Regional Patterns in Weed Composition of Maize Fields in Eastern Hungary: The Balance of Environmental and Agricultural Factors
Abstract
1. Introduction
2. Materials and Methods
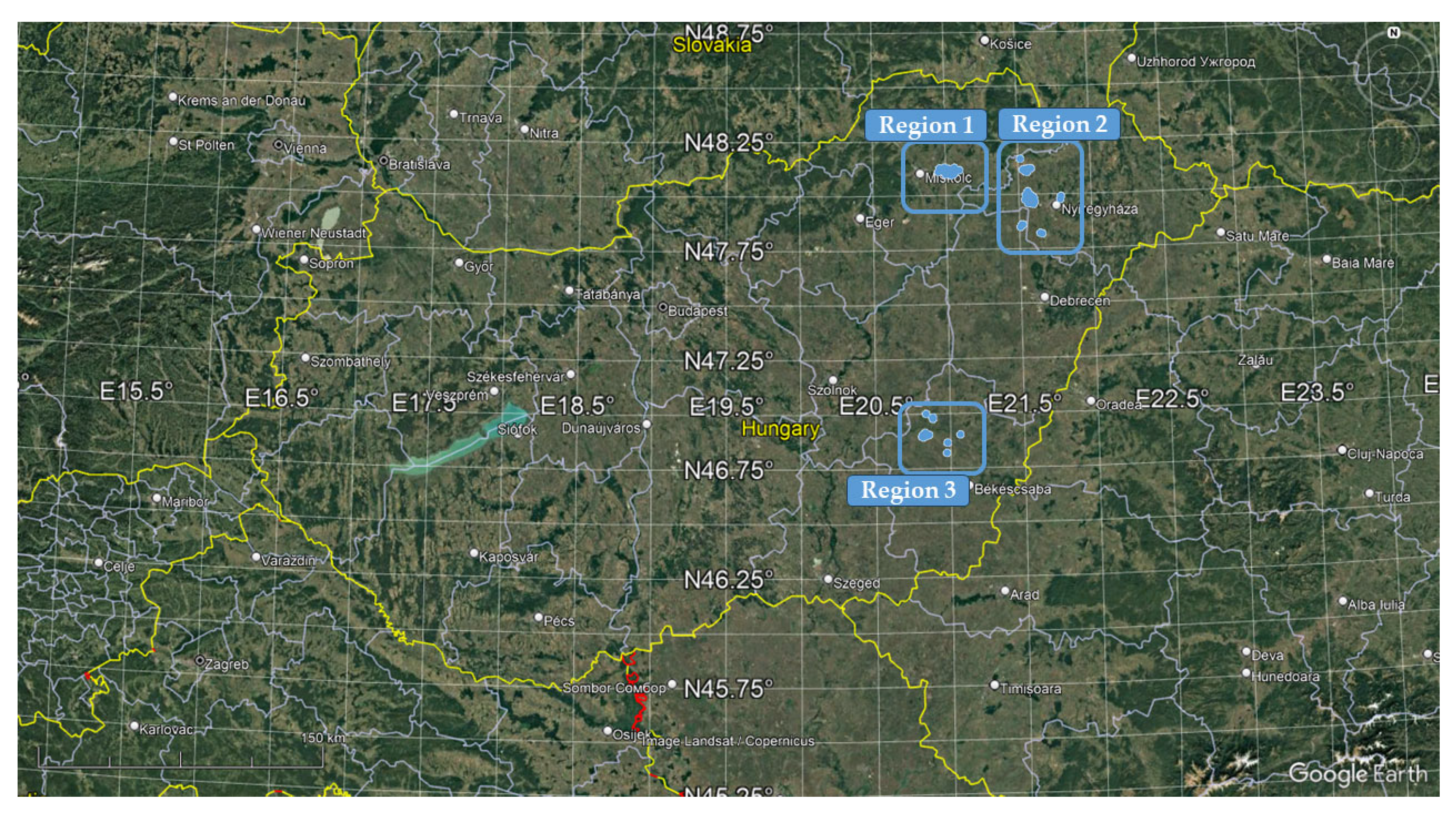
2.1. Description of the Regions Concerned
2.2. Methodology of Data Collection
2.3. Data Preparation
2.4. Statistical Analysis Procedure
3. Results
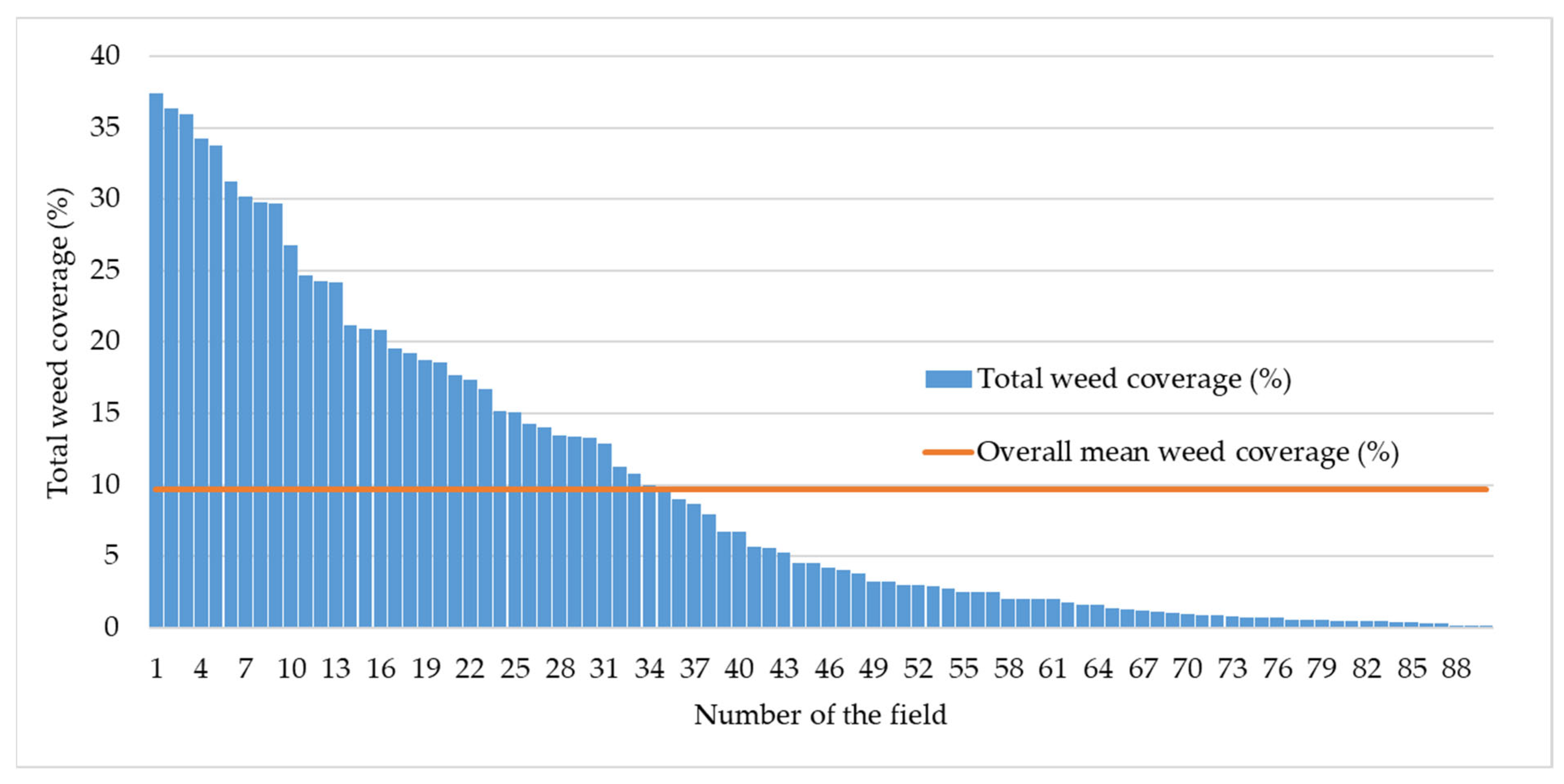
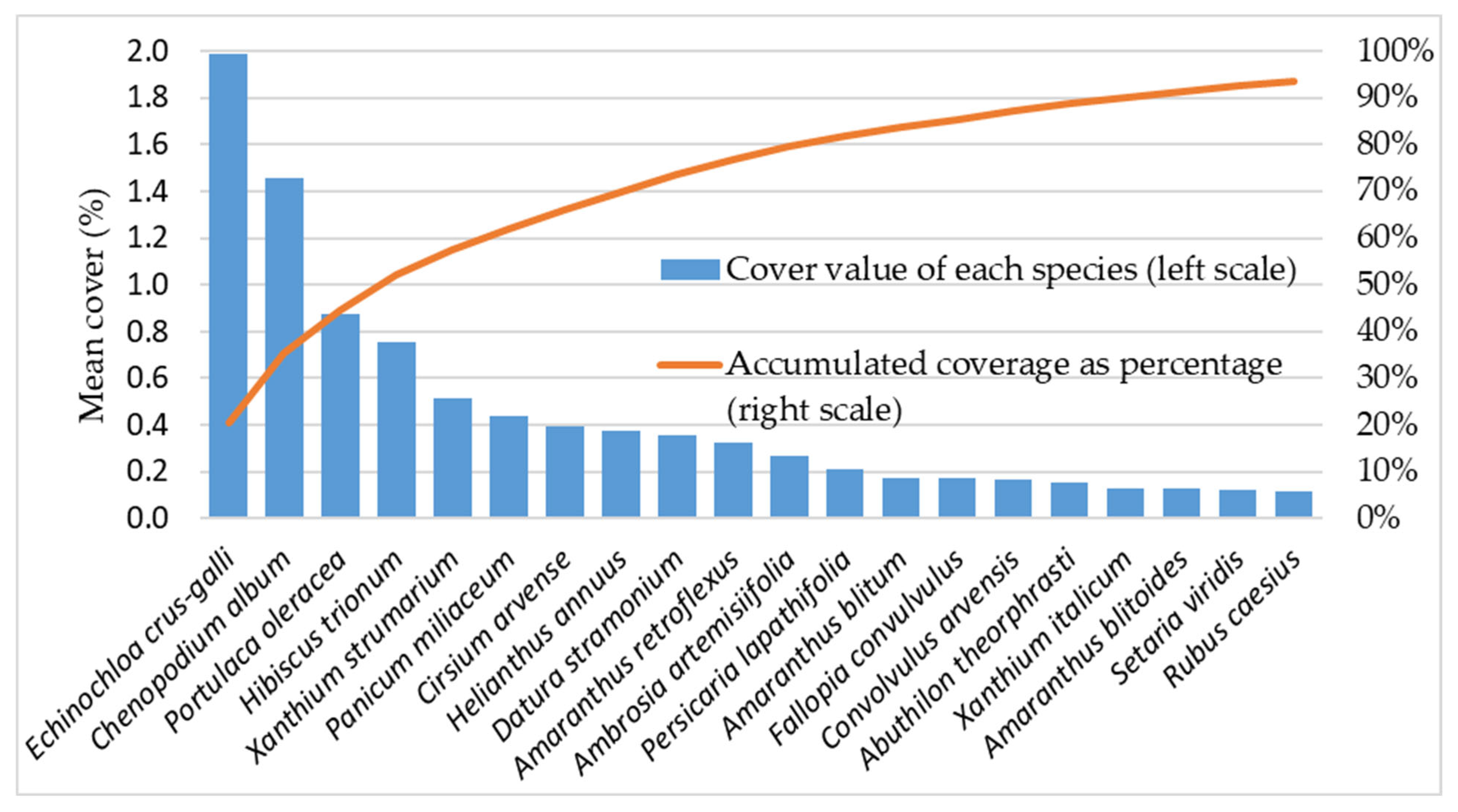
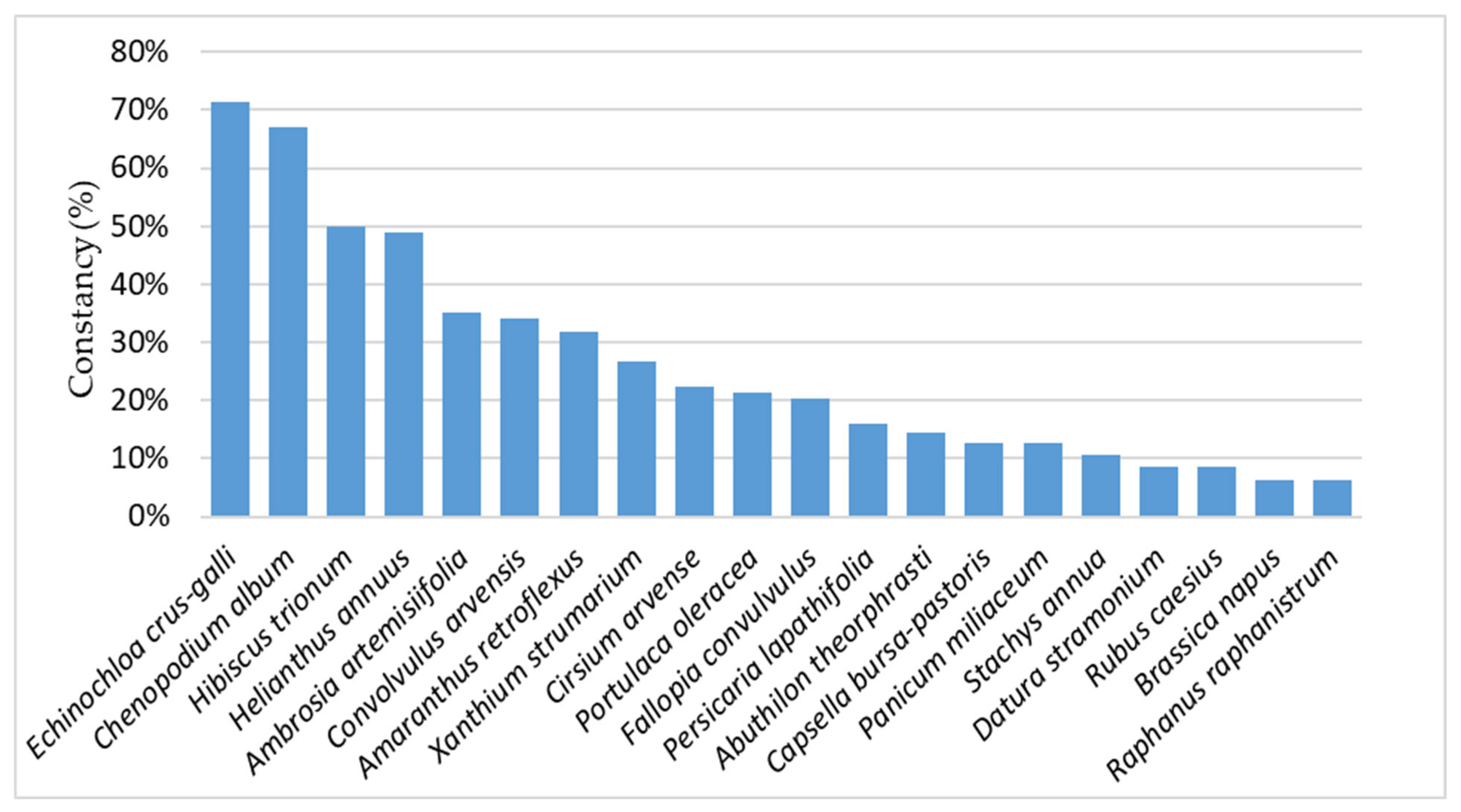
3.1. Weed Vegetation Characterization Across the Examined Regions
3.2. Influence of Environmental and Management Factors on Total Weed Cover, Species Richness, and Diversity in Surveyed Maize Fields
3.3. Impact of Environmental and Management Variables on Weed Species Composition
4. Discussion
4.1. Weed Composition of Maize Fieds Studied
4.2. Effect of Soil Conditions on Weed Population
4.3. Effect of Environmental Factors on Weed Population
4.4. Effect of Farming Factors on Weed Population
4.5. Connections Between Explanatory Variables
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| Taxonomy A | Predominant Photosynthetic Pathway B | Life- form C | Geographical Distribution | Mean Coverage (%) | Constancy (%) | |||
|---|---|---|---|---|---|---|---|---|
| Scientific Name | Family | Region 1 | Region 2 | Region 3 | ||||
| Abuthilon theorphrasti | Malvaceae | C3 | SA | × | × | × | 0.15 | 14 |
| Amaranthus blitoides | Amaranthaceae | C4 | SA | × | × | 0.13 | 3 | |
| Amaranthus blitum | Amaranthaceae | C4 | SA | × | 0.18 | 5 | ||
| Amaranthus chlorostachis | Amaranthaceae | C4 | SA | × | 0.01 | 2 | ||
| Amaranthus retroflexus | Amaranthaceae | C4 | SA | × | × | × | 0.33 | 32 |
| Ambrosia artemisiifolia | Asteraceae | C3 | SA | × | × | × | 0.27 | 35 |
| Aristolochia clematitis | Aristolochiaceae | C3 | G | × | 0.04 | 4 | ||
| Asclepias syriaca | Apocynaceae | C3 | G | × | <0.01 | 1 | ||
| Brassica napus | Brassicaceae | C3 | WA | × | × | × | 0.02 | 6 |
| Calamagrostis epigeios | Poaceae | C3 | G | × | 0.01 | 2 | ||
| Cannabis sativa | Cannabaceae | C3 | SA | × | 0.02 | 2 | ||
| Capsella bursa-pastoris | Brassicaceae | C3 | WA | × | × | × | 0.09 | 13 |
| Chenopodium album | Amaranthaceae | C3–C4 D | SA | × | × | × | 1.46 | 67 |
| Chenopodium hybridum | Amaranthaceae | C3 | SA | × | × | × | 0.02 | 4 |
| Chenopodium polyspermum | Amaranthaceae | C3 | SA | × | 0.02 | 5 | ||
| Cirsium arvense | Asteraceae | C3 | G | × | × | 0.39 | 22 | |
