Optimal Dark Tea Fertilization Enhances the Growth and Flower Quality of Tea Chrysanthemum by Improving the Soil Nutrient Availability in Simultaneous Precipitation and High-Temperature Regions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Substrates
2.2. Experimental Site and Design
2.3. Determination Items and Methods
2.3.1. Determination of Soil Physicochemical Properties
2.3.2. Measurement Method of Morphological Indexes
2.3.3. Determination Method of Photosynthetic Characteristics
2.3.4. Methods for Determination of Florescence Traits
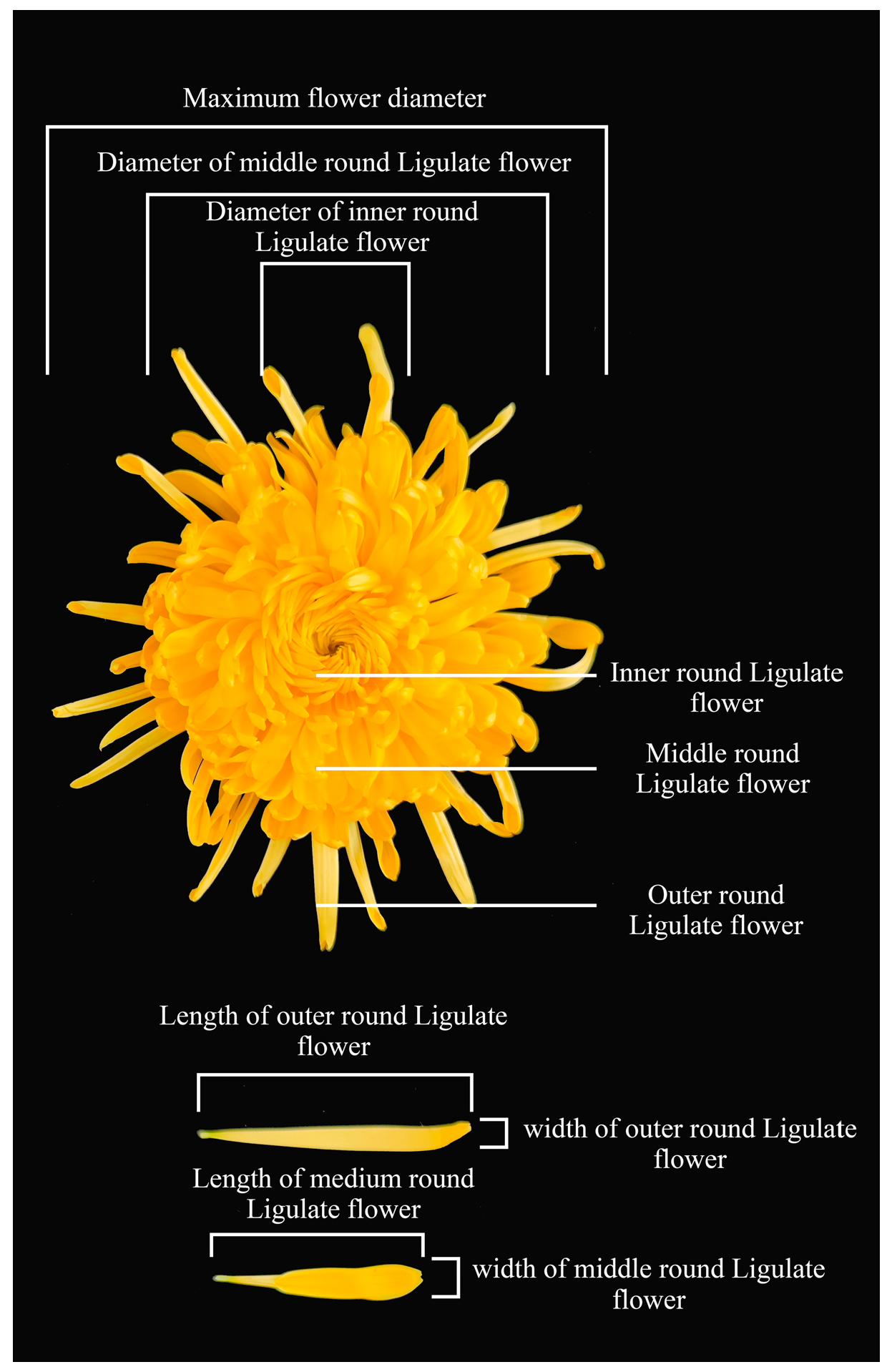
2.3.5. Determination Methods of Flower Components
2.4. Comprehensive Evaluation by Principal Component Analysis (PCA)
2.5. Statistical Analysis
3. Results
3.1. Different Fertilization Treatments Greatly Improved Soil Physicochemical Properties in SPH Regions
3.2. Different Fertilization Treatments Enhanced Morphological Growth of Dendranthema Morifolium ‘Jinsi Huang’
3.3. Different Fertilization Treatments Promoted the Photosynthetic Efficiency of Dendranthema Morifolium ‘Jinsi Huang’ Leaves in Summer
3.3.1. Changes in the Content of Photosynthetic Pigments of Dendranthema Morifolium ‘Jinsi Huang’ Leaves
3.3.2. Changes in Photosynthesis and Chlorophyll Fluorescence Parameters of Dendranthema Morifolium ‘Jinsi Huang’ Leaves
3.4. Different Fertilization Treatments Boosted Flower Performance and Compositions Accumulation of Dendranthema Morifolium ‘Jinsi Huang’
3.4.1. Effects on Flower Performance of Dendranthema Morifolium ‘Jinsi Huang’
3.4.2. Effects on Basic Nutritional and Medicinal Active Components of Dendranthema Morifolium ‘Jinsi Huang’ Flowers
3.5. Principal Component Analysis of Effects on Substrate-Plant System
4. Discussion
4.1. Dark Tea Biofertilizer Success in the Improvement of Soil Environment in SPH Regions
4.2. Dark Tea Biofertilizer Stimulated Light Energy Utilization of Dendranthema Morifolium ‘Jinsi Huang’ Leaves in SPH Weather
4.3. Dark Tea Biofertilizer Greatly Promoted the Accumulation of Chlorogenic Acid in Dendranthema Morifolium ‘Jinsi Huang’ Flowers in SPH Weather
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, G.; Zheng, K.; Shu, C.; Jiang, Y. Promote rural industry revitalization with supply China thinking. China Sustain. Trib. 2024, 3, 54–58. (In Chinese) [Google Scholar]
- Liu, Q.; Liu, Z. Exploration of treatment technology for seepage prevention and reinforcement of earth and rock DAMS. Heilongjiang Hydraul. Sci. Technol. 2020, 48, 172–174. (In Chinese) [Google Scholar] [CrossRef]
- Leng, C.; Ping, G.; Li, X.; Hu, G.; Yu, S. Harmless Cultivation Technology of Dendranthema morifolium ‘Jinsi Huang’ in Xiushui Huangshi. China Agric. Technol. Ext. 2017, 33, 39–40. (In Chinese) [Google Scholar]
