Abstract
The primary aim of this study was to explore the influence of abiotic factors on weed development in maize fields, with the goal of informing more effective weed management practices. We focused on identifying key environmental, edaphic, and agricultural variables that contribute to weed infestations, particularly before the application of spring herbicide treatments. Field investigations were conducted from 2018 to 2021 across selected maize-growing regions in Hungary. Over the four-year period, a total of 51 weed species were recorded, with Echinochloa crus-galli, Chenopodium album, Portulaca oleracea, and Hibiscus trionum emerging as the most prevalent taxa. Collectively, these four species accounted for more than half (52%) of the total weed cover. Altogether, the 20 most dominant species contributed 95% of the overall weed coverage. The analysis revealed that weed cover, species richness, and weed diversity were significantly affected by soil properties, nutrient levels, geographic location, and tillage systems. The results confirm that the composition of weed species was influenced by several environmental and management-related factors, including soil parameters, geographical location, annual precipitation, tillage method, and fertilizer application. Environmental factors collectively explained a slightly higher proportion of the variance (13.37%) than farming factors (12.66%) at a 90% significance level. Seasonal dynamics and crop rotation history also played a notable role in species distribution. Nutrient inputs, particularly nitrogen, phosphorus, and potassium, influenced both species diversity and floristic composition. Deep tillage practices favored the proliferation of perennial species, whereas shallow cultivation tended to promote annual weeds. Overall, the composition of weed vegetation proved to be a valuable indicator of site-specific soil conditions and agricultural practices. These findings underscore the need to tailor weed management strategies to local environmental and soil contexts for sustainable crop production.
1. Introduction
Maize (Zea mays) is the most widely cultivated cereal crop globally. Owing to its high yield and versatility, it serves as a cornerstone of the global agricultural economy. It functions as a staple food for billions of people, a primary source of livestock feed, and plays a crucial role in the production of biofuels, bioplastics, and pharmaceuticals. Its resilience to a wide range of climatic and soil conditions allows it to be cultivated across the world. According to FAOSTAT data from 2023 [1], the European Union (EU) is the fifth-largest producer of maize globally, following the United States, China, Brazil, and Argentina. Within the EU, Hungary ranks as the fourth-largest producer after France, Romania, and Poland. Despite maize being a major crop both globally and in Hungary, there is a notable lack of comprehensive data and research on how environmental and agricultural factors influence weed composition in maize fields. Although Teasdale et al. [2] already emphasized in 2010 the need for further research to quantify yield losses caused by weed interference under different cropping systems, little progress has been made in this regard for maize.
The weed flora of maize fields in Hungary is highly diverse. Weed species composition varies significantly by region, influenced by factors such as soil type, crop rotation practices, climatic conditions, and weed management strategies. Typically, late-spring and summer-emerging thermophilic annual weed species (with optimal germination temperatures ranging from 18 to 30 °C) pose the most serious threat. These weeds emerge en masse in early summer, especially following maize germination, and compete vigorously for water and nutrients. Notable examples include Chenopodium spp., Amaranthus spp., Datura stramonium, Setaria spp., and Echinochloa crus-galli [3].
Perennial weed species also present considerable challenges in Hungarian maize fields. Species such as Cirsium arvense, Convolvulus arvensis, and Phragmites australis (especially in moist areas) are problematic due to their deep root systems and their ability to regenerate, making them difficult to eradicate. These perennials not only reduce yield but can also impede harvesting operations. A particularly troublesome species is the perennial monocot Sorghum halepense, known for its aggressive spread and resistance to conventional control methods. The emergence of sulfonylurea-resistant S. halepense has exacerbated weed management difficulties [4]. Although resistance was first documented in Hungary in the early 2000s, it likely originated in the late 1990s, correlating with the intensive and repeated application of sulfonylurea herbicides such as rimsulfuron and nicosulfuron.
According to results from the Sixth National Arable Weed Survey conducted in 2018–2019, substantial shifts have occurred in the weed vegetation of Hungarian maize fields [5]. Although overall weed coverage has declined, several species continue to pose significant challenges. Currently, the three most dominant weed species in Hungarian maize crops remain Echinochloa crus-galli, Ambrosia artemisiifolia, and Chenopodium album. Among these, E. crus-galli is not only the most prominent weed in early summer but also maintains significant coverage later in the season. The spread of several annual monocotyledonous weed species has also been noted, including Setaria pumila, Setaria viridis, and Digitaria sanguinalis, which continue to exhibit high levels of abundance. Additionally, there has been a marked proliferation of Abutilon theophrasti and Sorghum halepense, further complicating weed control efforts.
Understanding the composition and distribution of weed communities is a central goal of weed ecology. These communities are shaped by a combination of abiotic factors such as climate and soil properties [6], biotic interactions like crop–weed competition [7], agricultural management practices [8], and landscape-level heterogeneity [9]. While the basic ecological principles behind weed dynamics are relatively well established, the complexity of these interacting variables makes it difficult to determine their individual effects [10]. Despite this, several studies have tried to rank the influence of these variables under specific environmental conditions [11,12].
Spatial scale and the extent to which management practices are incorporated into research models may explain why studies differ in attributing more weight to environmental versus anthropogenic factors in shaping weed communities. Fried et al. [13] studied weed flora composition in maize under monoculture and crop rotation systems. They found that weed communities respond to crop rotation in two principal ways: one promotes generalist species suited to monocultures, while the other supports specialist weeds that thrive under conditions specific to individual crops. These specialist species often alternate with the crops in rotation and persist in the seed bank during unfavorable seasons. The generalist Chenopodium album was dominant in both cropping systems. In monocultures, Echinochloa crus-galli and Amaranthus retroflexus were dominant specialist species, whereas in rotated systems, the generalist species Polygonum aviculare and Fallopia convolvulus were more abundant. Additionally, weed species richness declined markedly between 1970 and 2000, dropping from 16.56 to 9.34 species per field, while weed density decreased from 61.5 to 20.2 individuals per square meter during the same period.
In a separate study, Fried et al. [14] assessed weed community composition in spring-sown crops, including maize, based on 14 environmental and management variables. Nine of these variables showed significant relationships with weed composition. The cultivated crop was the strongest explanatory factor, followed by the crop from the previous year and soil pH. Other environmental variables—such as rainfall, soil texture, latitude, and altitude—had progressively less influence. Variables such as longitude, sowing date, and landscape structure showed minimal impact, while topography, tillage, and temperature had no significant effects.
Soil chemistry has also been shown to influence weed–crop competition. Weaver and Hamill [15] demonstrated that soil pH affects the growth and competitive ability of Amaranthus powellii, Abutilon theophrasti, and Setaria viridis in maize fields. Maize yields declined due to weed competition across all pH levels, though the dry matter of A. powelli and A. theophrasti was lowest at pH 4.8 and highest at pH 6.0–7.3. Conversely, S. viridis performed better at low pH (4.8), indicating species-specific responses to pH conditions. Pinke et al. [16] identified several soil parameters—such as magnesium, potassium, calcium, phosphorus, humus content, and pH—as major determinants of weed species presence. For instance, C. album was associated with high potassium, Echinochloa crus-galli with low magnesium, and Cirsium arvense with high calcium and low humus levels.
Field size has also been implicated in weed community structure. Some studies found that smaller fields tended to harbor higher abundances of Ambrosia artemisiifolia, suggesting that field-scale factors can influence weed flora in maize [17].
Nitrogen fertilization is a critical factor influencing early maize development. Increased N availability has been demonstrated to also enhance leaf area, plant height, and aboveground biomass, thereby improving the crop’s competitive ability against weeds. However, in weedy conditions, maize exhibited reduced maximum leaf area and plant height, particularly under lower nitrogen application rates [18]. Several authors have demonstrated that application of nitrogen and phosphorus can modify weed communities in maize crops [10,19]. Simard and Ziadi [20] conducted a long-term (24–25 years) study evaluating the effects of nitrogen and phosphorus fertilization and tillage on weed populations. They found that no-till plots had generally higher weed densities and biomass, including annual grasses, annual broadleaf, and perennial broadleaf species. Interestingly, they did not observe significant interactions between fertilization treatments and weed density, biomass, or species composition.
Tillage has a well-documented effect on weed communities [21,22]. Reduced or no-tillage systems usually require additional herbicide inputs due to the absence of mechanical weed suppression [23,24]. Such systems often lead to increased prevalence of grassy and perennial weed species [25]. In contrast, conventional tillage tends to favor annual broadleaf weeds, such as Chenopodium album and Abutilon theophrasti [26].
The impact of continuous herbicide use on weed populations is most pronounced under intensive farming practices [27]. In Hungary, herbicides represented the largest share of pesticide sales in 2024. Following glyphosate—which is extensively applied across all crop types and habitats—the herbicides with the highest market volumes were those containing a mixture of S-metolachlor and terbuthylazine (marketed as Gardoprim Plus Gold until 2024), tembotrione (marketed as Laudis), and a combination of pendimethalin and dimethenamid-P (marketed as Wing P) [28]. All of these products are (or were) registered for use in maize cultivation, for which a total of 28 active ingredients and 109 herbicide formulations were approved [29].
For both chemical and non-chemical weed control strategies, site-specific detection and management offer significant advantages. These include reductions in chemical and fuel usage, minimization of environmental impacts, and the potential to maintain or enhance crop yields [30,31,32].
Therefore, the objective of this study was to identify the most prevalent weed species in maize fields and to evaluate their spatial and temporal variability. Specifically, the study investigated how weed species richness, cover, and diversity varied across regions, years, and cultivation practices. The influence of soil characteristics (e.g., humus content, pH, nutrient availability), environmental conditions (e.g., climate variables, geographic location), and farming practices (e.g., tillage, fertilization, crop rotation) on weed community composition and abundance was examined in detail. Additionally, the potential of certain weed species to serve as bioindicators of specific environmental conditions or farming systems was assessed. Finally, the study aimed to evaluate the relative influence of farmer-managed variables versus broader climatic and regional factors on the structure of weed communities.
2. Materials and Methods
2.1. Description of the Regions Concerned
Each of the analyzed regions exhibits a significant prevalence of maize cultivation. The selection of these areas was guided by criteria related to soil properties, hydrological conditions, and geographic elevation (above sea level). Within each region, fields were chosen that reflect standard practices in crop rotation, soil tillage, and nutrient management.
Region 1: the Sajó-Hernád Plain microregion is part of the Great Plain and lies on the periphery of the North Great Hungarian Plain, extending deep into the Northern Central Mountains. The spatial distribution of the surveyed plots was between N 48.082778°–48.120278° and E 20.932056°–21.074250° (Figure A1). The landscape is characterized by a mix of gently rolling plains and slightly hilly terrain. The climate is moderately warm and dry, with meteorological data for the study period presented in Table 1. This loess-covered foothill region features a conical terrain and is predominantly used for arable farming. It has moderately deep groundwater levels and is composed of soil types such as chernozem, brown forest soil, and meadow chernozem. Human activity has significantly altered the natural environment, creating a heterogeneous, mosaic-like landscape. Extensive modifications have been made to the topography and hydrological network, leading to noticeable changes in all soil characteristics. Currently, natural vegetation covers only about 20% of the microregion. The Shannon diversity index ranges between 0.66 and 1.38, indicating moderate biodiversity. In light of projected climate change, the potential and necessity for altering existing land use patterns is considered moderate [33].

Table 1.
Yearly precipitation and average temperature data of surveyed regions [34].
