Abstract
Late sowing and spring low temperatures have a great impact on the growth and maturation of wheat in the rice–wheat rotation region. In order to analyze the impacts of cold stress in February in early spring on yield formation and agronomic traits of wheat on different sowing dates, a controlled pot experiment was performed using the widely promoted and applied spring-type wheat variety Yangmai23 (YM23). The yield of wheat treated with late sowing date II (SDII, 21 November) and overly late sowing date III (SDIII, 9 December) were both lower than that of wheat sown on the suitable date I (SDI, 1 November). The yield of late-sown wheat decreased by 40.82% for SDII and by 66.77% for SDIII, compared with SDI, and these three treatments of wheat all grew under the natural conditions as the control treatments. The plant height, stem diameter of the internode below the ear, flag leaf length and area, and total awn length of the spike, as well as the spike length of late-sown wheat, were all significantly lower than those of wheat in SDI treatment. Early spring low temperatures exacerbated the decline in yield of wheat sown on different dates, to some extent. Despite showing higher net photosynthetic rate, stomatal conductance, and transpiration rate in flag leaves of the SDIII treatment under low-temperature stress than those of the other treatments at anthesis, overly late sowing led to minimal leaf area, shorter plant height, fewer tillers, and smaller ears, ultimately resulting in the lowest yield. Our study suggested that additional focus and some regulation techniques are needed to be studied further to mitigate the combined negative impacts of late sowing and low-temperature stress in early spring on wheat production.
1. Introduction
Wheat (Triticum aestivum L. Poaceae) is the most extensively cultivated staple cereal crop worldwide. With the gradual development of modernization, in order to meet the demand of the wheat market, the yield and quality of wheat must be continuously improved [1]. In the 1990s, some provinces of China, for example, Jiangsu, made the important policy to adjust the agricultural cropping patterns: the implementation of the indica rice to japonica rice project because of the increase in the consumer requirements for grain quality and in farmers’ requirements for higher economic benefits of rice [2,3]. The growth period of japonica rice is longer than that of indica rice, resulting in later harvesting than before, so wheat could not be sown during the best suitable sowing period. In addition, wheat variety has a shorter optimal sowing period in the rice–wheat rotation region, and at the same time, to avoid frost injury in early spring, timely sowing becomes more necessary [4]. At present, the area of late-sown wheat accounts for more than 40% in Jiangsu province. With the delay of the sowing date, the duration from sowing to emergence of seedlings was greatly extended, while the wheat’s total growth period was shortened; the number of ears, the number of grains per ear, and the grain weight were all reduced, leading to the lower yield [5].
Global climate change has been affecting food security across the globe on a large scale over the last decades. Among climate changes, temperature is the primary factor restricting the growth of wheat in China, especially low temperature happening in the early spring. The occurrence probability of early spring coldness is greatly increased, which makes the probability of crops suffering from coldness stress and cold damage become more serious, and low-temperature stress causes crop reduction, production quality decline, and even the loss of economic efficiency of agricultural production [6]. Spring coldness caused yield loss due to the reduced number of effective tillers, stunted spikelet growth, reduced grain size, and reduced grain number [7,8,9]. With the change of morphology, spring coldness disrupted physiological activities of plants, resulting in decreased photosynthetic activity, nutrient uptake, and biomass accumulation [10,11,12]. Wheat varieties with stronger spring character had earlier sowing, a faster development rate of growth before overwintering, a faster development process of young ears, and more severe freezing damage during overwintering, while wheat varieties overwintering during the two-ridge primordium stage had a lower probability of freezing damage due to their stronger low-temperature resistance at this stage [13], and the later the wheat planting date, the shorter the differentiation period of the young ear of wheat, leading to the smaller ear size of wheat. The plant morphology of wheat was significantly affected by low-temperature injury at different periods, especially before the jointing stage. In some previous studies, the wheat plant height under low-temperature stress during ear differentiation was observed. It was found that plant height under low-temperature stress conditions decreased with the development of the growth period [14,15].
Leaves are an important organ for plants to carry out photosynthesis, and stomata are an important part of leaf epidermal structure and an important portal for plants to exchange water and gas with the outside world [16,17], and the regulating function of stomatal opening size, quantity, and distribution on leaves is a key mechanism for plants to adapt or to resist the external environment [18,19]. Abnormal temperature is one of the important external factors affecting stomatal opening. Studies have shown that stomatal conductance is positively correlated with stomatal size, and stomatal development can regulate leaf temperature by affecting stomatal conductance and transpiration rate. It was observed that the leaf temperature of young leaves was higher than that of mature leaves in the field [20], and the lower transpiration rate of young leaves was consistent with the higher leaf temperature, while the higher transpiration rate of mature leaves was consistent with the lower leaf temperature [21].
Low-temperature stress not only affects plant biomass accumulation but also affects many physiological–biochemical processes of plants, among which photosynthesis of leaves is an important physiological–biochemical process for plants to obtain nutrients and energy that are required for growth, and this process is extremely sensitive to low temperature [22]. Previous studies have found that spring coldness reduces transpiration rate (Tr), net photosynthetic rate (Pn), and stomatal conductance (Gs) of wheat leaves and increases intercellular CO2 concentration (Ci) [23]. After low-temperature stress treatment, the gas exchange parameters, chlorophyll fluorescence parameters (Fv/Fm), and chlorophyll content of wheat leaves were significantly reduced [24,25]. Additionally, under low-temperature stress both at stem elongation and at booting stages, the transport volume and transport rate of wheat photosynthate decreased with the decline of temperature, which ultimately resulted in poor stem quality and low grain weight of winter wheat [26].
