Spatiotemporal Evolution of Soil Quality Under Long-Term Apple Cultivation in the Taihang Mountains, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Soil Sampling
2.3. Soil Environmental Chemistry Index Analysis
2.4. Soil Microbial Community PLFA Analysis
2.5. Statistical Analysis
3. Results
3.1. Spatiotemporal Evolution of Soil Physical Properties
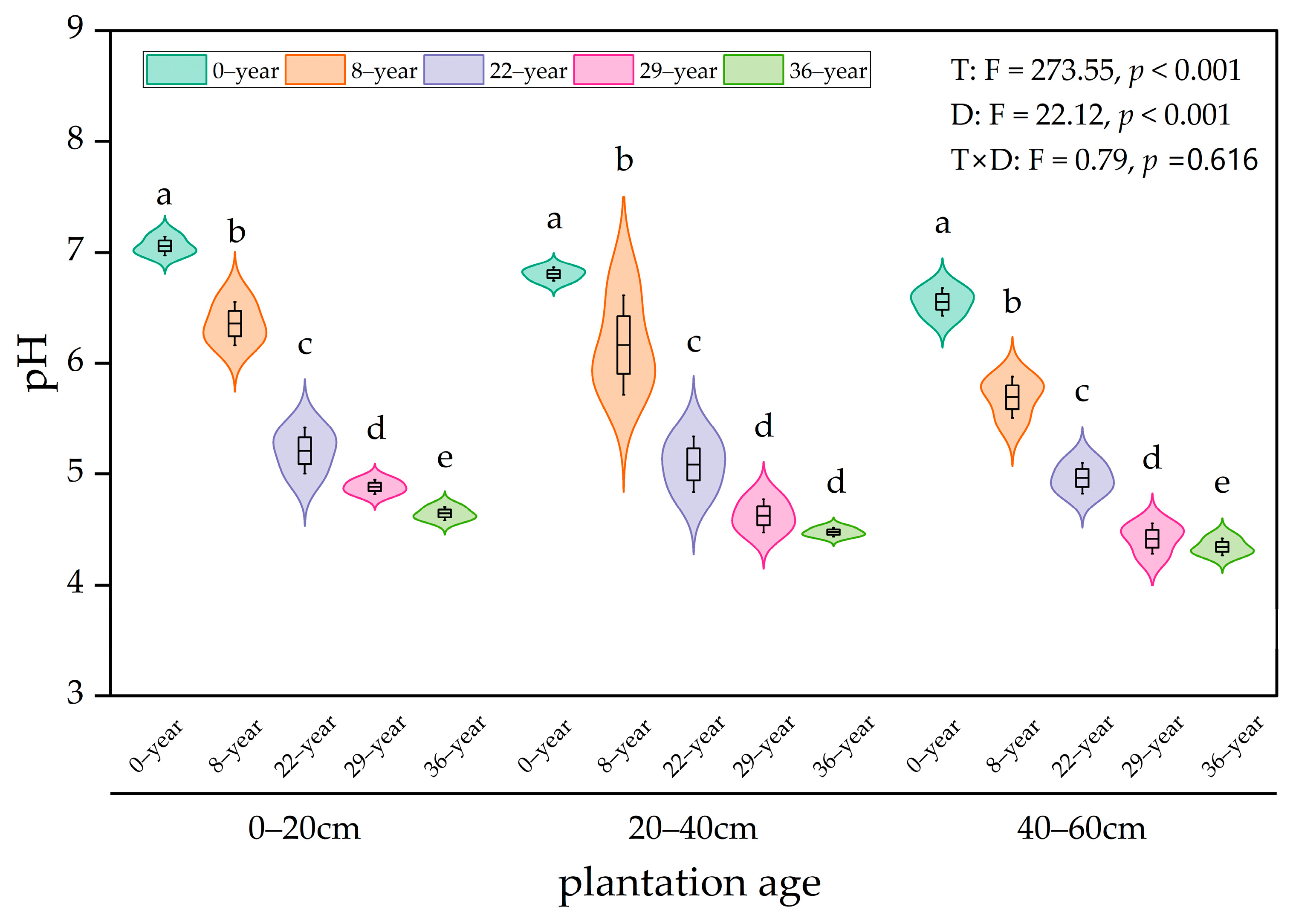
3.2. Evolution of Soil Acidity and Alkalinity
3.3. Spatiotemporal Evolution of Soil Fertility
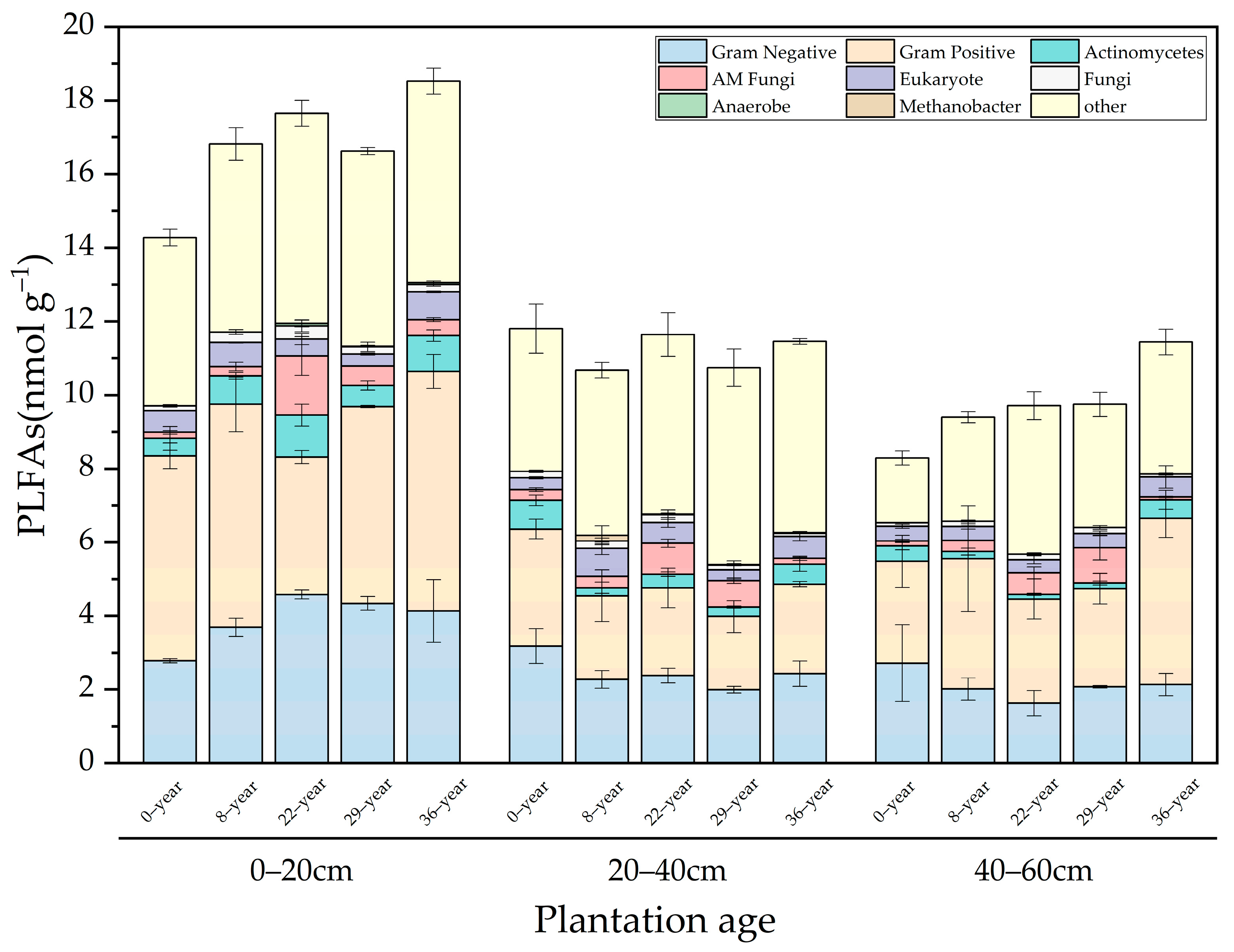
3.4. Spatiotemporal Evolution of Soil Microbial Quantity and Species
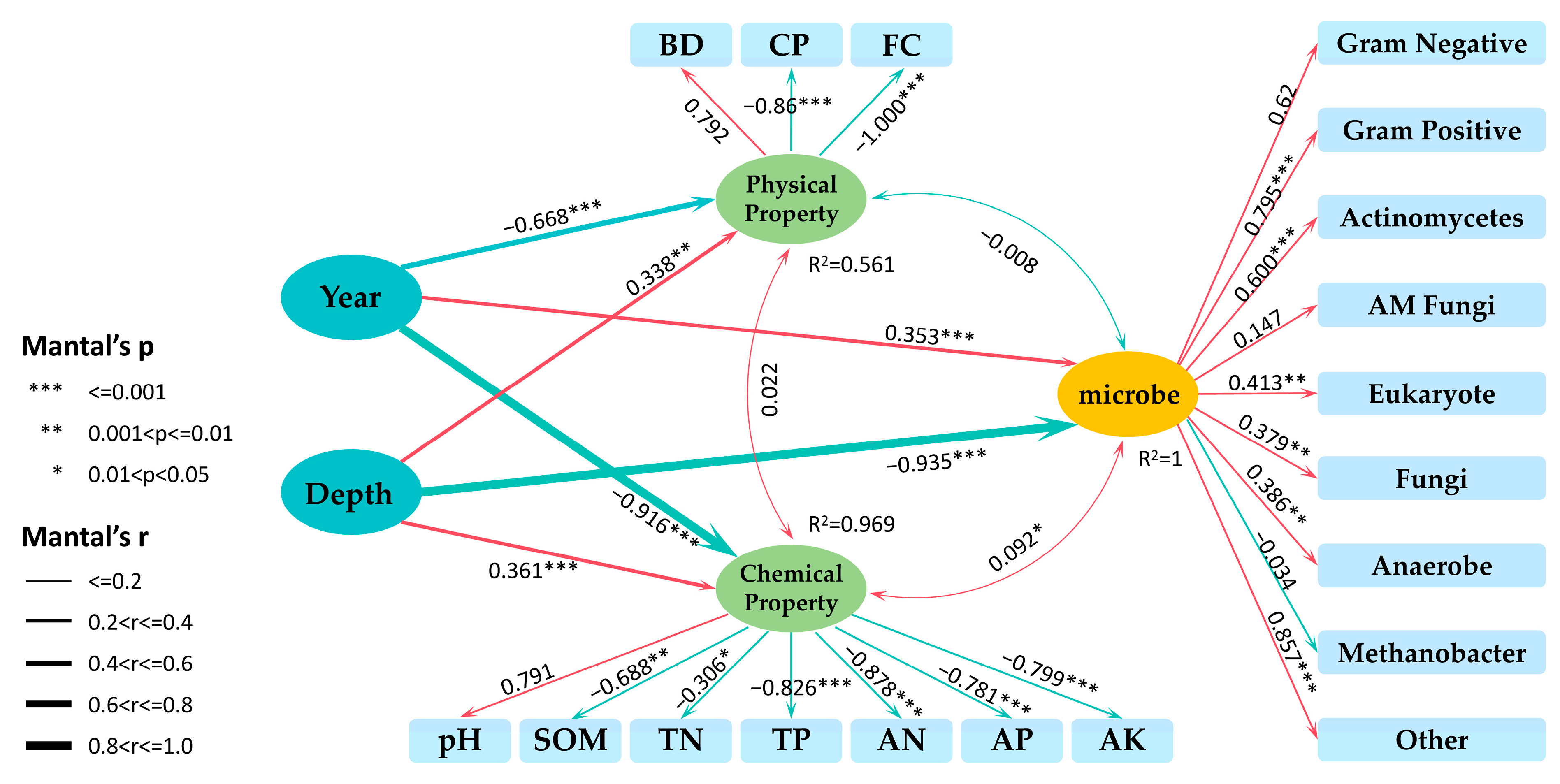
3.5. Correlations Between Soil Physical and Chemical Properties and Soil Microbial Communities
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, X.; Zhang, J.; Zhang, H.; Guo, S.; Xu, Y. Analysis on Species Diversity of Vegetation in Restoration and Efficiency of Water in With Gneissose of Taihangshan Mountian Region. J. Soil Water Conserv. 2007, 21, 133–136, 174. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, Z.; Zhao, S.; Qi, G.; Zhang, X.; Guo, S. Evolution of plant species diversity and soil characteristics on hillslopes during vegetation restoration in the gneiss region of the Taihang Mountains after reclamation. Acta Ecol. Sin. 2018, 38, 5331–5339. [Google Scholar] [CrossRef]
- Chaichi, M.R.; Keshavarz-Afshar, R.; Lu, B.; Rostamza, M. Growth and nutrient uptake of tomato in response to application of saline water, biological fertilizer, and surfactant. J. Plant Nutr. 2016, 40, 457–466. [Google Scholar] [CrossRef]
- Roy, R.; Wang, J.; Mostofa, M.G.; Fornara, D. Optimal water and fertilizer applications improve growth of Tamarix chinensis in a coal mine degraded area under arid conditions. Physiol. Plant. 2020, 172, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Worou, O.N.; Gaiser, T.; Saito, K.; Goldbach, H.; Ewert, F. Simulation of soil water dynamics and rice crop growth as affected by bunding and fertilizer application in inland valley systems of West Africa. Agric. Ecosyst. Environ. 2012, 162, 24–35. [Google Scholar] [CrossRef]
- Cesarano, G.; Zotti, M.; Antignani, V.; Marra, R.; Scala, F.; Bonanomi, G. Soil sickness and negative plant-soil feedback: A reappraisal of hypotheses. J. Plant Pathol. 2017, 99, 545–570. [Google Scholar]