- He, J.; Chen, L.; Chu, B.; Zhang, C. Determination of total polysaccharides and total flavonoids in Chrysanthemum morifolium using near-infrared hyperspectral imaging and multivariate analysis. Molecules 2018, 23, 2395. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, M.-Y.; Zafar, S.; Xie, Q.-L.; Jian, Y.-Q.; Yuana, H.-W.; Lia, B.; Penga, C.Y.; Chenb, W.M.; Liu, B.; et al. Structural elucidation, antioxidant and hepatoprotective activities of chemical composition from Jinsi Huangju (Chrysanthemum morifolium) flowers. Arab. J. Chem. 2022, 15, 104292. [Google Scholar] [CrossRef]
- Kim, I.S.; Koppula, S.; Park, P.-J.; Kim, E.H.; Kim, C.G.; Choi, W.S.; Lee, K.H.; Choi, D.-K. Chrysanthemum morifolium Ramat (CM) extract protects human neuroblastoma SH-SY5Y cells against MPP+-induced cytotoxicity. J. Ethnopharmacol. 2009, 126, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, R.; Wang, X.; Zhang, X.; Xiao, Z.; Wang, H.; Sun, W.; Yang, H.; Yu, P.; Hu, Q.; et al. Effects and mechanism of action of Chrysanthemum morifolium (Jinsi Huangju) on hyperlipidemia and non-alcoholic fatty liver disease. Eur. J. Med. Chem. 2023, 255, 115391. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-L.; Sun, P.; Feng, J.; Yuan, J.; Wang, Y.; Shang, Y.-F.; Niu, C.-L.; Yang, S.-H.; Wei, Z.-J. Solvent effect on phenolics and antioxidant activity of Huangshan Gongju (Dendranthema morifolium (Ramat) Tzvel. cv. Gongju) extract. Food Chem. Toxicol. 2021, 147, 111875. [Google Scholar] [CrossRef] [PubMed]
- Tsuji-Naito, K.; Saeki, H.; Hamano, M. Inhibitory effects of Chrysanthemum species extracts on formation of advanced glycation end products. Food Chem. 2009, 116, 854–859. [Google Scholar] [CrossRef]
- Ukiya, M.; Akihisa, T.; Yasukawa, K.; Kasahara, Y.; Kimura, Y.; Koike, K.; Nikaido, T.; Takido, M. Constituents of compositae plants. 2. Triterpene diols, triols, and their 3-O-fatty acid esters from edible chrysanthemum flower extract and their anti-inflammatory effects. J. Agric. Food Chem. 2001, 49, 3187–3197. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Nuerbiye, A.; Cheng, P.; Wang, J.-H.; Li, H. Analysis of floral volatile components and antioxidant activity of different varieties of Chrysanthemum morifolium. Molecules 2017, 22, 1790. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.C.; Lam, S.F.; Zhao, J.; Li, S.P. Rapid identification and comparison of compounds with antioxidant activity in Coreopsis tinctoria herbal tea by high-performance thin-layer chromatography coupled with DPPH bioautography and densitometry. J. Food Sci. 2016, 81, C2218–C2223. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Huang, J.; Wu, G.; Tong, J.; Xie, G.; Duan, J.-A.; Qin, M. Multiple responses optimization of ultrasonic-assisted extraction by response surface methodology (RSM) for rapid analysis of bioactive compounds in the flower head of Chrysanthemum morifolium Ramat. Ind. Crops Prod. 2015, 74, 192–199. [Google Scholar] [CrossRef]
- Yihui, D.; Chan, J.C. The East Asian summer monsoon: An overview. Meteorol. Atmos. Phys. 2005, 89, 117–142. [Google Scholar] [CrossRef]
- Liu, X.; Yin, C.; Chen, X.; Gan, D.; Yu, X.; Xu, L. Effects of Waste Substrates on the Growth of Chrysanthemum Morifolium ‘Huangju’ in High temperature Climate. Acta Agric. Univ. Jiangxiensis 2020, 42, 707–717. (In Chinese) [Google Scholar] [CrossRef]