The degree of variation of wheat photosynthetic characteristics under low-temperature conditions is determined not only by the degree of low-temperature stress but also by the growth phase of wheat when stress occurs. For instance, the photosynthetic parameters of wheat responded to cold stress in different days after jointing (DAJ) as follows: 10 DAJ > 15 DAJ > 5 DAJ, which indicated that winter wheat in the middle stage of stem jointing (10 DAJ) was the most sensitive to low temperature [27]. After low-temperature treatment during the elongation–booting period, wheat showed different photosynthetic characteristics due to different growth processes, and Pn, Gs, Tr, and Fv/Fm of leaves showed a trend of first going down and then increasing with the extension of low temperature duration at the jointing stage, while at the booting stage, they showed a trend of decreasing with the lasting of low-temperature duration and could not recover in a short time [28]. Some reports proved that the half-winter varieties had higher activity in the aspect of photosynthesis and a stronger self-defense mechanism under low-temperature stress than that of spring-type wheat varieties [29,30].
Low temperature in spring caused a significant decrease in yield when stress happened at different wheat growth stages. Compared with the wheat plant under the natural condition, the yield of wheat decreased by 49.42–54.80% after 24 h of low-temperature stress at the jointing stage and decreased by 59.54–61.50% after 48 h of stress, respectively. Under the above condition, the key reason for the decrease in yield was that low-temperature stress reduced the number of ears per plant and the number of grains per spike [31]. A significant decrease in the number of ears and grains and a decrease in grain weight were the respective reasons for lower yield when subjected to low-temperature stress at the anther or flowering stages [32,33].
To date, there are so many reports on the wheat injury due to low temperature, but few reports on the similar and different influences of low temperature in early spring on agronomic traits of wheat sown on different dates. Therefore, the objectives of this study were as follows: (1) identify the changes in the yield and morphological traits of wheat planted on different dates; (2) investigate the similar and different responses in cold resistance and yield of wheat sown on different dates to early spring low temperature and explore the potential factors contributing to yield reduction. The expected results could provide a theoretical basis and technical support for the low-temperature resistance technology of wheat in agricultural production.
2. Materials and Methods
The experiment was conducted at the pot experiment site of Yangzhou University, located in Jiangsu province in China, in the winter wheat growing seasons of 2018–2019.
The spring-type wheat YM 23 was used in the experiment. It was bred by the Jiangsu Lixiahe Agricultural Research Institute and widely cultivated in the Yangtze River Basin in China. All wheat was planted in the pot with 13 kg of soil and 2 L of water in each pot before sowing and with 1 kg of soil cover after sowing. We sowed 12 seeds first and then thinned the seedlings at the four-leaf stage and left 8 seedlings in each pot. Fertilizer was based on the amount of 320 kg of pure nitrogen, 130 kg of P2O5, and 130 kg of K2O applied per hectare. Each pot was treated with 1.26 g of urea and 3.90 g of compound fertilizer (containing 15% N, 15% P2O5, and 15% K2O) as base fertilizer. At the four-leaf stage, 0.51 g urea was applied to each pot as tillering fertilizer, and 0.76 g urea and 3.9 g compound fertilizer were applied to each pot at the jointing stage.
2.1. Experimental Design
The spring variety YM 23 was sown on 1 November (suitable sowing date I, SDI), 21 November (late sowing date II, SDII), and 9 December (overly late sowing date III, SDIII).
The artificial intelligence-controlled greenhouse was used to simulate natural low temperature. Wheat plants with different sowing dates were selected for low-temperature treatment (−6 °C/6 °C, night/day), and the treatment duration was divided into 24 h, 48 h, and 72 h. During the treatment, the soil moisture was controlled to 60–70% of the field water capacity. A 12 h photoperiod between 6:00 and 18:00, and light quantum flux density in the greenhouse was 600 μmol·m−2·s−1, room temperature control error was kept at ±0.5 °C, and atmospheric relative humidity was maintained at 70 ± 1%.
Low-temperature-treated wheat plants were transported to natural conditions to grow to maturity. At the same time, the seedlings always growing under the natural condition on the same sowing date were the controls, and the highest and lowest average daily air temperatures outside during the treatment period were 12 °C/0 °C (day/night).
When low-temperature treatment was performed on February 24 in early spring, the stages of wheat in SDI, SDII, and SDIII treatments were Zadck’s stage 19, Zadck’s stage 18, and Zadck’s stage 16, respectively.
2.2. Determination Items and Methods
2.2.1. Yield and Yield Components
At the dough stage, three pots were examined to determine the number of spikes per pot and the number of grains per spike. Additionally, at maturity, the grain weight was measured in order to calculate the theoretical yield. Five pots were harvested per treatment to measure the yield per pot. Moisture content of grain was measured to calculate the yield that was shown on a 13% moisture basis.
2.2.2. Plant Traits of Wheat
The diameters of the internodes below the ear, as well as the length of both the spike and the awns, were measured upon reaching maturity. A vernier caliper was utilized for measuring the diameters of both the upper and lower ends of the internode below the ear, and subsequently, an average value was obtained to determine the thickness of the uppermost internode. Plant heights were measured by a measuring scale of 100 cm.
2.2.3. Ear Traits of Wheat
At maturity, 5 pots were selected from each treatment to determine the number of fertile spikelets per ear and the number of degenerate spikelets per ear. The length and width of grain were measured by the SC-G analyzer (Wseen, Hangzhou, China). Cut all the awns off each wheat ear, and the awn length was scanned by a scanner (Epson Expression 1680, Tokyo, Japan). The total length of awn per ear was calculated by WinRHIZO analysis 2016 software (Regent Instruments Inc., Quebec city, QC, Canada).
2.2.4. Leaf Traits of Wheat
At the flowering stage, the length and width of the flag blade were measured with a measuring ruler with a measuring range of 40 cm. The length of the flag blade was taken from the top to the bottom of the flag blade, and the width was taken from the widest part in the middle of the flag blade.
2.2.5. Leaf Photosynthetic Parameter
After stress, the first fully expanded leaf of a single stem with uniform growth was randomly selected for each treatment, and the net photosynthetic rate (Pn), transpiration rate (Tr), stomata conductance (Gs), and intercellular CO2 concentration (Ci) of the leaves were measured by portable photosynthesis analyzer LI-6400XT (LI-COR Biosciences, Lincoln, NE, USA) in the morning when the treatment was completed and at anthesis, respectively. The average value was repeated three times for each leaf.
