Effects of Modified Atmosphere Packaging on Postharvest Physiology and Quality of ‘Meizao’ Sweet Cherry (Prunus avium L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sweet Cherry Fruit and Experimental Design
2.2. The Change in O2 and CO2 Gas Composition (%) in Different MAPs
2.3. Weight Loss
2.4. Pedicel Freshness, Respiration Rate, and Fruit Decay
2.5. Fruit Color Characteristics
2.6. Fruit Quality Parameters
2.7. Analysis of Ethanol and MDA Produced
2.8. Enzyme Extraction and Activity Assays
2.9. Statistical Analysis
3. Results
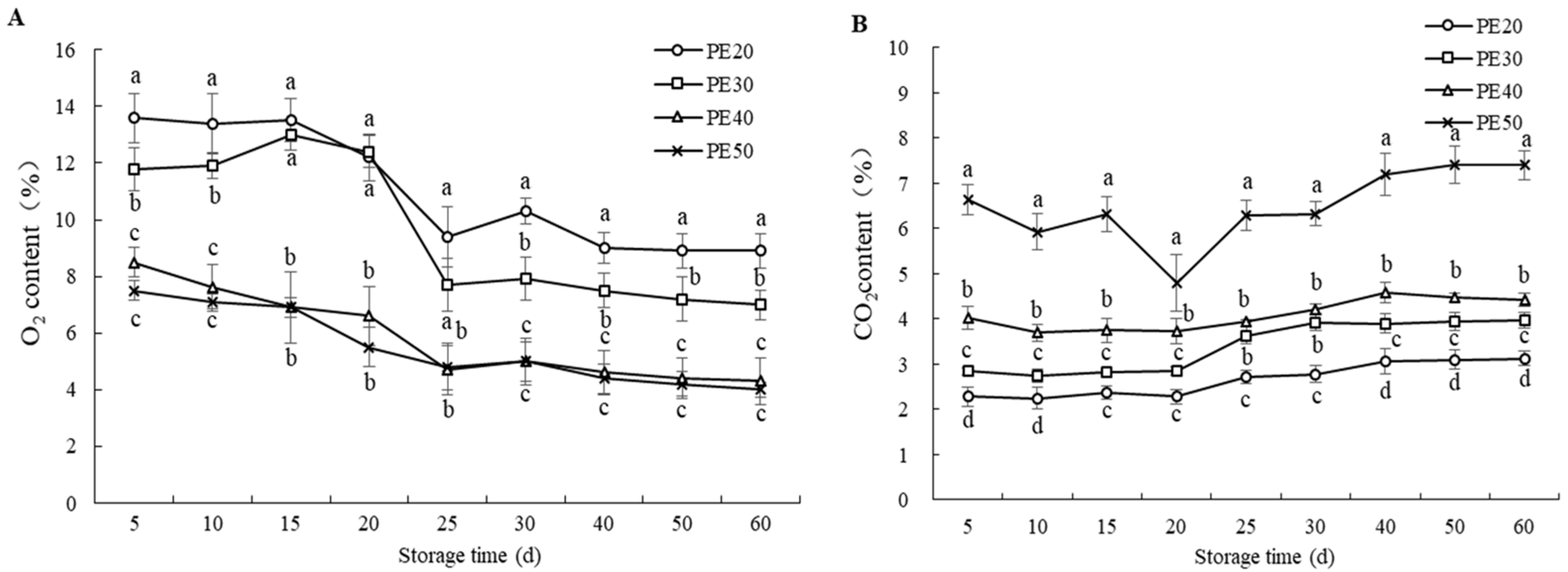
3.1. Changes in O2 and CO2 Gas Composition (%) in MAP
3.2. Weight Loss, Pedicel Freshness, and Decay Ratio
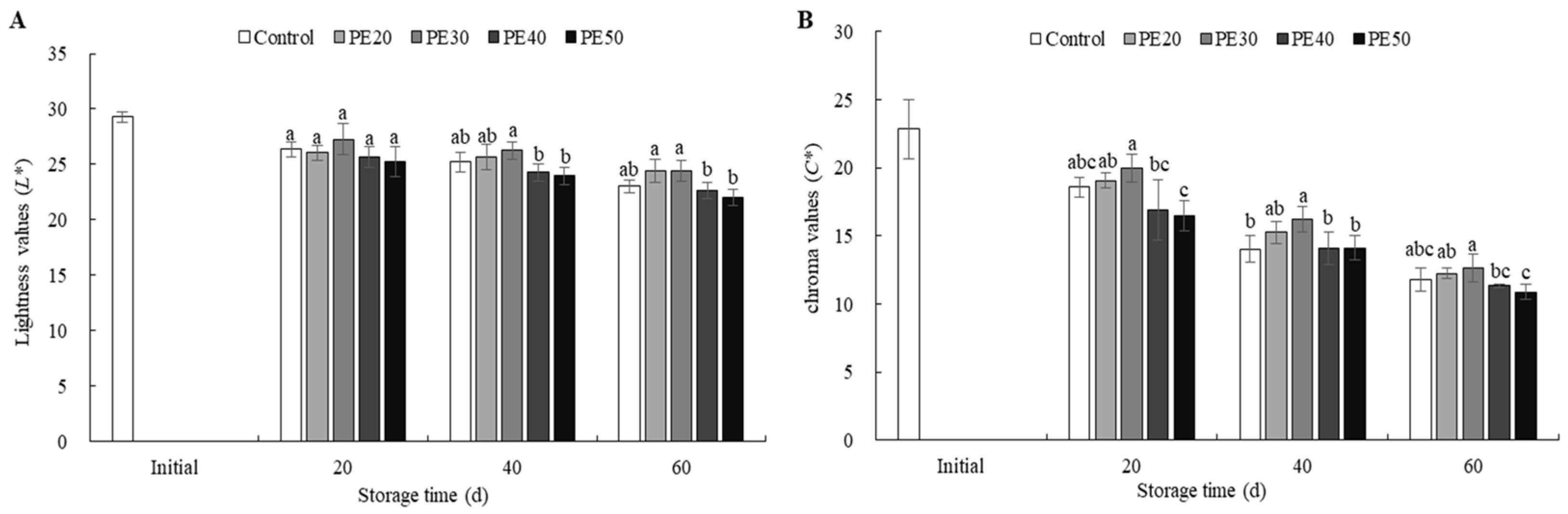
3.3. Fruit Color
3.4. Fruit Firmness, SSC, TA, and Vc Content
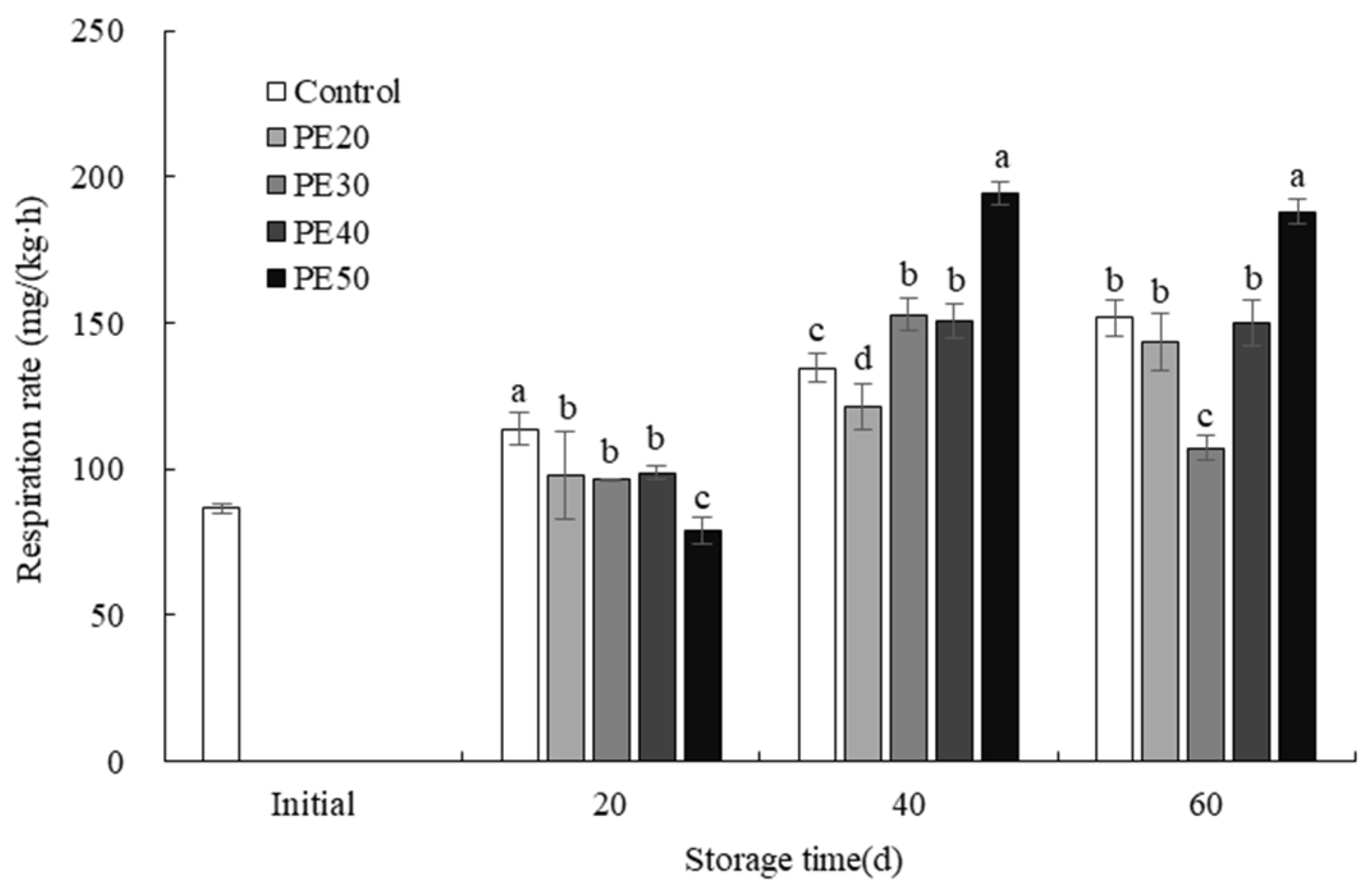
3.5. Fruit Respiratory Rate
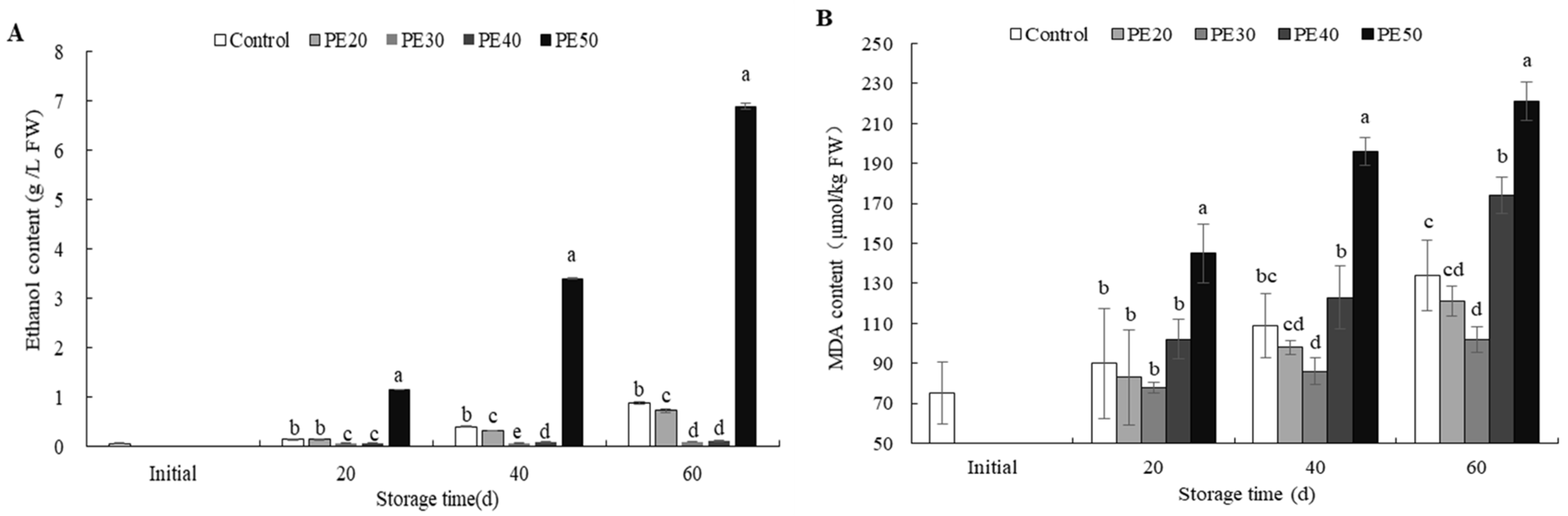
3.6. Fruit Ethanol and MDA
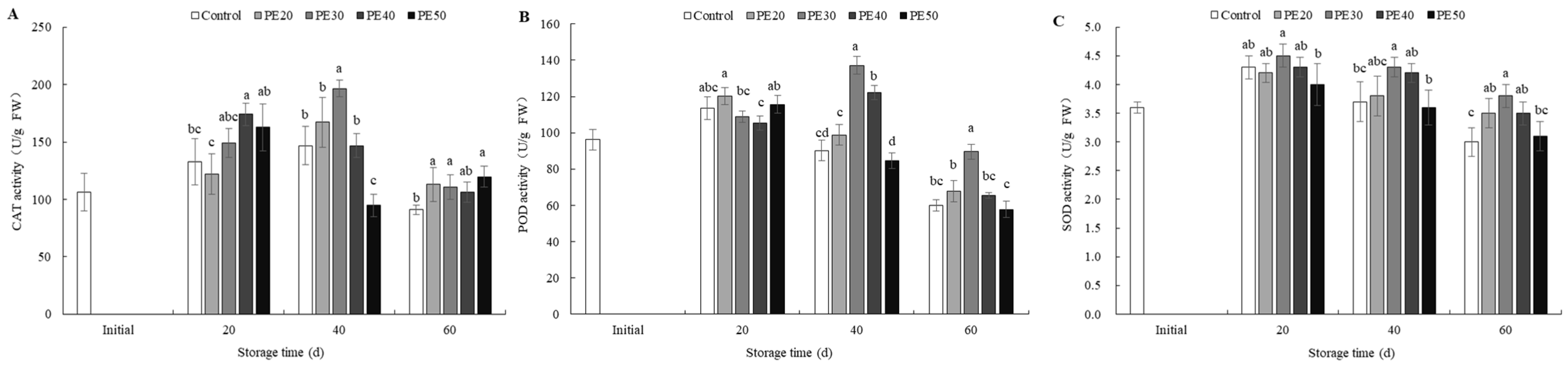
3.7. Antioxidant Enzyme Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frusciante, L.; Nyong’a, C.N.; Trezza, A.; Shabab, B.; Olmastroni, T.; Barletta, R.; Mastroeni, P.; Visibelli, A.; Orlandini, M.; Raucci, L.; et al. Bioactive potential of sweet cherry (Prunus avium L.) waste: Antioxidant and anti-inflammatory properties for sustainable applications. Foods 2025, 14, 1523. [Google Scholar] [CrossRef] [PubMed]
- Li, B.B.; Peng, T. Development status and high efficiency cultivation techniques of cherry in China. Tillage Cultiv. 2020, 40, 71–73. [Google Scholar]
- Cui, J.C.; Xu, H.J.; Jia, X.H.; Wang, Y.J.; Chen, X.H.; Wang, W.H. Isolation, identification and pathogenicity analysis of pathogenic fungus in sweet cherries during postharvest storage. J. Nucl. Agric. Sci. 2024, 38, 1968–1975. [Google Scholar]
- Zou, Y.X.; Li, H.; Teng, F.; Jin, Z.H. The trade status, challenges and coping strategies of sweet cherry export trade in China. China Fruits 2024, 9, 134–140, 150. [Google Scholar]
- Zheng, W. Development status, problem and suggestions of sweet cherry industry in Dalian. Hortic. Seed 2021, 41, 33–36. [Google Scholar]
- Mei, J.Z.; Li, X.L.; You, Y.; Fan, X.G.; Sun, C.C.; Guo, F.J.; Shan, M.; Zhang, J.J. Methyl salicylate affects fruit quality and aroma compounds of cherry during cold storage. Sci. Hortic. 2024, 333, 113291. [Google Scholar] [CrossRef]