- Mazzola, M.; Manici, L.M. Apple Replant Disease: Role of Microbial Ecology in Cause and Control. Annu. Rev. Phytopathol. 2012, 50, 45. [Google Scholar] [CrossRef]
- Weller, D.M. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu. Rev. Phytopathol. 2002, 40, 309–348. [Google Scholar] [CrossRef]
- Álvarez-Martín, A.; Hilton, S.L.; Bending, G.D.; Rodríguez-Cruz, M.S.; Sánchez-Martín, M.J. Changes in activity and structure of the soil microbial community after application of azoxystrobin or pirimicarb and an organic amendment to an agricultural soil. Appl. Soil Ecol. 2016, 106, 47–57. [Google Scholar] [CrossRef]
- Mbuthia, L.W.; Acosta-Martínez, V.; Debryun, J.; Schaeffer, S.; Tyler, D.; Odoi, E.; Mpheshea, M.; Walker, F.; Eash, N. Long term tillage, cover crop, and fertilization effects on microbial community structure, activity: Implications for soil quality. Soil Biol. Biochem. 2015, 89, 24–34. [Google Scholar] [CrossRef]
- Berg, B.R. Litter decomposition and organic matter turnover in northern forest soils. For. Ecol. Manag. 2000, 133, 13–22. [Google Scholar] [CrossRef]
- Elliott, E.T. Aggregate Structure and Carbon, Nitrogen, and Phosphorus in Native and Cultivated Soils. Soil Sci. Soc. Am. J. 1986, 50, 627. [Google Scholar] [CrossRef]
- Verma, S.; Sharma, P.K. Effect of long-term manuring and fertilizers on carbon pools, soil structure, and sustainability under different cropping systems in wet-temperate zone of northwest Himalayas. Biol. Fertil. Soils 2007, 44, 235–240. [Google Scholar] [CrossRef]
- Yi, J.; Qiu, W.; Hu, W.; Zhang, H.; Liu, M.; Zhang, D.; Wu, T.; Tian, P.; Jiang, Y. Effects of cultivation history in paddy rice on vertical water flows and related soil properties. Soil Tillage Res. 2020, 200, 104613. [Google Scholar] [CrossRef]
- de Oliveira, A.D.; Ribeiro, F.P.; Figueiredo, C.C.d.; Muller, A.G.; Vitoria Malaquias, J.; Santos, I.L.D.; Sá, M.A.C.d.; Soares, J.P.G.; Santos, M.V.A.D.; Carvalho, A.M.d. Effects of soil management, rotation and sequence of crops on soil nitrous oxide emissions in the Cerrado: A multi-factor assessment. J. Environ. Manag. 2023, 348, 119295. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.H.; Liang, Z.W.; Suarez, D.L.; Wang, Z.C.; Wang, M.M.; Yang, H.Y.; Liu, M. Impact of cultivation year, nitrogen fertilization rate and irrigation water quality on soil salinity and soil nitrogen in saline-sodic paddy fields in Northeast China. J. Agric. Sci. 2015, 154, 632–646. [Google Scholar] [CrossRef]