2.3. Data Analysis Methods
Data in this paper were analyzed and plotted by Excel 2021. All the data were subjected to an analysis of variances using DPS 7.05 Statistical Software (DPS, Hangzhou, Zhejiang, China) and Origin 2021 Software (OrilginLab, Northampyon, MA, USA). Treatment means of comparison were calculated and made at the 5% level of probability using the least significant difference test (LSD).
3. Results and Analysis
3.1. Yield Changes of Wheat Sown on Different Dates Under Spring Low-Temperature Stress
The number of spikes per pot significantly decreased in the late and super late sowing treatments for wheat variety YM23, and the yield of late-sown wheat decreased by 40.82% for SDII treatment and by 66.77% for SDIII treatment, compared with SDI treatment (Table 1). Moreover, the decrease in wheat yield was further increased after wheat was exposed to low-temperature treatment. When the low temperature was at −6/6 °C and lasted for 24 h on February 24, the yield of wheat plants in the SDI treatment decreased by 69.6% compared with the natural growth control (CKI), while those in SDII and SDIII decreased by 64.7% and 59.36% compared with CKII and CKIII, respectively. In conclusion, the yield of wheat in the three sowing dates decreased with the extension of cold stress time. The yield of SDI decreased by 84.93% after 72 h of low-temperature stress, compared with CKI, and that of SDIII decreased by 74.14% after the same duration of low-temperature stress, compared with CKIII. Sowing late caused a significant decrease in the number of ears, and wheat of SDI was just at the jointing stage when it encountered low-temperature stress in February; its tillers from the low node of the crown were seriously frozen, resulting in the lower final ear number and grain number per ear in maturity. Coupled with the decrease in grain weight, the yield declined more sharply than that of CKI.

Table 1.
Yield and its components of wheat sown on different dates under low-temperature stress (−6 °C/6 °C night/day).
The yield of SDI decreased by 69.90–86.49%, while that of SDII and SDIII treatments decreased by 38.29–48.71% and 19.72–24.64% due to low temperature alone, respectively. This indicated that the cold resistance of wheat in the SDII and SDIII treatments was stronger than that of wheat in the SDI treatment, due to their slow development progress and higher resistance to cold stress themselves. Path coefficient analysis indicates that, after early spring low-temperature stress, the direct impact of grain number per ear on the wheat yield of SDI (0.493), the direct impact of grain weight on the yield of SDII (0.511), and the direct impact of ear number on the yield of SDIII (0.732) are the greatest, respectively.
3.2. Agronomic Trait Changes of Wheat Sown on Different Dates Under Spring Low-Temperature Stress
The grain length and width of YM 23 showed a decreasing trend with the delay of sowing date (Table 2), and there were significant differences between SDIII and SDI under the natural condition. After being subjected to low temperature, the grain length exhibited a significant decrease, while there was no significant alteration observed in the grain width. Under the same duration of low-temperature treatment, the ratio of grain length to width decreased with the delay of sowing date. The lasting time of low-temperature treatment significantly affected the length and width of the flag leaf (Figure 1A–C). Significant differences in the flag leaf area and length among the three sowing dates were observed under the natural condition at anthesis. However, under low-temperature treatment lasting for 24 h, no significant differences were observed in the area, length, and width of flag leaf among the three sowing dates. But lasting for 48 h, there were also significant differences in flag leaf length and area between suitable sowing and late sowing, and the leaf area of SDI was 1.27 times that of SDIII. After 72 h of low-temperature treatment, flag leaf area was not significantly different among the three sowing dates at anthesis.

Table 2.
Flag leaf and grain traits of wheat sown on different dates under low-temperature stress (−6 °C/6 °C night/day).
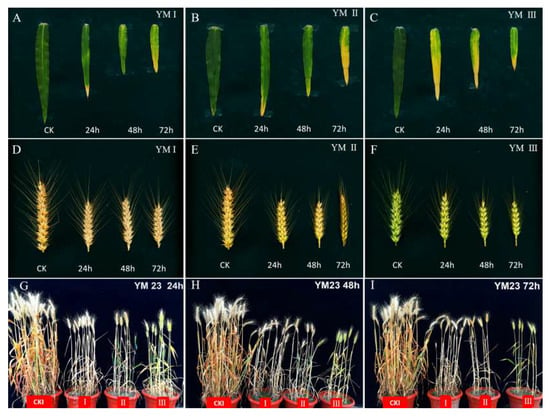
Figure 1.
Plant morphology of wheat sown on different dates under low-temperature stress (leaf photographed on 20 April, ear and plant photographed on 18 May 2019). (A–C) shows the flag leaves of wheat sown on the date I, II, and III after the different low-temperature duration. (D–F) shows the ears of wheat sown on different dates under early spring low temperature stress. (G–I) shows the wheat plants obtained after different sowing periods and subjected to low-temperature treatment for 0 h, 24 h, 48 h, and 72 h.
The number of fertile spikelets, total spikelets, and grains per spike of wheat grown under the natural outdoor conditions decreased progressively with the delayed sowing dates, while the number of degenerated spikelets increased (Table 3, Figure 1D–F). No obvious differences in fertile grain number per spikelet were observed among the sowing date treatments. Compared to outdoor-grown wheat on the same sowing date, YM 23 exhibited declining trends in total spikelet, fertile spikelet number, grains per ear, and average fertile grains per spikelet with the delayed sowing date under identical low-temperature treatment duration. The degenerated spikelet number of YM23 followed the order SDII > SDI > SDIII, though no notable differences were detected among stress treatments. The percentage of spikelet degeneration showed a pattern of SDII > SDIII > SDI, with degeneration rates ranging from 8.67% to 10.52%.

Table 3.
Spike fertility traits of wheat sown on different dates under low-temperature stress (−6 °C/6 °C night/day).
Plant height, the stem diameter of the internode below the spike, and ear length of YM 23 on late sowing dates were significantly decreased and decreased further under low-temperature treatment for 24 h, 48 h, and 72 h in early spring, compared with the outdoor controls (Table 4, Figure 1G–I). Compared with the control under the same sowing date, the average plant height of wheat decreased by 21.48%, and the average stem diameter of the internode below the spike decreased by 33.56% after 72 h of cold stress. After 24 h of low-temperature treatment, the change in diameter of the internode below the spike of SDIII was the lowest (18.15%), and there was no significant difference among the cold treatments.