| Convolvulus arvensis | Convolvulaceae | C3 | G | × | × | × | 0.16 | 34 |
| Datura stramonium | Solanaceae | C3 | SA | × | × | 0.36 | 9 | |
| Echinochloa crus-galli | Poaceae | C4 | SA | × | × | × | 1.99 | 71 |
| Equisetum arvense | Equisetaceae | C3 | G | × | × | 0.04 | 4 | |
| Fallopia convolvulus | Polygonaceae | C3 | SA | × | × | × | 0.17 | 20 |
| Galinsoga parviflora | Asteraceae | C3 | SA | × | <0.01 | 1 | ||
| Galium aparine | Rubiaceae | C3 | WA | × | 0.06 | 2 | ||
| Helianthus annuus | Asteraceae | C3 | SA | × | × | × | 0.37 | 49 |
| Hibiscus trionum | Malvaceae | C3 | SA | × | × | × | 0.76 | 50 |
| Iva xanthiifolia | Asteraceae | C3 | SA | × | 0.01 | 4 | ||
| Lamium purpureum | Lamiaceae | C3 | WA | × | <0.01 | 2 | ||
| Lathyrus tuberosus | Solanaceae | C3 | G | × | 0.01 | 5 | ||
| Medicago lupulina | Fabaceae | C3 | SA | × | 0.00 | 2 | ||
| Panicum miliaceum | Poaceae | C4 | SA | × | × | × | 0.44 | 13 |
| Papaver rhoeas | Papaveraceae | C3 | WA | × | <0.01 | 1 | ||
| Persicaria amphibia | Polygonaceae | C3 | G | × | <0.01 | 1 | ||
| Persicaria lapathifolia | Polygonaceae | C3 | SA | × | × | 0.21 | 16 | |
| Persicaria maculosa | Polygonaceae | C3 | SA | × | <0.01 | 1 | ||
| Phalaris canariensis | Poaceae | C3 | SA | × | <0.01 | 1 | ||
| Pisum sativum | Fabaceae | C3 | WA | × | 0.02 | 3 | ||
| Polygonum aviculare | Polygonaceae | C3 | SA | × | × | 0.03 | 4 | |
| Portulaca oleracea | Portulacaceae | C4 | SA | × | × | 0.87 | 21 | |
| Raphanus raphanistrum | Fabaceae | C3 | SA | × | × | 0.02 | 6 | |
| Rubus caesius | Rosaceae | C3 | G | × | × | 0.12 | 9 | |
| Setaria viridis | Poaceae | C4 | SA | × | × | 0.12 | 6 | |
| Silybum marianum | Asteraceae | C3 | HT | × | 0.01 | 1 | ||
| Solanum dulcamara | Solanaceae | C3 | Ph | × | 0.06 | 2 | ||
| Sonchus asper | Asteraceae | C3 | SA | × | <0.01 | 1 | ||
| Stachys annua | Lamiaceae | C3 | SA | × | × | 0.03 | 11 | |
| Stellaria media | Caryophyllaceae | C3 | WA | × | <0.01 | 1 | ||
| Tripleurospermum inodorum | Asteraceae | C3 | SA | × | 0.04 | 4 | ||
| Triticum aestivum | Poaceae | C3 | WA | × | 0.01 | 4 | ||
| Veronica hederifolia | Scrophulariaceae | C3 | WA | × | <0.01 | 1 | ||
| Xanthium italicum | Asteraceae | C3 | SA | × | × | 0.13 | 5 | |
| Xanthium strumarium | Asteraceae | C3 | SA | × | × | × | 0.51 | 27 |
References
- FAOSTAT Database. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 28 May 2025).
