Seed Priming Beyond Stress Adaptation: Broadening the Agronomic Horizon
Abstract
1. Introduction
2. Seed Priming and Its Mechanism
3. Classical View: Stress Adaptation Through Priming
4. Emerging Functions of Seed Priming Beyond Stress Tolerance
4.1. Improvement of Seed Vigor and Germination Uniformity
4.1.1. Improvements in Germination Parameters
4.1.2. Challenges of Low-Vigor and Poor-Quality Seeds
4.2. Yield Enhancement Under Optimal Conditions
- Early canopy closure;
- Improved light interception;
- More efficient nutrient and water uptake;
- Reduced intra-specific competition;
- Enhanced sink strength during reproductive phases.
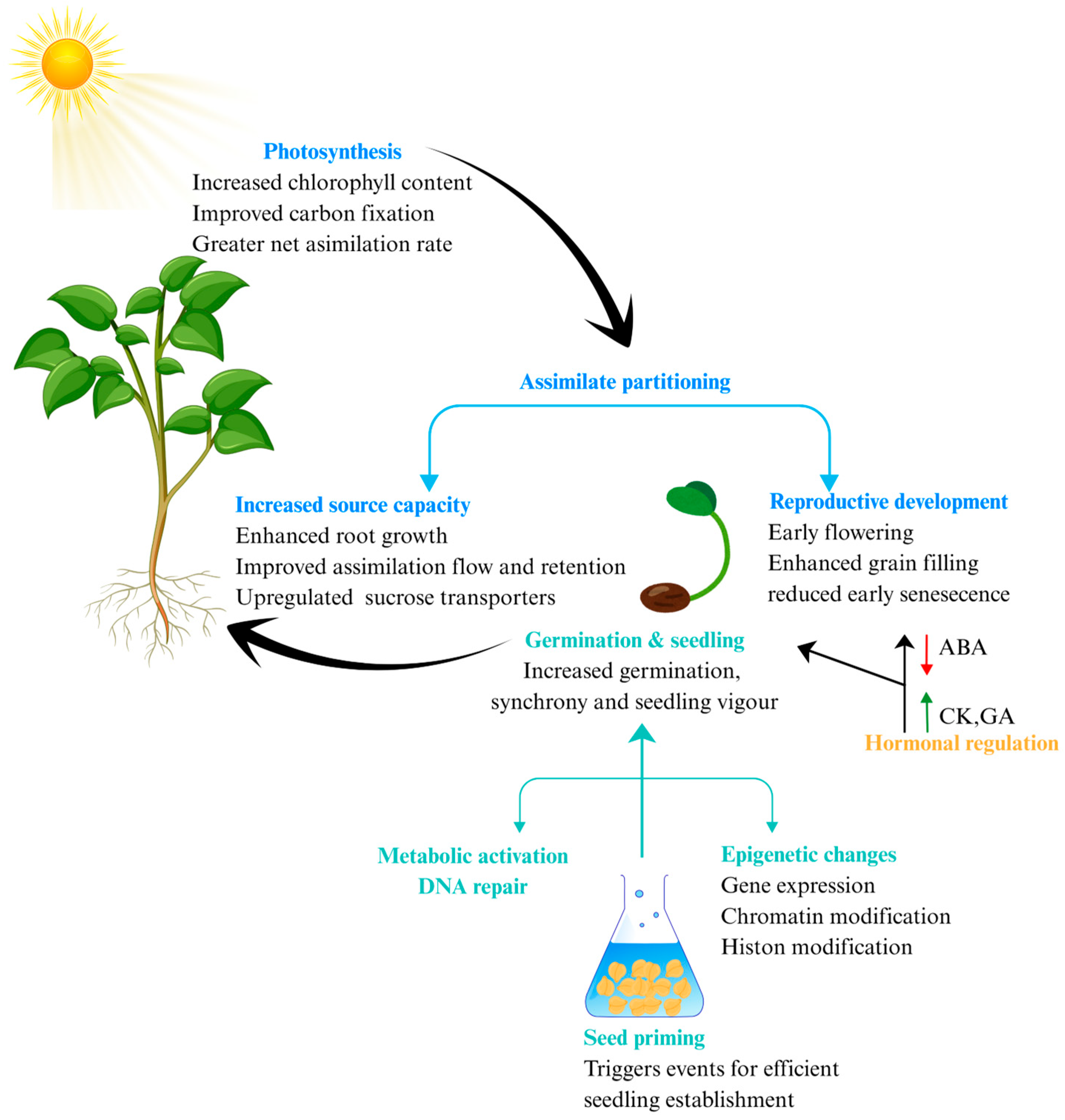
5. Metabolic and Developmental Reprogramming
5.1. Alteration of Source–Sink Dynamics
5.2. Hormonal Crosstalk and Growth Modulation
5.3. Accelerated Phenological Development
6. Applications in Climate-Smart and Precision Agriculture
6.1. Use in Marginal Soils and Ecological Restoration
6.2. Tailored Priming for Cropping Systems and Technologies
7. Integration of Omics for Mechanistic Insights and Marker Discovery
7.1. Transcriptomic Reprogramming and Priming Duration
7.2. Proteomic and Antioxidative Response
7.3. Metabolomic and Functional Insights
7.4. Epigenomic Regulation and Transgenerational Memory
7.5. Toward Biomarker-Guided Precision Priming
8. Seed Industry and Commercial Considerations
9. Future Directions and Research Gaps
- Thresholds of water imbibition during priming to avoid premature germination while activating beneficial metabolic responses;
- The interaction of primed seeds with soil microbiota, including effects on rhizosphere dynamics and the microbial community;
- Combinatorial priming protocols, involving dual stress simulations (e.g., salt + heat or drought + metal) or hybrid methods (e.g., nano-biopriming);
- Socioeconomic barriers to adoption in smallholder contexts, including accessibility, cost, and farmer awareness;
- Environmental impact assessment of novel priming agents (e.g., nanoparticles) to ensure sustainability and regulatory compliance.
