Fungal Pretreatment of Alperujo for Bioproduct Recovery and Detoxification: Comparison of Two White Rot Fungi †
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Alperujo
2.3. Experimental Set-Up and Design
2.4. Sampling Procedure and Analytical Methods
2.5. Phytotoxicity Tests After Fungal Treatment
2.6. Statistical Analysis
3. Results and Discussion
3.1. Value-Added Compounds Obtained in the Aqueous Extract
3.1.1. Ligninolytic Enzyme Activity by A. discolor and S. hirsutum
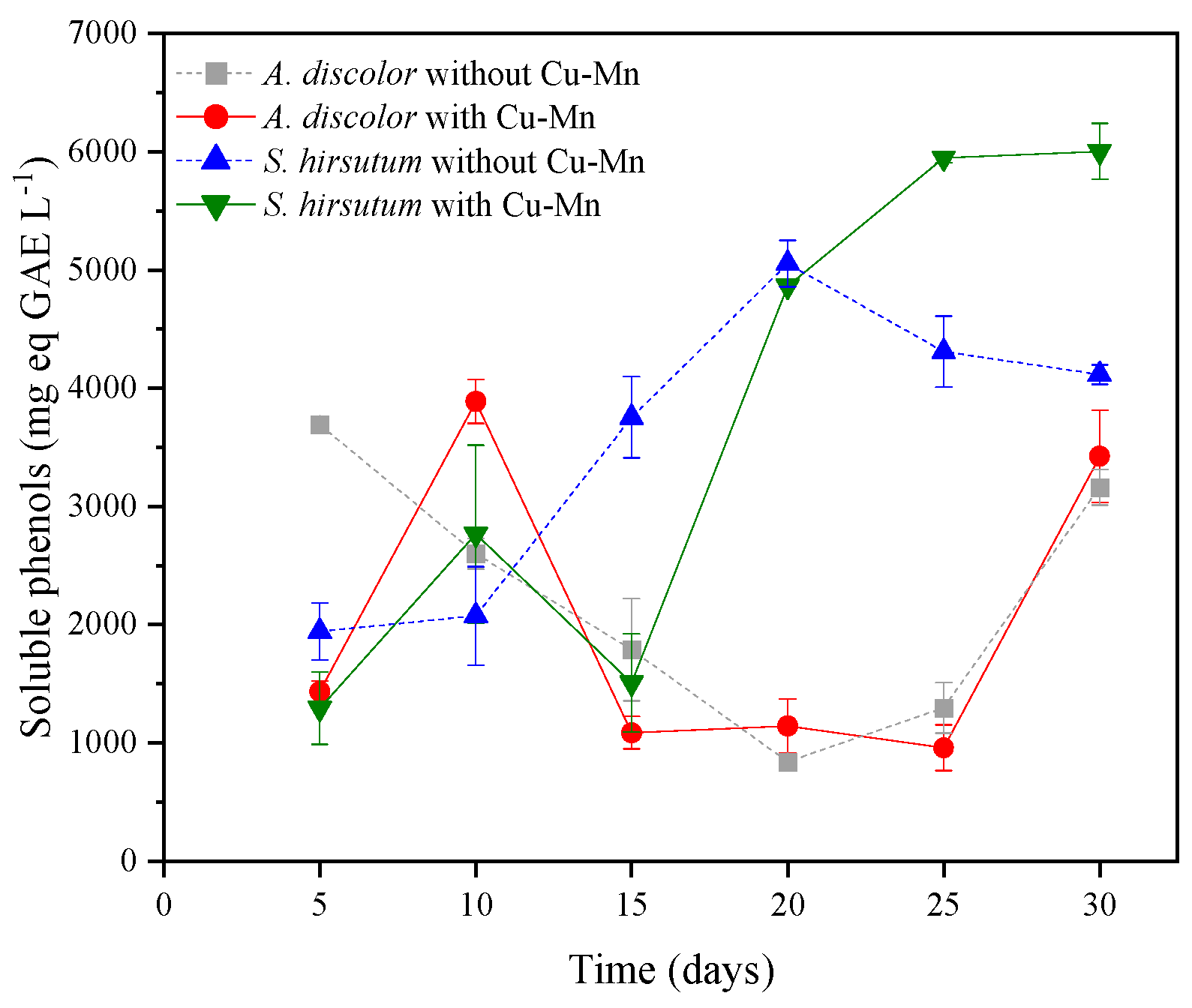
3.1.2. Phenolic Compounds Release from the Alperujo to the Aqueous Extract
3.1.3. Volatile Fatty Acid (VFA) Composition in the Aqueous Extract
3.2. Characterization of the Obtained Alperujo Solid Phase
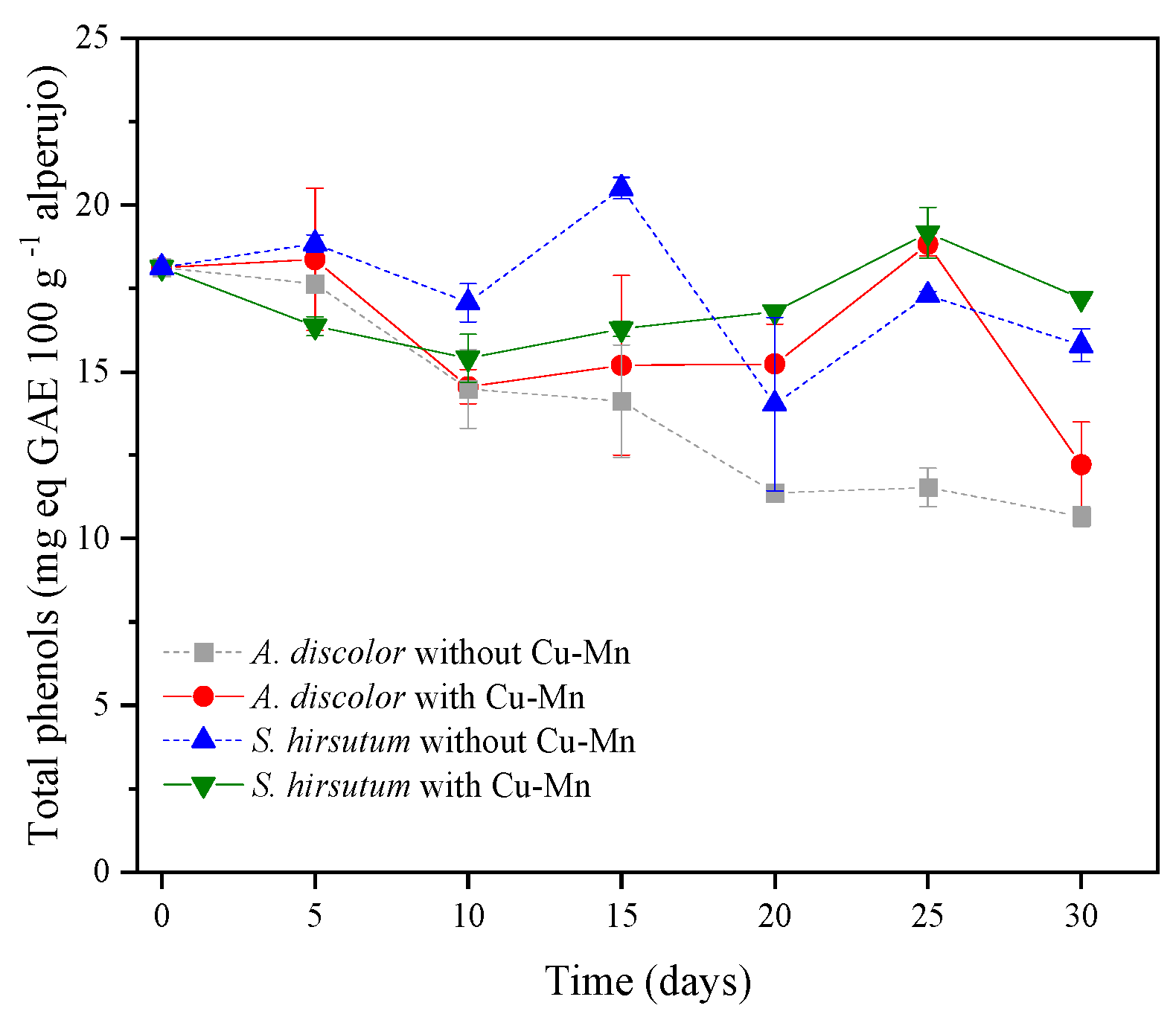
3.2.1. Phenolic Compounds in the Alperujo Solid Phase
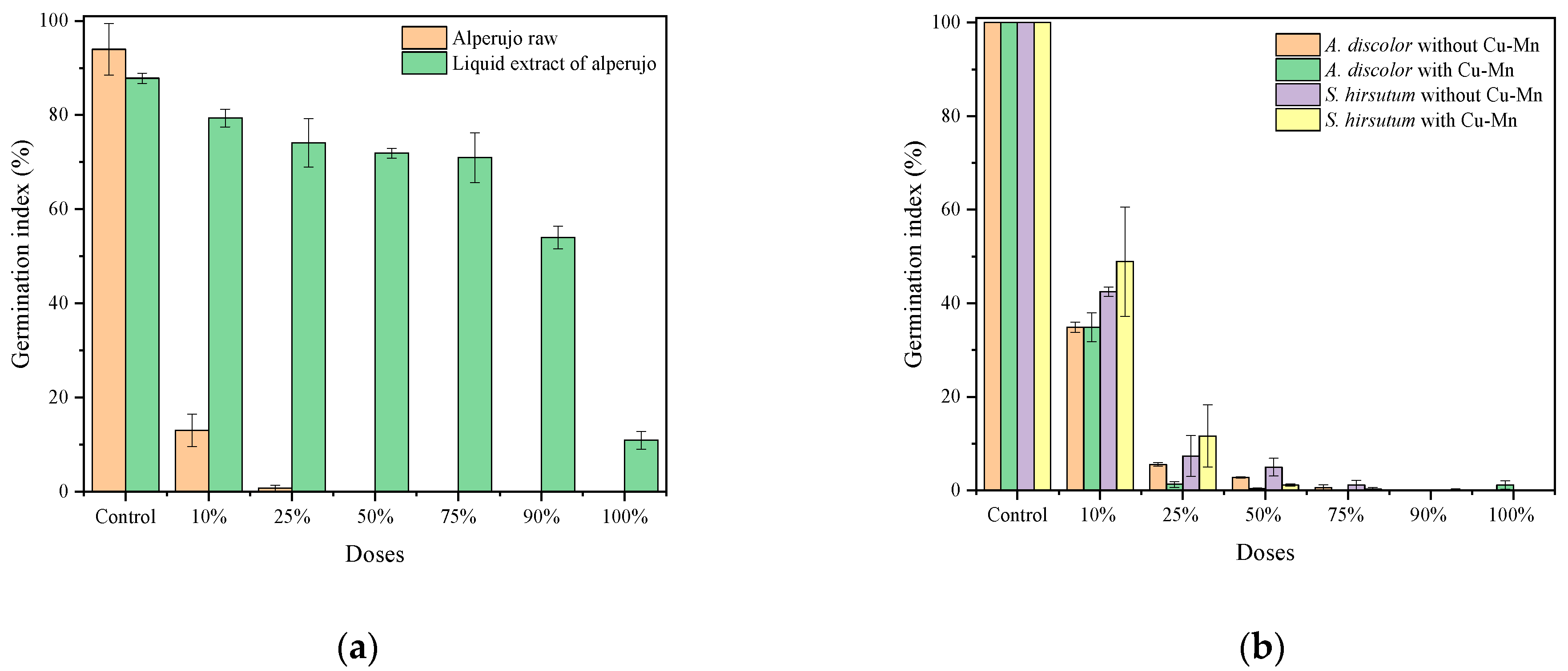
3.2.2. Effect of Alperujo on S. lycopersicum Seed Germination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COD | Chemical Oxygen Demand |
| FID | Flame Ionization Detector |
| GI | Germination Index |
| HPLC-DAD | High-Performance Liquid Chromatography with diode-array detection |
| Lac | Laccase |
| MnP | Manganese Peroxidase |
| MS | Mineral Solids |
| RRG | Relative Radicle Growth |
| RSG | Relative Seed Germination |
| SSF | Solid State Fermentation |
| TS | Total Solids |
| VFAs | Volatile Fatty Acids |
| VS | Volatile Solids |
| WRF | White Rot Fungi |
References
- Enaime, G.; Dababat, S.; Wichern, M.; Lübken, M. Olive mill wastes: From wastes to resources. Environ. Sci. Pollut. Res. 2024, 31, 20853–20880. [Google Scholar] [CrossRef]
- Lenzuni, M.; Converti, A.; Casazza, A.A. From laboratory- to industrial-scale plants: Future of anaerobic digestion of olive mill solid wastes. Bioresour. Technol. 2024, 394, 130317. [Google Scholar] [CrossRef] [PubMed]
- Genethliou, C.; Kornaros, M.; Dailianis, S. Biodegradation of olive mill wastewater phenolic compounds in a thermophilic anaerobic upflow packed bed reactor and assessment of their toxicity in digester effluents. J. Environ. Manag. 2020, 255, 109882. [Google Scholar] [CrossRef]