- Li, X.; Lewis, E.E.; Liu, Q.; Li, H.; Bai, C.; Wang, Y. Effects of long-term continuous cropping on soil nematode community and soil condition associated with replant problem in strawberry habitat. Sci. Rep. 2016, 6, 30466. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, H.; Zhang, X.; Li, C.; Yu, S. Vertical Distribution of Cu and Pd in Greenhouse Soils with Different Growing Years. J. Soil Water Conserv. 2016, 30, 321–325, 330. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Zhu, T.; Zucong, C. Characteristics of soil organic nitrogen components of orchards with different planting ages. Chin. J. Ecol. 2015, 34, 5. [Google Scholar] [CrossRef]
- Guo, X.-W.; Li, K.; Guo, Y.-S.; Li, C.-X.; Xie, H.-G.; Hu, X.-X.; Zhang, L.-H.; Sun, Y.-N. Root zone soil nutrient change of vineyard with different planting years and related effect on replanted grape growth. Chin. J. Eco-Agric. 2010, 18, 477–481. [Google Scholar] [CrossRef]
- Qu, J.-F.; Hou, Y.-L.; Ge, M.-Y.; Wang, K.; Liu, S.; Zhang, S.-L.; Li, G.; Chen, F. Carbon Dynamics of Reclaimed Coal Mine Soil under Agricultural Use: A Chronosequence Study in the Dongtan Mining Area, Shandong Province, China. Sustainability 2017, 9, 629. [Google Scholar] [CrossRef]
- Zuber, S.M.; Villamil, M.B. Meta-analysis approach to assess effect of tillage on microbial biomass and enzyme activities. Soil Biol. Biochem. 2016, 97, 176–187. [Google Scholar] [CrossRef]
- Jacobsen, C.S.; Hjelmsø, M.H. Agricultural soils, pesticides and microbial diversity. Curr. Opin. Biotechnol. 2014, 27, 15–20. [Google Scholar] [CrossRef]
- Wang, J.Y.; Xu, J.H.; Wu, L.K.; Wu, H.M.; Lin, W.X. Analysis of physicochemical properties and microbial diversity in rhizosphere soil of Achyranthes bidentata under different cropping years. Acta Ecol. Sin. 2017, 37, 5621–5629. [Google Scholar] [CrossRef][Green Version]
- Pang, X.; Chen, M.; Miao, P.; Cheng, W.; Zhou, Z.; Zhang, Y.; Zhang, Q.; Ye, J.; Jia, X.; Wang, H. Effects of Different Planting Years on Soil Physicochemical Indexes, Microbial Functional Diversity and Fruit Quality of Pear Trees. Agriculture 2024, 14, 226. [Google Scholar] [CrossRef]