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 8-oxo-dG | 8-hydroxy-2′-deoxyguanosine |
| ABA | Abscisic acid |
| APX | Ascorbate peroxidase |
| BAP | 6-benzylaminopurine |
| BER | Base excision repair |
| CAT | Catalase |
| Cd | Cadmium |
| DLP | Dehydrin-like proteins |
| FAO | Food and Agriculture Organization of the United Nations |
| GA | Gibberellic acid |
| GAs | Gibberellins |
| GI | Germination index |
| GP | Germination percentage |
| GRI | Germination rate index |
| Hg | Mercury |
| HI | Harvest index |
| HMs | Heavy metals |
| HR | Homologous recombination |
| HSPs | Heat shock proteins |
| IAA | Indole-3-acetic acid |
| IBA | Indole butyric acid |
| LEA | Late embryogenesis abundant |
| MDA | Malondialdehyde |
| MGT | Mean germination time |
| NER | Nucleotide excision repair |
| NHEJ | Non-homologous end joining |
| Ni | Nickel |
| NPs | Nanoparticles |
| PAH | Polycyclic aromatic hydrocarbons |
| Pb | Lead |
| PEG | Polyethylene glycol |
| PGPB | Plant growth-promoting bacteria |
| RH | Relative humidity |
| ROS | Reactive oxygen species |
| SA | Salicylic acid |
| SETs | Seed enhancement technologies |
| SiA | Silicic acid |
| SOD | Superoxide dismutase |
| SVI | Seedling vigor index |
References
- Obroucheva, N.V.; Antipova, O.V.; Lityagina, S.V.; Novikova, G.V. Relationship between initiation of cell elongation and cell division in radicles of germinating seeds. Plant Soil 1995, 173, 311–316. [Google Scholar] [CrossRef]
- Hasanović, M.; Durmić-Pašić, A.; Karalija, E. Enhancing nickel stress tolerance in Micro-Tom tomatoes through biopriming with Paraburkholderia phytofirmans PsJN: Insights into growth and physiological responses. Front. Microbiol. 2025, 16, 1561924. [Google Scholar] [CrossRef]
- Shil, S.; Ashwath, M.N.; Das, S.; Vats, P.; Raj, A.K.; Dash, U.; Bhardwaj, A. Seed biopriming: A sustainable solution for enhancing seed vigor and crop productivity. In Advances in Seed Quality Evaluation and Improvement; Springer: Singapore, 2025; pp. 127–168. [Google Scholar] [CrossRef]
- Tabassum, T.; Ahmad, R.; Farooq, M.; Basra, S.M.A. Improving salt tolerance in barley by osmopriming and biopriming. IJAB 2018, 20, 2455–2464. [Google Scholar]
- Sghayar, S.; Debez, A.; Lucchini, G.; Abruzzese, A.; Zorrig, W.; Negrini, N.; Morgutti, S.; Abdelly, C.; Sacchi, G.A.; Pecchioni, N.; et al. Seed priming mitigates high salinity impact on germination of bread wheat (Triticum aestivum L.) by improving carbohydrate and protein mobilization. Plant Direct 2023, 7, e497. [Google Scholar] [CrossRef]
- Hmissi, M.; Krouma, A.; García-Sánchez, F.; Chaieb, M. Potential of seed halopriming in the mitigation of salinity stress during germination and seedling establishment in durum wheat (Triticum durum Desf.). Plants 2024, 13, 66. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Moradi Dezfuli, P.; Sharifzadeh, F. Seed invigoration techniques to improve germination and early growth of inbred line of maize under salinity and drought stress. Gen. Appl. Plant Physiol. 2008, 34, 215–226. [Google Scholar]
- Nasir, M.W.; Yasmeen, A.; Imran, M.; Zoltan, T. Seed priming to alleviate drought stress in cotton. J. Environ. Agric. Sci. 2019, 21, 14–22. [Google Scholar]
- Farooq, M.; Basra, S.M.A.; Wahid, A.; Rehman, H. Exogenously applied nitric oxide enhances the drought tolerance in fine grain aromatic rice (Oryza sativa L.). J. Agron. Crop Sci. 2009, 195, 254–261. [Google Scholar] [CrossRef]
- Kaur, S.; Gupta, A.; Kaur, N. Effect of osmo- and hydropriming of chickpea seeds on seedling growth and carbohydrate metabolism under water deficit stress. Plant Growth Regul. 2002, 37, 17–22. [Google Scholar] [CrossRef]
- Ellouzi, H.; Zorrig, W.; Amraoui, S.; Oueslati, S.; Abdelly, C.; Rabhi, M.; Siddique, K.H.M.; Hessini, K. Seed priming with salicylic acid alleviates salt stress toxicity in barley by suppressing ROS accumulation and improving antioxidant defense systems, compared to halo- and gibberellin priming. Antioxidants 2023, 12, 1779. [Google Scholar] [CrossRef] [PubMed]
- Tamindžić, G.; Ignjatov, M.; Miljaković, D.; Červenski, J.; Milošević, D.; Nikolić, Z.; Vasiljević, S. Seed priming treatments to improve heat stress tolerance of garden pea (Pisum sativum L.). Agriculture 2023, 13, 439. [Google Scholar] [CrossRef]
- Mahmood ur Rehman, M.; Liu, J.; Nijabat, A.; Alsudays, I.M.; Saleh, M.A.; Alamer, K.H.; Attia, H.; Ziaf, K.; Zaman, Q.U.; Amjad, M. Seed priming with potassium nitrate alleviates the high temperature stress by modulating growth and antioxidant potential in carrot seeds and seedlings. BMC Plant Biol. 2024, 24, 606. [Google Scholar] [CrossRef]