- Ozturk, B.; Yılmaz, M.; Guler, S.K. Preharvest biofilm coating treatments affect the storage quality of sweet cherry fruit by maintaining bioactive components. Int. J. Fruit Sci. 2024, 24, 301–313. [Google Scholar] [CrossRef]
- Xin, Y.; Jin, Z.Y.; Chen, F.S.; Lai, S.J.; Yang, H.S. Effect of chitosan coatings on the evolution of sodium carbonate-soluble pectin during sweet cherry softening under non-isothermal conditions. Int. J. Biol. Macromol. 2020, 154, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Valizadehkaji, B.; Fakhri, N. Postharvest application of Aloe vera gel and thymol enhances shelf-life of duke cherries via altering physiochemical parameters. Chem. Biol. Technol. Agric. 2023, 10, 85. [Google Scholar] [CrossRef]
- Wang, Y.; Long, L.E. Respiration and quality responses of sweet cherry to different atmospheres during cold storage and shipping. Postharvest Biol. Technol. 2014, 92, 62–69. [Google Scholar] [CrossRef]
- Liu, L.; Lin, H.Y.; Zhou, X.X.; Zhang, Z.; Zhang, Y.; Deng, S.W.; Peng, S.Q.; Gong, S.K.; Guo, S.Y.; Fan, W. Application of modified atmosphere preservation technology in cherry storage: A review. Agriculture 2025, 15, 462. [Google Scholar] [CrossRef]
- Wani, A.A.; Singh, P.; Gul, K.; Wani, M.H.; Langowski, H.C. Sweet cherry (Prunus avium): Critical factors affecting the composition and shelf life. Food Packag. Shelf Life 2014, 1, 86–99. [Google Scholar] [CrossRef]
- Remón, S.; Ferrer, A.; Marquina, P.; Burgos, J.; Oria, R. Use of modified atmospheres to prolong the postharvest life of Burlat cherries at two different degrees of ripeness. J. Sci. Food Agric. 2000, 80, 1545–1552. [Google Scholar] [CrossRef]
- Zhang, M.; Meng, X.Y.; Bhandari, B.; Fang, Z.X. Recent development in film and gas research in modified atmosphere packaging of fresh foods. Crit. Rev. Food Sci. Nutr. 2016, 56, 2174–2182. [Google Scholar] [CrossRef] [PubMed]
- Kartal, S.; Aday, M.S.; Caner, C. Use of microperforated films and oxygen scavengers to maintain storage stability of fresh strawberries. Postharvest Biol. Technol. 2012, 71, 32–40. [Google Scholar] [CrossRef]