- Wang, Y.; Wenqing, L.I.; Binghai, D.U.; Hanhao, L.I. Effect of biochar applied with plant growth-promoting rhizobacteria(PGPR)on soil microbial community composition and nitrogen utilization in tomato. Pedosphere 2021, 31, 872–881. [Google Scholar] [CrossRef]
- Ji, W.; Han, K.; Cai, Y.; Mu, Y.; Zhao, L.; Zhang, M.; Hou, C.; Gao, M.; Zhao, Q. Characterization of rhizosphere bacterial community and berry quality of Hutai No.8 (Vitis vinifera L.) with different ages, and their relations. J. Sci. Food Agric. 2019, 99, 4532–4539. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, Y.; Hu, C.; Zhan, T.; Zhao, X. Soil Applied Ca, Mg and B altered Phyllosphere and Rhizosphere Bacterial Microbiome and Reduced Huanglongbing Incidence in Gannan Navel Orange. Sci. Total Environ. 2021, 791, 148046. [Google Scholar] [CrossRef]
- Uri, V.; Kukumägi, M.; Aosaar, J.; Varik, M.; Becker, H.; Aun, K.; Lõhmus, K.; Soosaar, K.; Astover, A.; Uri, M.; et al. The dynamics of the carbon storage and fluxes in Scots pine (Pinus sylvestris) chronosequence. Sci. Total Environ. 2022, 817, 152973. [Google Scholar] [CrossRef]
- Bokhorst, S.; Kardol, P.; Bellingham, P.J.; Kooyman, R.M.; Richardson, S.J.; Schmidt, S.; Wardle, D.A. Responses of communities of soil organisms and plants to soil aging at two contrasting long-term chronosequences. Soil Biol. Biochem. 2016, 106, 69–79. [Google Scholar] [CrossRef]
- Klute, A. (Ed.) Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1986; Volume 146, pp. 413–423. [Google Scholar] [CrossRef]
- Mebius, L.J. A Rapid Method for the Determination of Organic Carbon in Soil. Anal. Chim. Acta 1960, 22, 120–124. [Google Scholar] [CrossRef]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 678–681. [Google Scholar] [CrossRef]
- Sarma, B.; Farooq, M.; Gogoi, N.; Borkotoki, B.; Kataki, R.; Garg, A. Soil organic carbon dynamics in wheat—Green gram crop rotation amended with Vermicompost and Biochar in combination with inorganic fertilizers: A comparative study. J. Clean. Prod. 2018, 201, 471–480. [Google Scholar] [CrossRef]