Region 2: In this region, four micro-landscape units with similar characteristics were identified: Szoboszlói-Hajdúság, Hajdúhát, Löszös-Nyírség, and Nagykállói-Nyírség. All belong to the Great Hungarian Plain macroregion and are situated within the Danube–Tisza Interfluve. The spatial distribution of the surveyed plots was between N 47.811682°–48.160491° and E 21.485486°–21.762198° (Figure A1). These areas are characterized by a moderately continental climate and gently undulating plains. The meteorological conditions in the region are shown in Table 1. The predominant soil types include loess, chernozem, humus-rich sandy soils, and wind-blown (drifting) sand soils. While the original natural conditions remain partially intact, they have been moderately affected by human activity. Intensive arable farming has further disturbed the soil structure, with deflation and compaction commonly observed. These processes have negatively impacted water retention and soil aeration. Currently, natural vegetation covers only 10–20% of the area. The Shannon diversity index ranges from 0.61 to 1.72, indicating a moderately diverse ecosystem. The region is moderately vulnerable to natural hazards, particularly wind erosion and drought, while the risk of inland water flooding remains high. Projected climate change may lead to moderate changes in the current land use structure [33].
Region 3: The Körös Plain, part of the Great Hungarian Plain macroregion, is characterized by a moderately continental climate, high groundwater levels, and a formerly flooded landscape that has been extensively drained. It is predominantly an agricultural area, featuring meadow and, in some parts, saline alluvial meadow soils. The climate is warm and dry, with meteorological data for the study period presented in Table 1. This region is an entirely flat plain, defined by large arable fields interspersed with rows of trees and shrubs marking parcel boundaries. The natural landscape has been heavily modified by human activity, classifying it as polyhemerobic—meaning it has significantly deviated from its original state. Large-scale water management interventions have altered both the topography and soil conditions. As a result, the extent of natural vegetation is now very limited, covering less than 20% of the area. The Shannon diversity index for land use in this region is low, measured at 1.06, compared to the national average of 1.41, indicating limited landscape diversity. The area is highly vulnerable to environmental risks, particularly flooding, waterlogging, and drought. With projected climate change impacts, the sensitivity of current land use is expected to increase, suggesting a high probability of significant land use transformation [33]. The spatial distribution of the surveyed plots was between N 46.784400°–46.967608° and E 20.823639°–21.081198° (Figure A1).
Meteorological records were compiled for the regions and time periods under investigation. Although precipitation amounts differed across locations, seasonal patterns remained relatively consistent. In Region 1 and Region 3, the wettest years observed were 2018 and 2021, whereas 2020 experienced near-average rainfall. Conversely, within Region 2, the highest levels of precipitation were recorded in 2020, as well as in 2018 and 2021. The year 2019 was notably dry in all regions. Annual mean temperatures across the regions ranged from 10.6 °C to 12.2 °C. Drier conditions were generally associated with elevated average temperatures in all areas studied (Table 1).
2.2. Methodology of Data Collection
To characterize the typical weed flora in the target areas and to evaluate the influence of environmental conditions and agricultural practices on weed communities, a total of 90 maize fields were surveyed between 2018 and 2021 across three sub-regions in Eastern Hungary (ranging from 46.784400° to 48.160491° N, 20.823639° to 21.762198° E). Although the maize cultivars grown across the 90 surveyed fields differed, all belonged to the FAO maturity group 300. Weed species identification followed the nomenclature of the EPPO database [35]. In cases where multiple taxa fall under a broader species concept (sensu lato), the collective species name was used—for example, Veronica hederifolia. The locations and regional distribution of the studied maize fields are shown in Figure A1.
Weed composition was assessed by recording all occurring species and their cover values, defined as the percentage of the soil surface occupied by the above-ground parts of weeds [36]. This assessment was conducted in eight randomly distributed 1 × 1 m quadrats per field, placed at least 10 m from the field margins to avoid edge effects [37]. For all subsequent analyses, species data were averaged at the field level.
Weed presence was evaluated in relation to the life form of each species, categorized into therophytes (annuals—either winter or summer types), hemitherophytes (biennials), and perennials, including geophytes and phanerophytes, following established classifications [38,39]. Additionally, species were grouped based on their taxonomic identity [35] and photosynthetic mechanisms [40,41].
A range of variables influencing weed distribution—collectively termed as explanatory variables—was documented for all sampled fields. These included soil properties, environmental factors, and management practices, gathered through laboratory soil testing, GPS-based datasets, and structured interviews with farmers.
The timing of the weed surveys (conducted between 8 May and 10 July; BBCH 13–19 of the Maize) was aligned with the schedule of herbicide treatments. Species data were collected approximately one week prior to chemical weed control in order to capture the fullest extent of weed infestation within each field. Due to this direct link with agricultural management, the ‘survey date’ was categorized as a farming-related variable.
Field size was also documented, under the assumption that variations in the edge-to-area ratio could influence both weed community structure and management practices.
In addition, the type of preceding crop was classified into three categories: (1) ‘maize’, (2) ‘other wide-row crops’—including sunflower, tomato, oilseed pumpkin, and watermelon—and (3) ‘dense crops’, such as winter wheat, winter barley, canary grass, winter pea, and winter oilseed rape.
The type and depth of soil tillage implemented in the year prior to data collection were documented. Three distinct tillage practices were identified across the examined sites: (1) shallow tillage, carried out with disc harrows or tine cultivators to a depth of 15–20 cm; (2) conventional ploughing, reaching depths of 20–35 cm; and (3) deep loosening, performed at depths ranging from 30 to 40 cm. In the statistical analyses, tillage type was treated as a categorical factor, whereas tillage depth was incorporated as a continuous variable. Additionally, the quantities of nitrogen (N), phosphorus (P), and potassium (K) fertilizers—expressed in terms of active ingredient—applied during the preceding growing season were also included in the evaluation (see Table 2).

Table 2.
Units and value ranges of environmental and agricultural explanatory variables.
2.3. Data Preparation
Prior to statistical analysis, species cover data from the eight quadrats within each field were aggregated to calculate the mean weed composition per field. These average values were then subjected to a Hellinger transformation [42], which served as the basis for subsequent multivariate analyses.
To assess the overall significance of each weed species, both the untransformed mean cover values and their constancy (i.e., frequency of occurrence at the field level) were computed across all study sites.
Interrelationships among explanatory variables were evaluated using generalized variance inflation factors (GVIFs) and derived variance inflation factors
(VIF = GVIF^(1/(2 df))).
These assessments revealed strong correlations between geographical variables (altitude, latitude, longitude) and regional categories, as well as between tillage type and tillage depth. Despite these associations, all variables were retained in the statistical models to determine which component of each correlated pair exerted a greater influence. A moderate correlation was also found between regional classification and soil properties—understandable, as regional boundaries were partly defined based on edaphic characteristics—yet all corresponding VIF values remained below 5 [43], indicating acceptable levels of multicollinearity.
To quantify species diversity, the Shannon diversity index (H’) was calculated for each field using the raw (non-transformed) cover values of weed species. The index was computed with the following formula:
where R represents the total number of species in a given field and pi denotes the proportion of total cover attributed to the ith species [44].
2.4. Statistical Analysis Procedure
The initial phase of the statistical analysis involved conducting Analyses of Covariance (ANCOVA) to identify which explanatory variables—encompassing environmental factors, and farming practices—had a significant impact on total weed cover, species richness, and the Shannon diversity index, each analyzed independently [45]. When ANCOVA results indicated a significant influence of continuous variables, Pearson correlation coefficients were calculated to determine the strength and direction of these relationships [46]. The correlation strength was interpreted as follows: values between 0 and 0.19 indicated a very weak correlation, 0.20 and 0.39 weak, 0.40 and 0.59 moderate, 0.60 and 0.79 strong, and 0.80 and 1.00 very strong correlation. For categorical variables found to be significant in the ANCOVA, pairwise comparisons among factor levels were performed using the Tukey post hoc test to identify statistically meaningful differences [47].
In the subsequent phase of analysis, redundancy analysis (RDA) was employed to evaluate how soil, environmental, and agricultural factors collectively influence weed species composition [48]. To refine the model, a backward stepwise variable selection procedure was applied [49], ultimately producing a simplified model comprising twelve explanatory variables. This RDA also aimed to quantify both the gross and net contributions of each significant variable. A unified ranking of variable importance within the reduced model was then derived, based on the adjusted R2 values calculated from partial RDA models representing the net effects of individual variables. To further explore associations between key explanatory variables and weed taxa, the ten species demonstrating the highest levels of variation explained along the constrained axes were identified for each partial RDA as well as for the full RDA model. Species occurring in fewer than three fields were excluded from this selection.
All statistical computations were carried out using a 90% confidence threshold in the R statistical environment (R Development Core Team, version 4.5.0), utilizing the following R packages: car (v3.1–3), lattice (v0.22–7), MASS (v7.3–65), permute (v0.9–7), and vegan (v2.6–10).
3. Results
3.1. Weed Vegetation Characterization Across the Examined Regions
In the maize fields we examined, the average weed cover was 9.7%; however, substantial variation in weed infestation was observed among individual fields. The lowest recorded weed cover was 0.1%, while the highest reached 37.5%. Among the surveyed fields, 48% had a weed cover exceeding 5%, 38% exceeded 10%, 18% exceeded 20%, and 8% exceeded 30% (Figure 1).
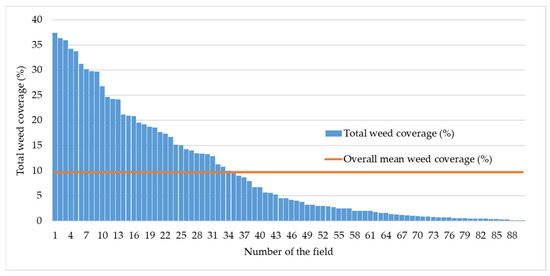
Figure 1.
Overall weed cover across the surveyed maize fields.
A total of 51 weed species were recorded in the maize fields surveyed between 2018 and 2021. On average, the highest cover percentages were exhibited by Echinochloa crus-galli (2.0%), Chenopodium album (1.4%), Portulaca oleracea (0.9%), and Hibiscus trionum (0.8%). Collectively, these four species accounted for more than half (52%) of the total weed cover. In total, the 20 most dominant species contributed 95% of the overall weed coverage (Figure 2).
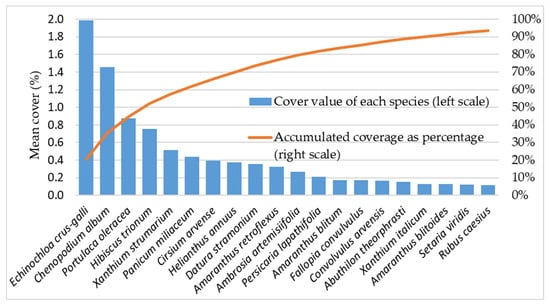
Figure 2.
Mean percentage cover of the most prevalent weed species observed in the surveyed maize fields.
In most cases, a strong correlation was found between weed cover and frequency of occurrence. This relationship is exemplified by the dominance of Echinochloa crus-galli and Chenopodium album, followed by Hibiscus trionum and volunteer Helianthus annuus. Conversely, certain species such as Convolvulus arvensis exhibited high constancy despite low cover values, while Portulaca oleracea, which had the third highest cover, was present in only 21% of the surveyed fields (Figure 3).
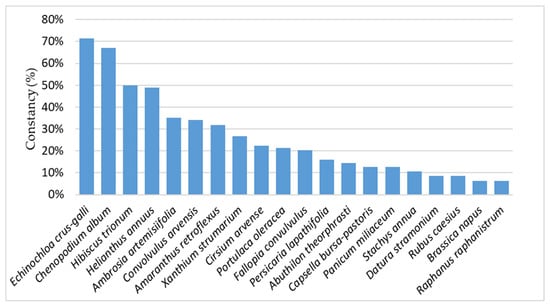
Figure 3.
