Abstract
Fusarium circinatum poses severe threats to agroforestry ecosystem as a globally significant pathogenic fungus. This study utilized multi-source species distribution data and environmental variables (climatic, topographic, and soil factors) to predict the global potential habitat suitability of F. circinatum and its response to future climate change using an optimized MaxEnt model (RM = 1, FC = LQ). The results indicate that the current total suitable area spans approximately 69.29 million km2, with highly suitable habitats (>0.493) accounting for 15.07%, primarily concentrated in East Asia, southwestern North America, western South America, the Mediterranean coast, and eastern Australia. The distribution of F. circinatum’s suitable habitats is primarily constrained by the following environmental factors, ranked by contribution rate: coldest quarter precipitation (29.4%), coldest quarter mean temperature (18.2%), annual mean temperature (17.2%), and annual precipitation (12%). Under future climate scenarios, the suitable habitats exhibited an overall contraction and poleward shift, with the most significant decline in highly suitable areas observed under SSP370-2050s (−52.1%). The centroid of suitable habitats continuously migrated northwestward from Gombe State, Nigeria, with the maximum displacement reaching 1077.6 km by SSP585-2090s. This study reveals a latitude gradient redistribution pattern of F. circinatum driven by climate warming, providing a scientific basis for transboundary biosecurity and early warning systems.
1. Introduction
Forests are a critical component of the global ecosystem, and their health directly impacts the maintenance of biodiversity and the stability of ecological balance [1]. Pine Pitch Canker (PPC), caused by Fusarium circinatum Nirenberg & O’Donnell, is a severe plant disease posing a significant biological threat to Pinus taeda, Pinus pinaster, Pinus radiata, and Pinus elliottii [2,3]. The pathogen was first discovered in the southeastern United States in 1945 and has since been reported in 14 countries worldwide, including the United States, Japan, South Korea, South Africa, Spain, and Portugal [4]. It has been successfully eradicated in some European countries, such as France and Italy [5]. Its distribution is closely linked to climatic conditions, with optimal regions encompassing Mediterranean and subtropical climate zones, where temperature and humidity are critical factors. Coastal areas with occult precipitation (e.g., fog and dew) facilitate its spread [6,7]. The pathogen is transmitted via vectors such as insects, water, and wind, while human activities, including plant trade and the movement of contaminated equipment, serve as the primary pathways for long-distance dispersal. In Europe, P. radiata and P. pinaster nurseries and plantations in Spain and Portugal have been affected, prompting local eradication and control programs [8]. Furthermore, CLIMEX modeling predicts that future climate change may expand suitable regions in Europe, though areas in Spain could experience reduced suitability due to declining precipitation [6].
Against the backdrop of intensifying global climate change and persistent anthropogenic disturbances to natural environments, the ecological distribution patterns of pathogenic microorganisms are undergoing significant transformations [9,10]. The multi-scale interactions within the soil–topography–climate system collectively regulate the survival, reproduction, and transmission dynamics of pathogens through intricate ecological mechanisms [11]. Specifically, as the foundational substrate for pathogen survival, soil properties—including texture, fertility, pH, and water retention capacity—directly modulate the physiological activity and metabolic pathways of pathogens [12]. Geomorphological parameters such as elevation gradients, topographic complexity, and slope aspect indices not only shape localized microenvironments but also constrain the dispersal dynamics and habitat suitability of pathogens by influencing biogeochemical cycling processes [13]. Climatic factors (temperature variability and precipitation patterns), as pivotal environmental drivers, determine the phenological traits, population dynamics, and transmission potential of pathogens through thermodynamic and hydrodynamic mechanisms [14]. These multidimensional environmental factors, via nonlinear interactions and cascading effects, collectively constitute the complex ecological context for pathogen survival and distribution [15].
The coupling analysis of environmental factors and species distribution data enables Species Distribution Models (SDMs) to simulate the spatial patterns of species’ potential suitable habitats, elucidate their niche characteristics, and assess the invasion risks associated with the spread dynamics of alien invasive species [16,17]. Among these, the Maximum Entropy model (MaxEnt), has gained widespread application in biogeographical studies as a species distribution model grounded in niche theory [18,19]. The MaxEnt model integrates known species occurrence records with environmental variables to predict the potential geographic distribution probability of species based on the principle of maximum entropy [20]. This model exhibits robust predictive performance, particularly when dealing with limited sample sizes and complex environmental variables, enabling effective simulation of species’ ecological niches and evaluation of the relative contributions of different environmental factors to species distributions [21]. Furthermore, the MaxEnt model can account for interactions among environmental variables, providing strong support for investigating the ecological mechanisms underlying species distribution patterns [22]. To enhance model reliability, we employed ENMTools to systematically evaluate various feature combinations (FCs) and regularization multipliers (RMs) through batch testing. Using delta AICc or omission rate as evaluation metrics, we efficiently identified the optimal parameter combination, thereby significantly improving the AUC and reducing prediction errors [23].
This study employs the MaxEnt model to systematically predict and evaluate the global potential distribution patterns of F. circinatum by integrating soil properties, topographic characteristics, and climatic factors [24]. Through quantitative analysis of the relative contribution rates of various environmental factors to the spatial distribution of the pathogen, we elucidate its ecological niche requirements and simulate the dynamic changes in suitable habitats under SSP126, SSP370, and SSP585 climate change scenarios [25,26]. The findings will provide theoretical support for the establishment of a global monitoring and early warning system for pine forest ecosystem diseases. Furthermore, by analyzing the coupling mechanisms between environmental gradients and pathogen distribution, this research not only expands the application paradigm of the MaxEnt model in the biogeographic study of plant pathogens but also offers valuable references for model construction and methodological innovation in forest disease ecology research [27,28].
2. Material and Methods
2.1. Acquire the Occurrence Records of Species
We integrated multi-source data from the following to obtain global distribution information of F. circinatum: the Centre for Agriculture and Bioscience International (CABI; accessed on 19 June 2025), the EPPO Global Database (EPPO; accessed on 19 June 2025), the Global Biodiversity Information Facility (GBIF [29]; accessed on 20 June 2025; occurrence dataset DOI: 10.15468/dl.mda9gp), and published literature data [30,31,32]. For records lacking geographic coordinates in the literature, we georeferenced precise latitude and longitude coordinates using Google Earth (v7.1) [33]. The initial dataset comprised 126 distribution points from Asia, North America, Europe, and Africa. To mitigate the effects of spatial autocorrelation and sampling bias on model predictions, we employed a 5 km buffer analysis in ArcGIS (version 10.8), ensuring that only one representative distribution point was retained per 5 km × 5 km grid cell. After spatial deduplication, a final set of 101 independent distribution points was obtained for subsequent model training and validation (Figure 1A) [34,35].