- Qu, P.; Zhang, M.; Fan, K.; Guo, Z.M. Microporous modified atmosphere packaging to extend shelf life of fresh foods: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 51–65. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, E.; Serradilla, M.J.; Ruiz-Moyano, S.; Córdoba, M.G.; Villalobos, M.C.; Casquete, R.; Hernández, A. Combined effect of antagonistic yeast and modified atmosphere to control Penicillium expansum infection in sweet cherries cv. Ambrunés. Int. J. Food Microbiol. 2017, 241, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Koutsimanis, G.; Harte, J.; Almenar, E. Freshness maintenance of cherries ready for consumption using convenient, microperforated, bio-based packaging. J. Sci. Food Agric. 2015, 95, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Frans, M.; Aerts, R.; Ceusters, N.; Luca, S.; Ceusters, J. Possibilities of modified atmosphere packaging to prevent the occurrence of internal fruit rot in bell pepper fruit (Capsicum annuum) caused by Fusarium spp. Postharvest Biol. Technol. 2021, 178, 111545. [Google Scholar] [CrossRef]
- Jiao, J.Q.; Guo, L.Y.; Liu, H.; Wang, X.L.; He, Y.H.; Jin, M.J.; Rao, J.P. Effect of different packaging film thicknesses on chilling injury in postharvest ‘Cuixiang’ Kiwifruit. N. Z. J. Crop Hortic. Sci. 2021, 49, 168–181. [Google Scholar] [CrossRef]
- Nasser, M.A.; Ei-Mogy, M.M.; Samaan, M.S.F.; Hassan, K.M.; El-Sayed, S.M.; Alsubeie, M.S.; Darwish, D.B.E.; Mahmoud, S.F.; Al-Harbi, N.A.; AI-Qahtani, S.M.; et al. Postharvest exogenous melatonin treatment of table grape berry enhances quality and maintains bioactive compound during refrigerated storage. Horticulturae 2022, 8, 860. [Google Scholar] [CrossRef]
- Kou, X.H.; Chen, Q.; Li, X.H.; Li, M.F.; Kan, C.; Chen, B.; Zhang, Y.; Xue, Z.H. Quantitative assessment of bioactive compounds and the antioxidant activity of 15 jujube cultivars. Food Chem. 2015, 173, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.H.; Wang, W.H.; Tong, W.; Du, Y.M.; Wang, Z.H.; Jiang, X.C. Effect of modified atmosphere packaging on postharvest physiology and quality of ‘Korla Xiangli’ pears during storage. Sci. Agric. Sin. 2016, 49, 4785–4796. [Google Scholar]
- Sharafi, Y.; Jannatizadeh, A.; Fard, J.R.; Aghdam, M.S. Melatonin treatment delays senescence and improves antioxidant potential of sweet cherry fruits during cold storage. Sci. Hortic. 2021, 288, 110304. [Google Scholar] [CrossRef]