- Pouramir-Dashtmian, F.; Khajeh-Hosseini, M.; Esfahani, M. Improving chilling tolerance of rice seedling by seed priming with salicylic acid. Arch. Agron. Soil Sci. 2014, 60, 1291–1302. [Google Scholar] [CrossRef]
- Afrouz, M.; Sayyed, R.Z.; Fazeli-Nasab, B.; Piri, R.; Almalki, W.; Fitriatin, B.N. Seed bio-priming with beneficial Trichoderma harzianum alleviates cold stress in maize. PeerJ 2023, 11, e15644. [Google Scholar] [CrossRef] [PubMed]
- Vidak, M.; Lazarević, B.; Nekić, M.; Šatović, Z.; Carović-Stanko, K. Effect of hormonal priming and osmopriming on germination of winter savory (Satureja montana L.) natural population under drought stress. Agronomy 2022, 12, 1288. [Google Scholar] [CrossRef]
- Ma, L.; Wei, J.; Han, G.; Sun, X.; Yang, X. Seed osmopriming with polyethylene glycol (PEG) enhances seed germination and seedling physiological traits of Coronilla varia L. under water stress. PLoS ONE 2024, 19, e0303145. [Google Scholar] [CrossRef] [PubMed]
- Patanè, C.; Saita, A.; Tubeileh, A. Seedling emergence response to early sowings in unprimed and osmoprimed seeds of fiber sorghums for energy biomass under semi-arid climate. Ital. J. Agron. 2012, 7, 30. [Google Scholar] [CrossRef]
- Kubala, S.; Wojtyla, Ł.; Quinet, M.; Lechowska, K.; Lutts, S.; Garnczarska, M. Enhanced expression of the proline synthesis gene P5CSA in relation to seed osmopriming improvement of Brassica napus germination under salinity stress. J. Plant Physiol. 2015, 183, 1–12. [Google Scholar] [CrossRef]
- Singha, A.; Soothar, R.K.; Wang, C.; Trujillo Marín, E.E.; Tankari, M.; Hao, W.; Wang, Y. Drought priming alleviated salinity stress and improved water use efficiency of wheat plants. Plant Growth Regul. 2022, 96, 357–368. [Google Scholar] [CrossRef]
- Ozbingol, N.; Corbineau, F.; Côme, D. Responses of tomato seeds to osmoconditioning as related to temperature and oxygen. Seed Sci. Res. 2008, 8, 377–384. [Google Scholar] [CrossRef]
- Nowicki, M.; Nowakowska, M.; Nowak, K.; Szczechura, W.; Kaminski, P. Seed priming and abiotic stress tolerance in carrot: Unraveling the mechanisms of improved germination. PLoS ONE 2025, 20, e0318753. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Jatana, B.S.; Grover, S.; Ram, H.; Baath, G.S. Seed priming: Molecular and physiological mechanisms underlying biotic and abiotic stress tolerance. Agronomy 2024, 14, 2901. [Google Scholar] [CrossRef]
- Heshmati, S.; Amini Dehaghi, M.; Farooq, M.; Wojtyla, Ł.; Maleki, K.; Heshmati, S. Role of melatonin seed priming on antioxidant enzymes and biochemical responses of Carthamus tinctorius L. under drought stress conditions. Plant Stress 2021, 2, 100023. [Google Scholar] [CrossRef]
- Qian, J.; Mo, X.; Wang, Y.; Li, Q. Seed priming with 2,4-epibrassionolide enhances seed germination and heat tolerance in rice by regulating the antioxidant system and plant hormone signaling pathways. Antioxidants 2025, 14, 242. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Gao, G.; Ali, I.; Wu, X.; Tang, M.; Chen, L.; Jiang, L.; Liang, T. Effects of various seed priming on morphological, physiological, and biochemical traits of rice under chilling stress. Front. Plant Sci. 2023, 14, 1146285. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Puthur, J.T. Influence of different seed priming techniques on oxidative and antioxidative responses during the germination of Oryza sativa varieties. Physiol. Mol. Biol. Plants 2020, 26, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, A.; Ghosh, S.; Paul, S.; Mazumdar, S.; Das, G.; Das, S. Pre-treatment of seeds with salicylic acid attenuates cadmium chloride-induced oxidative damages in the seedlings of mungbean (Vigna radiata L. Wilczek). Acta Physiol. Plant. 2016, 38, 130. [Google Scholar] [CrossRef]
- Khepar, V.; Ahuja, R.; Sidhu, A.; Samota, M.K. Nano-sulfides of Fe and Mn efficiently augmented the growth, antioxidant defense system, and metal assimilation in rice seedlings. ACS Omega 2023, 8, 30231–30238. [Google Scholar] [CrossRef]
- Donia, D.T.; Carbone, M. Seed Priming with Zinc Oxide Nanoparticles to Enhance Crop Tolerance to Environmental Stresses. Int. J. Mol. Sci. 2023, 24, 17612. [Google Scholar] [CrossRef]
- Jamil, M.; Ashraf, M.; Rehman, S.; Rha, E.S. Cell membrane stability (CMS): A simple technique to check salt stress alleviation through seed priming with GA3 in canola. In Salinity and Water Stress, 1st ed.; Ashraf, M., Ozturk, M., Athar, H., Eds.; Springer: Dordrecht, The Netherlands, 2009; Volume 44, pp. 167–176. [Google Scholar] [CrossRef]