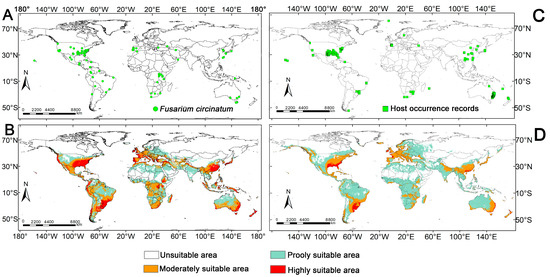
Figure 1.
Global distribution records and potential suitable habitat modeling for F. circinatum and its hosts. Notes: (A): the global distribution of F. circinatum; (B): the global distribution of F. circinatum hosts; (C): habitat suitability prediction for F. circinatum; (D): prediction of suitable habitats for F. circinatum hosts.
2.2. Environmental Factor Data
This study utilized environmental variable data obtained from internationally authoritative database platforms. The baseline climate data were sourced from the WorldClim global climate database (Version 2.1; data accessed on 7 June 2024), which includes 19 bioclimatic variables and digital elevation model (DEM) data at a spatial resolution of 5 km (equivalent to 2.5 arc-minutes in geographic coordinate systems) (see Supplementary Material Table S1 for details). [36]. We utilized ArcGIS software (Version 10.8) to process the DEM through spatial analysis methods, deriving two key topographic derivatives: slope and aspect [37]. This study utilizes simulation data from the BCC-CSM2-MR climate model (developed by the National Climate Center of China under the CMIP6 initiative) to analyze future trends in bioclimatic indicators across three representative shared socioeconomic pathways (SSP126, SSP370, and SSP585). The analysis focuses on the following four distinct periods: 2021–2040 (2030s), 2041–2060 (2050s), 2061–2080 (2070s), and 2081–2100 (2090s) [38]. Among these, SSP126 represents a low radiative forcing scenario, SSP370 corresponds to a medium-to-high radiative forcing scenario, and SSP585 characterizes a high radiative forcing scenario [39,40].
The soil attribute data utilized in this study were obtained from the Harmonized World Soil Database (version 1.2) released by the Food and Agriculture Organization of the United Nations (FAO) (https://data.apps.fao.org/; accessed on 8 June 2024). A total of 26 key soil parameters were extracted (see Table S1 for details) [41]. All environmental variables were processed using the ArcGIS software platform, which included spatial resampling (with a unified resolution of 5 km × 5 km), projection transformation (converted to the WGS 1984 coordinate system), and standardization (Z-score standardization). Ultimately, a comprehensive dataset comprising 35 environmental variables was constructed, categorized into the following four major groups: temperature, precipitation, topography, and soil characteristics (Table S1). Due to the limited availability of soil data under future scenarios, this study assumed that current soil conditions would remain relatively stable in future periods. Consequently, the present soil data were adopted as surrogate data for future scenarios.
2.3. Selection and Filtering of Environmental Variables
To construct the species distribution model, we first calculated a Pearson correlation coefficient matrix based on 48 climatic factors from 1970 to 2000 (Table S1; Figure 2A) [42]. To eliminate redundant information and select appropriate environmental variables for modeling, we employed the correlation analysis function in ENM Tools (version ENMTools.pl), setting the Pearson correlation coefficient threshold at |r| > 0.8 [43]. When two environmental variables exhibited high correlation, we prioritized retaining the variable with greater biological significance and higher model contribution to reduce multicollinearity among environmental variables. Subsequently, the filtered dataset of 102 effective species distribution points and environmental variables were imported into the MaxEnt model for 10 replicate runs [44]. By analyzing the percentage contribution of each environmental variable, we further excluded factors with contribution rates below 2%, retaining variables of greater ecological significance among the significantly correlated factors [45]. Ultimately, 10 key environmental variables were selected for the final modeling (Table 1).

Figure 2.
Evaluating how accurately the species distribution model predicts the potential geographic spread of F. circinatum. Notes: (A): Correlation of climatic factors. (B): Optimization of feature combination and regularization. (C): The average AUC values of the model run.

Table 1.
The filtered environmental variables’ contribution rate percentage and permutation importance.
2.4. Parameter Optimization of Models
The MaxEnt model is a complex model in machine learning that is sensitive to sampling bias, and its model complexity is significantly influenced by feature combinations (FCs) and the regularization multiplier (RM) [46]. Feature classes encompass the following five types: Linear (L), Quadratic (Q), Product (P), Threshold (T), and Hinge (H) features. To evaluate the impact of different parameter combinations on the model, we configured 8 FCs and 12 RM gradients (ranging from 0 to 6 at intervals of 0.5), resulting in a total of 96 parameter combinations. Model construction, calibration, and evaluation were conducted using the ENMeval package (version 2.0.5.2), an R package that implements species distribution modeling based on the MaxEnt algorithm [47]. By optimizing FC and RM combinations, the risk of model overfitting can be effectively mitigated, and structural complexity can be refined, thereby enhancing predictive accuracy [48]. Model selection was based on the corrected Akaike Information Criterion (delta AICc), with the optimal model determined by the minimum delta AICc value. Consequently, the best-performing RM and FC parameter combinations were identified [49].
2.5. Model Construction
This study employed the MaxEnt model (version 3.4.1) for species distribution predicting. The model parameters were configured as follows: a subsample replication run type was selected, with the dataset randomly partitioned into 75% for training and 25% for testing; to ensure adequate model convergence, the maximum number of iterations was set to 50,000; the output format was specified as logistic regression, with results saved in asc file format to facilitate spatial analysis in ArcGIS (version 10.8) [50,51]. Computational parameters were set to single-threaded operation, with 10 replicate runs performed to enhance the robustness of the results. Key environmental variables were identified through Jackknife analysis, and response curves were generated to elucidate the relationships between environmental variables and species occurrence probability [52]. The AUC (Area Under the Curve) method of ROC (Receiver Operating Characteristic) is used to evaluate the accuracy of MaxEnt models. Theoretically, an AUC value of 0.5–0.7 indicates poor model performance; 0.7–0.9 suggests moderate performance; and values above 0.9 represent good performance [53].
2.6. Hierarchical Classification and Spatial Pattern Changes in Species Habitats
Using ArcGIS we generated potential distribution prediction maps for the baseline period (1970–2000) and four future periods and quantitatively analyzed the spatiotemporal changes in suitable habitats. The specific workflow was as follows: First, we combined the species suitability distribution data generated by the optimized MaxEnt model with the global administrative boundary layer in ArcGIS. Through spatial analysis we determined the species’ global potential distribution areas by extracting masks [54]. Second, the Jenks natural breaks classification method was applied to determine the classification thresholds, dividing the potential distribution areas of F. circinatum into four suitability levels: unsuitable (0–0.101), low suitability (0.101–0.275), moderate suitability (0.275–0.493), and high suitability (>0.493) [55]. Subsequently, the area proportions of each suitability level and their spatiotemporal variation characteristics were calculated. To investigate the shifts in distribution centers, the “Centroid Change Trajectory” tool in SDMtoolbox (v2.10.4-9) was employed to compute the centroid migration paths of high-suitability areas from the baseline period to each future period [56]. The dynamic changes in the centroids of high-suitability areas for F. circinatum were then visualized.
