrusion Treatment to Improve the Mechanical Properties of Biodegradable Straw Fiber Mulching Films. Agronomy. 2025; 15(8):1923. https://doi.org/10.3390/agronomy15081923
Chicago/Turabian StyleJiang, Tao, Xing Wang, Haoyuan Yang, Chuang Gao, Mende Hongyang, Xinhang Xu, Shubai Cong, Yuanjun Sun, Tianzheng Pei, Bin Wang, and et al. 2025. "Biological Enzymatic Hydrolysis—Single Screw Co-Extrusion Treatment to Improve the Mechanical Properties of Biodegradable Straw Fiber Mulching Films" Agronomy 15, no. 8: 1923. https://doi.org/10.3390/agronomy15081923
APA StyleJiang, T., Wang, X., Yang, H., Gao, C., Hongyang, M., Xu, X., Cong, S., Sun, Y., Pei, T., Wang, B., Liu, S., Wang, Y., Li, R., Chen, H., & Li, L. (2025). Biological Enzymatic Hydrolysis—Single Screw Co-Extrusion Treatment to Improve the Mechanical Properties of Biodegradable Straw Fiber Mulching Films. Agronomy, 15(8), 1923. https://doi.org/10.3390/agronomy15081923