- Ben Youssef, R.; Jelali, N.; Boukari, N.; Albacete, A.; Martinez, C.; Alfocea, F.P.; Abdelly, C. The efficiency of different priming agents for improving germination and early seedling growth of local Tunisian barley under salinity stress. Plants 2021, 10, 2264. [Google Scholar] [CrossRef] [PubMed]
- Çalık Koç, G.; Rezaei, F.; Kahraman Ilıkkan, Ö.; Bağdat, E.Ş. Effect of seed priming with polyethylene glycol, distilled water, and sorbitol on physical, chemical quality parameters, and nodule microbiota of lentil. Braz. J. Microbiol. 2024, 55, 3997–4008. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Jia, X.; Wang, L.; McGrath, S.P.; Zhu, Y.-G.; Hu, Q.; Zhao, F.-J.; Bank, M.S.; O’Connor, D.; Nriagu, J. Global soil pollution by toxic metals threatens agriculture and human health. Science 2025, 388, 316–321. [Google Scholar] [CrossRef]
- Sneideris, L.C.; Gavassi, M.A.; Campos, M.L.; D’Amico-Damião, V.; Carvalho, R.F. Effects of hormonal priming on seed germination of pigeon pea under cadmium stress. An. Acad. Bras. Cienc. 2015, 87, 1847–1852. [Google Scholar] [CrossRef]
- Karalija, E.; Selović, A. The effect of hydro and proline seed priming on growth, proline and sugar content, and antioxidant activity of maize under cadmium stress. Environ. Sci. Pollut. Res. 2018, 25, 33370–33380. [Google Scholar] [CrossRef]
- Karalija, E.; Selović, A.; Dahija, S.; Demir, A.; Samardžić, J.; Vrobel, O.; Ćavar Zeljković, S.; Parić, A. Use of seed priming to improve Cd accumulation and tolerance in Silene sendtneri, novel Cd hyper-accumulator. Ecotoxicol. Environ. Saf. 2021, 210, 111882. [Google Scholar] [CrossRef]
- Karalija, E.; Subašić, M.; Selović, A. Priming, Cd tolerance, and phytoremediation. In Cadmium Toxicity Mitigation; Jha, A.K., Kumar, N., Eds.; Springer: Cham, Switzerland, 2024; pp. 273–296. [Google Scholar] [CrossRef]
- Ahmed, S.; Amjad, M.; Sardar, R.; Siddiqui, M.H.; Irfan, M. Seed priming with triacontanol alleviates lead stress in Phaseolus vulgaris L. (common bean) through improving nutritional orchestration and morpho-physiological characteristics. Plants 2023, 12, 1672. [Google Scholar] [CrossRef]
- Bankaji, I.; Hammouda, I.B.; Attia, H.; Sleimi, N. Effects of hydro-priming and hormonal priming on seedling growth and seed germination of Cucurbita pepo treated by mercury. Int. J. Life Sci. Eng. 2018, 3, 59–63. [Google Scholar]
- Khan, F.; Hussain, S.; Khan, S.; Geng, M. Seed priming improved antioxidant defense system and alleviated Ni-induced adversities in rice seedlings under N, P, or K deprivation. Front. Plant Sci. 2020, 11, 565647. [Google Scholar] [CrossRef]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Pagano, A.; Macovei, A.; Balestrazzi, A. Molecular dynamics of seed priming at the crossroads between basic and applied research. Plant Cell Rep. 2023, 42, 657–688. [Google Scholar] [CrossRef]
- Ashman, C.; Awty-Carroll, D.; Mos, M.; Robson, P.; Clifton-Brown, J. Assessing seed priming, sowing date, and mulch film to improve the germination and survival of direct-sown Miscanthus sinensis in the United Kingdom. GCB Bioenergy 2018, 10, 612–627. [Google Scholar] [CrossRef]
- Pawar, V.A.; Laware, S.L. Seed priming: A critical review. Int. J. Sci. Res. Biol. Sci. 2018, 5, 94–101. [Google Scholar] [CrossRef]
- Corbineau, F.; Taskiran-Özbingöl, N.; El-Maarouf-Bouteau, H. Improvement of seed quality by priming: Concept and biological basis. Seeds 2023, 2, 101–115. [Google Scholar] [CrossRef]
- El-Sanatawy, A.M.; Ash-Shormillesy, S.M.A.I.; Qabil, N.; Awad, M.F.; Mansour, E. Seed halo-priming improves seedling vigor, grain yield, and water use efficiency of maize under varying irrigation regimes. Water 2021, 13, 2115. [Google Scholar] [CrossRef]
- Ahmed, M.G.U.; Khatun, F.; Islam, Z. Effects of osmotic, thermal and plant growth regulators seed priming on different wheat varieties. J. Bio. Sci. 2021, 29, 111–122. [Google Scholar] [CrossRef]
- Singh, R.; Vij, L.; Kaur, N. Seed priming: Technique, applications and future prospects in vegetable crops. Seed Res. 2024, 52, 41–57. [Google Scholar]
- Nile, S.H.; Thiruvengadam, M.; Wang, Y.; Samynathan, R.; Shariati, M.A.; Rebezov, M.; Nile, A.; Sun, M.; Venkidasamy, B.; Xiao, J.; et al. Nano-priming as emerging seed priming technology for sustainable agriculture—Recent developments and future perspectives. J. Nanobiotechnol. 2022, 20, 254. [Google Scholar] [CrossRef]
- Hasanaklou, N.T.; Mohagheghi, V.; Hasanaklou, H.T.; Ma’mani, L.; Malekmohammadi, M.; Moradi, F.; Dalvand, Y. Seed nano-priming using silica nanoparticles: Effects in seed germination and physiological properties of Stevia rebaudiana Bertoni. Chem. Biol. Technol. Agric. 2023, 10, 96. [Google Scholar] [CrossRef]