3. Results
3.1. Model Parameter Optimization and Predictive Accuracy Validation
Through systematic parameter optimization we identified the optimal MaxEnt model configuration that satisfies all predefined screening criteria. As illustrated in Figure 2B, the model achieved optimal fitting performance when employing a regularization multiplier (RM = 1) combined with linear quadratic (LQ) feature classes, with its delta AICc value converging to zero (ΔAICc = 0). This configuration demonstrated significantly superior performance compared to other parameter combinations. The selected setup not only ensured model parsimony but also effectively mitigated overfitting risks while substantially enhancing predictive accuracy. Model validation results (Figure 2C) revealed that the average area under the receiver operating characteristic curve (AUC = 0.937) obtained through 10-fold cross-validation significantly exceeded random prediction levels (AUC = 0.5), confirming the model’s high reliability in predicting potential suitable habitats for F. circinatum.
3.2. Environmental Variables Influencing the Distribution of F. circinatum
Modeling analysis revealed that among the 10 environmental variables bio19 exhibited the highest relative contribution rate (29.41%) to the potential distribution of F. circinatum (Table 1). The secondary contributing variables were mean temperature of coldest quarter (18.2%), annual mean temperature (17.2%), and annual precipitation (12%), collectively accounting for 76.8% of the total contribution. However, the Jackknife test results (Figure 3A) indicated that the key climatic factors influencing the geographical distribution of this species, ranked by importance, were mean diurnal temperature range, mean temperature of warmest quarter, annual precipitation, precipitation of driest month, and elevation. Based on the habitat suitability probability threshold (>0.5) the potential distribution range was delineated, showing that the optimal ecological niche parameters for F. circinatum were as follows: annual mean temperature (10.30–22.20 °C), mean temperature of coldest quarter (−0.56–11.28 °C), annual precipitation (872.10–3299.04 mm), precipitation of driest quarter (103.32–553.10 mm), and precipitation of coldest quarter (157.18–805.57 mm) (Figure 3B–F). These parameter ranges provide a suitable survival probability for the species.

Figure 3.
The Jackknife tests of the importance of environmental variables in MaxEnt mode. Notes: (A): comparative analysis of regularization effects across seven environmental covariates in model training; (B): bio01, annual mean temperature; (C): bio11, mean temperature of coldest quarter; (D): bio12, annual precipitation; (E): bio17, precipitation of driest quarter; (F): bio19, precipitation of coldest quarter.
3.3. Global Potential Distribution of F. circinatum Under Current Environmental Variables
This study demonstrates that the global distribution of known hosts of F. circinatum and its potential suitable habitats have largely overlapped with the pathogen’s suitable range, thereby providing independent validation for the reliability of the model results. Current geographical distribution models indicate that the potential suitable habitats of F. circinatum exhibit a transcontinental distribution across all continents except Antarctica, with a distinct latitudinal zonation pattern. In the Southern Hemisphere, suitable areas are primarily concentrated between 0° and 50° S, while in the Northern Hemisphere, they are distributed within the 10° to 60° N latitudinal belt. Highly suitable areas are clustered in East Asia (Japan, South Korea, eastern China, Pakistan, and Nepal), North America (the western and southeastern United States), South America (the western Andes and southeastern coastal regions), Western Europe (the Mediterranean coast), and eastern Australia. Moderately suitable areas form a ring-like distribution around the periphery of highly suitable zones, encompassing regions such as northern and central China, central and southern Africa, the central United States, and southern Australia. In contrast, poorly suitable area or unsuitable areas are predominantly located in Arctic-adjacent regions (northern North America and the entirety of Russia), equatorial Africa, and arid/alpine ecosystems such as northwestern and northeastern China. This distribution pattern demonstrates significant spatial coupling with gradients of environmental factors, particularly temperature and precipitation, under current climatic conditions.
According to model predictions the total global potential suitable habitat for F. circinatum reaches approximately 6928.6 × 104 km2. Under current climatic conditions the distribution of different suitability levels is as follows: highly suitable habitat: covers an area of about 1043.95 × 104 km2, accounting for 15.07% of the total suitable habitat; moderately suitable habitat: spans approximately 2514.49 × 104 km2, representing 36.29% of the total; poorly suitable habitat: encompasses around 3370.16 × 104 km2, constituting 48.64% of the total suitable area.
3.4. Research on Predicting the Potential Suitable Habitat Distribution of F. circinatum Under Global Climate Change
Based on CMIP6 multi-scenario climate models (SSP126, SSP370, and SSP585), we analyzed the projected shifts in potential suitable habitats for F. circinatum across four time periods. The results indicate an overall decline in the total suitable habitat area compared to current conditions, with particularly significant reductions in highly suitable areas and moderately suitable areas.
The simulation results across different scenarios and time periods reveal significant variations in potential suitable areas (Table 2). Specifically, the SSP585 scenario in the 2090s projects the largest total suitable area (5222.92 × 104 km2), representing a 24.62% reduction compared to the baseline period. In contrast, the smallest total suitable area (3882.64 × 104 km2) is predicted under the SSP126 scenario in the 2070s, showing a more substantial decline of 43.96%. For highly suitable areas, the maximum value (680.28 × 104 km2) is projected under the SSP126 scenario in the 2030s, still reflecting a 34.84% decrease relative to the baseline. The minimum value (500.26 × 104 km2) occurs under the SSP370 scenario in the 2050s, with a notable reduction of 52.08%. Moderately suitable areas exhibit extremes in the SSP585 scenario of the 2090s (1521.97 × 104 km2, a 39.47% decline) and the SSP370 scenario of the 2070s (1164.06 × 104 km2, a 53.71% decline). Spatial analysis indicates that the distribution patterns of suitable areas under SSP126 and SSP370 scenarios follow similar trends (Table 2). Notably, a pronounced contraction of highly suitable areas is observed in parts of mainland China and Japan during the 2050s. As radiative forcing intensifies and time progresses, the spatial distribution of suitable areas exhibits a distinct latitudinal gradient shift. Under the SSP370 and SSP585 scenarios in the 2090s, suitable areas demonstrate a poleward migration tendency. This spatial reorganization is particularly evident in key regions such as East Asia, North America, Australia, and Africa, where highly suitable areas undergo the most significant redistribution (Figure 4).

Table 2.
Global distribution of suitable habitats for F. circinatum under current and future climate scenarios.

Figure 4.
Projected global distribution of F. circinatum in future climate scenarios.