- Sounni, F.; Elgnaoui, Y.; El Bari, H.; Merzouki, M.; Benlemlih, M. Effect of mixture ratio and organic loading rate during anaerobic co-digestion of olive mill wastewater and agro-industrial wastes. Biomass Convers. Biorefinery 2021, 13, 1223–1229. [Google Scholar] [CrossRef]
- Jiménez-Páez, E.; Serrano, A.; Purswani, J.; Correa-Galeote, D.; Cubero-Cardoso, J.; Fermoso, F.G. Impact on the microbial population during biological volatile fatty acid production from olive mill solid waste. Environ. Technol. Innov. 2023, 32, 103409. [Google Scholar] [CrossRef]
- Fernández-Prior, Á.; Bermúdez-Oria, A.; Millán-Linares, M.D.C.; Fernández-Bolaños, J.; Espejo-Calvo, J.A.; Rodríguez-Gutiérrez, G. Anti-inflammatory and antioxidant activity of hydroxytyrosol and 3,4-dihydroxyphenyglycol purified from table olive effluents. Foods 2021, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Jiang, Y.; Zhao, C. Hydroxytyrosol isolation, comparison of synthetic routes and potential biological activities. Food Sci. Nutr. 2024, 12, 6899–6912. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.I.; Ibañez, M.; Laca, A.; Díaz, M. Biodegradation of olive mill effluent by white-rot fungi. Appl. Sci. 2021, 11, 9930. [Google Scholar] [CrossRef]
- Reina, R.; Liers, C.; Ocampo, J.A.; García-Romera, I.; Aranda, E. Solid state fermentation of olive mill residues by wood- and dung-dwelling Agaricomycetes: Effects on peroxidase production, biomass development and phenol phytotoxicity. Chemosphere 2013, 93, 1406–1412. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; Rodríguez-Gutiérrez, G.; Lama-Muñoz, A.; Fernández-Bolaños, J. Phenolic extract obtained from steam-treated olive oil waste: Characterization and antioxidant activity. LWT 2013, 54, 114–124. [Google Scholar] [CrossRef]
- Serrano, A.; Fermoso, F.G.; Alonso-Fariñas, B.; Rodríguez-Gutierrez, G.; Fernandez-Bolaños, J.; Borja, R. Phenols recovery after steam explosion of Olive Mill Solid Waste and its influence on a subsequent biomethanization process. Bioresour. Technol. 2017, 243, 169–178. [Google Scholar] [CrossRef]