Weed species most frequently encountered across the maize field sites surveyed.
Weed species utilizing the C4 photosynthetic pathway—such as Echinochloa crus-galli, Chenopodium album (C3–C4 intermediate), and Portulaca oleracea—were particularly significant in terms of both cover and constancy across the study sites. Most of the recorded weeds were dicotyledonous species, with only a few monocotyledons and Equisetum arvense occurring in the maize fields. Regarding life form classifications, the majority of species were summer annuals. Nevertheless, winter annuals (e.g., Capsella bursa-pastoris) and perennials (e.g., Cirsium arvense, Convolvulus arvensis) were also identified. Table A1 summarizes the taxonomic and life form classifications, regional occurrences, cover percentages, and constancy values of all recorded species.
A strong correlation was observed between the average weed cover and species frequency ranking. However, some species showed high occurrence rates despite moderate cover (e.g., Helianthus annuus), whereas others exhibited substantial cover but occurred less frequently (e.g., Portulaca oleracea) (Figure 4).
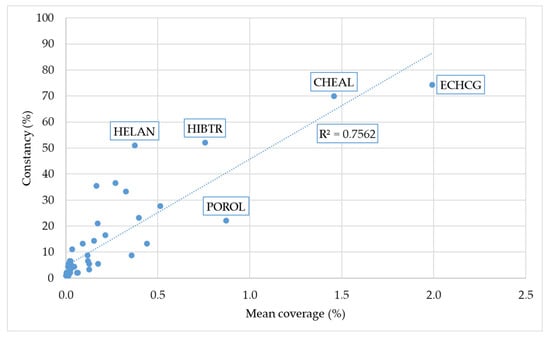
Figure 4.
Relationship between average weed species cover and their constancy across sites. (CHEAL, Chenopodium album; ECHCG, Echinochloa crus-galli; HELAN, Helianthus annuus; HIBTR, Hibiscus trionum; POROL, Portulaca oleracea).
3.2. Influence of Environmental and Management Factors on Total Weed Cover, Species Richness, and Diversity in Surveyed Maize Fields
The results of the statistical analysis showed that soil texture influenced both Shannon diversity and the number of weed species. The strength of the correlation was medium to weak; higher soil compaction was associated with increased weed species richness and greater diversity. Similarly, soil pH affected Shannon diversity and the number of weed species; alkaline soils exhibited higher species richness and diversity but did not significantly influence total weed coverage. Soil humus content had a weak positive correlation with the number of weed species only. Among the macronutrients, the effect of phosphorus could not be confirmed for any of the parameters studied. Nitrogen showed a negative correlation with species richness and Shannon diversity. Potassium had a low but positive correlation (r = 0.18) with species richness.
Soil lime content was negatively correlated with total weed coverage but positively associated with Shannon diversity. The meso- and micronutrient content of the soil also influenced weed growth in maize fields. Zinc and sodium levels were positively correlated with both species richness and Shannon diversity. High magnesium concentrations were associated with increased diversity, while elevated sulfur and copper levels positively affected the number of weed species. Manganese content showed a positive correlation with both total weed coverage and species richness.
The geographical location of the study sites also influenced the weed populations. Higher altitudes were associated with increased weed coverage. Both latitude and longitude were negatively correlated with weed diversity; fields located further south and west had higher species diversity. Longitude also exhibited a moderate negative correlation (r = −0.40) with the number of weed species. While Shannon diversity was not significantly affected by regional location, both total weed coverage and species richness were. Regions 1 and 3 showed similar weed characteristics, whereas fields in Region 2 had lower weed coverage and fewer weed species.
Regarding the temporal dimension of the study, differences in weed coverage were observed only between the years 2019 and 2020. The number of weed species was highest in 2019 compared to all other years. However, no significant differences in Shannon diversity were found across the years.
Subsequent weed surveys revealed higher total weed coverage but lower Shannon diversity values. Field size had no significant effect on weed coverage, species richness, or diversity. Tillage influenced both species number and Shannon diversity. Shallow cultivation resulted in lower species richness and diversity compared to deep loosening and plowing. This finding was also consistent with the quantified depth of cultivation.
Analysis of fertilization effects indicated that higher nitrogen input increased both species richness and diversity. Phosphorus fertilization positively affected total weed cover and species number, while potassium fertilization had a positive effect on all aspects: weed cover, species richness, and Shannon diversity (Table 3).

Table 3.
Summary of ANCOVA outcomes, Pearson correlation coefficients (for significant continuous predictors), and Tukey’s post hoc comparisons (for categorical predictors), examining how environmental and agronomic variables influence weed community characteristics in the Hungarian maize study (2018–2021).
3.3. Impact of Environmental and Management Variables on Weed Species Composition
At a 90% significance level, twelve measured variables were found to influence weed species composition, collectively explaining 26% of the total variance. Among these, soil-related factors—specifically humus, zinc, and copper content—together accounted for 5.35% of the total variance. Among all factors, the region alone explained the largest proportion of the variance (4.81%), followed by growing season (3.21%), tillage (3.17%), and the preceding crop (2.86%). The effects of weed survey date (1.38%) and fertilization (1.35% for nitrogen, 1.38% for phosphorus, and 1.25% for potassium) were relatively minor. Notably, the combined explanatory power of variables under farmers’ control (12.66%) was nearly equal to that of environmental factors (13.37%) (Table 4).

Table 4.
Redundancy analysis (RDA) results showing the influence of key environmental predictors on weed species composition in the maize study conducted in Hungary between 2018 and 2021.
As the humus content increased in the studied maize fields, the coverage of Chenopodium album, Amaranthus retroflexus, and Polygonum aviculare also increased. Conversely, the presence of other amaranth species (A. blitum and A. blitoides) and Ambrosia artemisiifolia decreased with higher humus content. Among the microelements, higher concentrations of copper had a positive effect on the occurrence of Helianthus annuus, Xanthium strumarium, Datura stramonium, and Ambrosia artemisiifolia, but reduced the presence of Polygonum aviculare, Amaranthus blitoides, and Cirsium arvense. Elevated zinc levels had a particularly beneficial impact on Chenopodium album and also promoted the growth of Convolvulus arvensis and Aristolochia clematitis. However, high zinc concentrations appeared to create unfavorable conditions for Datura stramonium, Panicum miliaceum, and Helianthus annuus.
Fertilizer application also significantly influenced weed composition. Convolvulus arvensis, Xanthium italicum, and Persicaria lapathifolia were more frequently observed with nitrogen fertilization, whereas Amaranthus retroflexus, Lathyrus tuberosus, and Echinochloa crus-galli showed reduced coverage under the same conditions. Higher phosphorus levels favored Hibiscus trionum, Capsella bursa-pastoris, and Equisetum arvense, while negatively affecting Amaranthus blitoides, Xanthium strumarium, and Ambrosia artemisiifolia. Increased potassium fertilization enhanced the coverage of Helianthus annuus, Abutilon theophrasti, and Chenopodium polyspermum, but decreased the presence of Chenopodium album, Hibiscus trionum, and Persicaria lapathifolia (Table 5).

Table 5.
Species identities, ordination scores, and model fit statistics for the taxa most strongly aligned with the primary constrained axis in the partial redundancy analysis (pRDA), focusing on soil and nutrient-adjusted variables in the Hungarian maize experiment (2018–2021).
Seasonal effects also revealed significant variations in weed composition over the study years. In 2018, the most prevalent weed species were Hibiscus trionum, Persicaria lapathifolia, and Amaranthus blitum. In 2019, Fallopia convolvulus was dominant; however, Rubus caesius and Equisetum arvense were also commonly observed. In 2020, Portulaca oleracea, Hibiscus trionum, and Setaria viridis were the most frequent species, whereas Ambrosia artemisiifolia and Chenopodium album were among the least common. In the following year, Panicum miliaceum and Ambrosia artemisiifolia were the most dominant, followed by Echinochloa crus-galli. Weed flora composition also varied across the studied regions. In Region 1, dominant species included Echinochloa crus-galli, Fallopia convolvulus, and Rubus caesius. In Region 2, the most significant species were Portulaca oleracea, Ambrosia artemisiifolia, and Amaranthus blitum. In Region 3, the most characteristic weeds were Cirsium arvense, Datura stramonium, and Lathyrus tuberosus (Table 6).

Table 6.
Species identities, ordination scores, and model fit statistics for the taxa most strongly aligned with the primary constrained axis in the partial redundancy analysis (pRDA), focusing on growing season and region variables in the Hungarian maize experiment (2018–2021).
The timing of weed surveys also influenced species prevalence. Helianthus annuus, Chenopodium album, and Persicaria lapathifolia were more abundant at later sampling dates, while Datura stramonium and Portulaca oleracea were more prevalent in earlier samples. Field size significantly influenced weed flora development. In larger fields, Helianthus annuus and Amaranthus blitoides were more abundant, whereas smaller fields were dominated by Cirsium arvense, Fallopia convolvulus, and Rubus caesius. The type of preceding crop also had a notable impact on weed composition. The abundance of Chenopodium album, Persicaria lapathifolia, and Amaranthus blitum increased following maize cultivation. Conversely, Portulaca oleracea, Cirsium arvense, and Convolvulus arvensis dominated fields previously sown with high-density winter crops. When the preceding crop had wide row spacing, the most abundant weed species were Panicum miliaceum, Abutilon theophrasti, and Xanthium italicum.
Tillage method also influenced weed presence. Deep loosening increased the occurrence of Xanthium strumarium, Lathyrus tuberosus, and Cirsium arvense, while reducing Persicaria lapathifolia and Portulaca oleracea. Plowing favored the emergence of Echinochloa crus-galli, Persicaria lapathifolia, and Abutilon theophrasti, while reducing Xanthium strumarium and Portulaca oleracea. Under shallow cultivation, Portulaca oleracea and Setaria viridis were dominant, whereas Ambrosia artemisiifolia, Echinochloa crus-galli, and Chenopodium species were less frequent (Table 7).

Table 7.
Species identities, ordination scores, and model fit statistics for the taxa most strongly aligned with the primary constrained axis in the partial redundancy analysis (pRDA), focusing on farming variables in the Hungarian maize experiment (2018–2021).
The gradient formed by the three study regions showed a strong correlation with soil nutrient availability (humus, Cu, Zn). In Region 1, weed species typical of nutrient-rich environments dominated, in contrast to Region 2.
4. Discussion
4.1. Weed Composition of Maize Fieds Studied
Our results indicate that the average weed cover in the surveyed maize fields was 9.7%, ranging from 0.1% to 37.5%. Given the competitive ability of maize, this level of weed infestation may already pose a risk of yield reduction [3]. However, to fully understand the yield-reducing effects of weed vegetation, it is essential to assess not only weed biomass (i.e., cover), but also weed density [50] and species composition [51].
The weed flora observed in the study area differed from the results of the Sixth National Weed Survey in Hungary [5], primarily due to pronounced regional variations in species composition. According to national data, Ambrosia artemisiifolia was identified as the most significant late-summer weed species; however, it exhibited only limited presence within the surveyed area. In contrast, Echinochloa crus-galli, which ranked third at the national level, emerged as the most prevalent species in our survey. Additionally, certain species—such as Chenopodium album, Hibiscus trionum and Datura stramonium—were also dominant both nationally and locally. Conversely, some weed species of national importance, including Sorghum halepense, and Setaria pumila, were not exhibited in the study region. Meanwhile, other species that are less significant or unregistered at the national level, such as Portulaca oleracea, Xanthium strumarium, and Rubus caesius, were frequently encountered locally.