3.5. Projected Changes in the Potential Distribution Range of F. circinatum Under Future Climate Scenarios
This study elucidates the dynamic changes in the potential distribution of F. circinatum under future climate scenarios through geospatial analysis (Figure 5; Figure S1), providing a scientific basis for the development of regional prevention and control strategies. The results demonstrate that under future climate scenarios the suitable habitats of this species exhibit distinct latitudinal gradient shifts, characterized by an overall contraction from low-latitude to high-latitude regions, with only sporadic local expansions. Under different shared socioeconomic pathways (SSPs) scenarios the changes in suitable habitats show clear gradient responses. For example, under the SSP126 scenario most regions remain stable, with only a few areas experiencing contraction or expansion; under the SSP370 scenario, the contraction area significantly increases in mid-to-high-latitude regions such as North America, Europe, and Australia, while expansions remain primarily confined to specific ecoregions in South America and Africa; by the SSP585 scenario, both expansion areas in the Southern Hemisphere and contraction areas in the Northern Hemisphere exhibit a marked increase, with the most pronounced expansions occurring in tropical regions of South America, Africa, and Southeast Asia, and significant range contractions in temperate regions of North America and Europe.
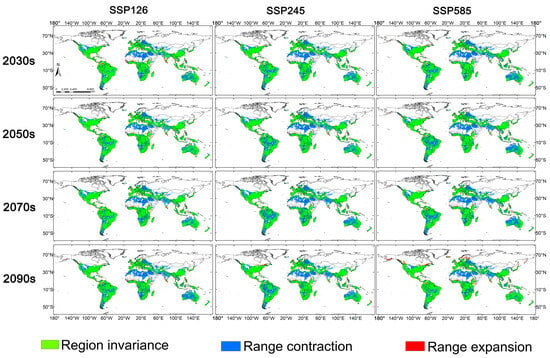
Figure 5.
Spatiotemporal dynamics of the potential suitable habitats for F. circinatum under climate change scenarios.
3.6. Center Distributional Shifts
Under different climate scenarios, the direction and distance of the centroid shift for F. circinatum’s suitable habitat exhibits significant spatial heterogeneity (Figure 6; Table 3). Compared to the current climate scenario (with the centroid located in Gombe State, Nigeria), future scenarios generally show a northwestward shift in the centroid, with migration distances ranging from 80.3 to 1077.6 km. Notably, across all scenarios the centroids remain consistently distributed within the border region between Niger and Nigeria. This spatial pattern highlights the potential impact of climate warming on the suitability distribution of the species’ habitat. Among these scenarios, the SSP585 scenario for the 2090s demonstrates the most pronounced migration trend (a northwestward shift of 1077.6 km), while the SSP370 scenario for the 2050s exhibits the smallest migration magnitude (a northwestward shift of 80.3 km).

Figure 6.
Spatiotemporal shifts in the geographic distribution center of F. circinatum.

Table 3.
Shifts in the geographic centroid of climatically suitable habitats for F. circinatum under projected future climates.
4. Discussion
4.1. Primary Drivers Governing the Potential Geographic Distribution of Fusarium circinatum
This study elucidated the key environmental variables influencing the potential distribution of F. circinatum and delineates its suitable ecological niche parameters. The results indicate that the precipitation of the coldest quarter has the highest contribution rate to the potential distribution of it. Additionally, the mean diurnal temperature range and the mean temperature of the warmest quarter also significantly impact its geographical distribution, which is consistent with previous research emphasizing the importance of temperature and precipitation [30,57]. These results are consistent with existing research on the sensitivity of pathogenic fungi to temperature and precipitation, as seasonal variations in these factors critically affect the pathogen’s growth rate, reproductive efficiency, and dispersal capacity, thereby determining its geographical range [58]. Moreover, the precipitation of the driest quarter and the coldest quarter also significantly affects the distribution of the fungus, likely because seasonal differences in precipitation can indirectly influence its distribution by affecting soil moisture and plant health [59]. Compared with previous studies, the ecological niche parameters defined in this study are more precise, providing more detailed data support for future research [60].
4.2. The Credibility of the Outputs Derived from the MaxEnt Model
Leveraging the ENMeval platform (ENMTools.pl) we systematically optimized MaxEnt model parameters, and through a multidimensional evaluation framework we selected the optimal configuration that satisfies the predefined criteria. The results demonstrate that the optimal parameter combination exhibits significant statistical superiority, with its delta AICc value substantially lower than other combinations. This validates the importance of parameter optimization in balancing model complexity and predictive accuracy while effectively mitigating overfitting risks by adhering to the principle of model parsimony [61]. During model validation the area under the receiver operating characteristic curve (AUC) exceeded 0.9, consistent with findings from prior MaxEnt model studies, further confirming the model’s robustness [62]. Compared to previous research, this study introduces the following methodological innovations: (1) utilization of the WorldClim v2.1 high-resolution climate dataset, whose enhanced spatial interpolation algorithms and expanded meteorological station data significantly improve the ecological representation accuracy of environmental variables [63]; (2) incorporation of a globally comprehensive F. circinatum distribution database, with spatial bias correction to strengthen the model’s extrapolation capability; (3) application of an information criterion-based model selection framework (AICc) combined with a grid search algorithm to determine the optimal combination of FC and RM parameters [64].
To further enhance the reliability of habitat suitability predictions for F. circinatum, this study recommends that future research incorporate multi-model ensemble approaches (e.g., BIOCLIM, Random Forest) to establish a complementary ecological niche modeling framework [65]. Such an integrated multi-model strategy would not only strengthen cross-validation among different modeling techniques but also effectively mitigate systematic biases inherent in single-model approaches, thereby significantly improving both the predictive accuracy of species distribution models and the generalizability of ecological interpretations.
4.3. Projected Shifts in the Climatically Suitable Niche of F. circinatum
This study reveals that the highly suitable habitats of F. circinatum are predominantly distributed across East Asia, North America, South America, Western Europe, and eastern Australia. This spatial pattern aligns with existing research on the global distribution of forest pathogens, further corroborating that warm and humid climatic conditions are most favorable for the establishment and dispersal of such pathogens [66].
Under future climate scenarios, the suitable habitats exhibit a pronounced trend of “low-latitude contraction and high-latitude expansion”, consistent with the IPCC Sixth Assessment Report (AR6) regarding poleward species migration [67]. Notably, the projected overall reduction in suitable habitat area (24.62% by the 2090s under SSP585) closely parallels the estimates by Godefroid et al. for European forest pathogens (15–30% decline) yet markedly exceeds those for certain tropical pathogens (e.g., Phytophthora spp., showing only a 5–10% reduction) [68]. This discrepancy likely reflects the heightened sensitivity of F. circinatum to extreme climatic events (e.g., drought and heat stress), particularly within core current habitats such as Mediterranean climate zones [69].