- Khalaki, M.A.; Moameri, M.; Lajayer, B.A.; Astatkie, T. Influence of nano-priming on seed germination and plant growth of forage and medicinal plants. Plant Growth Regul. 2021, 93, 13–28. [Google Scholar] [CrossRef]
- Mageshwaran, V.; Gupta, R.; Singh, S.; Sahu, P.K.; Singh, U.B.; Chakdar, H.; Bagul, S.Y.; Paul, S.; Singh, H.V. Endophytic Bacillus subtilis antagonize soil-borne fungal pathogens and suppress wilt complex disease in chickpea plants (Cicer arietinum L.). Front. Microbiol. 2022, 13, 994847. [Google Scholar] [CrossRef] [PubMed]
- Marques da Silva, D.M.; Santos, C.C.; Wagner, F.E.; Martins, L.O.M.; Ozório, J.P.A.; da Silva, O.A.; Ribeiro, D.M.; Scalon, S.P.Q. Seed biopriming with Parachlorella, Bacillus subtilis, and Trichoderma harzianum alleviates the effects of salinity in soybean. BMC Plant Biol. 2024, 24, 1149. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Ebone, L.A.; Caverzan, A.; Tagliari, A.; Chiomento, J.L.T.; Silveira, D.C.; Chavarria, G. Soybean seed vigor: Uniformity and growth as key factors to improve yield. Agronomy 2020, 10, 545. [Google Scholar] [CrossRef]
- Carrillo-Reche, J.; Newton, A.C.; Quilliam, R.S. Using seed respiration as a tool for calculating optimal soaking times for ‘on-farm’ seed priming of barley (Hordeum vulgare). Seed Sci. Res. 2021, 31, 116–124. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed germination and vigor: Ensuring crop sustainability in a changing climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef]
- Kong, C.K.Y.; Lee, R.S.; Hasan, K.; Wong, C.K.F.; Teh, C.Y. Proline priming enhances seed vigour and biochemical attributes of rice (Oryza sativa L.) during germination. Trop. Life Sci. Res. 2024, 35, 149–163. [Google Scholar] [CrossRef]
- Matsushima, K.I.; Sakagami, J.I. Effects of seed hydropriming on germination and seedling vigour during emergence of rice under different soil moisture conditions. Am. J. Plant Sci. 2013, 4, 1584–1593. [Google Scholar] [CrossRef]
- Saux, M.; Bleys, B.; André, T.; Bailly, C.; El-Maarouf-Bouteau, H. A correlative study of sunflower seed vigor components as related to genetic background. Plants 2020, 9, 386. [Google Scholar] [CrossRef]
- McDonald, M.B. Seed deterioration: Physiology, repair and assessment. Seed Sci. Technol. 1999, 27, 177–237. [Google Scholar]
- Walters, C.; Ballesteros, D.; Vertucci, V.A. Structural mechanics of seed deterioration: Standing the test of time. Plant Sci. 2005, 169, 545–550. [Google Scholar] [CrossRef]
- Yan, H.; Jia, S.; Mao, P. Melatonin priming alleviates aging-induced germination inhibition by regulating β-oxidation, protein translation, and antioxidant metabolism in oat (Avena sativa L.) seeds. Int. J. Mol. Sci. 2020, 21, 1898. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Rao, D.; Sushma, M.K.; Choudhary, R.; Yadav, S.K. Principles and methods of seed vigour testing. In Training Manual, Division of Seed Science and Technology; ICAR-IARI: New Delhi, India, 2024; pp. 167–171. Available online: https://www.researchgate.net/publication/379083320 (accessed on 3 June 2025).
- Shu, K.; Liu, X.D.; Xie, Q.; He, Z.H. Two faces of one seed: Hormonal regulation of dormancy and germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, H.; Mor, V.S.; Sharma, S.; Khan, M.; Bhuker, A.; Singh, V.; Yadav, J.; Sangwan, S.; Singh, J.; Yashveer, S.; et al. Optimization of ‘on farm’ hydropriming conditions in wheat: Soaking time and water volume have interactive effects on seed performance. PLoS ONE 2023, 18, e0280962. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; He, A.; Peng, S.; Huang, J.; Cui, K.; Nie, L. The effect of storage condition and duration on the deterioration of primed rice seeds. Front. Plant Sci. 2018, 9, 172. [Google Scholar] [CrossRef]
- Tu, K.; Cheng, Y.; Pan, T.; Wang, J.; Sun, Q. Effects of seed priming on vitality and preservation of pepper seeds. Agriculture 2022, 12, 603. [Google Scholar] [CrossRef]
- Rehman, H.U.; Iqbal, H.; Basra, S.M.A.; Afzal, I.; Farooq, M.; Wakeel, A.; Wang, N. Seed priming improves early seedling vigor, growth and productivity of spring maize. J. Integr. Agric. 2015, 14, 1745–1754. [Google Scholar] [CrossRef]
- Basra, S.M.A.; Farooq, M.; Tabassum, R. Physiological and biochemical aspects of seed vigor enhancement treatments in fine rice (Oryza sativa L.). Seed Sci. Technol. 2006, 34, 507–518. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Rehman, H.U.; Basra, S.M.A. Priming-induced hormonal regulation and seedling vigor in rice under normal field conditions. Agronomy 2019, 9, 234. [Google Scholar] [CrossRef]