- Ulery, A.L.; Drees, L.R.; Baltensperger, D.; Barbarick, K.; Editorinchief, A.S.A.; Roberts, C.; Editorinchief, C.; Logsdon, S.; Editorinchief, S. Methods of Soil Analysis Part 5—Mineralogical Methods; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Bottomley, P.S. Methods of Soil Analysis: Part 2—Microbiological and Biochemical Properties || Plasmid Profiles; SSSA Book Series; John Wiley & Sons: Hoboken, NJ, USA, 1994. [Google Scholar] [CrossRef]
- Dyer, E.G.; Bligh, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Siles, J.A.; Gomez-Perez, R.; Vera, A.; Garcia, C.; Bastida, F. A comparison among EL-FAME, PLFA, and quantitative PCR methods to detect changes in the abundance of soil bacteria and fungi. Soil Biol. Biochem. 2024, 198, 109557. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Y.; Guo, X.; Ning, D.; Zhou, X.; Feng, J.; Yuan, M.M.; Liu, S.; Guo, J.; Gao, Z.; et al. Reduction of microbial diversity in grassland soil is driven by long-term climate warming. Nat. Microbiol. 2022, 7, 1054–1062. [Google Scholar] [CrossRef]
- Frostegård, A.; Tunlid, A.; Bååth, E. Phospholipid Fatty Acid Composition, Biomass, and Activity of Microbial Communities from Two Soil Types Experimentally Exposed to Different Heavy Metals. Appl. Environ. Microbiol. 1993, 59, 3605–3617. [Google Scholar] [CrossRef]
- Wang, W.; Wu, H.; Wu, T.; Luo, Z.; Lin, W.; Liu, H.; Xiao, J.; Luo, W.; Li, Y.; Wang, Y. Soil microbial influences over coexistence potential in multispecies plant communities in a subtropical forest. Ecology 2024, 105, e4415. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2023, 22, 226–239. [Google Scholar] [CrossRef]
- Baumert, V.L.; Vasilyeva, N.A.; Vladimirov, A.A.; Meier, I.C.; Mueller, C.W. Root Exudates Induce Soil Macroaggregation Facilitated by Fungi in Subsoil. Front. Environ. Sci. 2018, 6, 140. [Google Scholar] [CrossRef]
- Pierret, A.; Moran, C.J. Plant Roots and Soil Structure. In Encyclopedia of Agrophysics; Springer: Dordrecht, The Netherlands, 2011; pp. 628–632. [Google Scholar] [CrossRef]
- Heras, M.D.L. Development of soil physical structure and biological functionality in mining spoils affected by soil erosion in a Mediterranean-Continental environment. Geoderma 2009, 149, 249–256. [Google Scholar] [CrossRef]