Furthermore, multi-scenario comparisons demonstrate a significant divergence between SSP126 (low emissions) and SSP585 (high emissions), suggesting that climate mitigation could effectively curb pathogen spread [70]. For instance, the contraction of highly suitable habitats under SSP585-2090s (52.08%) far exceeds that under SSP126-2030s (34.84%). This finding, when contrasted with earlier RCP-based projections, underscores the increasingly suppressive effect of elevated greenhouse gas concentrations on pathogen survival [71]. However, fragmented habitat expansions into higher latitudes (e.g., northern North America and Siberia) may still pose emerging ecological risks, particularly in plantation-dense yet naturally vulnerable regions (e.g., Northern Europe). This observation supports the hypothesis proposed that “climate change reshapes plant disease hotspots” [72].
4.4. Limitations and Future Directions in Ecological Niche Modeling of F. circinatum
Ecological niche models, while providing critical insights into the current and potential suitable habitats of F. circinatum, must be contextualized within the complex ecological processes governing disease emergence, which inherently involve synergistic interactions between biotic and abiotic factors [60]. Despite centering on climatic, topographic, and edaphic variables, prevailing modeling paradigms continue to exhibit pronounced inherent limitations. First, the pathogen displays a markedly heterogeneous host range that encompasses coniferous genera, including Pinus, Picea, and Abies [73]. The spatial heterogeneity of host stands—including age–class structure, mixed-species composition, and the frequency of resistant genotypes—plays a critical role in modulating pathogen infection dynamics [74]. Second, under globalized conditions, timber trade networks, seedling transportation systems, and forest management practices can substantially alter the pathogen’s dispersal pathways through anthropogenic vectors [75]. Notably, large-scale monoculture plantations have been empirically demonstrated to lower ecological thresholds for disease outbreaks [76]. Most critically, the regulatory mechanisms within the soil rhizosphere microecosystem are decisive. Antagonistic microorganisms such as Trichoderma spp. and myxobacteria (Myxobacteria) can directly suppress pathogen population expansion through niche competition or the secretion of secondary metabolites (e.g., chitinases) [77,78]. Meanwhile, arbuscular mycorrhizal fungi (AMF) contribute to systemic defense barriers in the tripartite “microbe–plant–pathogen” interaction by modulating host water-use efficiency and the biosynthesis of defense-related phytohormones (e.g., jasmonic acid) [79].
Due to limitations in the availability of host genetic resistance data, human-mediated dispersal pathways, and microbial functional trait datasets, the current model does not incorporate these critical factors. Future research paradigms must prioritize the development of a multi-scale data fusion framework, with a focus on integrating the following: (1) landscape genomics-based geographic distribution modeling of host resistance; (2) pathogen transmission route analysis incorporating complex network theory; (3) a meta-genomics-enhanced dynamic monitoring system for microbial functional guilds.
5. Conclusions
This study systematically evaluated the potential global distribution patterns of F. circinatum under current and future climate scenarios using the MaxEnt model. The results indicate that the current global potentially suitable habitat area for this pathogen is approximately 6.93 × 107 km2, primarily distributed within the latitudinal zones of 0–50° S and 10–60° N. High-suitability regions are concentrated in East Asia, the western and southeastern coasts of North America, the western and southeastern coastal areas of South America, the Mediterranean basin, and the eastern coast of Australia. Factor analysis revealed that annual precipitation (bio12), mean temperature of the coldest quarter (bio11), annual mean temperature (bio1), and precipitation of the coldest quarter (bio19) are the key climatic determinants influencing its distribution. Under future climate scenarios, simulations across three SSP scenarios consistently projected a contraction in the total suitable habitat area, with particularly significant reductions in high- and medium-suitability regions. Specifically, the smallest total suitable area was predicted under the SSP126-2070s scenario (3.88 × 107 km2, a 43.96% decrease compared to the current level), while the largest was under the SSP585-2090s scenario (5.22 × 107 km2, a 24.62% decrease). The spatial distribution patterns exhibited a distinct latitudinal shift characterized by “low-latitude contraction and high-latitude expansion”, with the most pronounced migration observed under the SSP585-2090s scenario. Centroid analysis of suitable habitats indicated an overall northwestward shift of 80.3–1077.6 km, consistently stabilizing near the Nigeria–Niger border region. This finding corroborates the phenomenon of niche migration driven by global warming.
The study provides critical spatiotemporal insights for global early monitoring, cross-border quarantine, and region-specific precision management of F. circinatum, particularly highlighting the need to focus on potential risk shifts in tropical expansion zones (e.g., South America, Africa, and Southeast Asia) and temperate contraction zones (e.g., North America and Europe). It should be emphasized that the present study incorporated only bioclimatic variables, soil properties, and topographic factors. Future efforts should further integrate anthropogenic disturbances and host distribution as additional drivers. With the continuous enrichment of data resources, the ongoing refinement of modeling techniques, and the substantial advancement of computational capabilities, the predictive accuracy of the future distribution patterns of F. circinatum—the causal agent of pine pitch canker—is expected to improve commensurately.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15081913/s1, Figure S1: Comparative analysis of potential geographic distribution patterns of F. circinatum under future and current climate scenarios; Table S1: Environmental variables and their names for MaxEnt model construction.