- Chen, M.; Luo, Z.; Zhao, X.; Li, S.; Wu, F.; Chen, J.Y.; Xiang, M.L. Exogenously applied methyl jasmonate increased the resistance of postharvest pear fruit to blue mold. Fruit Res. 2022, 2, 11. [Google Scholar] [CrossRef]
- Pan, L.Y.; Chen, X.R.; Xu, W.; Fan, S.S.; Wan, T.; Zhang, J.; Cai, Y.L. Methyl jasmonate induces postharvest disease resistance to decay caused by Alternaria alternata in sweet cherry fruit. Sci. Hortic. 2022, 292, 110624. [Google Scholar] [CrossRef]
- Gidado, M.J.; Gunny, A.A.N.; Subash, C.B.; Gopinath, S.C.B.; Asgar, A.; Wongs-Aree, C.; Salleh, N.H.M. Challenges of postharvest water loss in fruits: Mechanisms, influencing factors, and effective control strategies-a comprehensive review. J. Agric. Food Res. 2024, 17, 101249. [Google Scholar] [CrossRef]
- Linke, M.; Herppich, W.B.; Geyer, M. Green peduncles may indicate postharvest freshness of sweet cherries. Postharvest Biol. Technol. 2010, 58, 135–141. [Google Scholar] [CrossRef]
- Ricardo-Rodrigues, S.; Laranjo, M.; Agulheiro-Santos, A.C. Methods for quality evaluation of sweet cherry. J. Sci. Food Agric. 2023, 103, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Esturk, O.; Ayhan, Z.; Ustunel, M.A. Modified atmosphere packaging of “Napoleon” cherry: Effect of packaging material and storage time on physical, chemical, and sensory quality. Food Bioprocess Technol. 2012, 5, 1295–1304. [Google Scholar] [CrossRef]
- Sándor, E.; Mihály, K.; Nagy, A.; Pál, K.; Peles, F.; Zabiák, A.; Kovács, C.; Takács, F.; Romanazzi, G.; Holb, I.J. Effects of storage conditions, cultivars, and production systems on fruit decay incidence of sour cherry (Prunus cerasus L.) fruit after shelf-life conditions. Agronomy 2024, 14, 2212. [Google Scholar] [CrossRef]
- Li, H.Y.; Yu, T. Effect of chitosan on incidence of brown rot, quality and physiological attributes of postharvest peach fruit. J. Sci. Food Agric. 2000, 81, 269–274. [Google Scholar] [CrossRef]
- Ozturk, B.; Aglar, E.; Saracoglu, O.; Karakaya, O.; Gun, S. Effects of GA3, CaCl2 and modified atmosphere packaging (MAP) applications on fruit quality of sweet cherry at cold storage. Int. J. Fruit Sci. 2022, 22, 696–710. [Google Scholar] [CrossRef]