The fact that more than half of the total weed cover was accounted for by just four species—three of which (Echinochloa crus-galli, Chenopodium album, and Hibiscus trionum) exhibited the highest constancy values—suggests that a few dominant weed species characterize the weed flora of maize fields in the studied regions. Similar findings were reported by Vidotto et al. [52] in their weed surveys of maize fields in Italy, where Echinochloa crus-galli and Chenopodium album emerged as the most widespread weed species. Echinochloa crus-galli is prevalent in arable fields worldwide due to its ability to germinate and flower across a wide range of environmental conditions [53].
The widespread occurrence of Chenopodium album has also been confirmed by several previous studies [30,52]. This species is among the five most prevalent weeds globally, ranks as the seventh most common weed in maize, and has been one of the most problematic species in the U.S. Corn Belt for decades [54,55]. A report identified the most common weed species in maize in the following European countries in 2011: Belgium, the Czech Republic, Denmark, France, Germany, Hungary, Italy, Poland, Romania, Spain (in both the northern and southern regions), and the United Kingdom. A total of 203 different weed species were reported, 61 of which were classified as ‘very common’ in at least one of the countries surveyed. It is noteworthy that Chenopodium album was the only species to be categorized as ‘very common’ in all 11 countries [56].
Among the four most dominant species, Echinochloa crus-galli and Portulaca oleracea are C4 plants, like maize, while Chenopodium album exhibits intermediate C3–C4 photosynthesis. This may provide them with a competitive advantage over C3 weed species, particularly during hot summer months and under intense light conditions [57].
The more vigorous growth of the C4 species is also reflected in their cover values. The nine C4 species recorded (including Chenopodium album) accounted for a total of 5.51% of weed cover (0.61% per species), in contrast to the 42 C3 species, which collectively contributed 4.21% (0.10% per species).
4.2. Effect of Soil Conditions on Weed Population
Based on the results, it can be concluded that the physical and chemical properties of the soil, as well as agronomic interventions, significantly influence the composition and diversity of weed flora in maize fields. Soil texture (as indicated by the Arany-type plasticity index) and soil pH affected both Shannon diversity and weed species richness, suggesting that soil structure and chemical properties play a key role in shaping weed communities. Previous studies have also supported the relationship between soil physical and chemical properties and the composition of weed communities [58,59].
The role of micro- and meso-elements is also notable: in particular, magnesium, manganese, sulfur, and zinc showed a positive correlation with weed diversity and species richness, providing further evidence that weed vegetation responds sensitively to changes in soil mineral composition. The dual effect of lime content (a negative correlation with weed cover but a positive one with diversity) may indicate the suppression of highly competitive species. The concentration of micro- and meso-elements in the soil can influence nutrient uptake by both maize and weed species; however, direct evidence linking specific micronutrient levels to changes in weed species diversity in maize fields remains limited. Some studies [60] suggest that certain weed species may thrive in soils with higher micronutrient availability, potentially influencing which species become dominant. However, the direct effect on overall weed diversity has not been firmly established.
Weeds in maize fields often accumulate higher concentrations of micronutrients such as iron (Fe) and manganese (Mn) compared to maize itself. For example, species such as Convolvulus arvensis and Echinochloa crus-galli have been shown to accumulate higher Fe and Mn concentrations in their tissues [60].
Among the soil parameters, humus, copper, and zinc contents showed a correlation with weed species composition. Based on redundancy analysis (RDA), several indicator weed species were identified. The positive correlation between humus content and the presence of Chenopodium album and Amaranthus retroflexus suggests that these nitrophilous species prefer nutrient-rich soils with higher humus levels, which is consistent with previous findings [61]. In contrast, the more frequent occurrence of Ambrosia artemisiifolia and Amaranthus blitoides in samples with lower humus content indicates that these species are less associated with nutrient-rich habitats and are well adapted to degraded environments. The strong adaptability of Amaranthus blitoides to such conditions has also been confirmed by other studies. This species is capable of establishing in a wide range of habitats, frequently occurring in agricultural fields, urban areas, riverbanks, and dry, sandy sites, which demonstrates its high tolerance for disturbed environments [62,63]. Ambrosia artemisiifolia is likewise recognized for its ability to colonize and persist in degraded or contaminated soils. Previous research has investigated its growth responses, adaptation mechanisms, and spread across various challenging environments, including those affected by heavy metal contamination and altered soil conditions [64,65].
The positive correlation of Helianthus annuus, Xanthium strumarium, and Datura stramonium with higher soil copper content suggests that these species are capable of tolerating elevated levels of copper in the soil. Sunflower (Helianthus annuus) appears particularly tolerant to high copper concentrations. Experiments utilizing sunflower for the remediation of copper-contaminated soils have demonstrated removal efficiencies of up to 85%. Studies indicate that sunflower accumulates copper primarily in its roots, making it an effective candidate for the phytoremediation of copper-polluted soils [66]. Further research shows that Xanthium strumarium can accumulate substantial amounts of copper in its tissues—especially in the roots—and is able to maintain growth even in soils with high concentrations of copper and other heavy metals, such as cadmium and nickel. This suggests strong metal tolerance and adaptability to metalliferous environments [67,68]. Similarly, D. stramonium has been shown to tolerate and accumulate various heavy metals, including copper, cadmium, and chromium, highlighting its adaptability to polluted soils [69,70]. In contrast, the negative correlation of Cirsium arvense with soil copper content indicates its sensitivity to elevated copper levels, suggesting that its presence may serve as an indicator of copper-poor habitats. Other studies have reported a significant positive relationship between soil copper levels and weed frequency, particularly at specific soil depths and during rainy periods. This implies that copper availability may influence both the structure of weed communities and the composition of the soil seed bank [71,72].
High soil zinc concentrations serve as indicators of the favorable presence of Chenopodium album, and also promote the occurrence of Convolvulus arvensis and Aristolochia clematitis. The competitiveness of weed species in zinc uptake depends on both the weed species and the specific crop in which they occur. Research by Malicki and Berbeciowa [73] demonstrated that Chenopodium album is more competitive in zinc uptake than winter wheat and spring barley, yet accumulates less zinc compared to sugar beet and spring rape.
4.3. Effect of Environmental Factors on Weed Population
The most significant factors influencing species composition were the region and the year. Regional classification functions as a composite factor, reflecting the environmental conditions of the studied areas, which are shaped by soil properties, geographical location (altitude, longitude, latitude), annual precipitation, and average temperature. Our results revealed differences in total weed cover and species richness between regions; however, no significant differences were observed in species diversity.
Regarding the climatic conditions of the study sites, although the actual environmental factors (e.g., soil and water parameters, including their spatial and temporal distribution and quality) varied across geographically distinct areas, interannual variation exceeded regional variation. Year-to-year differences in temperature and precipitation likely contributed to the observed fluctuations in weed cover, species richness, and species dominance rankings. Dudic et al. [74] similarly reported temporal variation in the weed flora of maize fields in Serbia between two distinct years (2017 and 2024), confirming that both the specific year and associated weather conditions significantly influence weed coverage, species occurrence, and dominance patterns. For instance, in particularly hot and dry years, broadleaf weeds and therophytes tended to dominate, although the identity of the dominant species varied depending on annual temperature and precipitation conditions. In weed surveys conducted in fennel seed crops in Hungary, the year significantly affected total weed cover, but had no measurable effect on species richness or Shannon diversity at the field level [75]. The substantial influence of interannual weather variability and precipitation differences on weed flora composition has also been documented by Pinke et al. [16] and Nagy [37] in oil pumpkin, maize, and cereal cropping systems.
In addition to interannual variation, the timing of weed surveys within the same year also influenced the weed community. Surveys conducted later in the season typically recorded greater total weed cover but lower species diversity. Early-season surveys were dominated by species such as Portulaca oleracea and Datura stramonium, whereas late-season surveys were characterized by the dominance of Helianthus annuus and Chenopodium album. These patterns may be attributed to seasonal weather conditions, the developmental stage of maize, and the progression of the vegetation period. The findings align with previous studies, further supporting the notion that seasonality significantly affects weed community composition [37,52,76,77,78].
4.4. Effect of Farming Factors on Weed Population
Field size was also found to influence weed species composition. Larger fields favored the occurrence of Helianthus annuus and Amaranthus blitoides, while smaller fields were more frequently dominated by Cirsium arvense, Polygonum convolvulus, and Rubus caesius. The occurrence of Chenopodium album was not affected by field size. Smaller fields tend to have a greater proportion of poorly managed field margins, which are typically more susceptible to weed infestation [79], potentially influencing the weed flora even within the interior zones of the fields, as observed in this study. However, Nagy did not find a significant relationship between field size and species composition [37].
The preceding crop did not significantly affect total weed cover, species richness, or diversity. However, it did influence the dominance hierarchy of weed species. Fields that followed maize or had dense preceding crops exhibited distinct patterns of weed dominance, whereas those following “other wide-row” crops showed intermediate weed communities, more similar to those observed after maize. Fried et al. [14] demonstrated that both the crop type and the preceding crop primarily influence weed diversity and community structure. Other researchers have reported that crop rotation can affect Shannon diversity and the species richness of the soil weed seed bank [80].
Although specific herbicide applications were not recorded at the field level, the dominant herbicides used in preceding crops included sulfonylureas, imidazolinones (ALS inhibitors), and triketones (HPPD inhibitors). Different preceding crops typically require distinct herbicide regimes, which—when combined with the weed-suppressing effects of the previous crop (e.g., soil cover and competition)—shape the subsequent weed flora. Previous studies have shown that both the type of preceding crop and herbicide use significantly influence weed infestation in subsequent years [81]. A well-designed crop rotation, combined with effective herbicide management, which is common in Hungary [28,29], can substantially reduce weed populations [82] and reduce the risk of weed resistance [83].
Tillage type also affected species richness, with deeper tillage being associated with greater species richness and diversity. This is likely due to increased soil disturbance and improved germination conditions. Other studies have reported that while deep loosening generally does not significantly affect the density or biomass of annual weeds, it can reduce perennial weed populations and support higher crop yields [84].
Our RDA analysis confirmed that tillage methods significantly influence weed species composition. Different tillage systems generate varying physical and biological soil conditions—such as soil layer redistribution, seed bank disruption, and altered germination environments—that play a critical role in determining which species establish and dominate. Several studies have shown that long-term deep loosening, especially when integrated with other tillage systems, can reduce overall weed infestation [85].
Certain weed species responded positively to deep soil disturbance. Xanthium strumarium, Lathyrus tuberosus, and Convolvulus arvensis showed strong positive correlations with deep loosening, indicating their adaptability to such soil structures. Similarly, Datura stramonium and Chenopodium polyspermum were also favored by deep tillage.
The species composition associated with ploughing partially overlapped with that observed under deep loosening; however, several species exhibited opposing trends. Echinochloa crus-galli, Persicaria lapathifolia, and Abutilon theophrasti were positively associated with ploughing, suggesting a high regenerative capacity in disturbed environments. In contrast, Xanthium strumarium and Portulaca oleracea showed negative correlations with ploughing, indicating greater sensitivity to deep soil inversion or a preference for less disturbed conditions. Numerous studies have confirmed that dicot species tend to dominate in conventional plough-based systems [86,87].