Table 4.
Plant height, internode diameter, spike length, and total awn length per spike of wheat sown on different dates under low-temperature stress (−6 °C/6 °C night/day).
3.3. Leaf Photosynthetic Changes of Wheat Sown on Different Dates Under Spring Low-Temperature Stress
The first fully expanded leaf of wheat under natural growth conditions without low-temperature treatment exhibited a progressive decline in Pn, Gs, Ci, and Tr along with the delayed sowing date (Table 5). On the day of the low-temperature treatment ending in February, all treatments exhibited significantly lower Pn, Gs, and Tr compared to the control under the natural growth condition. The magnitude of decline increased progressively with prolonged chilling exposure. After 72 h of cold treatment, SDI showed reductions of 31.67% (Pn), 48.00% (Gs), and 27.00% (Tr) relative to the control on the same sowing date; SDII decreased by 34.05% (Pn), 55.21% (Gs), and 37.45% (Tr); SDIII declined by 33.24% (Pn), 51.76% (Gs), and 28.28% (Tr), respectively. Analysis of photosynthetic parameters revealed that low-temperature stress significantly impaired the photosynthetic capacity of wheat functional leaves and, in addition, exacerbated the inhibitory effects on leaf photosynthesis with the prolonged cold exposure. Specifically, the duration of cold exposure exhibited a positive correlation with the severity of photosynthetic inhibition, which demonstrated that extended low-temperature treatment damaged the photosynthesis of wheat leaves to some extent.

Table 5.
Leaf photosynthetic parameters of wheat sown on different dates under low-temperature stress (−6 °C/6 °C night/day).
After low-temperature stress, the Ci of all treatments was higher than that of natural growth control, and the increase was greater with the extension of low-temperature treatment duration. A comparison of the low-temperature treatment with the outdoor natural growth treatment revealed a decreasing trend in Pn, Gs, Ci, and Tr values with the delay of the sowing date under the same stress duration. From the day of low-temperature treatment ending to the day of flowering, the Pn, Gs, Ci, and Tr values of the flag leaves were still significantly lower than those of leaves under the natural growth condition, indicating that low-temperature stress in February also caused varying degrees of damage to the flag leaves that were born after low-temperature treatment. In addition, the Pn, Gs, and Tr values of flag leaves enhanced when the sowing date was postponed, indicating that the photosynthetic parameters of the first fully expanded leaf of SDII and SDIII decreased more than that of SDI after cold stress in February. However, at anthesis, the photosynthetic performance of flag leaves of SDII and SDIII exhibited a slight superiority compared to those of SDI, indicating that the impact of early low temperature on flag leaf growth of SDII and SDIII was relatively less than that of SDI.
3.4. Correlation Analysis in Leaf Parameters and Plant Traits as Well as Yield Parameters
Late-sown wheat was subjected to low-temperature stress in spring; its leaf length, width, and area, Pn, Gs, and Tr of leaf were significantly positively correlated with plant height, ear length, fertile spikelets per ear, grain weight, and yield, while these parameters were all notably negatively correlated with Ci of leaf. Degenerated spikelets per ear and grain length/width ratio were not significantly correlated with the above parameters (Figure 2).
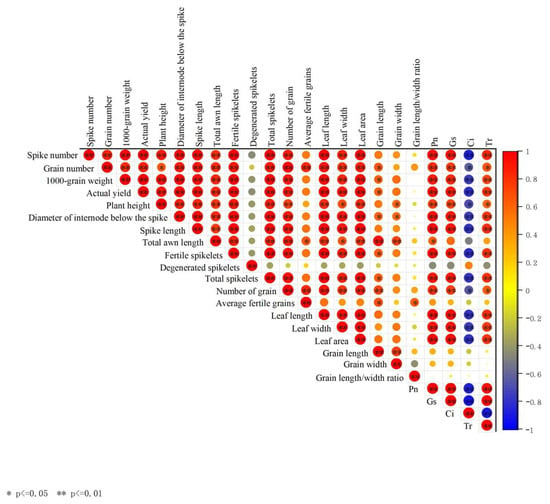
Figure 2.
Correlation analysis among the agronomic traits, leaf photosynthetic parameters, and yield components.
4. Discussion
4.1. Similarities and Differences in Agronomic Traits of Wheat Sown on Different Dates Under Low-Temperature Stress
Previous studies showed that wheat plant height, stem diameter, and ear length shortened along with the delay of sowing date [34,35] or under low-temperature stress at the jointing stage [36]. Low-temperature stress had the most significant influence on plant height at the differentiation stage of glume primordium, but the effect was relatively lower during both periods of gynostemium and androecium primordia as well as the formation stages of anther and tetrad [37]. Our results showed that the plant height of YM23 decreased notably with the delay of sowing date, low temperature in early spring inhibited internode elongation, and the plant height of wheat further decreased. The longer the duration of cold stress exposure, the greater the reduction in the plant height. Low temperature during the jointing stage mainly decreased the length of basal internodes I and II, while low temperature during meiosis mainly decreased the peduncle length, which resulted in lower plant height [31]. We also observed that the stem diameter of the internode below the spike was also affected by both planting time and the degree of temperature stress. The diameter of this internode was thinner along with the later sowing date and more significant, especially under late sowing together with early spring low-temperature conditions.