- Tian, S.P.; Fan, Q.; Xu, Y.; Wang, Y.; Jiang, A.L. Evaluation of the use of high CO2 concentrations and cold storage to control Monilinia fructicola on sweet cherries. Postharvest Biol. Technol. 2001, 22, 53–60. [Google Scholar] [CrossRef]
- Wang, J.J.; Zhu, Z.Q.; Moga, L.M.; Hu, J.Y. A holistic packaging efficiency evaluation method for loss prevention in fresh vegetable cold chain. Sustainability 2019, 11, 3874. [Google Scholar] [CrossRef]
- Pinto de Andrade, L.; Veloso, A.; Espírito Santo, C.; Dinis Gaspar, P.; Silva, P.D.; Resende, M.; Beato, H.; Baptista, C.; Pintado, C.M.; Paulo, L.; et al. Effect of controlled atmospheres and environmental conditions on the physicochemical and sensory characteristics of sweet cherry cultivar satin. Agronomy 2022, 12, 188. [Google Scholar] [CrossRef]
- Solomos, T.; Whitaker, B.; Lu, C.W. Deleterious effects of pure oxygen on ‘Gala’ and ‘Granny Smith’ apples. HortScience 1997, 32, 458. [Google Scholar] [CrossRef]
- Islam, A.; Acikalin, R.; Ozturk, B.; Aglar, E.; Clive, K. Combined effects of Aloe vera gel and modified atmosphere packaging treatments on fruit quality traits and bioactive compounds of jujube (Ziziphus jujube Mill.) fruit during cold storage and shelf life. Postharvest Biol. Technol. 2022, 187, 111855. [Google Scholar] [CrossRef]
- Basavaraju, M.; Lingegowdaru, J.; Thammaiah, N.; Jagadeesh, R.; Gangadharappa, P.M.; Noola, N. Pre-harvest application of azoxystrobin minimized anthracnose of mango (cv. ‘Alphonso’) both at field and postharvest level enhancing yield and quality of fruits. J. Pharmacogn. Phytochem. 2018, 7, 2962–2967. [Google Scholar]
- Gong, J.L.; Li, Y.C.; Shen, X.C.; Xu, Y.N.; Hu, X.L.; Shen, D.D.; Chen, C.W.; Sun, X.P. Diversity in plastids contributes to variation in fruit color. Sci. Hortic. 2024, 337, 113471. [Google Scholar] [CrossRef]
- Xiao, L.R.; Shibuya, T.; Kato, K.; Nishiyama, M.; Kanayama, Y. Effects of light quality on plant development and fruit metabolism and their regulation by plant growth regulators in tomato. Sci. Hortic. 2022, 300, 111076. [Google Scholar] [CrossRef]