- Alam, A.; Ullah, H.; Thuenprom, N.; Tisarum, R.; Cha-um, S.; Datta, A. Seed priming with salicylic acid enhances growth, physiological traits, fruit yield, and quality parameters of cantaloupe under water-deficit stress. S. Afr. J. Bot. 2022, 150, 1–12. [Google Scholar] [CrossRef]
- Islam, A.T.; Ullah, H.; Himanshu, S.K.; Tisarum, R.; Cha-um, S.; Datta, A. Effect of salicylic acid seed priming on morpho-physiological responses and yield of baby corn under salt stress. Sci. Hortic. 2022, 304, 111304. [Google Scholar] [CrossRef]
- Chen, K.; Arora, R. Priming memory invokes seed stress-tolerance. Environ. Exp. Bot. 2013, 94, 33–45. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Pre-sowing seed treatment—A shotgun approach to improve germination, plant growth, and crop yield under saline and non-saline conditions. Adv. Agron. 2005, 88, 223–271. [Google Scholar] [CrossRef]
- Chakma, R.; Saekong, P.; Biswas, A.; Ullah, H.; Datta, A. Growth, fruit yield, quality, and water productivity of grape tomato as affected by seed priming and soil application of silicon under drought stress. Agric. Water Manag. 2021, 256, 107055. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Kumar, J.S.; Suprasanna, P. Seed ‘primeomics’: Plants memorize their germination under stress. Biol. Rev. 2021, 96, 1723–1743. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Baskin, C.C. Seed priming with phytohormones: An effective approach for the mitigation of abiotic stress. Plants 2021, 10, 37. [Google Scholar] [CrossRef]
- Eisvand, H.R.; Tavakkol-Afshari, R.; Sharifzadeh, F.; Maddah Arefi, H.; Hesamzadeh Hejazi, S.M. Effects of hormonal priming and drought stress on activity and isozyme profiles of antioxidant enzymes in deteriorated seed of tall wheatgrass (Agropyron elongatum Host). Seed Sci. Technol. 2010, 38, 280–297. [Google Scholar] [CrossRef]
- Rivas, M.Á.; Friero, I.; Alarcón, M.V.; Salguero, J. Auxin-cytokinin balance shapes maize root architecture by controlling primary root elongation and lateral root development. Front. Plant Sci. 2022, 13, 836592. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M.; Jamil, A.; Ur-Rehman, S. Does seed priming induce changes in the levels of some endogenous plant hormones in hexaploid wheat plants under salt stress? J. Integr. Plant Biol. 2006, 48, 181–189. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Bakhtavar, M.A.; Afzal, I.; Basra, S.M.; Ahmad, A.U.; Noor, M.A. Physiological strategies to improve the performance of spring maize (Zea mays L.) planted under early and optimum sowing conditions. PLoS ONE 2015, 10, e0124441. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Wang, L.; Abbas, F.; Khan, I.; Saleem, M.F.; Akhtar, M.; Wang, X. Exogenously applied methyl jasmonate improves the drought tolerance in wheat imposed at early and late developmental stages. Acta Physiol. Plant. 2016, 38, 25. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Lima, E.C.; Zhang, S.; Shaheen, S.M.; Rinklebe, J. Global soil pollution by toxic elements: Current status and future perspectives on the risk assessment and remediation strategies—A review. J. Hazard. Mater. 2021, 417, 126039. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Castro, I.; Molina, L.; Prieto-Fernández, M.Á.; Segura, A. Past, present and future trends in the remediation of heavy-metal contaminated soil—Remediation techniques applied in real soil-contamination events. Heliyon 2023, 9, e16692. [Google Scholar] [CrossRef]
- Abubakar, M.; Alghanem, S.M.S.; Alhaithloul, H.A.S.; Alsudays, I.M.; Farid, M.; Zubair, M.; Farid, S.; Rizwan, M.; Yong, J.W.H.; Abeed, A.H.A. Microwave seed priming and ascorbic acid assisted phytoextraction of heavy metals from surgical industry effluents through spinach. Ecotoxicol. Environ. Saf. 2024, 282, 116731. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, L.; Wen, Z.; Fu, Y.; Liu, Q.; Xu, S.; Li, Z.; Liu, C.; Yu, C.; Feng, Y. Effects of intercropping on safe agricultural production and phytoremediation of heavy metal-contaminated soils. Sci. Total Environ. 2023, 875, 162700. [Google Scholar] [CrossRef] [PubMed]
- Nciizah, A.D.; Rapetsoa, M.C.; Wakindiki, I.I.; Zerizghy, M.G. Micronutrient seed priming improves maize (Zea mays) early seedling growth in a micronutrient deficient soil. Heliyon 2020, 6, e04766. [Google Scholar] [CrossRef]
- Tamindžić, G.; Ignjatov, M.; Milošević, D.; Nikolić, Z.; Kostić Kravljanac, L.; Jovičić, D.; Dolijanović, Ž.; Savić, J. Seed priming with zinc improves field performance of maize hybrids grown on calcareous chernozem. Ital. J. Agron. 2021, 16, 1795. [Google Scholar] [CrossRef]