Shallow tillage resulted in a distinctly different species composition. Notably, Portulaca oleracea exhibited a strong positive association and can be considered an indicator species for shallow tillage systems due to its ability to rapidly germinate from surface seed banks. Setaria viridis was also positively associated with shallow tillage. In contrast, Chenopodium album, Ambrosia elatior, and Cirsium arvense displayed negative correlations, suggesting a preference for deeper soil disturbance or a limited adaptability to shallow tillage conditions. Santín-Montanyá et al. [88] reported significant differences in weed abundance and Portulaca oleracea dominance between no-till and conventional tillage systems.
Overall, tillage significantly influenced weed species composition but had no measurable effect on total weed coverage.
Reducing tillage in corn and soybean production alters weed population dynamics, leading to increased populations of perennial species, summer annual grasses, biennials, and winter annuals; decreased densities of large-seeded dicot species; and reduced effectiveness of traditional weed control practices. These changes necessitate the development of new management strategies and control technologies [59].
Broader soil management practices, such as crop rotation and fertilization—which can alter micronutrient levels—have also been shown to affect weed community diversity and richness. Systems with diverse crop rotations and mineral fertilization tend to support higher weed biodiversity and more uniform weed communities compared to monocultures or systems with minimal fertilization [89].
Higher soil nitrogen levels were associated with reduced general weed species richness, though increased fertilizer application—particularly nitrogen—was correlated with higher weed species richness and diversity, potentially favoring the proliferation of fast-growing and competitive species. The application of potassium and phosphorus, especially in the form of fertilizers, had a positive effect on both species richness and weed cover, suggesting that different nutrients influence weed community composition through species-specific nutrient requirements or competitive abilities.
Fertilizer applications are most beneficial to crops when nutrient release is synchronized with peak crop demand and spatially targeted to crop rows. Otherwise, fertilization may promote weed growth (see [90,91]).
Long-term fertilization experiments have demonstrated that nitrogen-rich fertilization favors nitrophilous dicotyledonous species, whereas lower nitrogen levels tend to favor monocotyledons [92,93]. The highest nitrogen fertilizer rates were associated with the least heterogeneous weed communities [92].
The availability of key macronutrients—nitrogen, phosphorus, and potassium—also influenced the spatial distribution of weed species. Convolvulus arvensis, Xanthium strumarium, and Persicaria lapathifolia were positively associated with elevated nitrogen fertilizer levels, confirming their nitrophilous characteristics. In contrast, Lactuca tatarica and Echinochloa crus-galli were more commonly associated with low-nitrogen conditions.
The study by Jiaxiu Luo et al. [94] provides evidence that Xanthium strumarium possesses a high nitrate uptake capacity and significant biomass production. Conversely, other studies have indicated that Echinochloa crus-galli is highly tolerant of nitrogen-rich, low-oxygen environments, making it a particularly aggressive weed in rice cultivation systems [95]. Persicaria lapathifolia exhibits relatively limited nitrogen uptake compared to dominant species such as Phragmites communis and Phalaris arundinacea, suggesting a minimal role in nitrogen remediation within wetland ecosystems [96].
A similar pattern was observed for phosphorus, with Hibiscus trionum, Capsella bursa-pastoris, and Equisetum arvense indicative of areas with elevated phosphorus levels. Phosphorus plays a crucial role in the growth and reproductive success of C. bursa-pastoris. Studies have shown that when phosphorus is supplied in combination with nitrogen and potassium, C. bursa-pastoris produces a significantly greater number of seeds—exceeding 16,000 per plant under optimal conditions—with faster germination and higher germination rates. In contrast, phosphorus deficiency, particularly when coupled with high nitrogen and low potassium availability, results in reduced seed production and diminished germination success [97].
Helianthus annuus and Abutilon theophrasti were positively associated with higher potassium levels, corroborating their preference for potassium-rich environments. Potassium supplementation has been shown to enhance the growth of H. annuus [98].
Conversely, Hibiscus trionum and Persicaria lapathifolia were more frequently found in areas with low potassium input. The dominance of P. lapathifolia in wetland environments is closely tied to moderate potassium availability, which appears to shape its habitat preference and plant–soil interactions. P. lapathifolia demonstrates a strong relationship with soil potassium levels. In reservoir wetland ecosystems, the distribution and dominance of P. lapathifolia are significantly influenced by soil properties, particularly available potassium. Areas with moderate potassium concentrations (50–150 mg/kg) often support plant communities dominated by this species, suggesting that potassium availability is a key factor in its ecological niche and soil interactions [99].
In the RDA model, among the significant variables, it is noteworthy that fertilization (NPK) and shallow tillage had opposing effects on weed species composition (SC). This phenomenon has been previously demonstrated: long-term fertilization can reduce weed diversity and favor a few dominant species, particularly when high nutrient levels are applied [100], whereas shallow tillage can result in high weed densities and more diverse weed communities [101,102]. Similarly, the timing of the survey influenced weed community composition, as many new species may emerge later in the season [103,104], which was also observed in our study.
4.5. Connections Between Explanatory Variables
The effects of descriptive variables are often difficult to disentangle. For instance, soil quality parameters (humus, Cu, Zn) affected weed vegetation in a similar direction, but their effect contrasted with that of field size. This may be due to potentially insufficient nutrient replenishment in smaller fields [105]. Notably, Region 2, based on its independent explanatory effect, significantly contributed to the higher occurrence of Portulaca oleracea (Table 6). However, according to the full model (Figure 5), shallow tillage and survey date were the main explanatory variables for the presence of this species [88].
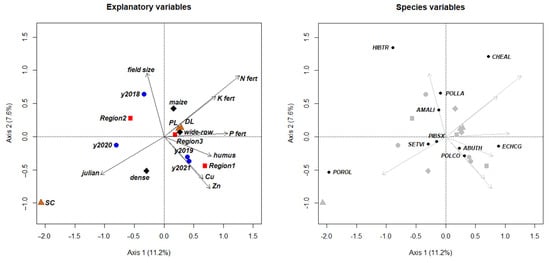
Figure 5.
Ordination plots from redundancy analysis (RDA), illustrating associations between significant environmental predictors (left panel) and weed species distribution patterns (right panel). (Arrow, numeric variable; blue cycles, year; brown triangle, tillage method; red square, region; black diamond, preceding crop; small black cycle, species; y2018–y2021, growing season between 2018 and 2021; DL, deep loosening; PL, ploughing; SC, shallow cultivation; maize, maize preceding crop; wide-row, other wide-row preceding crops; dense, dense preceding crops; humus, soil humus content; Cu, soil Cu content; Zn, soil Zn content; N fert, amount of N fertilizer; P fert, amount of P fertilizer; K fert, amount of K fertilizer; julian, date of weed survey; ABUTH, Abuthilon theophrasti; AMALI, Amaranthus blitum; CHEAL, Chenopodium album; ECHCG, Echinochloa crus-galli; HIBTR, Hibiscus trionum; PIBSX, Pisum sativum; POLCO, Fallopia convolvulus; POLLA, Persicaria lapathifolia; POROL, Portulaca oleracea; SETVI, Setaria viridis).
Overall, the correlations between the examined environmental and management parameters and total weed cover, species richness, and diversity were weak to moderate in strength (correlation coefficient < 0.6). Nevertheless, a large number of variables influenced the species composition of the weed community. Environmental factors accounted for a slightly higher proportion of the variance (13.37%) compared to farmer-dependent factors (12.66%), though the latter still had a considerable impact. Among the management-related variables, tillage practices and the effect of the previous crop emerged as the most influential. Several European studies have corroborated that climatic and edaphic factors generally exert a greater influence on weed community composition than land use [12,105,106,107].
5. Conclusions
The results clearly indicate that the weed flora is primarily shaped by site-specific environmental factors, particularly microclimatic conditions, soil management practices, and weed control methods. The findings suggest that different tillage systems are associated with district weed communities, and that certain weed species may even function as bioindicators of specific cultivation conditions.
A notable example is the occurrence of Portulaca oleracea and Xanthium strumarium, whose presence exhibited a strong correlation with shallow tillage practices. Weed flora can thus serve as an indicator in monitoring environmental conditions and assessing the impacts of agricultural management, potentially providing a valuable foundation for the development of more sustainable soil management systems and precision agriculture strategies.
Clear relationships were also observed between the presence of the examined weed species and specific chemical properties of the soil, particularly humus content and the concentrations of macro- and micronutrients (N, P, K, Cu, Zn). The varying tolerance levels and responses of weed species to these soil parameters suggest that weed flora composition is highly sensitive to changes in soil quality. Therefore, weed community analysis can offer valuable insights into the chemical status of soils.
An important finding is that both the quantitative and qualitative characteristics of weed flora are most strongly influenced by two factors—region and year—which are beyond the control of farmers. Nevertheless, the combined effect of soil management practices can surpass the influence of these uncontrollable external factors. This implies that by adopting appropriate tillage systems and management technologies, farmers may partially mitigate the adverse effects of climatic and regional variability.
For effective weed control in maize cultivation, the choice of preceding crop, as well as the type and quantity of applied nutrients, is of particular importance. Accordingly, the development of sustainable weed management systems necessitates careful consideration of site-specific soil characteristics and weed vegetation composition.
Future research should prioritize the identification and examination of bioindicator species, with particular emphasis on their indicative value and ecological stability. Investigating the mechanisms and dynamics underlying changes in weed communities under different tillage regimes and varying nutrient conditions would also provide valuable insights.
To improve the efficacy of weed control and support the long-term sustainability of soil systems, it is recommended that farmers adapt their tillage practices based on local weed population dynamics and soil characteristics. Shallow tillage should only be employed when it does not contribute to increased weed infestation. Additionally, crop rotation is advised, as it can interrupt the life cycles of dominant weed species. Furthermore, integrated weed management strategies should be prioritized over sole reliance on herbicides, particularly in regions characterized by high environmental variability.
Overall, it would be highly advantageous to utilize weed community composition more broadly in the future as a diagnostic tool for evaluating soil conditions and nutrient availability.
Author Contributions
Conceptualization, M.Z. and E.T.; methodology, M.Z.; software, M.Z.; validation, M.Z. and Z.D.; formal analysis, M.Z.; investigation, E.T.; resources, M.Z., J.G.N. and Z.D.; data curation, M.Z.; writing—original draft preparation, M.Z., E.T. and J.G.N.; writing—review and editing, M.Z., J.G.N. and Z.D.; visualization, M.Z.; supervision, M.Z.; project administration, M.Z.; funding acquisition, M.Z., J.G.N. and Z.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data on which the study is based are available on request from the corresponding author. The data are not publicly available.
Acknowledgments
We would like to thank the support of the Doctoral School of Plant Sciences of the Hungarian University of Agriculture and Life Sciences.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
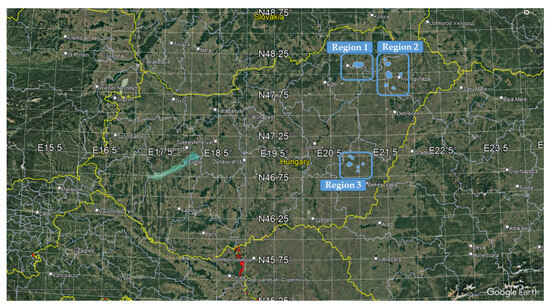
Figure A1.
Location and regional classification of the maize fields studied.
Appendix B

Table A1.
Taxonomic and ecological attributes, including scientific name, classification, dominant photosynthetic type, life form, regional occurrence, average cover, and constancy values for all recorded species in the study.
Table A1.