- Becerra, M.L.; Lizarazo, L.M.; Rojas, H.A.; Prieto, G.A.; Martinez, J.J. Biotransformation of 5-hydroxymethylfurfural and furfural with bacteria of bacillus genus. Biocatal. Agric. Biotechnol. 2022, 39, 102281. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Ntougias, S.; Baldrian, P.; Ehaliotis, C.; Nerud, F.; Merhautová, V.; Zervakis, G.I. Olive mill wastewater biodegradation potential of white-rot fungi—Mode of action of fungal culture extracts and effects of ligninolytic enzymes. Bioresour. Technol. 2015, 189, 121–130. [Google Scholar] [CrossRef]
- Shirkavand, E.; Baroutian, S.; Gapes, D.J.; Young, B.R. Pretreatment of radiata pine using two white rot fungal strains Stereum hirsutum and Trametes versicolor. Energy Convers. Manag. 2017, 142, 13–19. [Google Scholar] [CrossRef]
- Suryadi, H.; Judono, J.J.; Putri, M.R.; Eclessia, A.D.; Ulhaq, J.M.; Agustina, D.N.; Sumiati, T. Biodelignification of lignocellulose using ligninolytic enzymes from white-rot fungi. Heliyon 2022, 8, e08865. [Google Scholar] [CrossRef]
- El-Gendi, H.; Saleh, A.K.; Badierah, R.; Redwan, E.M.; El-Maradny, Y.A.; El-Fakharany, E.M. A Comprehensive Insight into Fungal Enzymes: Structure, Classification, and Their Role in Mankind’s Challenges. J. Fungi 2022, 8, 23. [Google Scholar] [CrossRef]
- Sampedro, I.; Cajthaml, T.; Marinari, S.; Stazi, S.R.; Grego, S.; Petruccioli, M.; Federici, F.; D’Annibale, A. Immobilized inocula of white-rot fungi accelerate both detoxification and organic matter transformation in two-phase dry olive-mill residue. J. Agric. Food Chem. 2009, 57, 5452–5460. [Google Scholar] [CrossRef]
- Durán, N.; Cuevas, R.; Cordi, L.; Rubilar, O.; Diez, M.C. Biogenic silver nanoparticles associated with silver chloride nanoparticles (Ag@AgCl) produced by laccase from Trametes versicolor. SpringerPlus 2014, 3, 645. [Google Scholar] [CrossRef]
- Benavides, V.; Pinto-Ibieta, F.; Serrano, A.; Rubilar, O.; Ciudad, G. Use of Anthracophyllum discolor and Stereum hirsutum as a Suitable Strategy for Delignification and Phenolic Removal of Olive Mill Solid Waste. Foods 2022, 11, 5438. [Google Scholar] [CrossRef]