- Jarrar, H.; El-Keblawy, A. Seed-enhancement technologies promote direct seeding and overcoming biotic and abiotic barriers in degraded dryland ecosystem. Environ. Sci. Proc. 2022, 16, 1. [Google Scholar] [CrossRef]
- Liniger, H.P.; Mekdaschi Studer, R.; Hauert, C.; Gurtner, M. Sustainable Land Management in Practice: Guidelines and Best Practices for Sub-Saharan Africa; TerrAfrica: Johannesburg, South Africa; WOCAT: Bern, Switzerland; FAO: Rome, Italy, 2011. [Google Scholar]
- FAO. Training Manual—Good Agricultural Practices (GAP) Guidelines; Vols. 1 and 2; FAO: Nay Pyi Taw, Myanmar, 2022. [Google Scholar] [CrossRef]
- Harris, D.; Pathan, A.K.; Gothkar, P.; Joshi, A.; Chivasa, W.; Nyamudeza, P. On-farm seed priming: Using participatory methods to revive and refine a key technology. Agric. Syst. 2001, 69, 151–164. [Google Scholar] [CrossRef]
- Sissoko, P.; Guindo, S.S.; Togola, S.; Dembélé, B.D.; Grimsby, L.K.; Aune, J.B. Effect of Adoption of Climate-Smart-Agriculture Technologies on Cereal Production, Food Security and Food Diversity in Central Mali. Agriculture 2023, 13, 1196. [Google Scholar] [CrossRef]
- Chopra, P.; Sapia, N.; Karami, O.; Kumar, P.; Honys, D.; Colombo, L.; Mendes, M.; Benhamed, M.; Fotopoulos, V.; Lieberman-Lazarovich, M.; et al. Priming thermotolerance: Unlocking heat resilience for climate-smart crops. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2025, 380, 20240234. [Google Scholar] [CrossRef]
- Catiempo, R.L.; Photchanachai, S.; Powell, A.F.; Strickler, S.R.; Wongs-Aree, C. Transcriptome analysis suggests the role of expansin genes in the improved germination of sunflower (Helianthus annuus L.) seeds after hydropriming. Crop Sci. 2024, 64, 1862–1873. [Google Scholar] [CrossRef]
- Chen, H.; Chu, P.; Zhou, Y.; Li, Y.; Liu, J.; Ding, Y.; Tsang, E.W.; Jiang, L.; Wu, K.; Huang, S. Overexpression of AtOGG1, a DNA glycosylase/AP lyase, enhances seed longevity and abiotic stress tolerance in Arabidopsis. J. Exp. Bot. 2012, 63, 4107–4121. [Google Scholar] [CrossRef]
- Sharma, S.N.; Maheshwari, A. Expression patterns of DNA repair genes associated with priming small and large chickpea (Cicer arietinum) seeds. Seed Sci. Technol. 2015, 43, 1–12. [Google Scholar] [CrossRef]
- Kiran, K.R.; Deepika, V.B.; Swathy, P.S.; Prasad, K.; Kabekkodu, S.P.; Murali, T.S.; Satyamoorthy, K.; Muthusamy, A. ROS-dependent DNA damage and repair during germination of NaCl primed seeds. J. Photochem. Photobiol. B Biol. 2020, 213, 112050. [Google Scholar] [CrossRef]
- Forti, C.; Shankar, A.; Singh, A.; Balestrazzi, A.; Prasad, V.; Macovei, A. Hydropriming and biopriming improve Medicago truncatula seed germination and upregulate DNA repair and antioxidant genes. Genes 2020, 11, 242. [Google Scholar] [CrossRef]
- Szlachtowska, Z.; Rurek, M. Plant dehydrins and dehydrin-like proteins: Characterization and participation in abiotic stress response. Front. Plant Sci. 2023, 14, 1213188. [Google Scholar] [CrossRef]
- Catusse, J.; Meinhard, J.; Job, C.; Strub, J.-M.; Fischer, U.; Peststova, E.; Westhof, P.; Van Dorsselaer, A.; Job, D. Proteomics reveals potential biomarkers of seed vigor in sugarbeet. Proteomics 2011, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
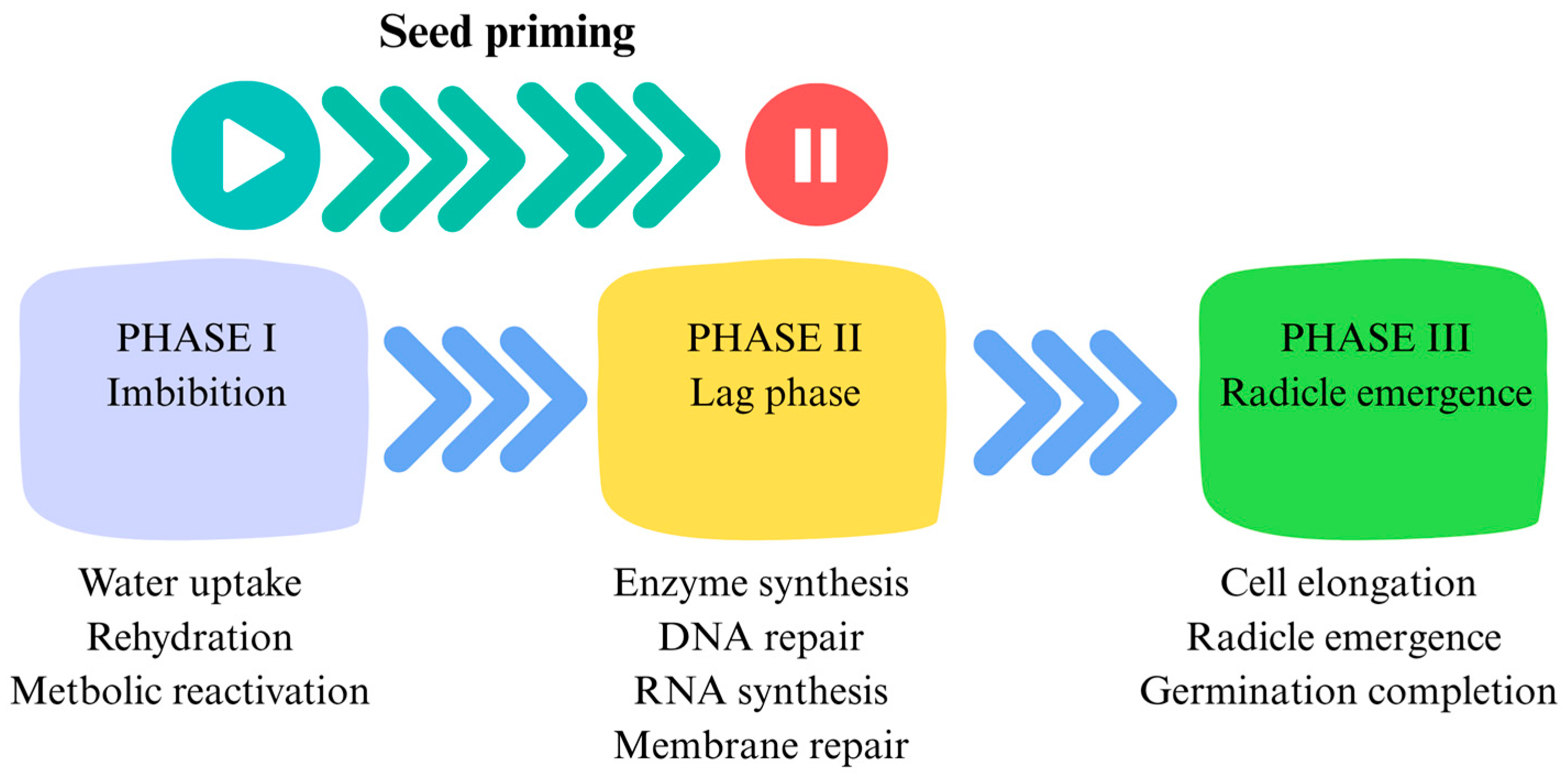

| Crop | Priming Method | Seed and Vigor Improvement | Synchrony of Germination | Seedling Establishment |
|---|---|---|---|---|
| Tomato (Solanum lycopersicum) [10] | Osmopriming (PEG −1.0 MPa) | ATP/ADP ratio, faster radicle emergence | Narrower emergence window, uniform stand | Improved shoot/root ratio |
| Leek (Allium porrum) [47] | Osmopriming (PEG −1.5 MPa) | Respiration rate, energy metabolism | Synchronized emergence across seed lot | Robust seedling biomass |
| Carrot (Daucus carota) [47] | Osmopriming (PEG −1.0 MPa) | Enhanced enzymatic activity, faster mobilization of reserves | Reduced variation in emergence time | Stronger hypocotyl elongation |
| Maize (Zea mays) [48] | Hydropriming and hardening | Shoot and root length, fresh and dry weight | Consistent establishment even in high-density plots | Better field emergence |
| Lettuce (Lactuca sativa) [47] | Osmopriming (PEG −1.2 MPa) | Improved membrane repair, reduced ROS damage | High uniformity in greenhouse trays | Vigorous transplant-ready seedlings |
| Sunflower (Helianthus annuus) [47] | Osmopriming (PEG −1.5 MPa) | Catalase and glutathione reductase activity | Stable emergence timing | Higher seedling survival and growth |
| Spinach (Spinacia oleracea) [47] | Osmopriming (PEG −0.6 MPa) | Antioxidant enzymes, faster germination | Uniform emergence in nursery conditions | Robust seedling architecture |