The area of the flag leaf showed a significantly positive correlation with the number of grains per ear and single spike weight, and the contribution rate of all leaves of the main shoot to its yield was up to 40.75% [38,39]. Additionally, it was observed that delayed sowing dates led to a reduction in the leaf area of over 59% [40]. Our experimental results also indicated that, compared to wheat sown on the optimal date, the length, width, and area of the flag leaf exhibited a declining trend with delayed sowing date, and the later the sowing time, the more serious the decline. This phenomenon is related to the rapid growth process in the middle and late stages of late-sown wheat, resulting in a shorter period from leaf differentiation to full expansion, leading to shorter leaf length and width. According to the previous research results, late spring low-temperature stress could lead to a reduction in the leaf area index, and the reduction was more serious with the aggravation of low-temperature stress [41]. Our experimental results showed that low-temperature stress in February also significantly decreases the length, width, and area of the flag leaves in wheat YM23, primarily because the flag leaves of SDI and SDII are in the stages of cell division and elongation, making them highly susceptible to the response of low temperatures in the aspect of leaf shape. This suggests that spring low-temperature stress, particularly in early spring, increases the differences in flag leaf area between late-sown and optimally-sown wheat, which potentially impacts the material source for grain filling.
Under low-temperature conditions, infertility in florets located in the middle and basal regions of the ear was 41% to 53% less than those in the uppermost one-third of the ear, which resulted in abnormal ears [42]. In this experiment, the delay of sowing date resulted in shorter ear length and total awns length per ear; the ear length of wheat in the SDIII treatment decreased by about 3.3 cm, and the total awns length of SDIII decreased by up to 16.34 cm. These results showed that late sowing caused smaller leaf area, shorter ear length and total awns length, shorter plant height, and thinner internode stem. In addition, after low-temperature stress in early spring, the change trend of the above values was intensified. Therefore, in wheat production, special attention should be paid to the fields that wheat is sown too late. When low temperature occurs in early spring, remedial measures should be taken in time to reduce the impact of low temperature on agronomic traits of wheat.
Some studies have shown that the length and width of wheat grains decreased with delayed sowing dates [43]. Our experimental results demonstrated that both the length and the width of grains from late-sown and excessively late-sown wheat were lower than those from optimally sown wheat, with a significant difference observed between excessively late-sown wheat and optimally sown wheat. Some researchers proposed that low-temperature treatment (−4 °C) during the early jointing stage changed the morphological characteristics of grains, increasing the length-to-width ratio and roundness while decreasing the grain diameter [44]. Our study found that early spring low-temperature stress reduced both the length and the width of grains compared to the naturally growing wheat but increased their length-to-width ratio, which influenced the formation of wheat yield.
4.2. Similarities and Differences in Leaf Photosynthesis and Yield Formation of Wheat Sown on Different Dates Under Low-Temperature Stress
According to the previous report, the postponed sowing date significantly reduced the Pn, Gs, and Tr of flag leaves, significantly reduced the Ci, and also reduced the accumulation of photosynthetic products, thus leading to lower yield [45]. Some reports showed that during low-temperature treatment at the jointing and booting stage, Pn, Gs, and Tr of wheat leaves all decreased with the decrease of temperature, and Ci of the leaf increased significantly [28]. Our results also indicated that early spring low-temperature significantly damaged the photosynthetic capacity of functional wheat leaves, and the longer the low-temperature stress time, the more significant the effects on the photosynthesis of wheat leaves. Under low-temperature stress, at the microscopic level, plant leaf epidermis fenestrated and spongy tissues thickened, chloroplast membrane structure was damaged, stromal vesicles leaked, and chlorophyll content decreased. The primary function of leaves was impaired, thus destroying the energy source for plant growth and ultimately inhibiting plant growth [46]. Under the same low-temperature treatment duration, Pn, Gs, and Tr of the first fully expanded leaf from the top all showed a decreasing trend with the postponed sowing date. Until the day of flowering, Pn, Gs, and Tr of the leaf increased with the delay of the sowing date, indicating that the effect of low temperature on the growth of leaves of late-sown wheat was less than that of suitable sown wheat. These results indicated that low temperature in spring not only damaged the emerged leaf of wheat plants during stress treatment but also affected the photosynthetic function of the undeveloped leaves that born after cold treatment, resulting in a decrease in Pn, Gs, Tr, and a significant increase in Ci of flag leaves at anthesis, which was not conducive to the accumulation of photosynthetic biomass in leaves during the grain-filling stage. A more considerable impact on leaves of wheat on the optimal sowing date was notable.
Due to the late harvest of rice, frequent rain, or drought during the wheat sowing period, it is difficult to sow wheat on an appropriate date, and the area of late-sown wheat is constantly increasing, which is the main production problem in current rice–wheat regions. Effective spike number, kernel number per spike, and grain weight of wheat decreased significantly under delayed sowing conditions, leading to yield reduction [47,48]. The results of this experiment showed that wheat yield, along with the delayed sowing date, decreased significantly. Compared with the suitable sowing time treatment, the yield of YM 23 under SDII and SDIII treatments decreased by 21.34% to 40.82%, mainly due to the decrease of effective spike number and grain number per spike. In recent years, global warming and frequent extreme weather, especially low-temperature disasters in winter and early spring, have adversely affected the plant growth and yield formation of wheat. The spikelet setting rate and yield of wheat decreased significantly compared with the outdoor growth control when the sowing date was delayed and then subjected to low-temperature injury [49]. This experiment showed that wheat yields on different sowing dates all decreased significantly under low-temperature stress, compared with the natural growth control. The differences in the wheat growth process and cold resistance itself were due to the inconsistency of sowing time, and so the main reasons for decreased yield caused by low-temperature stress were also different.
During low-temperature stress in early spring, suitable sowing wheat was at the jointing stage; the main stem and large tillers died seriously because of cold injury. Hence, the decrease of ear number and grain number per ear was the main reason for the lower yield of SDI. While wheat seedlings of SDII and SDIII treatments are younger at this time, they still possess a certain degree of cold resistance; therefore, their yield reduction due to low temperature only was less pronounced compared to SDI. Although the yield reduction caused only by low temperatures was lower for the late- or overly late-sown wheat, compared to wheat planted on a suitable date, the overall yields of SDII and SDIII were still lower than that of SDI because their yield reduction was mainly caused by the late sowing itself. In order to protect wheat from cold stress, researchers found that ascorbic acid priming enhances yield, antioxidant enzyme activities, and chlorophyll contents when wheat is subjected to low temperatures [50]. Therefore, some regulation techniques need to be studied further to alleviate the combined adverse effects of late sowing and low temperature on wheat yield.