Author Contributions
Formal analysis, X.Z.; Investigation, X.Z., C.C., F.W. and T.D.; Data curation, X.Z., C.C., F.W. and T.D.; Writing—original draft, X.Z.; Writing—review and editing, X.Z., C.C., F.W. and T.D.; Visualization, X.Z.; Project administration, T.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (32471873), the STI 2030-Major Projects (2023ZD0405605), the China Postdoctoral Science Foundation (2024M751426), the National Key R&D Program of China (2023YFD1401304), the Natural Science Foundation of Jiangsu Province, China (BK20231291), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Data Availability Statement
All data generated or analyzed during this study are included in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Betts, M.G.; Wolf, C.; Ripple, W.J.; Phalan, B.; Millers, K.A.; Duarte, A.; Butchart, S.H.M.; Levi, T. Global forest loss disproportionately erodes biodiversity in intact landscapes. Nature 2017, 547, 441–444. [Google Scholar] [CrossRef]
- Fernandes, L.; Paiva, D.S.; Silva, A.C.; Fernandes, C.; Fernandes, A.R.; Ribeiro, D.; Martins, L.; Bragança, H.; Portugal, A. From Lab to Nursery: Novel Approaches of Seed Disinfection for Managing Pine Pitch Canker Propagation. Forests 2024, 15, 1154. [Google Scholar] [CrossRef]
- De Vos, L.; van der Nest, M.A.; Santana, Q.C.; van Wyk, S.; Leeuwendaal, K.S.; Wingfield, B.D.; Steenkamp, E.T. Chromosome-level assemblies for the pine pitch canker pathogen Fusarium circinatum. Pathogens 2024, 13, 70. [Google Scholar] [CrossRef]
- Mullett, M.S. Global geographic distribution and host range of fusarium circinatum, the causal agent of pine pitch canker. Forests 2020, 11, 724. [Google Scholar] [CrossRef]
- EPPO. Gibberella Circinata Eradicated in France; EPPO Reporting Service; EPPO Global Database: Paris, France, 2008; p. 103. [Google Scholar]
- Ganley, R.J.; Watt, M.; Manning, L.; Iturritxa, E. A global climatic risk assessment of pitch canker disease. Can. J. For. Res. 2009, 39, 2246–2256. [Google Scholar] [CrossRef]
- Baker, R.; Candresse, T.S.; Erzsébet Dormannsné, E.; Gilioli, G.; Grégoire, J.; Jeger, M.J. Risk assessment of Gibberella circinata for the EU territory and identification and evaluation of risk management options. EFSA 2010, 8, 1620. [Google Scholar]
- EPPO. Update of the Situation of Fusarium circinatum in Spain; EPPO Reporting Service; EPPO Global Database: Paris, France, 2019; p. 196. [Google Scholar]
- Geng, Y.; Liu, Y.; Li, P.; Sun, J.Y.; Jiang, Y.; Pan, Z.; Li, Y.Z.; Zhang, Z. Anthropogenic activity and climate change exacerbate the spread of pathogenic bacteria in the environment. Sci. Adv. 2025, 11, eads4355. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Guerra, C.A.; Cano-Díaz, C.; Egidi, E.; Wang, J.T.; Eisenhauer, N.; Singh, B.K.; Maestre, F.T. The proportion of soil-borne pathogens increases with warming at the global scale. Nat. Clim. Change 2020, 10, 550–554. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Mostafa, M.; Ferdus, H.; Rahman, M.; Rana, J.A.; Islam, S.S.; Shanta, A.; Sannal, A.; Hosen, M.A.E.; et al. Plant disease dynamics in a changing climate: Impacts, molecular mechanisms, and climate-informed strategies for sustainable management. Discov. Agric. 2024, 2, 132. [Google Scholar] [CrossRef]
- Xing, Y.Y.; Wang, X.K.; Mustafa, A. Exploring the link between soil health and crop productivity. Ecotoxicol. Environ. Saf. 2025, 289, 117703. [Google Scholar] [CrossRef]
- Li, H.F.; Zhao, N.; Zhang, Q.Q.; Huang, L.; Zhang, H.; Gao, L.; Chen, W.Q.; Liu, T.G. Genetic and wind field analysis of wheat leaf rust (Puccinia triticina) dispersal: From winter sources in Gansu and Shaanxi to summer epidemics in China. Front. Plant Sci. 2025, 16, 1558898. [Google Scholar] [CrossRef]
- Balta, I.; Lemon, J.; Murnane, C.; Pet, I.; Vintila, T.; McCleery, D.; Callaway, T.; Douglas, A.; Stef, L.; Corcionivoschi, N. The One Health aspect of climate events with impact on foodborne pathogens transmission. One Health 2024, 19, 100926. [Google Scholar] [CrossRef]
- Sena, K.L.; Yang, J.; Kohlbrand, A.J.; Dreaden, T.J.; Barton, C.D. Landscape variables influence Phytophthora cinnamomi distribution within a forested Kentucky watershed. For. Ecol. Manag. 2019, 436, 39–44. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Xu, S.Y.; Capinha, C.; Weterings, R.; Gao, T.X. Using species distribution model to predict the impact of climate change on the potential distribution of Japanese whiting Sillago japonica. Ecol. Indic. 2019, 104, 333–340. [Google Scholar] [CrossRef]
- Mahmoud, A.R.; Farahat, E.A.; Hassan, L.M.; Halmy, M.W.A. Remotely sensed data contribution in predicting the distribution of native Mediterranean species. Sci. Rep. 2025, 15, 12475. [Google Scholar] [CrossRef]
- Liao, D.; Zhou, B.; Xiao, H.; Zhang, Y.; Zhang, S.; Su, Q.; Yang, X.H. Maxent modeling of the impacts of human activities and climate change on the potential distribution of plantago in china. Biology 2025, 14, 564. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, R.N.; Cantillo-Pérez, T.; Franca Rocha, W.J.; Aguiar, W.M.; Mendes, D.T.; de Jesus, T.B.; de Santana, C.O.; de Santana, M.M.M.; Oliveira, R.P. Advances and Challenges in Species Ecological Niche Modeling: A Mixed Review. Earth 2024, 5, 963–989. [Google Scholar] [CrossRef]
- Luo, M.; Yang, P.F.; Yang, L.L.; Zheng, Z.H.; Chen, Y.Y.; Li, H.; Wu, M.K. Predicting potentially suitable Bletilla striata habitats in China under future climate change scenarios using the optimized MaxEnt model. Sci. Rep. 2025, 15, 21231. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, C. Effects of sample size, sample accuracy and environmental variables on predictive performance of MaxEnt model. Pol. J. Ecol. 2016, 64, 303–312. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Li, Y.; Wu, C.; Zhao, B.; Peng, M.; Chen, W.; Wang, C. Response of extremely small populations to climate change—A case of Trachycarpus nanus in Yunnan, China. Biology 2024, 13, 240. [Google Scholar] [CrossRef]
- He, Z.; Ali, H.; Wu, J.; Liu, Z.; Wei, X.; Zhuo, Z. Impact of climate change on the distribution of Isaria cicadae Miquel in China: Predictions based on the MaxEnt model. Front. Microbiol. 2025, 16, 1509882. [Google Scholar] [CrossRef]
- Xiao, Y.; Guo, Q.Q.; Xie, N.; Yuan, G.Y.; Liao, M.G.; Gui, Q.; Ding, G.J. Predicting the global potential distribution of Bursaphelenchus xylophilus using an ecological niche model: Expansion trend and the main driving factors. BMC Ecol. Evol. 2024, 24, 48. [Google Scholar] [CrossRef]
- Hu, X.G.; Chen, J.H.; Chen, Q.Y.; Yang, Y.; Lin, Y.H.; Jin, Z.L.; Sha, L.Q.; Lin, E.; Yousry, E.-K.; Huang, H. The Spatial Shifts and Vulnerability Assessment of Ecological Niches under Climate Change Scenarios for Betula luminifera, a Fast-Growing Precious Tree in China. Plants 2024, 13, 1542. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, J.; König-Ries, B.; Recknagel, F. Proceedings of the 10th International Conference on Ecological Informatics: Translating ecological data into knowledge and decisions in a rapidly changing world. In Proceedings of the 10th International Conference on Ecological Informatics, Jena, Germany, 24–28 September 2018. [Google Scholar]
- Aidoo, O.F.; Souza, P.G.C.; da Silva, R.S.; Santana, P.A., Jr.; Picanço, M.C.; Osei-Owusu, J.; Sétamou, M.; Ekesi, S.; Borgemeister, C. A machine learning algorithm-based approach (MaxEnt) for predicting invasive potential of Trioza erytreae on a global scale. Ecol. Inform. 2022, 71, 101792. [Google Scholar] [CrossRef]
- GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.mda9gp (accessed on 20 June 2025).