- Rubilar, O.; Tortella, G.; Cea, M.; Acevedo, F.; Bustamante, M.; Gianfreda, L.; Diez, M.C. Bioremediation of a Chilean Andisol contaminated with pentachlorophenol (PCP) by solid substrate cultures of white-rot fungi. Biodegradation 2011, 22, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Atilano-Camino, M.M.; Álvarez-Valencia, L.H.; García-González, A.; García-Reyes, R.B. Improving laccase production from Trametes versicolor using lignocellulosic residues as cosubstrates and evaluation of enzymes for blue wastewater biodegradation. J. Environ. Manag. 2020, 275, 111231. [Google Scholar] [CrossRef]
- Benavides, V.; Serrano, A.; Pinto-Ibieta, F.; Rubilar, O.; Ciudad, G. Biodegradation of olive mill solid waste by Anthracophyllum discolor and Stereum hirsutum: Effect of copper and manganese supplementation. Bioresour. Bioprocess. 2025, 12, 18. [Google Scholar] [CrossRef]
- Mishra, V.; Jana, A.K.; Jana, M.M.; Gupta, A. Enhancement in multiple lignolytic enzymes production for optimized lignin degradation and selectivity in fungal pretreatment of sweet sorghum bagasse. Bioresour. Technol. 2017, 236, 49–59. [Google Scholar] [CrossRef]
- Siddeeg, S.M.; Tahoon, M.A.; Mnif, W.; Ben Rebah, F. Iron Oxide/Chitosan Magnetic Nanocomposite Immobilized Manganese Peroxidase for Decolorization of Textile Wastewater. Processes 2020, 8, 5. [Google Scholar] [CrossRef]
- Hseu, Z.Y. Evaluating heavy metal contents in nine composts using four digestion methods. Bioresour. Technol. 2004, 95, 53–59. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Moscoviz, R.; Astals, S.; Robles; Steyer, J.P. Unraveling the literature chaos around free ammonia inhibition in anaerobic digestion. Renew. Sustain. Energy Rev. 2020, 117, 109487. [Google Scholar] [CrossRef]
- Tortella, G.R.; Rubilar, O.; Gianfreda, L.; Valenzuela, E.; Diez, M.C. Enzymatic characterization of Chilean native wood-rotting fungi for potential use in the bioremediation of polluted environments with chlorophenols. World J. Microbiol. Biotechnol. 2008, 24, 2805–2818. [Google Scholar] [CrossRef]