5. Conclusions
Late sowing and low temperature in spring are the important factors of influence in wheat yield formation in the rice–wheat rotation region in China. The plant height, the thickness of the internode below the ear, shorter leaf length, smaller leaf area, and the Pn, Gs, and Tr of wheat leaves all showed a decreasing trend with the delayed sowing date, which affected grain filling and resulted in the total awn length per ear and ear length, as well as thinner and lighter grains. The above parameters were aggravated by low temperature in early spring. The influences of cold stress in February on the net photosynthetic rate of flag leaves of late-sowing wheat were relatively more minor than that of suitable-sowing wheat, which indicated that late-sown wheat had better cold resistance than the suitable-sown wheat. However, the leaf area of late-sown wheat was lower and finally affected the formation of its grain. Wheat yield decreased because of the delayed sowing date and decreased further combined with low-temperature stress in spring. The later the sowing, the more severe the low-temperature stress and the more significant the yield reduction. The decrease of ear number and grain number per ear caused by early spring low temperature was the primary reason for the reduction of yield. The yield of wheat under overly late sowing declined more seriously than that under cold stress. Therefore, how to increase seed amount and nitrogen application to mitigate the yield loss of late-sown wheat and enhance cold resistance to low-temperature stress needs to be further studied.
Author Contributions
Conceptualization, C.L., M.Z., J.D., X.Z. and W.G.; formal analysis, Y.Z. (Yangyang Zhu), Y.G., Z.Z. and X.Z.; investigation, Y.G., Z.Z., Y.Z. (Yueping Zhou), J.W. and S.Y.; methodology, Y.Z. (Yangyang Zhu), Y.G., Z.Z., Y.Z. (Yueping Zhou), S.Y. and J.W.; project administration, C.L. and W.G.; resources, J.D. and M.Z.; writing-original draft, Y.G., Y.Z. (Yangyang Zhu) and Y.Z. (Yueping Zhou); writing-review and editing, C.L., M.Z., J.D., X.Z. and W.G.; supervision, C.L.; validation, J.W. and S.Y.; funding acquisition, C.L. and W.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by China National Key R&D Program (2023YDF2300201), the Jiangsu Provincial Key R&D Program (BE2020319), the Independent Innovative Agricultural Project of Jiangsu Province (CX(22)1001), the Jiangsu Special Program for Morden Agriculture (2021), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Data Availability Statement
All the data included in this study are available upon request by contacting the corresponding author.
Acknowledgments
We thank the editor and anonymous reviewers for their constructive comments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kucharik, C.J.; Ramiadantsoa, T.; Zhang, J.; Ives, A.R. Spatiotemporal trends in crop yields, yield variability, and yield gaps across the USA. Crop Sci. 2020, 60, 2085–2101. [Google Scholar] [CrossRef]
- Bian, J.L.; Ren, G.L.; Xu, F.F.; Zhang, H.C.; Wei, H.Y. Comparison of grain yield and quality of different types of japonica rice cultivars in the northern Jiangsu plain, China. J. Integr. Agric. 2021, 20, 2065–2076. [Google Scholar] [CrossRef]
- Nai, J.; Zhang, H.C.; Lu, J.F. Regional pattern changes of rice production in thirty years and its influencing factors in Jiangsu Province. Sci. Agric. Sin. 2012, 45, 3446–3452. (In Chinese) [Google Scholar]
- Tanio, M.; Tateishi, K.; Fukami, K.; Sasaki, Y.; Watanabe, T. Pseudostem length as an indicator of the start of internode elongation in spring and winter wheat cultivars. Jpn. J. Farm. Work. Res. 2016, 51, 1–9. [Google Scholar] [CrossRef]
- Tian, Z.W.; Yin, Y.Y.; Li, B.W.; Zhong, K.T.; Liu, X.X.; Jiang, D.; Cao, W.X.; Dai, T.B. Optimizing planting density and nitrogen application to mitigate yield loss and improve grain quality of late-sown wheat under rice-wheat rotation. J. Integr. Agric. 2025, 24, 2558–2574. [Google Scholar] [CrossRef]
- Chen, X.; Lin, T.; Lin, F.F.; Zhang, Y.; Su, H.; Hu, Y.M.; Song, Y.H.; Wei, F.Z.; Li, J.C. Research progress on damage mechanism and prevention and measures of late spring coldness of wheat in Huanghuai Region. J. Triticeae Crops 2020, 40, 243–250. (In Chinese) [Google Scholar]
- Rapacz, M.; Jurczyk, B.; Bani, I.; Wόjcik-Jagla, M. Phenotyping the effects of simulated spring frost on the yield of barley (Hordeum vulgare L.) and wheat (Triticum aestivum L.). J. Agron. Crop Sci. 2025, 211, e70054. [Google Scholar] [CrossRef]
- Lin, F.F.; Li, C.; Xu, B.; Chen, J.; Chen, A.H.; Hassan, M.A.; Liu, B.B.; Xu, H.; Chen, X.; Sun, J.Q.; et al. Late spring cold reduces grain number at various spike positions by regulating spike growth and assimilate distribution in winter wheat. Crop J. 2023, 11, 1272–1278. [Google Scholar] [CrossRef]
- Liu, L.L.; Xia, Y.M.; Liu, B.; Chang, C.Y.; Xiao, L.J.; Shen, J.; Tang, L.; Cao, W.X.; Zhu, Y. Individual and combined effects of jointing and booting low-temperature stress on wheat yield. Eur. J. Agron. 2020, 113, 125989. [Google Scholar] [CrossRef]