- Šourková, M.; Frouz, J.; Fettweis, U.; Bens, O.; Hüttl, R.F.; Šantrůčková, H. Soil development and properties of microbial biomass succession in reclaimed post mining sites near Sokolov (Czech Republic) and near Cottbus (Germany). Geoderma 2005, 129, 73–80. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, C.; Song, Y.; Peng, T.; Zhang, C.; Li, K.; Pu, M.; Sun, H.; Li, J.; He, X.; et al. Key Soil Abiotic Factors Driving Soil Sickness in Lycium barbarum L. Under Long-Term Monocropping. Agronomy 2024, 14, 2525. [Google Scholar] [CrossRef]
- Hou, J.; Cheng, X.; Li, J.; Dong, Y. Magnesium and nitrogen drive soil bacterial community structure under long-term apple orchard cultivation systems. Appl. Soil Ecol. 2021, 167, 104103. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Liu, J.; Lu, X.; Li, J. Research on the Evolution Trend of Soil Nutrient of Different Tree Ages in Weibei Dry Plateau. J. Anhui Agric. Sci. 2008, 36, 4. [Google Scholar] [CrossRef]
- Huang, L.-M.; Thompson, A.; Zhang, G.-L. Long-term paddy cultivation significantly alters topsoil phosphorus transformation and degrades phosphorus sorption capacity. Soil Tillage Res. 2014, 142, 32–41. [Google Scholar] [CrossRef]
- Li, Q.; Andom, O.; Li, Y.; Cheng, C.; Deng, H.; Sun, L.; Li, Z. Responses of grape yield and quality, soil physicochemical and microbial properties to different planting years. Eur. J. Soil Biol. 2023, 120, 103587. [Google Scholar] [CrossRef]
- Neog, R. Assessing the impact of chemical fertilizers on soil acidification: A study on Jorhat district of Assam, India. Agric. Sci. Dig. 2018, 38, 270–274. [Google Scholar] [CrossRef]
- Wallace, A. Soil acidification from use of too much fertilizer. Commun. Soil Sci. Plant Anal. 1994, 25, 87–92. [Google Scholar] [CrossRef]
- Peruzzi, E.; Franke-Whittle, I.H.; Kelderer, M.; Ciavatta, C.; Insam, H. Microbial indication of soil health in apple orchards affected by replant disease. Appl. Soil Ecol. 2017, 119, 115–127. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Luan, H.; Liu, Y.; Huang, S.; Qiao, W.; Chen, J.; Guo, T.; Zhang, X.; Guo, S.; Zhang, X.; Qi, G. Successive walnut plantations alter soil carbon quantity and quality by modifying microbial communities and enzyme activities. Front. Microbiol. 2022, 13, 953552. [Google Scholar] [CrossRef]
- Arafat, Y.; Tayyab, M.; Khan, M.U.; Chen, T.; Lin, S. Long-Term Monoculture Negatively Regulates Fungal Community Composition and Abundance of Tea Orchards. Agronomy 2019, 9, 466. [Google Scholar] [CrossRef]