- Reina, R.; Liers, C.; García-Romera, I.; Aranda, E. Enzymatic mechanisms and detoxification of dry olive-mill residue by Cyclocybe aegerita, Mycetinis alliaceus and Chondrostereum purpureum. Int. Biodeterior. Biodegrad. 2017, 117, 89. [Google Scholar] [CrossRef]
- Wariishi, H.; Valli, K.; Gold, M.H. Manganese(II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium: Kinetic mechanism and role of chelators. J. Biol. Chem. 1992, 267, 23688–23695. [Google Scholar] [CrossRef]
- Parenti, A.; Muguerza, E.; Redin Iroz, A.; Omarini, A.; Conde, E.; Alfaro, M.; Castanera, R.; Santoyo, F.; Ramírez, L.; Pisabarro, A.G. Induction of laccase activity in the white rot fungus Pleurotus ostreatus using water polluted with wheat straw extracts. Bioresour. Technol. 2013, 133, 142–149. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Ravents, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; pp. 152–178. [Google Scholar]
- García, A.; Rodríguez-Juan, E.; Rodríguez-Gutiérrez, G.; Rios, J.J.; Fernández-Bolaños, J. Extraction of phenolic compounds from virgin olive oil by deep eutectic solvents (DESs). Food Chem. 2016, 197, 554–561. [Google Scholar] [CrossRef]
- Vargas-Muñoz, M.A.; Cerdà, V.; Cadavid-Rodríguez, L.S.; Palacio, E. Automated method for volatile fatty acids determination in anaerobic processes using in-syringe magnetic stirring assisted dispersive liquid-liquid microextraction and gas chromatography with flame ionization detector. J. Chromatogr. A 2021, 1643, 462034. [Google Scholar] [CrossRef]
- Pinho, I.A.; Lopes, D.V.; Martins, R.C.; Quina, M.J. Phytotoxicity assessment of olive mill solid wastes and the influence of phenolic compounds. Chemosphere 2017, 185, 258–267. [Google Scholar] [CrossRef]
- Zucconi, F.; Pera, A.; Forte, M.; DeBertolli, M. Evaluating toxicity of immature compost. Biocycle 1981, 22, 54–57. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Versión 2020; de Transferencia InfoStat, FCA; Universidad Nacional de Córdoba: Córdoba, Argentina, 2020; Available online: http://www.infostat.com.ar (accessed on 13 June 2024).
- Slavens, S.; Marek, S.M.; Wilkins, M.R. Effects of copper, manganese, and glucose on the induction of ligninolytic enzymes produced by Pleurotus ostreatus during fungal pretreatment of switchgrass. Trans. ASABE 2019, 62, 1673–1681. [Google Scholar] [CrossRef]