- Venzhik, Y.V.; Moshkov, I.E. The role of ultrastructural organization of cells in adaptation of winter wheat to low temperature. Russ. J. Plant Physiol. 2023, 70, 100. [Google Scholar] [CrossRef]
- Rose, T.J.; Raymond, C.A.; Bloomfield, C.; King, G.J. Perturbation of nutrient source-sink relationships by post-anthesis stresses results in differential accumulation of nutrients in wheat grain. J. Plant Nutr. Soil Sci. 2015, 178, 89–98. [Google Scholar] [CrossRef]
- Venzhik, Y.V.; Talanova, V.V.; Titov, A.F.; Kholoptseva, E.S. Similarities and differences in wheat plant responses to low temperature and cadmium. Biol. Bull. 2015, 42, 508–514. [Google Scholar] [CrossRef]
- Gao, Q.L.; Xue, X.; Liang, Y.J.; Wu, Y.; Ru, Z.G. Studies on regulating sowing time of wheat under the warm winter conditions. J. Triticeae Crops 2002, 22, 46–50. (In Chinese) [Google Scholar]
- Marcińska, I.; Biesaga-Koscielniak, J.; Dubert, F.; Kozdόj, J. Effect of length and developmental stage of spike on the induction and differentiation efficiency of callus tissue in winter wheat.: Evidence for generative development of regenerated plants. Acta Physiol. Plant. 1999, 21, 355–363. [Google Scholar] [CrossRef]
- Follmann, D.N.; Vendrame, M.; Rosa, G.B.; Santors, E.D.; Pereira, A.C. Agronomic performance associated with the incidence of frost on wheat cultivars in Brazil. Bulg. J. Agric. Sci. 2024, 30, 323–332. [Google Scholar]
- Guo, C.; Tao, R.R.; Zhu, M.; Zhou, M.X.; Zhao, C.C. An enhanced method for studying wheat stomata physiology. J. Plant Growth Regul. 2024, 43, 4886–4893. [Google Scholar] [CrossRef]
- Mehri, N.; Fotovat, R.; Saba, J.; Jabbari, F. Variation of stomata dimensions and densities in tolerant and susceptible wheat cultivars under drought stress. J. Food Agric. Environ. 2009, 7, 167–170. [Google Scholar]
- Xue, W.; Otieno, D.; Ko, J.; Werner, C.; Tenhunen, J. Conditional variations in temperature response of photosynthesis, mesophyll and stomatal control of water use in rice and winter wheat. Field Crops Res. 2016, 199, 77–88. [Google Scholar] [CrossRef]
- Sangha, J.S.; Wang, W.W.; Knox, R.; Ruan, Y.F.; Cuthbert, R.D.; Isidro-Sánchez, J.; Li, L.; He, Y.; Depauw, R.; Singh, A.; et al. Phenotypic plasticity of bread wheat contributes to yield reliability under heat and drought stress. PLoS ONE 2025, 20, e0312122. [Google Scholar] [CrossRef] [PubMed]
- Snider, J.L.; Choinski, J.S.; Wise, R.R. Juvenile Rhus glabra leaves have higher temperatures and lower gas exchange rates than mature leaves when compared in the field during periods of high irradiance. J. Plant Physiol. 2009, 166, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.J.; Chow, W.S.; Liu, Y.J.; Shi, L.; Jiang, C.D. Effects of stomatal development on stomatal conductance and on stomatal limitation of photosynthesis in Syringa oblata and Euonymus japonicus Thunb. Plant Sci. 2014, 229, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Xiang, C.; Farooq, M.; Muhammad, N.; Yan, Z.; Hui, X.; Ke, Y.Y.; Bruno, A.K.; Zhang, L.L.; Li, J.C. Cold stress in wheat: Plant acclimation responses and management strategies. Front. Plant Sci. 2021, 12, 676884. [Google Scholar] [CrossRef] [PubMed]
- Li, X.N.; Cai, J.; Liu, F.L.; Dai, T.B.; Cao, W.X.; Jiang, D. Cold priming drives the sub-cellular antioxidant systems to protect photosynthetic electron transport against subsequent low temperature stress in winter wheat. Plant Physiol. Biochem. 2014, 82, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Venzhik, Y.V.; Talanova, V.V.; Ignatenko, A.A.; Repkina, N.S.; Kholoptseva, E.S.; Titov, A.F. Features of wheat adaptation to frost under low-temperature exposure of different intensity. Russ. J. Plant Physiol. 2022, 69, 105. [Google Scholar] [CrossRef]
- Taskin, B.G.; Özbek, Ö.; San, S.K.; Eser, V.; Nachit, M.M.; Kaya, Z. Variation in cold tolerance in F6 durum wheat [Triticum turgidum (L.) Tell. convar. durum (Desf.) Mackey] RILs and the relationships of cold tolerance with some quality parameters and genetic markers. J. Agric. Sci. 2020, 158, 47–56. [Google Scholar]
- Liu, L.L.; Ji, H.T.; An, J.P.; Shi, K.J.; Ma, J.F.; Liu, B.; Tang, L.; Cao, W.X.; Zhu, Y. Response of biomass accumulation in wheat to low-temperature stress at jointing and booting stages. Environ. Exp. Bot. 2019, 157, 46–57. [Google Scholar] [CrossRef]
- Zhang, X.H.; Liu, R.H.; Li, Z.; Cheng, L. Effects of low temperature stress on photosynthetic characteristics of winter wheat in different jointing processes. J. Seed Ind. Guide 2019, 7, 6–12. (In Chinese) [Google Scholar]
- Liu, L.L.; Ji, H.T.; Liu, B.; Ma, J.F.; Xiao, L.J.; Tang, L.; Cao, W.X.; Zhu, Y. Effects of jointing and booting low temperature treatments on photosynthetic and chlorophyll flourscence characteristic in wheat leaf. Sci. Agric. Sin. 2018, 51, 4434–4448. (In Chinese) [Google Scholar]
- Zhang, W.J.; Huang, Z.L.; Wang, Q.; Guan, Y.N. Effects of low temperature on leaf anatomy and photosynthetic performance in different genotypes of wheat following a rice crop. Int. J. Agric. Biol. 2015, 17, 1165–1171. [Google Scholar] [CrossRef]