- Molaeafard, S.; Jamei, R.; Marjani, A.P. Co-pigmentation of anthocyanins extracted from sour cherry (Prunus cerasus L.) with some organic acids: Color intensity, thermal stability, and thermodynamic parameters. Food Chem. 2021, 339, 128070. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, R.N.; Santos, D.T.; Meireles, M.A.A. Non-thermal stabilization mechanisms of anthocyanins in model and food systems—An overview. Food Res. Int. 2011, 44, 499–509. [Google Scholar] [CrossRef]
- Pott, D.M.; Vallarino, J.G.; Osorio, S. Metabolite changes during postharvest storage: Effects on fruit quality traits. Metabolites 2020, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.C.; Lin, M.H.; Yang, X.Z.; Zhu, C.Q.; Wu, D.; Chen, K.S. Mechanisms of the response of apple fruit to postharvest compression damage analyzed by integrated transcriptome and metabolome. Food Chem. X 2023, 20, 100972. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Y.; Zhang, Y.; Qi, D.; Huo, H.L.; Dong, X.G.; Tian, L.M.; Zhang, X.S.; Liu, C.; Cao, Y.F. Postharvest metabolomic changes in Pyrus ussuriensis Maxim. wild accession ‘Zaoshu Shanli’. J. Sep. Sci. 2018, 41, 4001–4013. [Google Scholar] [CrossRef] [PubMed]
- We, Y.; Liu, Z.; Lv, T.X.; Xu, Y.X.; Wei, Y.J.; Liu, W.T.; Liu, L.; Wang, A.D.; Li, T. Ethylene enhances MdMAPK3-mediated phosphorylation of MdNAC72 to promote apple fruit softening. Plant Cell 2023, 35, 2887–2909. [Google Scholar] [CrossRef] [PubMed]
- Giné-Bordonaba, J.; Echeverria, G.; Ubach, D.; Aguiló-Aguayo, I.; López, M.L.; Larrigaudière, C. Biochemical and physiological changes during fruit development and ripening of two sweet cherry varieties with different levels of cracking tolerance. Plant Physiol. Biochem. 2017, 111, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.K.; Li, S.L. Cell wall hydrolytic enzyme activity during development of nonclimacteric sweet cherry (Prunus avium L.) fruit. J. Hortic. Sci. 1995, 70, 561–567. [Google Scholar] [CrossRef]
- Lufu, R.; Ambaw, A.; Opara, U. Water loss of fresh fruit: Influencing pre-harvest, harvest and postharvest factors. Sci. Hortic. 2020, 272, 109519. [Google Scholar] [CrossRef]
- Zhang, W.L.; Pan, Y.G.; Jiang, Y.M.; Zhang, Z.K. Advances in control technologies and mechanisms to treat peel browning in postharvest fruit. Sci. Hortic. 2023, 311, 111798. [Google Scholar] [CrossRef]