- Teasdale, J.R.; Cavigelli, M.A. Subplots facilitate assessment of corn yield losses from weed competition in a long-term systems experiment. Agron. Sustain. Dev. 2010, 30, 445–453. [Google Scholar] [CrossRef]
- Hunyadi, K.; Béres, I.; Kazinczi, G. Gyomnövények, Gyomirtás, Gyombiológia; Mezőgazda Kiadó: Budapest, Hungary, 2011; pp. 24–28. (In Hungarian) [Google Scholar]
- Gracza, L.; Gyulai, B.; Novák, R.; Szabó, L.; Simon, J.; Lang, B.; Doma, C.S.; Nagy, M.; Kovács, A.; Grünwaldné Almási, A.; et al. Egyes szulfonil-karbamidokkal szemben rezisztencia gyanús fenyércirok (Sorghum halepense L.) populációk vizsgálata Magyarországon. Magy. Gyomkutatás és Technológia 2015, 16, 76–78, (In Hungarian with an English Summary). [Google Scholar]
- Novák, R.; Magyar, M.; Simon, G.; Kadaravek, B.; Kadaravekné Guttyán, A.; Blazsek, K.; Erdélyi, K.; Farkas, G.; Gyulai, B.; Hornyák, A.; et al. A Hatodik Országos Szántóföldi Gyomfelvételezés előzetes eredményei. Magy. Gyomkutatás és Technológia 2019, 20, 55–58, (In Hungarian with an English Summary). [Google Scholar]
- Andreasen, C.; Streibig, J.C.; Haas, H. Soil properties affecting the distribution of 37 weed species in Danish fields. Weed Res. 1991, 31, 181–187. [Google Scholar] [CrossRef]
- Caussanel, J.P. Nuisibilite’ et seuil de nuisibilite’ des mauvaises herbes dans une culture annuelle: Situation de concurrence bispe´cifique. Agronomie 1989, 9, 219–240. [Google Scholar] [CrossRef]
- Dale, M.R.T.; Thomas, A.G.; John, E.A. Environmental factors including management practices as correlates of weed community composition in spring seeded crops. Can. J. Bot. 1992, 70, 1931–1939. [Google Scholar] [CrossRef]
- Boutin, C.; Baril, A.; Martin, P.A. Plant diversity in crop fields and woody hedgerows of organic and conventional farms in contrasting landscapes. Agric. Ecosyst. Environ. 2008, 123, 185–193. [Google Scholar] [CrossRef]
- Pysek, P.; Leps, J. Response of a weed community to nitrogen fertilization: A multivariate analysis. Veg. Sci. 1991, 2, 237–244. [Google Scholar] [CrossRef]
- Andersson, T.N.; Milberg, P. Weed flora and the relative importance of site, crop, crop rotation, and nitrogen. Weed Sci. 1998, 46, 30–38. [Google Scholar] [CrossRef]
- Lososova, Z.; Chytry, M.; Cimalova, S.; Kropac, Z.; Otypkova, Z.; Pysek, P.; Tichy, L. Weed vegetation of arable land in Central Europe: Gradients of diversity and species composition. J. Veg. Sci. 2004, 15, 415–422. [Google Scholar] [CrossRef]
- Fried, G.; Petit, S.; Reboud, X. A specialist-generalist classification of the arable flora and its response to changes in agricultural practices. BMC Ecol. 2010, 10, 20. [Google Scholar] [CrossRef]
- Fried, G.; Norton, L.R.; Reboud, X. Environmental and management factors determining weed species composition and diversity in France. Agric. Ecosyst. Environ. 2008, 128, 68–76. [Google Scholar] [CrossRef]
- Weaver, S.E.; Hamill, A.S. Effects of Soil pH on Competitive Ability and Leaf Nutrient Content of Corn (Zea mays L.) and Three Weed Species. Weed Sci. 1985, 33, 447–451. [Google Scholar] [CrossRef]
- Pinke, G.; Karácsony, P.; Czúcz, B.; Botta-Dukát, Z. When herbicides don’t really matter: Weed species composition of oil pumpkin (Cucurbita pepo L.) fields in Hungary. Crop Prot. 2018, 110, 236–244. [Google Scholar] [CrossRef]
- Pinke, G.; Karácsony, P.; Czúcz, B.; Botta-Dukát, Z. Environmental and land-use variables determining the abundance of Ambrosia artemisiifolia in arable fields in Hungary. Presila 2011, 83, 219–235. [Google Scholar]
- Evans, S.P.; Knezevic, S.Z.; Lindquist, J.L.; Shapiro, C.A. Influence of nitrogen and duration of weed interference on corn growth and development. Weed Sci. 2003, 51, 546–556. [Google Scholar] [CrossRef]
- Yin, L.C.; Cai, Z.C.; Zhong, W.H. Changes in weed community diversity of maize crops due to long-term fertilization. Crop Protect. 2006, 25, 910–914. [Google Scholar] [CrossRef]
- Simard, M.J.; Ziadi, N. Weed communities after decades of mineral fertilization and tillage treatments in a corn—soybean rotation. Weed Technol. 2024, 38, e5. [Google Scholar] [CrossRef]
- Légère, A.; Stevenson, F.C.; Ziadi, N. Contrasting responses of weed communities and crops to 12 years of tillage and fertilization treatments. Weed Technol. 2008, 22, 309–317. [Google Scholar] [CrossRef]
- Swanton, C.J.; Shrestha, A.; Roy, R.C.; Ball-Coelho, B.R.; Knezevic, S.Z. Effect of tillage systems, N, and cover crop on the composition of weed flora. Weed Sci. 1999, 47, 454–461. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Singh, R.G.; Mahajan, G. Ecology and management of weeds under conservation agriculture: A review. Crop Protect. 2012, 38, 57–65. [Google Scholar] [CrossRef]
- Locke, M.A.; Reddy, K.N.; Zablotowicz, R.M. Weed management in conservation crop production systems. Weed Biol. Manag. 2002, 2, 123–132. [Google Scholar] [CrossRef]
- Buhler, D.D.; Hatfield, J.L.; Stewart, B.A. Tillage systems and weed population dynamics and management. In Integrated Weed and Soil Management; CRC Press: Boca Raton, FL, USA, 1998; pp. 223–246. [Google Scholar]
- Buhler, D.D.; Oplinger, E.S. Influence of tillage systems on annual weed densities and control in solid-seeded soybean (Glycine max). Weed Sci. 1990, 38, 158–165. [Google Scholar] [CrossRef]
- Das, T.K.; Behera, B.; Nath, C.P.; Ghosh, S.; Sen, S.; Raj, R.; Ghosh, S.; Sharma, A.R.; Yaduraju, N.T.; Nalia, A.; et al. Herbicides use in crop production: An analysis of cost-benefit, non-target toxicities and environmental risks. Crop Prot. 2024, 181, 106691. [Google Scholar] [CrossRef]
- Medináné Lázár, V. Statisztikai Jelentések. Növényvédő Szerek Értékesítése 2024. Év; Agrárközgazdasági Intézet: Budapest, Hungary, 2025; pp. 3–10. Available online: https://www.aki.gov.hu/termek/novenyvedo-szerek-ertekesitese-2024-ev/ (accessed on 16 July 2025).
- NÉBIH Növényvédő Szerek Adatbázisa. Available online: https://novenyvedoszer.nebih.gov.hu/Engedelykereso/kereso (accessed on 16 July 2025).
- Reuter, T.; Nahrstedt, K.; Wittstruck, L.; Jarmer, T.; Broll, G.; Trautz, D. Site-specific mechanical weed management in maize (Zea mays) in North-West Germany. Crop Prot. 2025, 190, 107–123. [Google Scholar] [CrossRef]
- Maillot, T.; Vioix, J.-P.; Colbach, N. Site-specific herbicide spraying can control weeds as well as full spraying in the long-term. A simulation study. Comput. Electron. Agric. 2023, 214, 108338. [Google Scholar] [CrossRef]
- Gerhards, R.; Sanchez, D.A.; Hamouz, P.; Peteinatos, G.G.; Christensen, S.; Fernández-Quintanilla, C. Advances in site-specific weed management in agriculture—A review. Weed Res. 2022, 62, 12526. [Google Scholar] [CrossRef]
- Csorba, P. Magyarország Kistájai, 1st ed.; Meridián Táj-és Környezetföldrajzi Alapítvány: Debrecen, Hungary, 2021; p. 416. [Google Scholar]
- HungaroMet Meteorological Data Archive: Historical Data of Automatic Meteorological Stations. Available online: https://odp.met.hu/climate/observations_hungary/monthly/historical/ (accessed on 7 May 2025).