| Index | Plantation Age (T) | Soil Depths (D) | Effects | ||||
|---|---|---|---|---|---|---|---|
| 0–20 cm | 20–40 cm | 40–60 cm | T | D | T × D | ||
| BD (g cm−3) | 0-year | 1.49 ± 0.06 a | 1.55 ± 0.14 a | 1.60 ± 0.23 a | ** | * | NS |
| 8-year | 1.36 ± 0.09 b | 1.48 ± 0.05 ab | 1.42 ± 0.13 ab | ||||
| 22-year | 1.32 ± 0.06 b | 1.37 ± 0.05 bc | 1.47 ± 0.05 ab | ||||
| 29-year | 1.25 ± 0.08 b | 1.37 ± 0.08 bc | 1.35 ± 0.08 ab | ||||
| 36-year | 1.25 ± 0.04 b | 1.30 ± 0.04 c | 1.33 ± 0.08 b | ||||
| CP (%) | 0-year | 27.26 ± 1.02 a | 26.42 ± 0.96 a | 24.48 ± 1.50 b | ** | NS | NS |
| 8-year | 28.98 ± 1.23 a | 28.55 ± 0.85 a | 25.99 ± 1.10 b | ||||
| 22-year | 31.48 ± 2.21 a | 27.27 ± 0.95 a | 26.68 ± 2.73 a | ||||
| 29-year | 31.85 ± 2.07 ab | 30.71 ± 2.82 a | 31.41 ± 3.02 b | ||||
| 36-year | 28.35 ± 1.79 a | 28.78 ± 4.49 a | 31.54 ± 1.63 a | ||||
| FC (%) | 0-year | 18.29 ± 0.24 d | 17.17 ± 2.09 b | 15.54 ± 2.70 b | ** | ** | NS |
| 8-year | 21.38 ± 0.57 c | 18.56 ± 2.24 ab | 18.50 ± 2.28 ab | ||||
| 22-year | 23.88 ± 0.59 ab | 22.40 ± 2.79 a | 21.33 ± 1.85 a | ||||
| 29-year | 25.43 ± 1.32 a | 19.87 ± 0.30 ab | 19.81 ± 3.13 ab | ||||
| 36-year | 22.72 ± 0.81 bc | 22.17 ± 3.19 a | 23.79 ± 2.08 a | ||||
| Index | Plantation Age (T) | Soil Depths (D) | Effects | ||||
|---|---|---|---|---|---|---|---|
| 0–20 cm | 20–40 cm | 40–60 cm | T | D | T × D | ||
| SOM (g kg−1) | 0-year | 9.50 ± 0.80 e | 8.23 ± 0.53 c | 6.72 ± 0.80 b | ** | ** | ** |
| 8-year | 14.60 ± 1.39 d | 10.43 ± 1.39 bc | 7.88 ± 0.80 ab | ||||
| 22-year | 21.32 ± 1.45 c | 11.36 ± 2.12 bc | 7.88 ± 0.80 ab | ||||
| 29-year | 36.14 ± 1.21 b | 12.75 ± 2.89 ab | 8.69 ± 0.35 a | ||||
| 36-year | 32.67 ± 1.39 a | 15.99 ± 2.51 a | 8.46 ± 0.72 a | ||||
| TN (g kg−1) | 0-year | 0.45 ± 0.04 a | 0.51 ± 0.03 a | 0.48 ± 0.07 a | ** | ** | ** |
| 8-year | 0.68 ± 0.09 ab | 0.55 ± 0.03 a | 0.59 ± 0.07 ab | ||||
| 22-year | 0.82 ± 0.03 bc | 0.48 ± 0.03 ab | 0.37 ± 0.02 bc | ||||
| 29-year | 0.77 ± 0.05 c | 0.57 ± 0.01 ab | 0.68 ± 0.07 c | ||||
| 36-year | 0.57 ± 0.08 d | 0.52 ± 0.06 b | 0.46 ± 0.05 c | ||||
| TP (g kg−1) | 0-year | 0.43 ± 0.03 c | 0.49 ± 0.11 c | 0.40 ± 0.06 b | ** | NS | NS |
| 8-year | 0.72 ± 0.13 b | 0.53 ± 0.06 c | 0.49 ± 0.46 ab | ||||
| 22-year | 0.97 ± 0.20 ab | 0.70 ± 0.26 bc | 0.64 ± 0.03 ab | ||||