Taxonomic and ecological attributes, including scientific name, classification, dominant photosynthetic type, life form, regional occurrence, average cover, and constancy values for all recorded species in the study.
| Taxonomy A | Predominant Photosynthetic Pathway B | Life- form C | Geographical Distribution | Mean Coverage (%) | Constancy (%) | |||
|---|---|---|---|---|---|---|---|---|
| Scientific Name | Family | Region 1 | Region 2 | Region 3 | ||||
| Abuthilon theorphrasti | Malvaceae | C3 | SA | × | × | × | 0.15 | 14 |
| Amaranthus blitoides | Amaranthaceae | C4 | SA | × | × | 0.13 | 3 | |
| Amaranthus blitum | Amaranthaceae | C4 | SA | × | 0.18 | 5 | ||
| Amaranthus chlorostachis | Amaranthaceae | C4 | SA | × | 0.01 | 2 | ||
| Amaranthus retroflexus | Amaranthaceae | C4 | SA | × | × | × | 0.33 | 32 |
| Ambrosia artemisiifolia | Asteraceae | C3 | SA | × | × | × | 0.27 | 35 |
| Aristolochia clematitis | Aristolochiaceae | C3 | G | × | 0.04 | 4 | ||
| Asclepias syriaca | Apocynaceae | C3 | G | × | <0.01 | 1 | ||
| Brassica napus | Brassicaceae | C3 | WA | × | × | × | 0.02 | 6 |
| Calamagrostis epigeios | Poaceae | C3 | G | × | 0.01 | 2 | ||
| Cannabis sativa | Cannabaceae | C3 | SA | × | 0.02 | 2 | ||
| Capsella bursa-pastoris | Brassicaceae | C3 | WA | × | × | × | 0.09 | 13 |
| Chenopodium album | Amaranthaceae | C3–C4 D | SA | × | × | × | 1.46 | 67 |
| Chenopodium hybridum | Amaranthaceae | C3 | SA | × | × | × | 0.02 | 4 |
| Chenopodium polyspermum | Amaranthaceae | C3 | SA | × | 0.02 | 5 | ||
| Cirsium arvense | Asteraceae | C3 | G | × | × | 0.39 | 22 | |
| Convolvulus arvensis | Convolvulaceae | C3 | G | × | × | × | 0.16 | 34 |
| Datura stramonium | Solanaceae | C3 | SA | × | × | 0.36 | 9 | |
| Echinochloa crus-galli | Poaceae | C4 | SA | × | × | × | 1.99 | 71 |
| Equisetum arvense | Equisetaceae | C3 | G | × | × | 0.04 | 4 | |
| Fallopia convolvulus | Polygonaceae | C3 | SA | × | × | × | 0.17 | 20 |
| Galinsoga parviflora | Asteraceae | C3 | SA | × | <0.01 | 1 | ||
| Galium aparine | Rubiaceae | C3 | WA | × | 0.06 | 2 | ||
| Helianthus annuus | Asteraceae | C3 | SA | × | × | × | 0.37 | 49 |
| Hibiscus trionum | Malvaceae | C3 | SA | × | × | × | 0.76 | 50 |
| Iva xanthiifolia | Asteraceae | C3 | SA | × | 0.01 | 4 | ||
| Lamium purpureum | Lamiaceae | C3 | WA | × | <0.01 | 2 | ||
| Lathyrus tuberosus | Solanaceae | C3 | G | × | 0.01 | 5 | ||
| Medicago lupulina | Fabaceae | C3 | SA | × | 0.00 | 2 | ||
| Panicum miliaceum | Poaceae | C4 | SA | × | × | × | 0.44 | 13 |
| Papaver rhoeas | Papaveraceae | C3 | WA | × | <0.01 | 1 | ||
| Persicaria amphibia | Polygonaceae | C3 | G | × | <0.01 | 1 | ||
| Persicaria lapathifolia | Polygonaceae | C3 | SA | × | × | 0.21 | 16 | |
| Persicaria maculosa | Polygonaceae | C3 | SA | × | <0.01 | 1 | ||
| Phalaris canariensis | Poaceae | C3 | SA | × | <0.01 | 1 | ||
| Pisum sativum | Fabaceae | C3 | WA | × | 0.02 | 3 | ||
| Polygonum aviculare | Polygonaceae | C3 | SA | × | × | 0.03 | 4 | |
| Portulaca oleracea | Portulacaceae | C4 | SA | × | × | 0.87 | 21 | |
| Raphanus raphanistrum | Fabaceae | C3 | SA | × | × | 0.02 | 6 | |
| Rubus caesius | Rosaceae | C3 | G | × | × | 0.12 | 9 | |
| Setaria viridis | Poaceae | C4 | SA | × | × | 0.12 | 6 | |
| Silybum marianum | Asteraceae | C3 | HT | × | 0.01 | 1 | ||
| Solanum dulcamara | Solanaceae | C3 | Ph | × | 0.06 | 2 | ||
| Sonchus asper | Asteraceae | C3 | SA | × | <0.01 | 1 | ||
| Stachys annua | Lamiaceae | C3 | SA | × | × | 0.03 | 11 | |
| Stellaria media | Caryophyllaceae | C3 | WA | × | <0.01 | 1 | ||
| Tripleurospermum inodorum | Asteraceae | C3 | SA | × | 0.04 | 4 | ||
| Triticum aestivum | Poaceae | C3 | WA | × | 0.01 | 4 | ||
| Veronica hederifolia | Scrophulariaceae | C3 | WA | × | <0.01 | 1 | ||
| Xanthium italicum | Asteraceae | C3 | SA | × | × | 0.13 | 5 | |
| Xanthium strumarium | Asteraceae | C3 | SA | × | × | × | 0.51 | 27 |
A Source: [35]; B Source: [40,41,108]; C HT, hemitherophytes/biennials; G, geophyte perennials; Ph, phanerophyte perenniels; SA, summer annuals; WA, winter annuals. Source: [38,39]; D C3–C4 intermediate pathway. Source: [109,110].
References
- FAOSTAT Database. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 28 May 2025).
- Teasdale, J.R.; Cavigelli, M.A. Subplots facilitate assessment of corn yield losses from weed competition in a long-term systems experiment. Agron. Sustain. Dev. 2010, 30, 445–453. [Google Scholar] [CrossRef]
- Hunyadi, K.; Béres, I.; Kazinczi, G. Gyomnövények, Gyomirtás, Gyombiológia; Mezőgazda Kiadó: Budapest, Hungary, 2011; pp. 24–28. (In Hungarian) [Google Scholar]
- Gracza, L.; Gyulai, B.; Novák, R.; Szabó, L.; Simon, J.; Lang, B.; Doma, C.S.; Nagy, M.; Kovács, A.; Grünwaldné Almási, A.; et al. Egyes szulfonil-karbamidokkal szemben rezisztencia gyanús fenyércirok (Sorghum halepense L.) populációk vizsgálata Magyarországon. Magy. Gyomkutatás és Technológia 2015, 16, 76–78, (In Hungarian with an English Summary). [Google Scholar]
- Novák, R.; Magyar, M.; Simon, G.; Kadaravek, B.; Kadaravekné Guttyán, A.; Blazsek, K.; Erdélyi, K.; Farkas, G.; Gyulai, B.; Hornyák, A.; et al. A Hatodik Országos Szántóföldi Gyomfelvételezés előzetes eredményei. Magy. Gyomkutatás és Technológia 2019, 20, 55–58, (In Hungarian with an English Summary). [Google Scholar]
- Andreasen, C.; Streibig, J.C.; Haas, H. Soil properties affecting the distribution of 37 weed species in Danish fields. Weed Res. 1991, 31, 181–187. [Google Scholar] [CrossRef]
- Caussanel, J.P. Nuisibilite’ et seuil de nuisibilite’ des mauvaises herbes dans une culture annuelle: Situation de concurrence bispe´cifique. Agronomie 1989, 9, 219–240. [Google Scholar] [CrossRef]
- Dale, M.R.T.; Thomas, A.G.; John, E.A. Environmental factors including management practices as correlates of weed community composition in spring seeded crops. Can. J. Bot. 1992, 70, 1931–1939. [Google Scholar] [CrossRef]
- Boutin, C.; Baril, A.; Martin, P.A. Plant diversity in crop fields and woody hedgerows of organic and conventional farms in contrasting landscapes. Agric. Ecosyst. Environ. 2008, 123, 185–193. [Google Scholar] [CrossRef]
- Pysek, P.; Leps, J. Response of a weed community to nitrogen fertilization: A multivariate analysis. Veg. Sci. 1991, 2, 237–244. [Google Scholar] [CrossRef]
- Andersson, T.N.; Milberg, P. Weed flora and the relative importance of site, crop, crop rotation, and nitrogen. Weed Sci. 1998, 46, 30–38. [Google Scholar] [CrossRef]
- Lososova, Z.; Chytry, M.; Cimalova, S.; Kropac, Z.; Otypkova, Z.; Pysek, P.; Tichy, L. Weed vegetation of arable land in Central Europe: Gradients of diversity and species composition. J. Veg. Sci. 2004, 15, 415–422. [Google Scholar] [CrossRef]
- Fried, G.; Petit, S.; Reboud, X. A specialist-generalist classification of the arable flora and its response to changes in agricultural practices. BMC Ecol. 2010, 10, 20. [Google Scholar] [CrossRef]
- Fried, G.; Norton, L.R.; Reboud, X. Environmental and management factors determining weed species composition and diversity in France. Agric. Ecosyst. Environ. 2008, 128, 68–76. [Google Scholar] [CrossRef]
- Weaver, S.E.; Hamill, A.S. Effects of Soil pH on Competitive Ability and Leaf Nutrient Content of Corn (Zea mays L.) and Three Weed Species. Weed Sci. 1985, 33, 447–451. [Google Scholar] [CrossRef]
- Pinke, G.; Karácsony, P.; Czúcz, B.; Botta-Dukát, Z. When herbicides don’t really matter: Weed species composition of oil pumpkin (Cucurbita pepo L.) fields in Hungary. Crop Prot. 2018, 110, 236–244. [Google Scholar] [CrossRef]
- Pinke, G.; Karácsony, P.; Czúcz, B.; Botta-Dukát, Z. Environmental and land-use variables determining the abundance of Ambrosia artemisiifolia in arable fields in Hungary. Presila 2011, 83, 219–235. [Google Scholar]
- Evans, S.P.; Knezevic, S.Z.; Lindquist, J.L.; Shapiro, C.A. Influence of nitrogen and duration of weed interference on corn growth and development. Weed Sci. 2003, 51, 546–556. [Google Scholar] [CrossRef]
- Yin, L.C.; Cai, Z.C.; Zhong, W.H. Changes in weed community diversity of maize crops due to long-term fertilization. Crop Protect. 2006, 25, 910–914. [Google Scholar] [CrossRef]
- Simard, M.J.; Ziadi, N. Weed communities after decades of mineral fertilization and tillage treatments in a corn—soybean rotation. Weed Technol. 2024, 38, e5. [Google Scholar] [CrossRef]
- Légère, A.; Stevenson, F.C.; Ziadi, N. Contrasting responses of weed communities and crops to 12 years of tillage and fertilization treatments. Weed Technol. 2008, 22, 309–317. [Google Scholar] [CrossRef]
- Swanton, C.J.; Shrestha, A.; Roy, R.C.; Ball-Coelho, B.R.; Knezevic, S.Z. Effect of tillage systems, N, and cover crop on the composition of weed flora. Weed Sci. 1999, 47, 454–461. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Singh, R.G.; Mahajan, G. Ecology and management of weeds under conservation agriculture: A review. Crop Protect. 2012, 38, 57–65. [Google Scholar] [CrossRef]
- Locke, M.A.; Reddy, K.N.; Zablotowicz, R.M. Weed management in conservation crop production systems. Weed Biol. Manag. 2002, 2, 123–132. [Google Scholar] [CrossRef]
- Buhler, D.D.; Hatfield, J.L.; Stewart, B.A. Tillage systems and weed population dynamics and management. In Integrated Weed and Soil Management; CRC Press: Boca Raton, FL, USA, 1998; pp. 223–246. [Google Scholar]
- Buhler, D.D.; Oplinger, E.S. Influence of tillage systems on annual weed densities and control in solid-seeded soybean (Glycine max). Weed Sci. 1990, 38, 158–165. [Google Scholar] [CrossRef]
- Das, T.K.; Behera, B.; Nath, C.P.; Ghosh, S.; Sen, S.; Raj, R.; Ghosh, S.; Sharma, A.R.; Yaduraju, N.T.; Nalia, A.; et al. Herbicides use in crop production: An analysis of cost-benefit, non-target toxicities and environmental risks. Crop Prot. 2024, 181, 106691. [Google Scholar] [CrossRef]
- Medináné Lázár, V. Statisztikai Jelentések. Növényvédő Szerek Értékesítése 2024. Év; Agrárközgazdasági Intézet: Budapest, Hungary, 2025; pp. 3–10. Available online: https://www.aki.gov.hu/termek/novenyvedo-szerek-ertekesitese-2024-ev/ (accessed on 16 July 2025).