- EPPO Global Database. Available online: https://gd.eppo.int/search (accessed on 28 May 2025).
- Martin, J.R.; Green, J.D. Weed Management, 1st ed.; University of Kentucky: Lexington, KY, USA, 2009; pp. 30–41. [Google Scholar]
- Nagy, K. Investigation of Arable Weed Vegetation in Mures County. Ph.D. Dissertation, Széchenyi István University, Mosonmagyaróvár, Hungary, 2017. [Google Scholar]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Oxford University Press: London, UK, 1934. [Google Scholar]
- Ujvárosi, M. Gyomnövények, Gyomirtás; Mezőgazdasági Kiadó: Budapest, Hungary, 1957; pp. 93–708. (In Hungarian) [Google Scholar]
- Kalapos, T. C3 and C4 grasses of Hungary: Environmental requirements, phenology and role in the vegetation. Abstr. Bot. 1991, 15, 83–88. [Google Scholar]
- Kalapos, T.; Baloghné-Nyakas, A.; Csontos, P. Occurrence and ecological characteristics of C4 dicot and Cyperaceae species in the Hungarian flora. Phytosynthetica 1997, 33, 227–240. [Google Scholar] [CrossRef]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R.; Springer: New York, NY, USA, 2011; pp. 34–50. [Google Scholar]
- Fox, J. Applied Regression Analysis and Generalized Linear Models, 3rd ed.; Sage Publications: Thousand Oaks, CA, USA, 2016; pp. 342–358. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Chambers, J.M.; Freeny, A.; Heiberger, R.M. Analysis of variance, designed experiments. In Statistical Models in S, 1st ed.; Chambers, J.M., Hastie, T.J., Eds.; Wadsworth & Brooks/Cole: Pacific Grove, CA, USA, 1992; pp. 145–194. [Google Scholar]
- Soper, H.E.; Young, A.W.; Cave, B.M.; Lee, A.; Pearson, K. On the distribution of the correlation coefficient in small samples. Appendix II to the papers of “Student” and R.A. Fisher. A co-operative study. Biometrika 1917, 11, 328–413. [Google Scholar]
- Keselman, H.J.; Rogan, J.C. The Tukey multiple comparison test: 1953–1976. Psychol. Bull. 1977, 84, 1050–1056. [Google Scholar] [CrossRef]
- Mueller-Dombois, D.; Ellenberg, H. Aims and methods of vegetation ecology. Geogr. Rev. 1976, 66, 114–116. [Google Scholar] [CrossRef]
- Sutter, J.M.; Kalivas, J.H. Comparison of Forward Selection, Backward Elimination, and Generalized Simulated Annealing for Variable Selection. Microchem. J. 1993, 47, 60–66. [Google Scholar] [CrossRef]
- Soltani, N.; Dille, J.A.; Burke, I.C.; Everman, W.J.; VanGessel, M.J.; Davis, V.M.; Sikkema, P.H. Potential Corn Yield Losses from Weeds in North America. Weed Technol. 2016, 30, 979–984. [Google Scholar] [CrossRef]
- Adeux, G.; Vieren, E.; Carlesi, S.; Barberi, P.; Munier-Jolain, N.; Cordeau, S. Mitigating crop yield losses through weed diversity. Nat. Sustain. 2019, 2, 1018–1026. [Google Scholar] [CrossRef]
- Vidotto, F.; Fogliatto, S.; Milan, M.; Ferrero, A. Weed communities in Italian maize fields as affected by pedo-climatic traits and sowing time. Eur. J. Agron. 2016, 74, 38–46. [Google Scholar] [CrossRef]
- Keeley, P.E.; Thullen, R.J. Influence of planting date on growth of Barnyardgrass (Echinochloa crus-galli). Weed Sci. 1989, 37, 557–561. [Google Scholar] [CrossRef]
- Forcella, F.; Wilson, R.G.; Renner, K.A.; Dekker, J.; Harvey, R.G.; Alm, D.A.; Buhler, D.D.; Cardina, J. Weed seedbanks of the U.S. corn belt: Magnitude, variation, emergence, and application. Weed Sci. 1992, 40, 636–644. [Google Scholar] [CrossRef]
- Clements, D.R.; Benott, D.L.; Murphy, S.D.; Swanton, C.J. Tillage effects on weed seed return and seedbank composition. Weed Sci. 1996, 44, 314–322. [Google Scholar] [CrossRef]
- Jensen, P.K.; Bibard, V.; Czembor, E.; Dumitru, S.; Foucart, G.; Froud-Williams, R.J.; Jensen, J.E.; Saavedra, M.; Sattin, M.; Soukup, J.; et al. Survey of Weeds in Maize Crops in Europe; Department of Integrated Pest Management, Aarhus University: Aarhus, Denmark, 2011; pp. 7–49. [Google Scholar]
- Bernardo, E.L.; Sales, C.R.G.; Cubas, L.A.; Vath, R.L.; Kromdijk, J. A comparison of stomatal conductance responses to blue and red light between C3 and C4 photosynthetic species in three phylogenetically-controlled experiments. Front. Plant Sci. 2023, 14, 1253976. [Google Scholar] [CrossRef]
- Pyšek, P.; Jarošík, V.; Kropáč, Z.; Chytrý, M.; Wild, J.; Tichý, L. Effects of abiotic factors on species richness and cover in Central European weed communities. Agric. Ecosyst. Environ. 2005, 109, 1–8. [Google Scholar] [CrossRef]
- Buhler, D.D. Influence of tillage systems on weed population dynamics and management in corn and soybean in the Central USA. Crop Sci. 1995, 35, 1247–1258. [Google Scholar] [CrossRef]
- Ghasemi-Fasaei, R.; Mansoorpoor, Y. Metal micronutrients relationships in crop, soil, and common weeds of two maize (Zea mays L.) fields. Arch. Agron. Soil. Sci. 2015, 61, 1733–1741. [Google Scholar] [CrossRef]
- Moreau, D.; Milard, G.; Munier-Jolain, N. A plant nitrophily index based on plant leaf area response to soil nitrogen availability. Agron. Sustain. Dev. 2013, 33, 809–815. [Google Scholar] [CrossRef]
- Desserud, P.; Hugenholtz, C. Do Three Invasive Species: Amaranthus blitoides, Descurainia sophia and Bassia scoparia, Respond to Soil Properties? Ecol. Restor. 2015, 33, 127–130. [Google Scholar] [CrossRef]
- Abuhadra, M.; Essokne, R.; Mahklouf, M. A New Record Amaranthus blitoides S. Watson. (Amaranthaceae) For the Flora of Libya. Am. J. Life Sci. Res. 2016, 4, 89–91. [Google Scholar] [CrossRef][Green Version]
- Nataliia, R.; Abdelhak, E.; Michelle, G.; Tian, F.; Laptev, V. Bioaccumulation of As, Cd, Cr, Cu, Pb, Zn in Ambrosia artemisiifolia L. in the polluted area by enterprise for the production and processing of batteries. Ann. Civil. Environ. Eng. 2022, 6, 026–030. [Google Scholar] [CrossRef]
- Citterio, S.; Gentili, R.; Montagnani, C.; Smith, M. The Worldwide Spread, Success, and Impact of Ragweed (Ambrosia spp.). Crit. Rev. Plant Sci. 2017, 36, 139–178. [Google Scholar] [CrossRef]
- Rinanti, A.; Fachrul, M.; Mahardika, G. Phytoremediation of heavy metal copper (Cu2+) by sunflower (Helianthus annuus L.). IOP Conf. Ser. Earth Environ. Sci. 2018, 106, 012120. [Google Scholar] [CrossRef]
- Xue, C.; Sun, L.; Liu, Z.; Qu, B.; Gao, Y.; Tai, P.; Guo, C.; Liu, W.; Chang, W. Grafting with an invasive Xanthium strumarium improves tolerance and phytoremediation of native congener X. sibiricum to cadmium/copper/nickel tailings. Chemosphere 2022, 308, 136561. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, N.; Ali, K.; Khan, M.E.H.; Jones, D.A. Screening of Xanthium strumarium (IAPS) Growing on Abandoned Habitats in Khyber Pakhtunkhwa, Pakistan: Perspectives for Phytoremediation. Appl. Sci. 2021, 11, 11704. [Google Scholar] [CrossRef]
- Ngole-Jeme, V.; Makuleke, P. Soil Heavy Metal Distribution with Depth around a Closed Landfill and Their Uptake by Datura stramonium. Appl. Environ. Soil. Sci. 2020, 1, 872475. [Google Scholar] [CrossRef]
- Varun, M.; D’souza, R.; Pratas, J.; Paul, M. Metal contamination of soils and plants associated with the glass industry in North Central India: Prospects of phytoremediation. Environ. Sci. Pollut. Res. 2011, 19, 269–281. [Google Scholar] [CrossRef]
- Babst-Kostecka, A.; Cornu, J.; Pošćić, F.; Mattiello, A.; Novello, N. Copper accumulation in five weed species commonly found in the understory vegetation of Mediterranean vineyards. Environ. Pollut. 2023, 329, 121675. [Google Scholar] [CrossRef]
- Ogazie, C.A.; Ochekwu, E.B.; Agbagwa, I.O.; Ugiomoh, I.G. Relationship between soil weed seed bank and soil properties in arable farmlands in University of Port Harcourt and environs in rivers state—Nigeria. J. Agripreneurship Sustain. Dev. 2023, 6, 9–20. [Google Scholar] [CrossRef]
- Malicki, L.; Berbeciowa, C.z. Uptake of more important mineral components by common field weeds on loess soil. Acta Agrobot. 1986, 39, 129–141. [Google Scholar] [CrossRef]
- Dudić, M.; Begović, R.; Meseldžija, M.; Vranešević, M. Weed flora composition in exceptionally hot and dry years. In Proceedings of the 12th Jeep International Scientific Agribusiness Conference—MAK 2025, Kopaonik, Serbia, 30 January–2 February 2025. [Google Scholar] [CrossRef]