- Lu, S.; Yang, Z.; Zhang, Y.; Zheng, H.; Yang, I. Effect of Photoperiod on Fluorescence Characteristics of Photosynthetic System of Fresh-cut Chrysanthemum Leaves under High temperature. Chin. J. Agrometeorol. 2020, 41, 632–643. (In Chinese) [Google Scholar]
- Ahmed, R.; Hussain, M.; Ahmed, S.; Karim, M.; Siddiky, M. Effect of N, P and K fertilizer on the flower yield of chrysanthemum. Agriculturists 2017, 15, 58–67. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Jiang, Z.; Yue, X.; Liang, J.; Yang, Q.; Li, J.; Li, N. Micro-moistening irrigation combined with bio-organic fertilizer: An adaptive irrigation and fertilization strategy to improve soil environment, edible Rose yield, and nutritional quality. Ind. Crops Prod. 2023, 196, 116487. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, M.; Ding, H.; Xing, J.; Zhu, X.; Liu, C.; Jing, D.; Hong, L. Research Progress of Continuous Cropping Obstacles of Medicinal Chrysanthemum and Its Mitigation Measures. J. Anhui Agric. Sci. 2016, 44, 150–152. (In Chinese) [Google Scholar] [CrossRef]
- Kumar, S.; Sindhu, S.S.; Kumar, R. Biofertilizers: An ecofriendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microb. Sci. 2022, 3, 100094. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Panayiotou, C.; Tzortzakis, N. Nitrogen and phosphorus levels affected plant growth, essential oil composition and antioxidant status of lavender plant (Lavandula angustifolia Mill.). Ind. Crops Prod. 2016, 83, 577–586. [Google Scholar] [CrossRef]
- Edenborn, S.L.; Johnson, L.M.; Edenborn, H.M.; Albarran-Jack, M.R.; Demetrion, L.D. Amendment of a hardwood biochar with compost tea: Effects on plant growth, insect damage and the functional diversity of soil microbial communities. Biol. Agric. Hortic. 2018, 34, 88–106. [Google Scholar] [CrossRef]
- Sheng, Z.; Qian, Y.; Meng, J.; Tao, J.; Zhao, D. Rice hull biochar improved the growth of tree peony (Paeonia suffruticosa Andr.) by altering plant physiology and rhizosphere microbial communities. Sci. Hortic. 2023, 322, 112204. [Google Scholar] [CrossRef]
- Skrzypczak, D.; Izydorczyk, G.; Taf, R.; Moustakas, K.; Chojnacka, K. Cellulose-based fertilizers for sustainable agriculture: Effective methods for increasing crop yield and soil health. Ind. Crops Prod. 2023, 205, 117500. [Google Scholar] [CrossRef]
- Zheng, W.; Ma, Y.; Wang, X.; Wang, X.; Li, J.; Tian, Y.; Zhang, X. Producing high-quality cultivation substrates for cucumber production by in-situ composting of corn straw blocks amended with biochar and earthworm casts. Waste Manag. 2022, 139, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Matłok, N.; Piechowiak, T.; Królikowski, K.; Balawejder, M. Mechanism of reduction of drought-induced oxidative stress in maize plants by fertilizer seed coating. Agriculture 2022, 12, 662. [Google Scholar] [CrossRef]