- NÉBIH Növényvédő Szerek Adatbázisa. Available online: https://novenyvedoszer.nebih.gov.hu/Engedelykereso/kereso (accessed on 16 July 2025).
- Reuter, T.; Nahrstedt, K.; Wittstruck, L.; Jarmer, T.; Broll, G.; Trautz, D. Site-specific mechanical weed management in maize (Zea mays) in North-West Germany. Crop Prot. 2025, 190, 107–123. [Google Scholar] [CrossRef]
- Maillot, T.; Vioix, J.-P.; Colbach, N. Site-specific herbicide spraying can control weeds as well as full spraying in the long-term. A simulation study. Comput. Electron. Agric. 2023, 214, 108338. [Google Scholar] [CrossRef]
- Gerhards, R.; Sanchez, D.A.; Hamouz, P.; Peteinatos, G.G.; Christensen, S.; Fernández-Quintanilla, C. Advances in site-specific weed management in agriculture—A review. Weed Res. 2022, 62, 12526. [Google Scholar] [CrossRef]
- Csorba, P. Magyarország Kistájai, 1st ed.; Meridián Táj-és Környezetföldrajzi Alapítvány: Debrecen, Hungary, 2021; p. 416. [Google Scholar]
- HungaroMet Meteorological Data Archive: Historical Data of Automatic Meteorological Stations. Available online: https://odp.met.hu/climate/observations_hungary/monthly/historical/ (accessed on 7 May 2025).
- EPPO Global Database. Available online: https://gd.eppo.int/search (accessed on 28 May 2025).
- Martin, J.R.; Green, J.D. Weed Management, 1st ed.; University of Kentucky: Lexington, KY, USA, 2009; pp. 30–41. [Google Scholar]
- Nagy, K. Investigation of Arable Weed Vegetation in Mures County. Ph.D. Dissertation, Széchenyi István University, Mosonmagyaróvár, Hungary, 2017. [Google Scholar]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Oxford University Press: London, UK, 1934. [Google Scholar]
- Ujvárosi, M. Gyomnövények, Gyomirtás; Mezőgazdasági Kiadó: Budapest, Hungary, 1957; pp. 93–708. (In Hungarian) [Google Scholar]
- Kalapos, T. C3 and C4 grasses of Hungary: Environmental requirements, phenology and role in the vegetation. Abstr. Bot. 1991, 15, 83–88. [Google Scholar]
- Kalapos, T.; Baloghné-Nyakas, A.; Csontos, P. Occurrence and ecological characteristics of C4 dicot and Cyperaceae species in the Hungarian flora. Phytosynthetica 1997, 33, 227–240. [Google Scholar] [CrossRef]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R.; Springer: New York, NY, USA, 2011; pp. 34–50. [Google Scholar]
- Fox, J. Applied Regression Analysis and Generalized Linear Models, 3rd ed.; Sage Publications: Thousand Oaks, CA, USA, 2016; pp. 342–358. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Chambers, J.M.; Freeny, A.; Heiberger, R.M. Analysis of variance, designed experiments. In Statistical Models in S, 1st ed.; Chambers, J.M., Hastie, T.J., Eds.; Wadsworth & Brooks/Cole: Pacific Grove, CA, USA, 1992; pp. 145–194. [Google Scholar]
- Soper, H.E.; Young, A.W.; Cave, B.M.; Lee, A.; Pearson, K. On the distribution of the correlation coefficient in small samples. Appendix II to the papers of “Student” and R.A. Fisher. A co-operative study. Biometrika 1917, 11, 328–413. [Google Scholar]
- Keselman, H.J.; Rogan, J.C. The Tukey multiple comparison test: 1953–1976. Psychol. Bull. 1977, 84, 1050–1056. [Google Scholar] [CrossRef]
- Mueller-Dombois, D.; Ellenberg, H. Aims and methods of vegetation ecology. Geogr. Rev. 1976, 66, 114–116. [Google Scholar] [CrossRef]
- Sutter, J.M.; Kalivas, J.H. Comparison of Forward Selection, Backward Elimination, and Generalized Simulated Annealing for Variable Selection. Microchem. J. 1993, 47, 60–66. [Google Scholar] [CrossRef]
- Soltani, N.; Dille, J.A.; Burke, I.C.; Everman, W.J.; VanGessel, M.J.; Davis, V.M.; Sikkema, P.H. Potential Corn Yield Losses from Weeds in North America. Weed Technol. 2016, 30, 979–984. [Google Scholar] [CrossRef]
- Adeux, G.; Vieren, E.; Carlesi, S.; Barberi, P.; Munier-Jolain, N.; Cordeau, S. Mitigating crop yield losses through weed diversity. Nat. Sustain. 2019, 2, 1018–1026. [Google Scholar] [CrossRef]
- Vidotto, F.; Fogliatto, S.; Milan, M.; Ferrero, A. Weed communities in Italian maize fields as affected by pedo-climatic traits and sowing time. Eur. J. Agron. 2016, 74, 38–46. [Google Scholar] [CrossRef]
- Keeley, P.E.; Thullen, R.J. Influence of planting date on growth of Barnyardgrass (Echinochloa crus-galli). Weed Sci. 1989, 37, 557–561. [Google Scholar] [CrossRef]
- Forcella, F.; Wilson, R.G.; Renner, K.A.; Dekker, J.; Harvey, R.G.; Alm, D.A.; Buhler, D.D.; Cardina, J. Weed seedbanks of the U.S. corn belt: Magnitude, variation, emergence, and application. Weed Sci. 1992, 40, 636–644. [Google Scholar] [CrossRef]
- Clements, D.R.; Benott, D.L.; Murphy, S.D.; Swanton, C.J. Tillage effects on weed seed return and seedbank composition. Weed Sci. 1996, 44, 314–322. [Google Scholar] [CrossRef]
- Jensen, P.K.; Bibard, V.; Czembor, E.; Dumitru, S.; Foucart, G.; Froud-Williams, R.J.; Jensen, J.E.; Saavedra, M.; Sattin, M.; Soukup, J.; et al. Survey of Weeds in Maize Crops in Europe; Department of Integrated Pest Management, Aarhus University: Aarhus, Denmark, 2011; pp. 7–49. [Google Scholar]
- Bernardo, E.L.; Sales, C.R.G.; Cubas, L.A.; Vath, R.L.; Kromdijk, J. A comparison of stomatal conductance responses to blue and red light between C3 and C4 photosynthetic species in three phylogenetically-controlled experiments. Front. Plant Sci. 2023, 14, 1253976. [Google Scholar] [CrossRef]
- Pyšek, P.; Jarošík, V.; Kropáč, Z.; Chytrý, M.; Wild, J.; Tichý, L. Effects of abiotic factors on species richness and cover in Central European weed communities. Agric. Ecosyst. Environ. 2005, 109, 1–8. [Google Scholar] [CrossRef]
- Buhler, D.D. Influence of tillage systems on weed population dynamics and management in corn and soybean in the Central USA. Crop Sci. 1995, 35, 1247–1258. [Google Scholar] [CrossRef]
- Ghasemi-Fasaei, R.; Mansoorpoor, Y. Metal micronutrients relationships in crop, soil, and common weeds of two maize (Zea mays L.) fields. Arch. Agron. Soil. Sci. 2015, 61, 1733–1741. [Google Scholar] [CrossRef]
- Moreau, D.; Milard, G.; Munier-Jolain, N. A plant nitrophily index based on plant leaf area response to soil nitrogen availability. Agron. Sustain. Dev. 2013, 33, 809–815. [Google Scholar] [CrossRef]
- Desserud, P.; Hugenholtz, C. Do Three Invasive Species: Amaranthus blitoides, Descurainia sophia and Bassia scoparia, Respond to Soil Properties? Ecol. Restor. 2015, 33, 127–130. [Google Scholar] [CrossRef]
- Abuhadra, M.; Essokne, R.; Mahklouf, M. A New Record Amaranthus blitoides S. Watson. (Amaranthaceae) For the Flora of Libya. Am. J. Life Sci. Res. 2016, 4, 89–91. [Google Scholar] [CrossRef][Green Version]
- Nataliia, R.; Abdelhak, E.; Michelle, G.; Tian, F.; Laptev, V. Bioaccumulation of As, Cd, Cr, Cu, Pb, Zn in Ambrosia artemisiifolia L. in the polluted area by enterprise for the production and processing of batteries. Ann. Civil. Environ. Eng. 2022, 6, 026–030. [Google Scholar] [CrossRef]
- Citterio, S.; Gentili, R.; Montagnani, C.; Smith, M. The Worldwide Spread, Success, and Impact of Ragweed (Ambrosia spp.). Crit. Rev. Plant Sci. 2017, 36, 139–178. [Google Scholar] [CrossRef]
- Rinanti, A.; Fachrul, M.; Mahardika, G. Phytoremediation of heavy metal copper (Cu2+) by sunflower (Helianthus annuus L.). IOP Conf. Ser. Earth Environ. Sci. 2018, 106, 012120. [Google Scholar] [CrossRef]
- Xue, C.; Sun, L.; Liu, Z.; Qu, B.; Gao, Y.; Tai, P.; Guo, C.; Liu, W.; Chang, W. Grafting with an invasive Xanthium strumarium improves tolerance and phytoremediation of native congener X. sibiricum to cadmium/copper/nickel tailings. Chemosphere 2022, 308, 136561. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, N.; Ali, K.; Khan, M.E.H.; Jones, D.A. Screening of Xanthium strumarium (IAPS) Growing on Abandoned Habitats in Khyber Pakhtunkhwa, Pakistan: Perspectives for Phytoremediation. Appl. Sci. 2021, 11, 11704. [Google Scholar] [CrossRef]
- Ngole-Jeme, V.; Makuleke, P. Soil Heavy Metal Distribution with Depth around a Closed Landfill and Their Uptake by Datura stramonium. Appl. Environ. Soil. Sci. 2020, 1, 872475. [Google Scholar] [CrossRef]
- Varun, M.; D’souza, R.; Pratas, J.; Paul, M. Metal contamination of soils and plants associated with the glass industry in North Central India: Prospects of phytoremediation. Environ. Sci. Pollut. Res. 2011, 19, 269–281. [Google Scholar] [CrossRef]
- Babst-Kostecka, A.; Cornu, J.; Pošćić, F.; Mattiello, A.; Novello, N. Copper accumulation in five weed species commonly found in the understory vegetation of Mediterranean vineyards. Environ. Pollut. 2023, 329, 121675. [Google Scholar] [CrossRef]
- Ogazie, C.A.; Ochekwu, E.B.; Agbagwa, I.O.; Ugiomoh, I.G. Relationship between soil weed seed bank and soil properties in arable farmlands in University of Port Harcourt and environs in rivers state—Nigeria. J. Agripreneurship Sustain. Dev. 2023, 6, 9–20. [Google Scholar] [CrossRef]