- Kovács, E.B. Fénymag És Szárazborsó Kultúrák Gyomviszonyainak Elemzése Gyomaendrőd És Szarvas Térségében, Ökológiai És Konvencionális Területeken. Ph.D. Dissertation, Magyar Agrár-és Élettudományi Egyetem, Gödöllő, Hungary, 2024. [Google Scholar]
- Pinke, G.; Pál, R.W.; Tóth, K.; Karácsony, P.; Czúcz, B.; Botta-Dukát, Z. Weed vegetation of poppy (Papaver somniferum) fields in Hungary: Effects of management and environmental factors on species composition. Weed Res. 2011, 51, 621–630. [Google Scholar] [CrossRef]
- Marshall, E.J.P.; Brown, V.K.; Boatman, N.D.; Lutman, P.J.W.; Squire, G.R.; Ward, L.K. The role of weeds in supporting biological diversity within crop fields. Weed Res. 2003, 43, 77–89. [Google Scholar] [CrossRef]
- Fried, G.; Kazakou, E.; Gaba, S. Trajectories of weed communities explained by traits associated with species response to management practices. Agric. Ecosyst. Environ. 2012, 158, 147–155. [Google Scholar] [CrossRef]
- Gaba, S.; Chauvel, B.; Dessaint, F.; Bretagnolle, V.; Petit, S. Weed species richness in winter wheat increases with landscape heterogeneity. Agric. Ecosyst. Environ. 2010, 138, 318–323. [Google Scholar] [CrossRef]
- Menalled, U.D.; Ann Bybee-Finley, K.; Smith, R.G.; DiTomasso, A.; Darby, H.M.; Pethybridge, J.S.; Ryan, M.R. Legacy effects of crop diversity on weed-crop competition in maize production. Npj Sustain. Agric. 2024, 2, 28. [Google Scholar] [CrossRef]
- Doram, R.; Moyer, J.; Huang, H.; Entz, T.; Blackshaw, R. Effect of previous crop and herbicides on weed growth and wheat yield. Can. J. Plant Sci. 2005, 85, 735–746. [Google Scholar] [CrossRef]
- Oreja, F.H.; Mahoney, D.J.; Jordan, L.D.; Jennings, K.M.; Vann, M.; Leon, R.G. Crop rotation and herbicide program effects on Palmer amaranth and common ragweed population growth rate. Crop Forage Turfgrass Manag. 2023, 9, e20232. [Google Scholar] [CrossRef]
- Oreja, F.H.; Inman, M.D.; Jordan, D.L.; Bardhan, D.; León, R.G. Modeling weed community diversity based on species population density dynamics and herbicide use intensity. Eur. J. Agron. 2022, 138, 126533. [Google Scholar] [CrossRef]
- Trufanov, A.; Gornich, E.; Voronin, A.; Vaganova, N.; Shchukin, S. Effect of minimum tillage, fertilizers and herbicides on weed abundance and crop yields. In IOP Conference Series: Earth and Environmental Science, Proceedings of the II International Scientific and Practical Conference “Ensuring Sustainable Development in the Context of Agriculture, Green Energy, Ecology and Earth Science” Smolensk, Russia, 23–27 January 2022; IOP Publishing: Bristol, UK, 2022. [Google Scholar]
- Stupnicka-Rodzynkiewicz, E.; Szylak, A. Effect of deep loosening of soil on weed infestation of winter wheat cultivated in few crop rotations. Acta Agrobot. 1995, 48, 5–14. [Google Scholar] [CrossRef]
- Froud-Williams, R.J.; Drennan, D.S.H.; Chancellor, R.J. Influence of cultivation regime on weed floras of arable cropping systems. J. Appl. Ecol. 1983, 20, 187–197. [Google Scholar] [CrossRef]
- Bàrberi, P.; Cozzani, A.; Macchia, M.; Bonari, E. Size and composition of the weed seedbank under different management systems for continuous maize cropping. Weed Res. 1998, 38, 319–334. [Google Scholar] [CrossRef]
- Santín-Montanyá, M.I.; Zambrana-Quesada, E.; Tenorio-Pasamón, J.L. Weed abundance and soil seedbank responses to tillage systems in continuous maize crops. Arch. Agron. Soil Sci. 2018, 64, 1705–1713. [Google Scholar] [CrossRef]
- Đalović, I.; Božić, D.; Saulić, M.; Oveisi, M.; Vrbničanin, S. Soil weed seed bank in the function of biodiversity. Acta Herbol. 2024, 33, 141–149. [Google Scholar] [CrossRef]
- DiTomaso, J.M. Approaches for Improving Crop Competitiveness through the Manipulation of Fertilization Strategies. Weed Sci. 1995, 43, 491–497. [Google Scholar] [CrossRef]
- Little, N.G.; DiTommaso, A.; Westbrook, A.S.; Ketterings, Q.M.; Mohler, C.L. Effects of Fertility Amendments on Weed Growth and Weed—Crop Competition: A Review. Weed Sci. 2021, 69, 132–146. [Google Scholar] [CrossRef]
- Sadeghpour, A.; Pelzer, C.; Cordeau, S.; Ketterings, Q.; Ryan, M.; Wayman, S. Long-Term Soil Nutrient Management Affects Taxonomic and Functional Weed Community Composition and Structure. Front. Agron. 2021, 3, 636179. [Google Scholar] [CrossRef]
- Kordbacheh, F.; Flaten, D.N.; Gulden, R.H. Weed community dynamics under repeated fertilization with different nutrient sources over 5 years. Agric. Ecosyst. Environ. 2023, 346, 108328. [Google Scholar] [CrossRef]
- Luo, J.J.; Gao, Y.; Feng, W.; Liu, M.; Qu, B.; Zhang, C.; Feng, Y. Stronger ability to absorb nitrate and associated transporters in the invasive plant Xanthium strumarium compared with its native congener. Environ. Exp. Bot. 2022, 198, 104851. [Google Scholar] [CrossRef]
- Kennedy, R.; Zee, D.; Rumpho, M.; Barrett, S. Germination and seedling growth under anaerobic conditions in Echinochloa crus-galli (barnyard grass). Plant Cell Environ. 1980, 3, 243–248. [Google Scholar] [CrossRef]
- Wu, Y.; Li, P.; Wu, C.; Fu, W.; Hao, J. The distribution characteristics of nitrogen and phosphorus in the ecological system of Mt. Beigu wetland. Chin. J. Geochem. 2009, 28, 55–60. [Google Scholar] [CrossRef]
- Yang, W. Effect of nitrogen, phosphorus and potassium fertilizer on growth and seed germination of Capsella bursa-pastoris (L.) Medikus. J. Plant Nutr. 2018, 41, 636–644. [Google Scholar] [CrossRef]
- Murthy, I.; Anjaiah, T.; Jyothi, P.; Hussain, S.; Naik, R. Seed Yield and Nutrient Uptake of Sunflower (Helianthus annuus L.) as Influenced by Different Levels of Boron and Potassium in Sandy Loam Soil. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3684–3692. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, Y.; Liu, Y.; Liu, Y.; Miao, C.; Zhang, Y.; Li, W.; Yi, X. Response of plants and soils to inundation duration and construction of the plant—soil association mode in the hydro-fluctuation belt of the reservoir wetland. J. Environ. Manag. 2024, 357, 120776. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, T.; Huang, N.; Shen, X.; Shen, M.; Dai, Q. Effect of long-term fertilisation on the weed community of a winter wheat field. Sci. Rep. 2018, 8, 4017. [Google Scholar] [CrossRef]
- Matyukha, V.L.; Semenov, S.S. Influence of cultivation methods on the soil aggregate state in the context of weed development in winter wheat plantations. Agrology 2024, 7, 14–20. [Google Scholar] [CrossRef]
- Tóth, E.; Dorner, Z.; Nagy, J.G.; Zalai, M. How Weed Flora Evolves in Cereal Fields in Relation to the Agricultural Environment and Farming Practices in Different Sub-Regions of Eastern Hungary. Agronomy 2025, 15, 1033. [Google Scholar] [CrossRef]
- Hazarika, J.R.; Deka, A.M.; Borah, B.; Gogoi, B.; Gogoi, A.; Kalita, B.; Bordoloi, P.K.; Deva Nath, H. Weed flora shift as affected by cropping systems. Int. J. Res. Agron. 2024, 7, 415–420. [Google Scholar] [CrossRef]
- Grassini, P.; Lim, Y.L.; Tenorio, A.F.; Monzon, J.P.; Sugianto, H.; Donough, R.C.; Rahutomo, S.; Agus, F.; Slingerland, M.A.; Darlan, N.H.; et al. Too little, too imbalanced: Nutrient supply in smallholder oil palm fields in Indonesia. Agric. Syst. 2023, 210, 103729. [Google Scholar] [CrossRef]
- Pinke, G.; Karácsony, P.; Botta-Dukát, Z.; Czúcz, B. Relating Ambrosia artemisiifolia and other weeds to the management of Hungarian sunflower crops. J. Pest. Sci. 2013, 86, 621–631. [Google Scholar] [CrossRef]
- Pinke, G.; Blazsek, K.; Magyar, L.; Nagy, K.; Karácsony, P.; Czúcz, B.; Botta-Dukát, Z. Weed species composition of conventional soyabean crops in Hungary is determined by environmental, cultural, weed management and site variables. Weed Res. 2016, 56, 470–481. [Google Scholar] [CrossRef]
- de Mol, F.; von Redwicz, C.; Gerowitt, B. Weed species composition of maize fields in Germany is influenced by site and crop sequence. Weed Res. 2015, 55, 574–585. [Google Scholar] [CrossRef]
- Angiosperm Phylogeny Website. Available online: http://www.mobot.org/MOBOT/research/APweb/ (accessed on 9 May 2025).