| Rice (Oryza sativa) [10] | Hydropriming (12 h in water) | Amylase, improved reserve mobilization | Improved germination timing under nursery set-ups | Improved seedling biomass and shoot elongation |
| Barley (Hordeum vulgare) [47] | Hydropriming (30 °C, 52% moisture) | ABA levels, cell cycle activity | Faster and synchronized emergence | Stronger seedlings with early vigor |
| Sugar beet (Beta vulgaris) [47] | Osmopriming (PEG −2.0 MPa) | Respiration rate, improved membrane repair | Uniform emergence even under mechanical sowing | Better stand density in rows |
| Pepper (Capsicum annuum) [10] | Osmopriming (PEG −1.5 MPa) | Germination enzymes, improved uniformity | Synchronized emergence across variable seeds | Stronger hypocotyl and early leaf development |
| Primrose (Primula spp.) [48] | Osmopriming (PEG −1.5 MPa) | Homogenized emergence across genotypes | Consistent emergence among different colors | Uniform seedling size for transplanting |
| Wheat (Triticum aestivum) [49] | Osmopriming (PEG −1.0 MPa, 12 h) | Metabolic enzyme activity and seedling dry weight | Reduced time to 50% germination (T50) | Higher early growth vigor and uniform stands |
| Chickpea (Cicer arietinum) [50] | Hydropriming (12 h soak in water) | Germination energy and root/shoot length | Uniform emergence across replicates | Stronger root development and early shoot expansion |
| Cucumber (Cucumis sativus) [50] | Osmopriming (PEG −1.2 MPa) | Enhanced SOD and catalase activity | Synchronized germination in nursery trays | Improved seedling fresh and dry mass |
| Okra (Abelmoschus esculentus) [50] | Hydropriming (6 h at 25 °C) | Improved membrane integrity and reserve utilization | Reduced variability in emergence time | Robust seedlings with uniform morphology |
| Eggplant (Solanum melongena) [50] | Osmopriming with KNO3 (1%) | Antioxidant potential and seedling establishment rate | Faster and more uniform germination | Improved seedling length and biomass |
| Rice (Oryza sativa) [30] | Nanopriming (FeS and MnS nanoparticles) | Improved antioxidant defense and metal assimilation | Enhanced germination kinetics | Increased seedling biomass and shoot elongation |
| Various crops (e.g., rice, wheat, maize) [51] | Nanopriming with metal and metal oxide nanoparticles | Improved enzymatic activity, water uptake, stress tolerance | Accelerated and more uniform germination | Enhanced seedling vigor and abiotic stress resilience |
| Stevia (Stevia rebaudiana) [52] | Nanopriming with silica nanoparticles | Increased germination percentage, chlorophyll content, enzymatic activity | Reduced variation in germination timing | Improved physiological status and early growth performance |
| Forage and medicinal plants (e.g., Trigonella, Nigella, Plantago) [53] | Nanopriming with various nanoparticles (ZnO, TiO2, Fe3O4) | Boosted antioxidant capacity, seed enzyme activity, stress resilience | Accelerated and synchronized germination under abiotic stress | Improved biomass accumulation and stress-adaptive traits |
| Chickpea (Cicer arietinum) [54] | Biopriming with Bacillus subtilis | Increased root length, improved stress tolerance | More synchronized germination under saline conditions | Stronger and healthier seedlings |
| Tomato (Solanum lycopersicum “Micro-Tom”) [2] | Biopriming with Paraburkholderia phytofirmans PsJN | Improved chlorophyll content, reduced oxidative stress, enhanced shoot and root growth under Ni stress | Better uniformity under nickel stress | Robust seedlings with enhanced tolerance and physiological performance |
| Soybean (Glycine max) [55] | Biopriming with Parachlorella, B. subtilis, T. harzianum | Enhanced root and shoot growth, reduced salinity-induced stress markers | More uniform germination under salt stress | Increased seedling vigor and salt tolerance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasanović, M.; Durmić-Pašić, A.; Karalija, E. Seed Priming Beyond Stress Adaptation: Broadening the Agronomic Horizon. Agronomy 2025, 15, 1829. https://doi.org/10.3390/agronomy15081829
Hasanović M, Durmić-Pašić A, Karalija E. Seed Priming Beyond Stress Adaptation: Broadening the Agronomic Horizon. Agronomy. 2025; 15(8):1829. https://doi.org/10.3390/agronomy15081829
Chicago/Turabian StyleHasanović, Mujo, Adaleta Durmić-Pašić, and Erna Karalija. 2025. "Seed Priming Beyond Stress Adaptation: Broadening the Agronomic Horizon" Agronomy 15, no. 8: 1829. https://doi.org/10.3390/agronomy15081829
APA StyleHasanović, M., Durmić-Pašić, A., & Karalija, E. (2025). Seed Priming Beyond Stress Adaptation: Broadening the Agronomic Horizon. Agronomy, 15(8), 1829. https://doi.org/10.3390/agronomy15081829