- Liu, S.-H.; Tsai, S.-L.; Guo, P.-Y.; Lin, C.-W. Inducing laccase activity in white rot fungi using copper ions and improving the efficiency of azo dye treatment with electricity generation using microbial fuel cells. Chemosphere 2020, 243, 125304. [Google Scholar] [CrossRef] [PubMed]
- Kannaiyan, R.; Mahinpey, N.; Kostenko, V.; Martinuzzi, R.J. Nutrient media optimization for simultaneous enhancement of the laccase and peroxidases production by coculture of Dichomitus squalens and Ceriporiopsis subvermispora. Biotechnol. Appl. Biochem. 2015, 62, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P. Interactions of heavy metals with white-rot fungi. Enzyme Microb. Technol. 2003, 32, 78–91. [Google Scholar] [CrossRef]
- Flórez-Restrepo, M.A.; López-Legarda, X.; Segura-Sánchez, F. Bioremediation of emerging pharmaceutical pollutants acetaminophen and ibuprofen by white-rot fungi—A review. Sci. Total Environ. 2025, 977, 179379. [Google Scholar] [CrossRef]
- Sybuia, P.A.; Contato, A.G.; de Araújo, C.A.V.; Zanzarin, D.M.; Maciel, G.M.; Pilau, E.J.; Peralta, R.M.; de Souza, C.G.M. Application of the white-rot fungus Trametes sp. (C3) laccase in the removal of acetaminophen from aqueous solutions. J. Water Process Eng. 2024, 57, 104677. [Google Scholar] [CrossRef]
- Alokpa, K.; Lafortune, F.; Cabana, H. Application of laccase and hydrolases for trace organic contaminants removal from contaminated water. Environ. Adv. 2022, 8, 100243. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, J.; Zhang, X.; Hou, C.; Wu, D. Catalytic removal of emerging contaminant and phenolic compounds by laccase: Transformation mechanisms in aquiatic environments-polymerization or degradation? Sep. Purif. Technol. 2024, 355, 129544. [Google Scholar] [CrossRef]