- Yorimitsu, Y.; Kadosono, A.; Hatakeyama, Y.; Yabiku, T.; Ueno, O. Transition from C3 to proto-Kranz to C3–C4 intermediate type in the genus Chenopodium (Chenopodiaceae). J. Plant Res. 2019, 132, 839–855. [Google Scholar] [CrossRef]
- Oono, J.; Hatakeyama, Y.; Yabiku, T.; Ueno, O. Effects of growth temperature and nitrogen nutrition on expression of C3–C4 intermediate traits in Chenopodium album. J. Plant Res. 2022, 135, 15–27. [Google Scholar] [CrossRef]
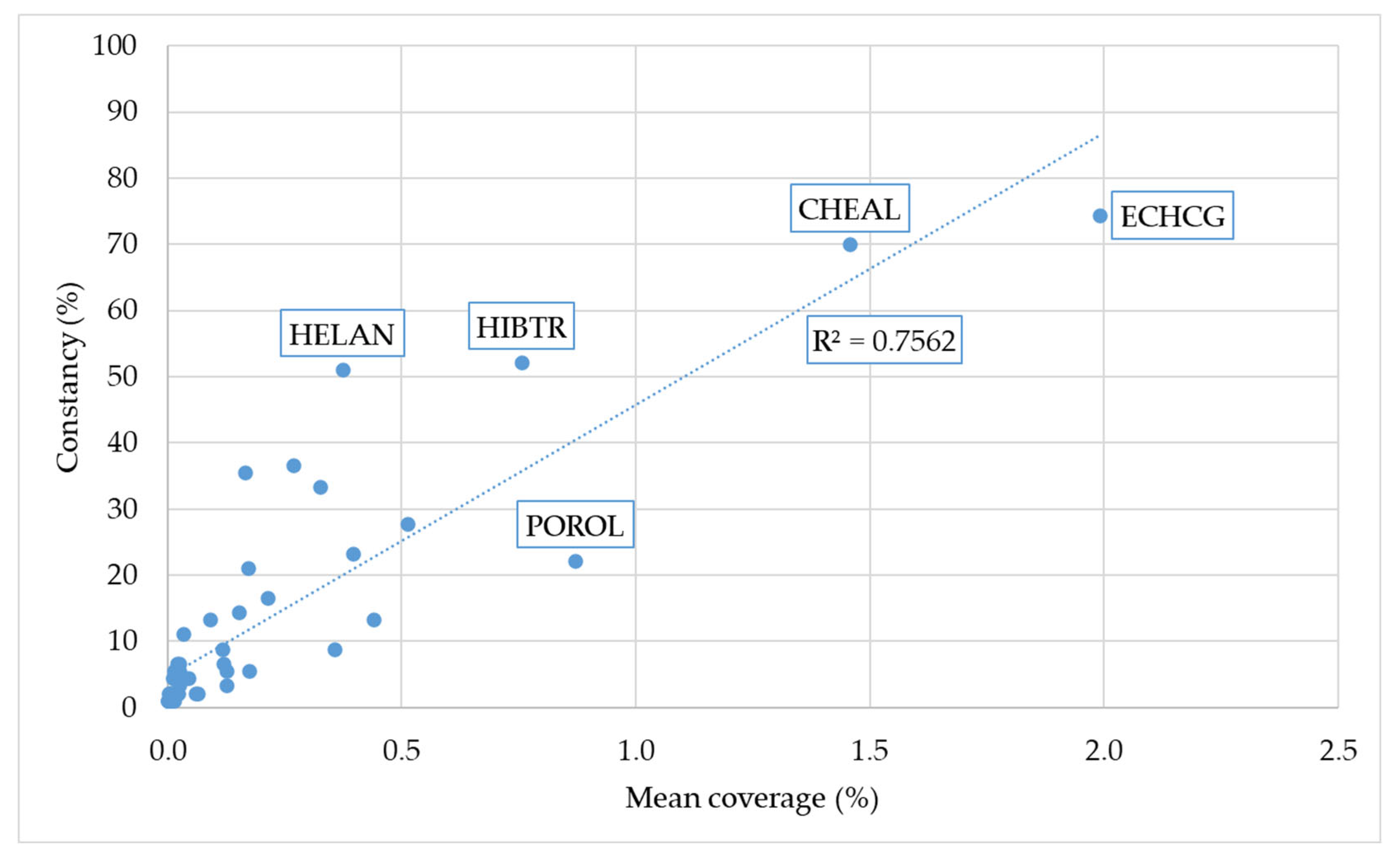
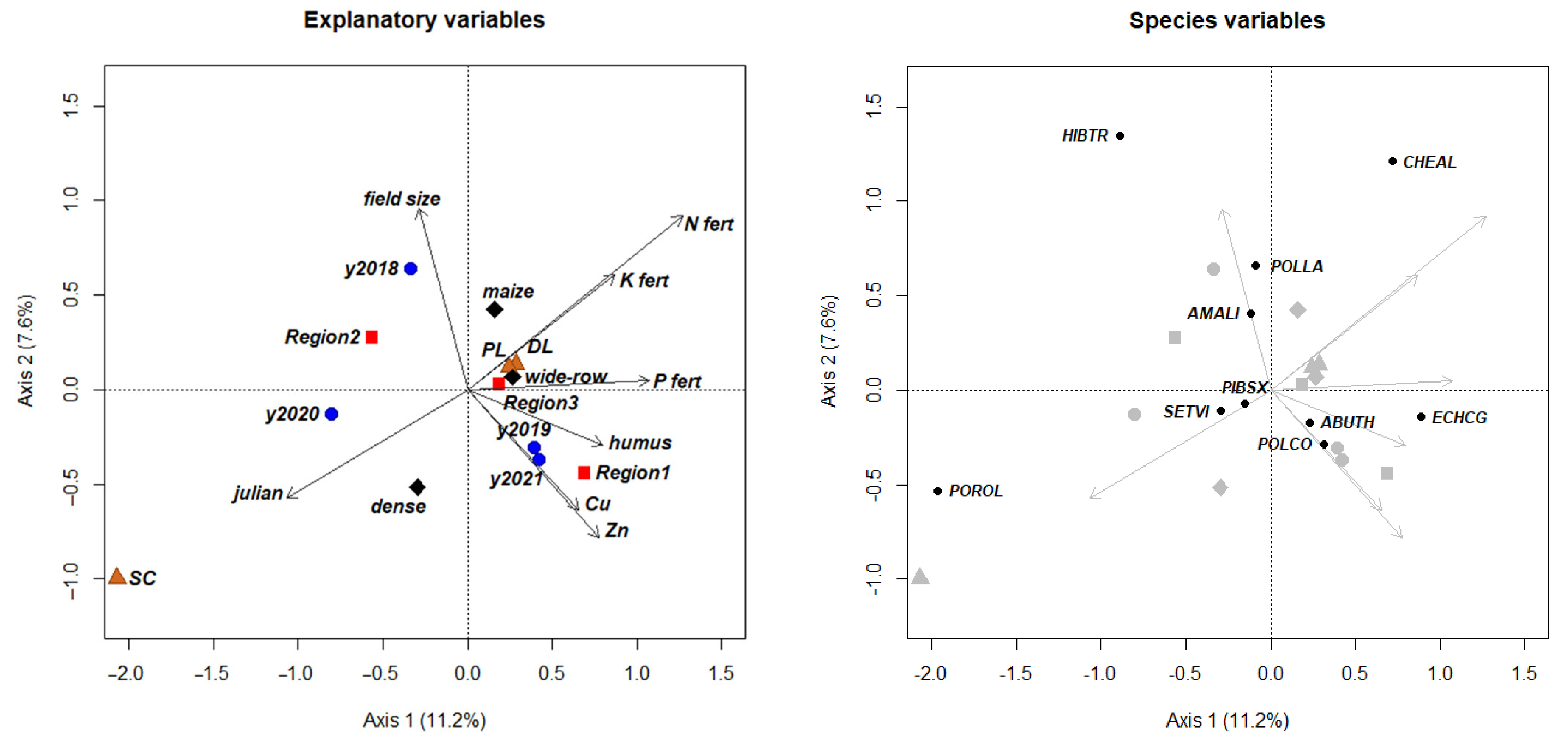
| Year A | Region 1 B | Region 2 C | Region 3 D | |||
|---|---|---|---|---|---|---|
| Rainfall (mm) | Avg. Temp. ·(°C) | Rainfall E (mm) | Avg. Temp. ·(°C) | Rainfall (mm) | Avg. Temp. ·(°C) | |
| 2018 | 604.5 | 11.0 | 594.5 | 11.6 | 771.5 | 12.0 |
| 2019 | 452.8 | 11.3 | 417.6 | 12.0 | 553.7 | 12.2 |
| 2020 | 541.5 | 11.0 | 660.0 | 11.7 | 545.7 | 12.0 |
| 2021 | 597.3 | 10.6 | 629.5 | 11.2 | 634.6 | 11.6 |
| 2018–2021 avg. | 549.0 | 11.0 | 575.4 | 11.6 | 626.4 | 12.0 |
| Variable (Unit) | Range/Recorded or Calculated Values |
|---|---|
| Environmental variables | |
| Soil texture (KArany) | 27–59 |
| Soil pH (KCl) | 3.89–7.41 |
| Soil properties (m/m %) | |
| Salt (m/m %) | 0.01–1.13 |
| Humus (m/m %) | 0.58–4.00 |
| N (mg kg−1) | 2.0–48.8 |
| P2O5 (mg kg−1) | 31–380 |
| K2O (mg kg−1) | 89–501 |
| CaCO3 (m/m %) | 0.01–5.30 |
| Na (mg kg−1) | 0.0–94.4 |
| Mg (mg kg−1) | 36–674 |
| S (mg kg−1) | 0.5–25.4 |
| Cu (mg kg−1) | 0.86–8.9 |
| Mn (mg kg−1) | 21–467 |
| Zn (mg kg−1) | 0.25–9.1 |
| Altitude (m, AMSL) | 79–167 |
| Latitude (°) | 46.784400–48.160491 |
| Longitude (°) | 20.823639–21.762198 |
| Region A | Region 1, Region 2, Region 3 |
| Growing season | 2018–2021 |
| Farming variables | |
| Date of weed survey (Julian day) A | 78–128 |
| Field size (ha) | 1.0–38.2 |
| Preceding crops | Maize, other wide-row crops B, dense crops C |
| Tillage method | Shallow cultivation, ploughing, deep loosening |
| Tillage depth (cm) | 15–40 |
| Amount of Nitrogen fertilizer (kg a.i. ha−1) | 0–163 |
| Amount of Phosphorus fertilizer (kg a.i. ha−1) | 0–104 |
| Amount of Potassium fertilizer (kg a.i. ha−1) | 0–60 |
| Variables | Total Weed Coverage [%] | Species Richness | Shannon Diversity |
|---|---|---|---|
| p-Values of ANCOVAs A (Pearson Correlation Coefficients)/[Tukey Post Hoc Test B] | |||
| Environmental variables | |||
| Soil texture | ns | <0.001 (+0.33) *** | <0.001 (+0.40) *** |
| Soil reaction | ns | 0.006 (+0.29) *** | 0.019 (+0.32) ** |
| Soil properties | |||
| Salt | ns | ns | ns |
| Humus | ns | 0.019 (+0.25) ** | ns |
| N | ns | 0.025 (−0.24) ** | 0.045 (−0.21) ** |
| P2O5 | ns | ns | ns |
| K2O | ns | 0.090 (+0.18) * | ns |
| CaCO3 | 0.059 (−0.20) * | ns | 0.062 (+0.20) * |
| Na | ns | 0.002 (+0.33) *** | 0.092 (+0.18) * |
| Mg | ns | ns | 0.007 (+0.28) *** |
| S | ns | 0.028 (+0.23) ** | ns |
| Cu | ns | 0.007 (+0.28) *** | ns |
| Mn | 0.016 (+0.25) ** | 0.068 (+0.19) * | ns |
| Zn | ns | <0.001 (+0.46) *** | 0.081 (+0.19) * |
| Altitude | 0.010 (+0.27) | ns | ns |
| Latitude | ns | ns | 0.090 (−0.18) * |
| Longitude | ns | <0.001 (−0.40) *** | 0.064 (−0.20) * |
| Region R1—Region 1 R2—Region 2 R3—Region 3 | 0.012 ** [R1—10.77 b R2—7.32 a R3—8.96 b] | <0.001 *** [R1—7.56 b R2—5.15 a R3—7.26 b] | ns |
| Growing season | 0.028 ** [2018—8.00 ab 2019—13.18 b 2020—3.24 a 2021—11.50 ab] | <0.001 *** [2018—6.86 a 2019—8.51 b 2020—5.40 a 2021—5.49 a] | ns |
| Farming variables | |||
| Date of weed survey | <0.001 (+0.35) *** | ns | 0.003 (−0.31) *** |
| Field size | ns | ns | ns |
| Preceding crop | ns | ns | ns |
| Tillage method SC—Shallow cultivation PL—Ploughing DL—Deep loosening | ns | <0.001 *** [SC—4.40 a PL—7.72 b DL—7.93 b] | <0.001 *** [SC—0.63 a PL—1.28 b DL—1.35 b] |
| Tillage depth | ns | 0.020 (+0.25) ** | 0.072 (+0.19) * |
| Amount of N fertilizer | ns | 0.003 (+0.31) *** | 0.010 (+0.27) ** |
| Amount of P fertilizer | 0.048 (+0.21) ** | <0.001 (+0.43) *** | ns |
| Amount of K fertilizer | 0.008 (+0.28) *** | <0.001 (+0.38) *** | 0.055 (+0.20) * |
| Significant Explanatory Variables | Df | Gross Effect | Net Effect | ||||
|---|---|---|---|---|---|---|---|
| Explained Variation (%) | R2adj | Explained Variation (%) | R2adj | F | p-Value A | ||
| Soil humus content | 1 | 4.40 | 0.033 | 1.99 | 0.014 | 2.470 | 0.003 *** |
| Soil Cu content | 1 | 3.79 | 0.027 | 1.22 | 0.005 | 1.516 | 0.087 * |
| Soil Zn content | 1 | 4.15 | 0.031 | 2.14 | 0.016 | 2.667 | 0.001 ** |
| Region | 2 | 10.00 | 0.079 | 4.81 | 0.038 | 2.990 | <0.001 *** |
| Growing season | 3 | 9.17 | 0.060 | 3.21 | 0.009 | 1.329 | 0.060 * |
| Date of weed survey | 1 | 4.15 | 0.031 | 1.38 | 0.070 | 1.718 | 0.026 ** |
| Field size | 1 | 2.92 | 0.018 | 1.27 | 0.006 | 1.579 | 0.059 * |
| Preceding crop | 2 | 4.95 | 0.028 | 2.86 | 0.015 | 1.779 | 0.007 *** |
| Tillage method | 2 | 11.40 | 0.094 | 3.17 | 0.019 | 1.970 | 0.002 *** |
| Amount of N fertilizer | 1 | 6.38 | 0.053 | 1.35 | 0.007 | 1.675 | 0.041 ** |
| Amount of P fertilizer | 1 | 5.37 | 0.043 | 1.38 | 0.007 | 1.717 | 0.036 ** |
| Amount of K fertilizer | 1 | 4.36 | 0.033 | 1.25 | 0.005 | 1.551 | 0.064 * |
| Total | - | - | - | 26.03 | - | - | - |