- Malicki, L.; Berbeciowa, C.z. Uptake of more important mineral components by common field weeds on loess soil. Acta Agrobot. 1986, 39, 129–141. [Google Scholar] [CrossRef]
- Dudić, M.; Begović, R.; Meseldžija, M.; Vranešević, M. Weed flora composition in exceptionally hot and dry years. In Proceedings of the 12th Jeep International Scientific Agribusiness Conference—MAK 2025, Kopaonik, Serbia, 30 January–2 February 2025. [Google Scholar] [CrossRef]
- Kovács, E.B. Fénymag És Szárazborsó Kultúrák Gyomviszonyainak Elemzése Gyomaendrőd És Szarvas Térségében, Ökológiai És Konvencionális Területeken. Ph.D. Dissertation, Magyar Agrár-és Élettudományi Egyetem, Gödöllő, Hungary, 2024. [Google Scholar]
- Pinke, G.; Pál, R.W.; Tóth, K.; Karácsony, P.; Czúcz, B.; Botta-Dukát, Z. Weed vegetation of poppy (Papaver somniferum) fields in Hungary: Effects of management and environmental factors on species composition. Weed Res. 2011, 51, 621–630. [Google Scholar] [CrossRef]
- Marshall, E.J.P.; Brown, V.K.; Boatman, N.D.; Lutman, P.J.W.; Squire, G.R.; Ward, L.K. The role of weeds in supporting biological diversity within crop fields. Weed Res. 2003, 43, 77–89. [Google Scholar] [CrossRef]
- Fried, G.; Kazakou, E.; Gaba, S. Trajectories of weed communities explained by traits associated with species response to management practices. Agric. Ecosyst. Environ. 2012, 158, 147–155. [Google Scholar] [CrossRef]
- Gaba, S.; Chauvel, B.; Dessaint, F.; Bretagnolle, V.; Petit, S. Weed species richness in winter wheat increases with landscape heterogeneity. Agric. Ecosyst. Environ. 2010, 138, 318–323. [Google Scholar] [CrossRef]
- Menalled, U.D.; Ann Bybee-Finley, K.; Smith, R.G.; DiTomasso, A.; Darby, H.M.; Pethybridge, J.S.; Ryan, M.R. Legacy effects of crop diversity on weed-crop competition in maize production. Npj Sustain. Agric. 2024, 2, 28. [Google Scholar] [CrossRef]
- Doram, R.; Moyer, J.; Huang, H.; Entz, T.; Blackshaw, R. Effect of previous crop and herbicides on weed growth and wheat yield. Can. J. Plant Sci. 2005, 85, 735–746. [Google Scholar] [CrossRef]
- Oreja, F.H.; Mahoney, D.J.; Jordan, L.D.; Jennings, K.M.; Vann, M.; Leon, R.G. Crop rotation and herbicide program effects on Palmer amaranth and common ragweed population growth rate. Crop Forage Turfgrass Manag. 2023, 9, e20232. [Google Scholar] [CrossRef]
- Oreja, F.H.; Inman, M.D.; Jordan, D.L.; Bardhan, D.; León, R.G. Modeling weed community diversity based on species population density dynamics and herbicide use intensity. Eur. J. Agron. 2022, 138, 126533. [Google Scholar] [CrossRef]
- Trufanov, A.; Gornich, E.; Voronin, A.; Vaganova, N.; Shchukin, S. Effect of minimum tillage, fertilizers and herbicides on weed abundance and crop yields. In IOP Conference Series: Earth and Environmental Science, Proceedings of the II International Scientific and Practical Conference “Ensuring Sustainable Development in the Context of Agriculture, Green Energy, Ecology and Earth Science” Smolensk, Russia, 23–27 January 2022; IOP Publishing: Bristol, UK, 2022. [Google Scholar]
- Stupnicka-Rodzynkiewicz, E.; Szylak, A. Effect of deep loosening of soil on weed infestation of winter wheat cultivated in few crop rotations. Acta Agrobot. 1995, 48, 5–14. [Google Scholar] [CrossRef]
- Froud-Williams, R.J.; Drennan, D.S.H.; Chancellor, R.J. Influence of cultivation regime on weed floras of arable cropping systems. J. Appl. Ecol. 1983, 20, 187–197. [Google Scholar] [CrossRef]
- Bàrberi, P.; Cozzani, A.; Macchia, M.; Bonari, E. Size and composition of the weed seedbank under different management systems for continuous maize cropping. Weed Res. 1998, 38, 319–334. [Google Scholar] [CrossRef]
- Santín-Montanyá, M.I.; Zambrana-Quesada, E.; Tenorio-Pasamón, J.L. Weed abundance and soil seedbank responses to tillage systems in continuous maize crops. Arch. Agron. Soil Sci. 2018, 64, 1705–1713. [Google Scholar] [CrossRef]
- Đalović, I.; Božić, D.; Saulić, M.; Oveisi, M.; Vrbničanin, S. Soil weed seed bank in the function of biodiversity. Acta Herbol. 2024, 33, 141–149. [Google Scholar] [CrossRef]
- DiTomaso, J.M. Approaches for Improving Crop Competitiveness through the Manipulation of Fertilization Strategies. Weed Sci. 1995, 43, 491–497. [Google Scholar] [CrossRef]
- Little, N.G.; DiTommaso, A.; Westbrook, A.S.; Ketterings, Q.M.; Mohler, C.L. Effects of Fertility Amendments on Weed Growth and Weed—Crop Competition: A Review. Weed Sci. 2021, 69, 132–146. [Google Scholar] [CrossRef]
- Sadeghpour, A.; Pelzer, C.; Cordeau, S.; Ketterings, Q.; Ryan, M.; Wayman, S. Long-Term Soil Nutrient Management Affects Taxonomic and Functional Weed Community Composition and Structure. Front. Agron. 2021, 3, 636179. [Google Scholar] [CrossRef]
- Kordbacheh, F.; Flaten, D.N.; Gulden, R.H. Weed community dynamics under repeated fertilization with different nutrient sources over 5 years. Agric. Ecosyst. Environ. 2023, 346, 108328. [Google Scholar] [CrossRef]
- Luo, J.J.; Gao, Y.; Feng, W.; Liu, M.; Qu, B.; Zhang, C.; Feng, Y. Stronger ability to absorb nitrate and associated transporters in the invasive plant Xanthium strumarium compared with its native congener. Environ. Exp. Bot. 2022, 198, 104851. [Google Scholar] [CrossRef]
- Kennedy, R.; Zee, D.; Rumpho, M.; Barrett, S. Germination and seedling growth under anaerobic conditions in Echinochloa crus-galli (barnyard grass). Plant Cell Environ. 1980, 3, 243–248. [Google Scholar] [CrossRef]
- Wu, Y.; Li, P.; Wu, C.; Fu, W.; Hao, J. The distribution characteristics of nitrogen and phosphorus in the ecological system of Mt. Beigu wetland. Chin. J. Geochem. 2009, 28, 55–60. [Google Scholar] [CrossRef]
- Yang, W. Effect of nitrogen, phosphorus and potassium fertilizer on growth and seed germination of Capsella bursa-pastoris (L.) Medikus. J. Plant Nutr. 2018, 41, 636–644. [Google Scholar] [CrossRef]
- Murthy, I.; Anjaiah, T.; Jyothi, P.; Hussain, S.; Naik, R. Seed Yield and Nutrient Uptake of Sunflower (Helianthus annuus L.) as Influenced by Different Levels of Boron and Potassium in Sandy Loam Soil. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3684–3692. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, Y.; Liu, Y.; Liu, Y.; Miao, C.; Zhang, Y.; Li, W.; Yi, X. Response of plants and soils to inundation duration and construction of the plant—soil association mode in the hydro-fluctuation belt of the reservoir wetland. J. Environ. Manag. 2024, 357, 120776. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, T.; Huang, N.; Shen, X.; Shen, M.; Dai, Q. Effect of long-term fertilisation on the weed community of a winter wheat field. Sci. Rep. 2018, 8, 4017. [Google Scholar] [CrossRef]
- Matyukha, V.L.; Semenov, S.S. Influence of cultivation methods on the soil aggregate state in the context of weed development in winter wheat plantations. Agrology 2024, 7, 14–20. [Google Scholar] [CrossRef]
- Tóth, E.; Dorner, Z.; Nagy, J.G.; Zalai, M. How Weed Flora Evolves in Cereal Fields in Relation to the Agricultural Environment and Farming Practices in Different Sub-Regions of Eastern Hungary. Agronomy 2025, 15, 1033. [Google Scholar] [CrossRef]
- Hazarika, J.R.; Deka, A.M.; Borah, B.; Gogoi, B.; Gogoi, A.; Kalita, B.; Bordoloi, P.K.; Deva Nath, H. Weed flora shift as affected by cropping systems. Int. J. Res. Agron. 2024, 7, 415–420. [Google Scholar] [CrossRef]
- Grassini, P.; Lim, Y.L.; Tenorio, A.F.; Monzon, J.P.; Sugianto, H.; Donough, R.C.; Rahutomo, S.; Agus, F.; Slingerland, M.A.; Darlan, N.H.; et al. Too little, too imbalanced: Nutrient supply in smallholder oil palm fields in Indonesia. Agric. Syst. 2023, 210, 103729. [Google Scholar] [CrossRef]
- Pinke, G.; Karácsony, P.; Botta-Dukát, Z.; Czúcz, B. Relating Ambrosia artemisiifolia and other weeds to the management of Hungarian sunflower crops. J. Pest. Sci. 2013, 86, 621–631. [Google Scholar] [CrossRef]
- Pinke, G.; Blazsek, K.; Magyar, L.; Nagy, K.; Karácsony, P.; Czúcz, B.; Botta-Dukát, Z. Weed species composition of conventional soyabean crops in Hungary is determined by environmental, cultural, weed management and site variables. Weed Res. 2016, 56, 470–481. [Google Scholar] [CrossRef]
- de Mol, F.; von Redwicz, C.; Gerowitt, B. Weed species composition of maize fields in Germany is influenced by site and crop sequence. Weed Res. 2015, 55, 574–585. [Google Scholar] [CrossRef]
- Angiosperm Phylogeny Website. Available online: http://www.mobot.org/MOBOT/research/APweb/ (accessed on 9 May 2025).
- Yorimitsu, Y.; Kadosono, A.; Hatakeyama, Y.; Yabiku, T.; Ueno, O. Transition from C3 to proto-Kranz to C3–C4 intermediate type in the genus Chenopodium (Chenopodiaceae). J. Plant Res. 2019, 132, 839–855. [Google Scholar] [CrossRef]
- Oono, J.; Hatakeyama, Y.; Yabiku, T.; Ueno, O. Effects of growth temperature and nitrogen nutrition on expression of C3–C4 intermediate traits in Chenopodium album. J. Plant Res. 2022, 135, 15–27. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).