- Fattoum, H.; Cherif, A.O.; Trabelsi, S.; Ben Messaouda, M. Identification of Phenolic Compounds Extracted from OMW Using LC-MS. J. Oleo Sci. 2023, 72, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; Fermoso, F.G.; Alonso-Fariñas, B.; Rodríguez-Gutierrez, G.; Fernandez-Bolaños, J.; Borja, R. Olive mill solid waste biorefinery: High-temperature thermal pre-treatment for phenol recovery and biomethanization. J. Clean. Prod. 2017, 148, 314–323. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Yang, Y.H. Microbial production of volatile fatty acids: Current status and future perspectives. Rev. Environ. Sci. Biotechnol. 2017, 16, 327–345. [Google Scholar] [CrossRef]
- He, G.Q.; Kong, Q.; Chen, Q.H.; Ruan, H. Batch and fed-batch production of butyric acid by Clostridium butyricum ZJUCB. J. Zhejiang Univ. Sci. B 2005, 6, 1076–1080. [Google Scholar] [CrossRef]
- Zigova, J. Butyric acid production by. Biotechnol. Bioeng. 1999, 34, 835–843. [Google Scholar]
- Capasso, R.; Cristinzio, G.; Evidente, A.; Scognamiglio, F. Isolation, spectroscopy and selective phytotoxic effects of polyphenols from vegetable waste waters. Phytochemistry 1992, 31, 4125–4128. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benavides, V.; Ciudad, G.; Pinto-Ibieta, F.; Aranda, E.; Ramos-Muñoz, V.; Rao, M.A.; Serrano, A. Fungal Pretreatment of Alperujo for Bioproduct Recovery and Detoxification: Comparison of Two White Rot Fungi. Agronomy 2025, 15, 1851. https://doi.org/10.3390/agronomy15081851
Benavides V, Ciudad G, Pinto-Ibieta F, Aranda E, Ramos-Muñoz V, Rao MA, Serrano A. Fungal Pretreatment of Alperujo for Bioproduct Recovery and Detoxification: Comparison of Two White Rot Fungi. Agronomy. 2025; 15(8):1851. https://doi.org/10.3390/agronomy15081851
Chicago/Turabian StyleBenavides, Viviana, Gustavo Ciudad, Fernanda Pinto-Ibieta, Elisabet Aranda, Victor Ramos-Muñoz, Maria A. Rao, and Antonio Serrano. 2025. "Fungal Pretreatment of Alperujo for Bioproduct Recovery and Detoxification: Comparison of Two White Rot Fungi" Agronomy 15, no. 8: 1851. https://doi.org/10.3390/agronomy15081851
APA StyleBenavides, V., Ciudad, G., Pinto-Ibieta, F., Aranda, E., Ramos-Muñoz, V., Rao, M. A., & Serrano, A. (2025). Fungal Pretreatment of Alperujo for Bioproduct Recovery and Detoxification: Comparison of Two White Rot Fungi. Agronomy, 15(8), 1851. https://doi.org/10.3390/agronomy15081851