| 29-year | 1.10 ± 0.10 a | 0.95 ± 0.13 a | 0.90 ± 0.14 a | ||||
| 36-year | 1.03 ± 0.15 a | 0.92 ± 0.10 ab | 0.90 ± 0.05 a | ||||
| AN (mg kg−1) | 0-year | 73.97 ± 11.85 b | 68.13 ± 0.40 b | 45.27 ± 4.28 b | ** | ** | NS |
| 8-year | 80.73 ± 7.18 b | 70.00 ± 4.90 b | 63.93 ± 15.48 b | ||||
| 22-year | 91.47 ± 14.57 b | 76.30 ± 7.95 b | 65.33 ± 6.80 b | ||||
| 29-year | 158.20 ± 4.20 a | 131.25 ± 5.95 a | 96.60 ± 13.50 a | ||||
| 36-year | 144.90 ± 3.50 a | 127.17 ± 15.08 a | 98.23 ± 25.34 a | ||||
| AP (mg kg−1) | 0-year | 47.60 ± 9.30 c | 40.93 ± 0.40 b | 20.41 ± 1.39 d | ** | ** | NS |
| 8-year | 77.14 ± 12.69 bc | 52.10 ± 4.37 b | 33.43 ± 2.91 c | ||||
| 22-year | 79.40 ± 59.41 bc | 65.41 ± 6.30 ab | 39.73 ± 1.62 c | ||||
| 29-year | 122.33 ± 9.93 ab | 71.93 ± 48.99 ab | 54.67 ± 6.41 b | ||||
| 36-year | 140.67 ± 4.24 a | 105.19 ± 7.64 a | 79.60 ± 13.45 a | ||||
| AK (mg kg−1) | 0-year | 230.43 ± 12.75 b | 203.47 ± 22.05 c | 208.37 ± 3.45 b | ** | NS | NS |
| 8-year | 198.57 ± 11.75 c | 205.90 ± 13.70 c | 208.37 ± 1.45 b | ||||
| 22-year | 252.50 ± 9.30 b | 262.30 ± 20.60 b | 237.77 ± 26.95 b | ||||
| 29-year | 281.90 ± 8.30 a | 299.07 ± 18.65 a | 291.70 ± 17.20 a | ||||
| 36-year | 286.80 ± 25.50 a | 296.60 ± 18.10 a | 281.87 ± 27.45 a | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, X.; Li, Z.; Liang, X.; Chi, M.; Ge, F. Spatiotemporal Evolution of Soil Quality Under Long-Term Apple Cultivation in the Taihang Mountains, China. Agronomy 2025, 15, 1922. https://doi.org/10.3390/agronomy15081922
Liu Y, Zhang X, Li Z, Liang X, Chi M, Ge F. Spatiotemporal Evolution of Soil Quality Under Long-Term Apple Cultivation in the Taihang Mountains, China. Agronomy. 2025; 15(8):1922. https://doi.org/10.3390/agronomy15081922
Chicago/Turabian StyleLiu, Yang, Xingrui Zhang, Zhuo Li, Xiaoyi Liang, Meidan Chi, and Feng Ge. 2025. "Spatiotemporal Evolution of Soil Quality Under Long-Term Apple Cultivation in the Taihang Mountains, China" Agronomy 15, no. 8: 1922. https://doi.org/10.3390/agronomy15081922
APA StyleLiu, Y., Zhang, X., Li, Z., Liang, X., Chi, M., & Ge, F. (2025). Spatiotemporal Evolution of Soil Quality Under Long-Term Apple Cultivation in the Taihang Mountains, China. Agronomy, 15(8), 1922. https://doi.org/10.3390/agronomy15081922