- Recuenco, M.E.; Cacciola, S.O.S.O.; Sanz-Ros, A.V.; Garbelotto, M.; Diez, J.J. Potential interactions between invasive fusarium circinatum and other pine pathogens in europe. Forests 2020, 11, 7. [Google Scholar] [CrossRef]
- Ortiz, B.; Mendoza, K.; Enríquez, L.; Aguilar, K.; Yánez-Euceda, Y.; Amaya-Funes, K.; Umaña-Valle, I.; Fontecha, G. First Molecular Characterization of Fusarium circinatum Isolated from Pinus oocarpa in Honduras. Forest 2024, 9, 10. [Google Scholar]
- Chen, C.; Wang, B.; Li, J.; Xiao, Y.M.; Chen, K.Y.; Liu, N.; Zhou, G.Y. Predicting potential and quality distribution of Anisodus tanguticus (Maxim.) Pascher under different climatic conditions in the Qinghai–Tibet plateau. Front. Plant Sci. 2024, 15, 1369641. [Google Scholar] [CrossRef]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Dormann, C.F.; McPherson, J.M.; Araújo, M.B.; Bivand, R.; Bolliger, J.; Carl, G.; Davies, R.G.; Hirzel, A.; Jetz, W.; Kissling, W.D.; et al. Methods to account for spatial autocorrelation in the analysis of species distributional data: A review. Ecography 2007, 30, 609–628. [Google Scholar] [CrossRef]
- Ancillotto, L.; Santini, L.; Ranc, N.; Maiorano, L.; Russo, D. Extraordinary range expansion in a common bat: The potential roles of climate change and urbanisation. Sci. Nat. 2016, 103, 15. [Google Scholar] [CrossRef] [PubMed]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Alba-Fernández, M.V.; Ariza-López, F.J.; Jiménez-Gamero, M.D. A new approach to the quality control of slope and aspect classes derived from digital elevation models. Remote Sens. 2021, 13, 2069. [Google Scholar] [CrossRef]
- Wang, J.G.; Wu, J.W.; Li, W.J. Ecological Niche Changes and Risk Regionalization of the Invasive Plant Praxelis clematidea. Ecol. Evol. 2025, 15, e71546. [Google Scholar] [CrossRef]
- Lee, J.Y.; Marotzke, J.; Bala, G.; Cao, L.; Corti, S.; Dunne, J.P.; Engelbrecht, F.; Fischer, E.; Fyfe, J.C.; Jones, C.; et al. Future Global Climate: Scenario-Based Projections and Near-Term Information; Cambridge University Press: Cambridge, UK, 2021; pp. 553–672. [Google Scholar]
- Wang, H.; Wang, L.; Yan, G.Y.; Bai, H.Z.; Zhao, Y.X.; Ju, M.M.; Xu, X.T.; Yan, J.; Xiao, D.P.; Chen, L. Assessment and prediction of extreme temperature indices in the North China Plain by CMIP6 climate model. Appl. Sci. 2022, 12, 7201. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, X.W.; Xiang, W.H.; Chen, L.; Ouyang, S. Predicting potential suitable habitats of Chinese fir under current and future climatic scenarios based on Maxent model. Ecol. Inform. 2021, 64, 101393. [Google Scholar] [CrossRef]
- Mosisa, G.B.; Tassie, N.; Adula, M. Current and future distribution of Eucalyptus globulus under changing climate in Ethiopia: Implications for forest management. Environ. Syst. Res. 2024, 13, 4. [Google Scholar] [CrossRef]
- Shen, S.C.; Zheng, F.P.; Zhang, W.; Xu, G.F.; Li, D.Y.; Yang, S.S.; Jin, G.M.; Clements, D.R.; Nikkel, E.; Chen, A.D.; et al. Potential distribution and ecological impacts of Acmella radicans (Jacquin) RK Jansen (a new Yunnan invasive species record) in China. BMC Plant Biol. 2024, 24, 494. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Chen, S.T.; Gao, Y.; Yang, L.; Yu, H. Prediction of global potential suitable habitats of Nicotiana alata Link et Otto based on MaxEnt model. Sci. Rep. 2023, 13, 4851. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, L.; Zhang, X.; Guo, Z. Predicting the potential distribution of Hylomecon japonica in China under current and future climate change based on Maxent model. Sustainability 2021, 13, 11253. [Google Scholar] [CrossRef]
- van Steenderen, C.J.; Sutton, G.F. Climate covariate selection influences MaxEnt model predictions and predictive accuracy under current and future climates. Ecol. Model. 2024, 498, 110872. [Google Scholar] [CrossRef]
- Wang, Y.H.; Ren, X.Y.; Wang, K.; Lin, W.; Wang, P.; Liu, Z.Y.; Zhang, H.; Zhou, N. Maxent model-based prediction of the potential distribution of Fritillaria taipaiensis P.Y Li. Sci. Rep. 2025, 15, 20837. [Google Scholar]
- Kang, Y.; Lin, F.; Yin, J.; Han, Y.; Zhu, M.; Guo, Y.; Tang, F.; Li, Y. Projected distribution patterns of Alpinia officinarum in China under future climate scenarios: Insights from optimized Maxent and Biomod2 models. Front. Plant Sci. 2025, 16, 1517060. [Google Scholar] [CrossRef] [PubMed]
- Radosavljevic, A.; Anderson, R.P. Making better Maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Yue, J.; Li, Z.; Zuo, Z.; Wang, Y. Evaluation of ecological suitability and quality suitability of panax notoginseng under multi-regionalization modeling theory. Front. Plant Sci. 2022, 13, 818376. [Google Scholar] [CrossRef]
- Wu, W.; Li, Y.; Hu, Y. Simulation of potential habitat overlap between red deer (Cervus elaphus) and roe deer (Capreolus capreolus) in northeastern China. PeerJ 2016, 4, e1756. [Google Scholar] [CrossRef]
- Tepker, J.H.; Strugnell, J.M.; Silva, C.N. Environmental determinants of suitable habitat and the prediction of a southern shift in the future distribution of spiny lobsters, genus Jasus. BioRxiv 2023. [Google Scholar] [CrossRef]
- Garcia-Lopez, Y.J.; Marquez, P.H.; Morales, N.N. Microfinance institutions failure prediction in emerging countries, a machine learning approach. PLoS ONE 2025, 20, e0321989. [Google Scholar] [CrossRef]
- Abrantes, J.F.V.; Cariño, Z.A.P.; Mercado, H.L.S.; Vicencio, F.N.; Sosa, G.R.S.; Habaña, M.A.M.; Dagamac, N.H.A. Identification of environmental determinants involved in the distribution of Burkholderia pseudomallei in Southeast Asia Using Maxent Software. PLOS Neglected Trop. Dis. 2025, 19, e0012684. [Google Scholar] [CrossRef]