- Miller, K.; Hall, D.; Kramer, D.; Olson, E.; Merewitz, E. Photosynthetic health of winter wheat following overwintering stresses in controlled conditions. Grass Res. 2024, 4, e0024. [Google Scholar] [CrossRef]
- Li, C.Y.; Yang, J.; Zhu, M.; Ding, J.F.; Zhu, X.K.; Zhou, G.S.; Guo, W.S. Urea amendment alleviated morphological and physiological damages and yield loss of winter wheat subjected to low temperature stress at jointing stage. Plant Growth Regul. 2022, 98, 589–598. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.Z.; Chen, X.; Li, J.C. Effects of low-temperature stress during the anther differentiation period on winter wheat photosynthetic performance and spike-setting characteristics. Plants 2022, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.D.; Chen, Q.H.; Agathokleous, E.; Zhang, J.Q.; Yang, Z.Q.; Takin, M. Effects of low-temperature stress during anthesis stage on dry matter accumulation and yield of winter wheat. Agronomy 2025, 15, 761. [Google Scholar] [CrossRef]
- Basheir, S.M.O.; Hong, Y.; Lv, C.; Xu, H.W.; Zhu, J.; Guo, B.J.; Wang, F.F.; Xu, R.G. Identification of wheat germplasm resistance to late sowing. Agronomy 2023, 13, 1010. [Google Scholar] [CrossRef]
- Baloch, M.S.; Shah, I.T.H.; Nadim, M.A.; Khan, M.I.; Khakwani, A.A. Effect of seeding density and planting time on growth and yield attributes of wheat. J. Anim. Plant Sci. 2010, 20, 239–242. [Google Scholar]
- Wang, W.L.; Wang, X.; Huang, M.; Cal, J.; Zhou, Q.; Dai, T.B.; Jiang, D. Alleviation of field low-temperature stress in winter wheat by exogenous application of salicylic acid. J. Plant Growth Regul. 2021, 40, 811–823. [Google Scholar] [CrossRef]
- Cheng, C.; Wu, L.; Yu, W.D.; Yang, F.Y.; Feng, L.P. Dynamic evaluation of winter wheat’s freezing resistance under different low-tempearture periods and durations. Sci. Rep. 2025, 15, 8488. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Hussain, M.; Khan, M.I.; Ali, Z.; Zulkiffal, M.; Anwar, J.; Sabir, W.; Zeeshan, M. Source-sink relationship between photosynthetic organs and grain yield attributes during grain filling stage in spring wheat (Triticum aestivum). Int. J. Agric. Biol. 2010, 12, 509–515. [Google Scholar]
- Bijanzadeh, E.; Emam, Y. Effect of source-sink manipulation on yield components and photosynthetic characteristic of wheat cultivars (Triticum aestivum and T.durum L.). J. Appl. Sci. 2010, 10, 564–569. [Google Scholar] [CrossRef]
- Chun, J.F.; Wang, J.L.; Wang, J.C.; Wang, Z.; Zhao, L.H.; Yan, Y.H.; Li, J.Y.; Xu, H.Z.Y.; Sun, C.M.; Liu, T. Investigating the impact of sowing date on wheat leaf morphology through image analysis. Agriculture 2025, 15, 770. [Google Scholar] [CrossRef]
- Xiao, L.J.; Asseng, S.; Wang, X.T.; Xia, J.X.; Zhang, P.; Liu, L.L.; Tang, L.; Cao, W.X.; Zhu, Y.; Liu, B. Simulating the effects of low-temperature stress on wheat biomass growth and yield. Agric. For. Meteorol. 2022, 326, 109191. [Google Scholar] [CrossRef]
- Subedi, K.D.; Gregory, P.J.; Summerfield, R.J.; Gooding, M.J. Pattern of grain set in boron-deficient and cold-stressed wheat (Triticum aestivum L.). J. Agric. Sci. 2000, 134, 25–31. [Google Scholar] [CrossRef]
- Chaubey, R.K.; Bhutia, D.D.; Navathe, S.; Mishra, V.K.; Singh, A.K.; Chand, R. Interrelationships among different grain characteristics of wheat grown under optimum and late sowing date conditions in the Eastern Indo-Gangetic plains of India. Cereal Res. Commun. 2021, 49, 449–455. [Google Scholar] [CrossRef]
- Wang, S.G.; Wang, Z.L.; Wang, P.; Wang, H.W.; Huang, W.; Wu, Y.G.; Yin, Y.P. Freeze resistance analysis of different wheat cultivars based on the relationships between physiological indices and grain yield. Chin. J. Appl. Ecol. 2011, 22, 1477–1484. (In Chinese) [Google Scholar]
- Sattar, A.; Cheema, M.A.; Abbas, T.; Sher, A.; Ijaz, M.; Wahid, M.A.; Hussain, M. Physiological response of late sown wheat to exogenous application of silicon. Cereal Res. Commun. 2017, 45, 202–213. [Google Scholar] [CrossRef]
- Zhang, F.H.; Lu, K.; Gu, Y.Y.; Zhang, L.; Li, W.Y.; Li, Z. Effects of low-temperature stress and brassinolide application on the photosynthesis and leaf structure of tung tree seedling. Front. Plant Sci. 2020, 31, 1767. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shen, Y.; Zeng, C.W.; Zhang, J.S.; Shi, S.B.; Xue, L.H.; Jia, Y.H.; Li, J.J.; Liang, X.D. Effects of sowing time on yield and quality of winter and spring wheat varieties. Sustainability 2025, 17, 2479. [Google Scholar] [CrossRef]
- Gupta, N.K.; Shukla, D.S.; Pande, P.C. Interaction of yield determining parameters in late sown wheat genotypes. Indian J. Plant Physiol. 2002, 7, 264–269. [Google Scholar]
- Farooq, M.; Basra, S.M.A.; Rehman, H.; Saleem, B.A. Seed priming enhances the performance of late sown wheat (Triticum aestivum L.) by improving chilling tolerance. J. Agron. Crop Sci. 2008, 194, 55–60. [Google Scholar] [CrossRef]
- Shah, T.; Latif, S.; Khan, H.; Munsif, F.; Nie, L.X. Ascorbic acid priming enhances seed germination and seedling growth of winter wheat under low temperature due to late sowing in Pakistan. Agronomy 2019, 9, 757. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).