| Species | Fit | Ax 1 Score | Species | Fit | Ax 1 Score |
|---|---|---|---|---|---|
| Soil humus content (+ high; − low) | Amount of N fertilizer (+ high; − low) | ||||
| Chenopodium album | 0.305 | 0.125 | Convolvulus arvensis | 0.144 | 0.082 |
| Amaranthus retroflexus | 0.083 | 0.019 | Xanthium italicum | 0.092 | 0.088 |
| Polygonum aviculare | 0.035 | 0.054 | Persicaria lapathifolia | 0.082 | 0.036 |
| Pisum sativum | 0.021 | 0.039 | Fallopia convolvulus | 0.060 | 0.018 |
| Tripleurospermum inodorum | −0.024 | 0.033 | Stachys annua | 0.040 | 0.021 |
| Chenopodium hybridum | −0.028 | 0.029 | Aristolochia clematitis | 0.029 | 0.020 |
| Triticum aestivum | −0.028 | 0.077 | Datura stramonium | −0.061 | 0.019 |
| Amaranthus blitum | −0.045 | 0.018 | Amaranthus retroflexus | −0.064 | 0.011 |
| Amaranthus blitoides | −0.081 | 0.063 | Lathyrus tuberosus | −0.114 | 0.115 |
| Ambrosia artemisiifolia | −0.091 | 0.024 | Echinochloa crus–galli | −0.140 | 0.026 |
| Soil Cu content (+ high; − low) | Amount of P fertilizer (+ high; − low) | ||||
| Helianthus annuus | 0.157 | 0.041 | Hibiscus trionum | 0.179 | 0.045 |
| Xanthium strumarium | 0.113 | 0.031 | Capsella bursa–pastoris | 0.123 | 0.207 |
| Datura stramonium | 0.096 | 0.048 | Equisetum arvense | 0.075 | 0.056 |
| Ambrosia artemisiifolia | 0.071 | 0.015 | Convolvulus arvensis | 0.068 | 0.018 |
| Stachys annua | 0.057 | 0.043 | Stachys annua | 0.037 | 0.018 |
| Brassica napus | 0.043 | 0.017 | Triticum aestivum | −0.015 | 0.022 |
| Pisum sativum | −0.013 | 0.015 | Tripleurospermum inodorum | −0.024 | 0.033 |
| Polygonum aviculare | −0.035 | 0.054 | Amaranthus blitoides | −0.044 | 0.019 |
| Amaranthus blitoides | −0.044 | 0.019 | Xanthium strumarium | −0.081 | 0.016 |
| Cirsium arvense | −0.131 | 0.043 | Ambrosia artemisiifolia | −0.139 | 0.055 |
| Soil Zn content (+ high; − low) | Amount of K fertilizer (+ high; − low) | ||||
| Chenopodium album | 0.128 | 0.022 | Helianthus annuus | 0.134 | 0.030 |
| Convolvulus arvensis | 0.072 | 0.020 | Abutilon theophrasti | 0.053 | 0.023 |
| Aristolochia clematitis | 0.041 | 0.041 | Chenopodium polyspermum | 0.048 | 0.017 |
| Polygonum aviculare | 0.031 | 0.042 | Raphanus raphanistrum | 0.033 | 0.027 |
| Brassica napus | −0.066 | 0.040 | Pisum sativum | 0.019 | 0.031 |
| Abutilon theophrasti | −0.086 | 0.061 | Aristolochia clematitis | −0.028 | 0.019 |
| Stachys annua | −0.087 | 0.102 | Amaranthus blitum | −0.081 | 0.057 |
| Datura stramonium | −0.107 | 0.059 | Chenopodium album | −0.099 | 0.013 |
| Panicum miliaceum | −0.120 | 0.031 | Hibiscus trionum | −0.121 | 0.021 |
| Helianthus annuus | −0.266 | 0.119 | Persicaria lapathifolia | −0.124 | 0.082 |
| Species | Fit | Ax 1 Score | Species | Fit | Ax 1 Score |
|---|---|---|---|---|---|
| 2018 (+ high; − low) | Region 1 (+ high; − low) baz | ||||
| Hibiscus trionum | 0.311 | 0.135 | Echinochloa crus–galli | 0.338 | 0.150 |
| Persicaria lapathifolia | 0.132 | 0.093 | Fallopia convolvulus | 0.240 | 0.290 |
| Amaranthus blitum | 0.122 | 0.130 | Rubus caesius | 0.122 | 0.109 |
| Chenopodium polyspermum | 0.097 | 0.070 | Abutilon theophrasti | 0.103 | 0.088 |
| Raphanus raphanistrum | 0.046 | 0.051 | Capsella bursa–pastoris | 0.076 | 0.080 |
| Pisum sativum | 0.028 | 0.072 | Aristolochia clematitis | 0.061 | 0.089 |
| Capsella bursa–pastoris | −0.061 | 0.052 | Tripleurospermum inodorum | 0.038 | 0.081 |
| Abutilon theophrasti | −0.091 | 0.068 | Triticum aestivum | 0.033 | 0.104 |
| Fallopia convolvulus | −0.135 | 0.092 | Portulaca oleracea | −0.237 | 0.078 |
| Panicum miliaceum | −0.162 | 0.057 | Hibiscus trionum | −0.267 | 0.100 |
| 2019 (+ high; − low) | Region 2 (+ high; − low) h-ny | ||||
| Fallopia convolvulus | 0.135 | 0.091 | Portulaca oleracea | 0.373 | 0.192 |
| Rubus caesius | 0.099 | 0.071 | Ambrosia artemisiifolia | 0.157 | 0.071 |
| Equisetum arvense | 0.090 | 0.081 | Amaranthus blitum | 0.092 | 0.073 |
| Capsella bursa–pastoris | 0.084 | 0.096 | Stachys annua | −0.064 | 0.056 |
| Xanthium italicum | 0.082 | 0.071 | Datura stramonium | −0.103 | 0.055 |
| Stachys annua | 0.062 | 0.051 | Fallopia convolvulus | −0.142 | 0.101 |
| Polygonum aviculare | 0.046 | 0.091 | Convolvulus arvensis | −0.147 | 0.086 |
| Tripleurospermum inodorum | 0.038 | 0.081 | Helianthus annuus | −0.204 | 0.070 |
| Triticum aestivum | 0.023 | 0.051 | Echinochloa crus–galli | −0.243 | 0.077 |
| Hibiscus trionum | −0.191 | 0.051 | Cirsium arvense | −0.284 | 0.202 |
| 2020 (+ high; − low) | Region 3 (+ high; − low) bek | ||||
| Portulaca oleracea | 0.277 | 0.106 | Cirsium arvense | 0.226 | 0.128 |
| Hibiscus trionum | 0.141 | 0.028 | Datura stramonium | 0.188 | 0.183 |
| Setaria viridis | 0.110 | 0.153 | Lathyrus tuberosus | 0.138 | 0.169 |
| Fallopia convolvulus | 0.070 | 0.025 | Convolvulus arvensis | 0.129 | 0.066 |
| Lathyrus tuberosus | 0.060 | 0.032 | Chenopodium polyspermum | 0.111 | 0.092 |
| Aristolochia clematitis | 0.026 | 0.016 | Stachys annua | 0.092 | 0.113 |
| Convolvulus arvensis | −0.079 | 0.025 | Raphanus raphanistrum | 0.055 | 0.074 |
| Panicum miliaceum | −0.099 | 0.021 | Iva xanthiifolia | 0.035 | 0.097 |
| Ambrosia artemisiifolia | −0.136 | 0.053 | Persicaria lapathifolia | −0.106 | 0.060 |