- Zhiyan, R.; Zhu, Y.X.; Liu, M.T.; Zhou, Y.J.; Su, X.G.; Jiang, Y.M.; Jiang, G.X. Tyr-Asp treatment delays senescence in litchi fruit by enhancing redox balance and maintaining energy homeostasis. Postharvest Biol. Technol. 2025, 223, 113420. [Google Scholar] [CrossRef]
- Wei, W.Y.; Liu, Z.S.; Pan, X.J.; Yang, T.Y.; An, C.T.; Wang, Y.H.; Li, L.; Liao, W.B.; Wang, C.L. Effects of reactive oxygen species on fruit ripening and postharvest fruit quality. Plant Sci. 2025, 352, 112391. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Q.; Zhang, S.S.; Yang, H.Y.; Wu, W.L.; Lyu, L.F.; Zhang, C.H.; Cao, F.L.; Li, W.L. Methyl jasmonate and salicylic acid treatment changes the nutritional quality, antioxidant profile and gene expression of postharvest blackberry fruit. Postharvest Biol. Technol. 2025, 219, 113205. [Google Scholar] [CrossRef]
- Aktaruzzaman, M.; Afroz, T.; Kim, B.S.; Lee, Y.G. Occurrence of postharvest gray mold rot of sweet cherry due to Botrytis cinerea in Korea. J. Plant Dis. Prot. 2017, 124, 93–96. [Google Scholar] [CrossRef]
| Attribute | Treatments | Storage Time (d) | ||
|---|---|---|---|---|
| 20 | 40 | 60 | ||
| Weight loss (%) | Control | 12.2 ± 1.60 a | 17.1 ± 2.50 a | 33.4 ± 3.71 a |
| PE20 | 5.3 ± 1.52 b | 5.5 ± 1.94 b | 10.3 ± 3.81 b | |
| PE30 | 0.4 ± 1.34 c | 1.2 ± 1.39 c | 4.0 ± 3.36 d | |
| PE40 | 1.5 ± 1.19 c | 5.8 ± 1.61 b | 15.5 ± 3.53 b | |
| PE50 | 1.6 ± 1.13 c | 6.9 ± 2.78 b | 8.5 ± 0.00 b | |
| Control | 83.1 ± 1.93 c | 76.3 ± 2.97 c | 68.9 ± 1.63 d | |
| Pedicel preservation index (PPI) | PE20 | 96.7 ± 1.14 a | 92.7 ± 1.95 a | 88.8 ± 0.87 a |
| PE30 | 94.0 ± 1.65 ab | 93.3 ± 1.15 a | 91.1 ± 1.78 a | |
| PE40 | 92.9 ± 1.93 b | 87.8 ± 2.10 b | 78.3 ± 2.87 c | |
| PE50 | 94.3 ± 1.51 ab | 88.7 ± 1.44 b | 83.8 ± 2.96 b | |
| Control | 4.9 ± 0.40 a | 17.3 ± 2.15 a | 20.9 ± 2.19 a | |
| Decayed (%) | PE20 | 3.5 ± 0.82 b | 9.3 ± 1.80 b | 15.3 ± 2.17 b |
| PE30 | 1.8 ± 0.30 c | 2.7 ± 0.44 c | 7.2 ± 1.18 d | |
| PE40 | 2.1 ± 0.36 c | 9.5 ± 2.35 b | 13.4 ± 3.65 bc | |
| PE50 | 2.3 ± 0.30 c | 5.0 ± 1.37 c | 10.9 ± 1.45 cd | |
| Storage Time (d) | Treatments | Firmness (kg/cm2) | Soluble Solid (%) | Titratable Acid (%) | VC Content (mg/100 g FW) |
|---|---|---|---|---|---|
| 0 | 2.53 ± 0.14 | 15.58 ± 0.23 | 0.550 ± 0.002 | 7.78 ± 0.15 | |
| Control | 1.74 ± 0.10 b | 13.15 ± 0.27 b | 0.383 ± 0.004 b | 7.28 ± 0.12 d | |