- Duret, C.; Bartet, T.; Hambuckers, A.; Kishida, O.; Okada, S.; Taguchi, Y.; Takahashi, M.K.; Denoël, M. Loss of habitat suitability and distribution range of the endangered Japanese giant salamander under climate change. Front. Biogeogr. 2025, 18, e133105. [Google Scholar] [CrossRef]
- Yan, C.C.; Hao, H.T.; Wang, Z.; Sha, S.S.; Zhang, Y.W.; Wang, Q.P.; Kang, Z.S.; Huang, L.L.; Wang, L.; Feng, H. Prediction of Suitable Habitat Distribution of Cryptosphaeria pullmanensis in the World and China under Climate Change. J. Fungi 2023, 9, 739. [Google Scholar] [CrossRef] [PubMed]
- Dvořák, M.; Janoš, P.; Botella, L.; Rotková, G.; Zas, R. Spore dispersal patterns of Fusarium circinatum on an infested Monterey pine forest in North-Western Spain. Forests 2017, 8, 432. [Google Scholar] [CrossRef]
- Garbelotto, M.; Smith, T.; Schweigkofler, W. Variation in rates of spore deposition of Fusarium circinatum, the causal agent of pine pitch canker, over a 12-month-period at two locations in Northern California. Phytopathology 2008, 98, 137–143. [Google Scholar] [CrossRef]
- Díaz, R.; Poveda, J.; Torres-Sánchez, E.; Sánchez-Gómez, T.; Martín-García, J.; Diez, J.J. Relation between morphology and native climate in the resistance of different Pinus pinaster populations to pitch canker disease caused by Fusarium circinatum. For. Ecol. Manag. 2024, 561, 121909. [Google Scholar] [CrossRef]
- Möykkynen, T.; Capretti, P.; Pukkala, T. Modelling the potential spread of Fusarium circinatum, the causal agent of pitch canker in Europe. Ann. For. Sci. 2015, 72, 169–181. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENM eval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Khwarahm, N.R. MaxEnt-based distribution modeling of the invasive species Phragmites australis under climate change conditions in Iraq. Plants 2025, 14, 768. [Google Scholar] [CrossRef] [PubMed]
- Jihan, M.A.T.; Popy, S.; Kayes, S.; Rasul, G.; Maowa, A.S.; Rahman, M.M. Climate change scenario in Bangladesh: Historical data analysis and future projection based on CMIP6 model. Sci. Rep. 2025, 15, 7856. [Google Scholar] [CrossRef]
- Gyeltshen, S.; Wangdi, N.; Jurmey, K.; Loday, P.; Rai, P.; Jamtsho, D.; Dema, K. Social and Environmental Drivers of Black-Necked Crane (BNC) Habitat Suitability in Bhutan: Insights from Maxent Modelling and Conservation Implications. Qeios 2024. [Google Scholar] [CrossRef]
- Moreno-Arzate, C.N.; Martínez-Meyer, E. A retrospective approach for evaluating ecological niche modeling transferability over time: The case of Mexican endemic rodents. PeerJ 2024, 12, e18414. [Google Scholar] [CrossRef]
- Ogris, N.; Drenkhan, R.; Vahalík, P.; Cech, T.; Mullett, M.; Tubby, K. The potential global distribution of an emerging forest pathogen, Lecanosticta acicola, under a changing climate. Front. For. Glob. Change 2023, 6, 1221339. [Google Scholar] [CrossRef]
- Dong, W.; Bai, X.; Zhao, L.; Dong, H.; Liu, C. Comparative analysis of climate-induced habitat shift of economically significant species with diverse ecological preferences in the Northwest Pacific. Front. Mar. Sci. 2024, 11, 1476097. [Google Scholar] [CrossRef]
- Collot, D.; Robinet, C. Probability of outbreaks of forest insects in Europe: A generic model calibrated on six forest insect profiles. BioRxiv 2024. [Google Scholar] [CrossRef]
- Essa, Y.H.; Hirschi, M.; Thiery, W.; El-Kenawy, A.M.; Yang, C. Drought characteristics in Mediterranean under future climate change. Npj Clim. Atmos. Sci. 2023, 6, 133. [Google Scholar] [CrossRef]
- Adusei, G.; Aidoo, M.K.; Srivastava, A.K.; Asibuo, J.Y.; Gaiser, T. Model-based climate change adaptational potential and productivity of some cowpea genotypes and its sensitivity to bias adjustment. Front. Agron. 2023, 5, 1144219. [Google Scholar] [CrossRef]
- Sanchez-Lucas, R.; Luna, E. Elevated CO2: A double-edged sword for plant defence against pathogens. New Phytol. 2025, 246, 2380. [Google Scholar] [CrossRef]
- Angelotti, F.; Hamada, E.; Bettiol, W. A Comprehensive Review of Climate Change and Plant Diseases in Brazil. Plants 2024, 13, 2447. [Google Scholar] [CrossRef]
- Woodward, S.; Flores-Pacheco, J.A.; Muñoz-Adalia, E.J.; Martínez-Álvarez, P.; Martín-García, J.; Diez, J.J. Susceptibility of germinating seedlings of European and Eurasian populations of Pinus sylvestris to dam**-off caused by Fusarium circinatum. For. Pathol. 2022, 52, e12749. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, X.; Jiang, Y.; Zhang, J.; Qi, F.H.; Jing, T.Z. Spatial Pattern of Host Tree Size, Rather than of Host Tree Itself, Affects the Infection Likelihood of a Fungal Stem Disease. Biology 2024, 13, 616. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Narine, L.L.; Willoughby, J.R.; Eckhardt, L.G. Remote sensing-based detection of brown spot needle blight: A comprehensive review, and future directions. PeerJ 2025, 13, e19407. [Google Scholar] [CrossRef]
- Widyati, E.; Nuroniah, H.S.; Tata, H.L.; Mindawati, N.; Lisnawati, Y.; van Noordwijk, M. Soil degradation due to conversion from natural to plantation forests in Indonesia. Forests 2022, 13, 1913. [Google Scholar] [CrossRef]
- Bharti, L.; Yadav, K.; Kumar Chaubey, A. Trichoderma spp.: Approach for bio-control agent. Challenges in Plant Disease Detection and Recent Advancements; IntechOpen: London, UK, 2024; pp. 1–30. [Google Scholar]
- Zhang, L.; Bao, L.; Li, S.; Liu, Y.; Liu, H. Active substances of myxobacteria against plant diseases and their action mechanisms. Front. Microbiol. 2024, 14, 1294854. [Google Scholar] [CrossRef] [PubMed]
- Delaeter, M.; Magnin-Robert, M.; Randoux, B.; Lounès-Hadj Sahraoui, A. Arbuscular mycorrhizal fungi as biostimulant and biocontrol agents: A review. Microorganisms 2024, 12, 1281. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).