| Chenopodium album | −0.146 | 0.029 | Portulaca oleracea | −0.175 | 0.043 |
| 2021 (+ high; − low) | |||||
| Panicum miliaceum | 0.244 | 0.128 | |||
| Ambrosia artemisiifolia | 0.228 | 0.148 | |||
| Echinochloa crus–galli | 0.139 | 0.025 | |||
| Amaranthus retroflexus | 0.117 | 0.037 | |||
| Abutilon theophrasti | 0.091 | 0.069 | |||
| Convolvulus arvensis | −0.075 | 0.022 | |||
| Persicaria lapathifolia | −0.103 | 0.056 | |||
| Cirsium arvense | −0.110 | 0.030 | |||
| Portulaca oleracea | −0.174 | 0.042 | |||
| Hibiscus trionum | −0.247 | 0.085 | |||
| Species | Fit | Ax 1 Score | Species | Fit | Ax 1 Score |
|---|---|---|---|---|---|
| Date of weed survey (+ late; − early) | Field size (+ high; − low) baz | ||||
| Helianthus annuus | 0.179 | 0.054 | Helianthus annuus | 0.193 | 0.063 |
| Chenopodium album | 0.131 | 0.023 | Amaranthus blitoides | 0.090 | 0.079 |
| Persicaria lapathifolia | 0.088 | 0.042 | Chenopodium hybridum | 0.036 | 0.046 |
| Convolvulus arvensis | 0.062 | 0.015 | Tripleurospermum inodorum | 0.035 | 0.068 |
| Setaria viridis | 0.052 | 0.034 | Triticum aestivum | 0.029 | 0.081 |
| Polygonum aviculare | 0.022 | 0.021 | Capsella bursa–pastoris | −0.051 | 0.036 |
| Lathyrus tuberosus | −0.039 | 0.014 | Amaranthus blitum | −0.060 | 0.031 |
| Pisum sativum | –0.039 | 0.138 | Rubus caesius | −0.061 | 0.027 |
| Datura stramonium | −0.069 | 0.024 | Fallopia convolvulus | −0.066 | 0.022 |
| Portulaca oleracea | −0.145 | 0.029 | Cirsium arvense | −0.088 | 0.020 |
| Maize preceding crop (+ high; − low) | Shallow cultivation (+ high; − low) h-ny | ||||
| Chenopodium album | 0.262 | 0.092 | Portulaca oleracea | 0.691 | 0.661 |
| Persicaria lapathifolia | 0.173 | 0.159 | Setaria viridis | 0.130 | 0.212 |
| Amaranthus blitum | 0.092 | 0.073 | Pisum sativum | 0.054 | 0.261 |
| Tripleurospermum inodorum | 0.028 | 0.043 | Abutilon theophrasti | −0.048 | 0.019 |
| Raphanus raphanistrum | −0.045 | 0.050 | Persicaria lapathifolia | −0.064 | 0.022 |
| Setaria viridis | −0.046 | 0.026 | Convolvulus arvensis | −0.092 | 0.034 |
| Stachys annua | −0.064 | 0.056 | Cirsium arvense | −0.112 | 0.032 |
| Lathyrus tuberosus | −0.072 | 0.046 | Ambrosia artemisiifolia | −0.117 | 0.039 |
| Cirsium arvense | −0.148 | 0.055 | Echinochloa crus–galli | −0.227 | 0.068 |
| Portulaca oleracea | −0.173 | 0.042 | Chenopodium album | −0.320 | 0.138 |
| Other wide-row preceding crop (+ high; − low) | Ploughing (+ high; − low) | ||||
| Panicum miliaceum | 0.169 | 0.061 | Echinochloa crus–galli | 0.222 | 0.065 |
| Abutilon theophrasti | 0.123 | 0.126 | Persicaria lapathifolia | 0.151 | 0.121 |
| Xanthium italicum | 0.088 | 0.081 | Abutilon theophrasti | 0.095 | 0.075 |
| Xanthium strumarium | 0.069 | 0.012 | Pisum sativum | −0.023 | 0.047 |
| Lathyrus tuberosus | 0.052 | 0.024 | Stachys annua | −0.058 | 0.046 |
| Aristolochia clematitis | 0.022 | 0.012 | Setaria viridis | −0.064 | 0.052 |
| Raphanus raphanistrum | −0.023 | 0.013 | Chenopodium polyspermum | −0.078 | 0.045 |
| Amaranthus blitum | −0.037 | 0.012 | Lathyrus tuberosus | −0.097 | 0.083 |
| Convolvulus arvensis | −0.107 | 0.045 | Xanthium strumarium | −0.199 | 0.097 |
| Portulaca oleracea | −0.125 | 0.022 | Portulaca oleracea | −0.262 | 0.095 |
| Dense preceding crop (+ high; − low) | Deep loosening (+ high; − low) | ||||
| Portulaca oleracea | 0.272 | 0.102 | Xanthium strumarium | 0.223 | 0.122 |
| Cirsium arvense | 0.151 | 0.057 | Lathyrus tuberosus | 0.124 | 0.135 |
| Convolvulus arvensis | 0.131 | 0.068 | Convolvulus arvensis | 0.121 | 0.058 |
| Chenopodium polyspermum | 0.082 | 0.050 | Chenopodium polyspermum | 0.099 | 0.074 |
| Raphanus raphanistrum | 0.063 | 0.099 | Datura stramonium | 0.098 | 0.050 |
| Setaria viridis | 0.058 | 0.042 | Stachys annua | 0.080 | 0.086 |
| Pisum sativum | 0.024 | 0.051 | Raphanus raphanistrum | 0.047 | 0.055 |
| Abutilon theophrasti | −0.073 | 0.044 | Iva xanthiifolia | 0.027 | 0.055 |
| Persicaria lapathifolia | −0.144 | 0.110 | Persicaria lapathifolia | −0.118 | 0.074 |
| Chenopodium album | −0.319 | 0.137 | Portulaca oleracea | −0.192 | 0.051 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zalai, M.; Tóth, E.; Nagy, J.G.; Dorner, Z. Regional Patterns in Weed Composition of Maize Fields in Eastern Hungary: The Balance of Environmental and Agricultural Factors. Agronomy 2025, 15, 1814. https://doi.org/10.3390/agronomy15081814
Zalai M, Tóth E, Nagy JG, Dorner Z. Regional Patterns in Weed Composition of Maize Fields in Eastern Hungary: The Balance of Environmental and Agricultural Factors. Agronomy. 2025; 15(8):1814. https://doi.org/10.3390/agronomy15081814
Chicago/Turabian StyleZalai, Mihály, Erzsébet Tóth, János György Nagy, and Zita Dorner. 2025. "Regional Patterns in Weed Composition of Maize Fields in Eastern Hungary: The Balance of Environmental and Agricultural Factors" Agronomy 15, no. 8: 1814. https://doi.org/10.3390/agronomy15081814
APA StyleZalai, M., Tóth, E., Nagy, J. G., & Dorner, Z. (2025). Regional Patterns in Weed Composition of Maize Fields in Eastern Hungary: The Balance of Environmental and Agricultural Factors. Agronomy, 15(8), 1814. https://doi.org/10.3390/agronomy15081814