| 20 | PE20 | 2.10 ± 0.11 a | 13.24 ± 0.67 b | 0.384 ± 0.006 b | 8.39 ± 0.04 a |
| PE30 | 2.19 ± 0.19 a | 14.49 ± 0.33 a | 0.398 ± 0.005 a | 8.18 ± 0.07 b | |
| PE40 | 1.93 ± 0.10 ab | 13.57 ± 0.71 ab | 0.381 ± 0.002 b | 7.50 ± 0.16 c | |
| PE50 | 2.07 ± 0.23 a | 13.93 ± 0.39 ab | 0.376 ± 0.007 c | 6.84 ± 0.05 e | |
| Control | 1.63 ± 0.08 b | 13.34 ± 0.21 d | 0.262 ± 0.002 d | 5.03 ± 0.17 c | |
| 40 | PE20 | 1.99 ± 0.03 a | 13.58 ± 0.20 cd | 0.278 ± 0.002 b | 6.54 ± 0.03 a |
| PE30 | 2.01 ± 0.16 a | 14.60 ± 0.21 a | 0.297 ± 0.003 a | 5.78 ± 0.09 b | |
| PE40 | 1.87 ± 0.12 a | 14.21 ± 0.24 ab | 0.268 ± 0.000 c | 5.61 ± 0.08 b | |
| PE50 | 1.92 ± 0.07 a | 13.90 ± 0.35 bc | 0.257 ± 0.000 d | 4.98 ± 0.08 c | |
| Control | 1.55 ± 0.06 d | 13.80 ± 0.26 d | 0.169 ± 0.002 b | 4.55 ± 0.22 b | |
| 60 | PE20 | 1.82 ± 0.07 ab | 13.82 ± 0.23 d | 0.166 ± 0.005 b | 5.61 ± 0.66 a |
| PE30 | 1.90 ± 0.10 a | 15.40 ± 0.19 a | 0.216 ± 0.002 a | 4.56 ± 0.16 b | |
| PE40 | 1.67 ± 0.08 cd | 14.86 ± 0.18 b | 0.169 ± 0.001 b | 3.63 ± 0.08 c | |
| PE50 | 1.75 ± 0.06 bc | 14.21 ± 0.12 c | 0.169 ± 0.002 b | 3.62 ± 0.10 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Jia, X.; Wang, W.; Fan, L.; Zhao, W.; He, L.; Xu, H. Effects of Modified Atmosphere Packaging on Postharvest Physiology and Quality of ‘Meizao’ Sweet Cherry (Prunus avium L.). Agronomy 2025, 15, 1774. https://doi.org/10.3390/agronomy15081774
Cui J, Jia X, Wang W, Fan L, Zhao W, He L, Xu H. Effects of Modified Atmosphere Packaging on Postharvest Physiology and Quality of ‘Meizao’ Sweet Cherry (Prunus avium L.). Agronomy. 2025; 15(8):1774. https://doi.org/10.3390/agronomy15081774
Chicago/Turabian StyleCui, Jianchao, Xiaohui Jia, Wenhui Wang, Liying Fan, Wenshi Zhao, Limin He, and Haijiao Xu. 2025. "Effects of Modified Atmosphere Packaging on Postharvest Physiology and Quality of ‘Meizao’ Sweet Cherry (Prunus avium L.)" Agronomy 15, no. 8: 1774. https://doi.org/10.3390/agronomy15081774
APA StyleCui, J., Jia, X., Wang, W., Fan, L., Zhao, W., He, L., & Xu, H. (2025). Effects of Modified Atmosphere Packaging on Postharvest Physiology and Quality of ‘Meizao’ Sweet Cherry (Prunus avium L.). Agronomy, 15(8), 1774. https://doi.org/10.3390/agronomy15081774