- Toundou, O.; Pallier, V.; Feuillade-Cathalifaud, G.; Tozo, K. Impact of agronomic and organic characteristics of waste composts from Togo on Zea mays L. nutrients contents under water stress. J. Environ. Manag. 2021, 285, 112158. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jiang, X.; Agathokleous, E.; Lu, X.; Zaiqiang, Y.; Li, R. High temperature inhibits photosynthesis of chrysanthemum (Chrysanthemum morifolium Ramat.) seedlings more than relative humidity. Front. Plant Sci. 2023, 14, 1272013. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Chen, L.; Wang, W.; Xu, Y.; Zhang, W.; Zhang, H.; Liu, L.; Wang, Z.; Gu, J.; Yang, J. Effects of Application of Rapeseed Cake as Organic Fertilizer on Rice Quality at High Yield Level. J. Sci. Food Agric. 2022, 102, 1832–1841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, R.; Xian, X. Research Progress of Functional Chrysanthemum in China. Chin. Agric. Sci. Bull. 2022, 38, 38–46. (In Chinese) [Google Scholar]
- Zhou, S. Progress in the High-value Comprehensive Utilization of Tea Residue. China Tea Process. 2019, 4, 54–60. (In Chinese) [Google Scholar] [CrossRef]
- Pane, C.; Palese, A.M.; Spaccini, R.; Piccolo, A.; Celano, G.; Zaccardelli, M. Enhancing sustainability of a processing tomato cultivation system by using bioactive compost teas. Sci. Hortic. 2016, 202, 117–124. [Google Scholar] [CrossRef]
- Ramírez-Gottfried, R.I.; Preciado-Rangel, P.; Carrillo, M.G.; García, A.B.; González-Rodríguez, G.; Espinosa-Palomeque, B. Compost Tea as Organic Fertilizer and Plant Disease Control: Bibliometric Analysis. Agronomy 2023, 13, 2340. [Google Scholar] [CrossRef]
- Mei, Y.; Liang, X. Analysis of China’s Tea Production and Domestic Sales in 2021. China Tea 2022, 44, 17–22. (In Chinese) [Google Scholar]
- Meng, Z.; Xiang, S.; Wang, X.; Zhang, J.; Bai, G.; Liu, H.; Li, R.; Shen, Q. Turning Waste into Wealth: Utilizing Trichoderma’s Solid-State Fermentation to Recycle Tea Residue for Tea Cutting Production. Agronomy 2024, 14, 526. [Google Scholar] [CrossRef]
- Lv, Y.-Z.; Li, B.-G. Soil Science Experiments; China Agriculture Press: Beijing, China, 2010. [Google Scholar]
- Canbay, H.S.; Bardakci, B. Determination of fatty acid, C, H, N and trace element composition in grape seed by GC/MS, FTIR, elemental analyzer and ICP/OES. Süleyman Demirel Univ. Fac. Arts Sci. J. Sci. 2011, 6, 140–148. [Google Scholar]
- Bao, S. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Pribyl, D.W. A critical review of the conventional SOC to SOM conversion factor. Geoderma 2010, 156, 75–83. [Google Scholar] [CrossRef]
- Gu, Q.; Chen, Z.; Yu, X.; Cui, W.; Pan, J.; Zhao, G.; Xu, S.; Wang, R.; Shen, W. Melatonin confers plant tolerance against cadmium stress via the decrease of cadmium accumulation and reestablishment of microRNA-mediated redox homeostasis. Plant Sci. 2017, 261, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.; Srivastava, A.; Tsimilli-Michael, M.; Yunus, M.; Pathre, U.; Mohanty, P. Probing Photosynthesis: Mechanisms, Regulation and Adaptation; Taylor and Francis: London, UK; New York, NY, USA, 2000; pp. 445–483. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Huang, Y.Q.; Ling, M.O.; You, Y.M.; Jiao, J.F. Comparison of Two Photosynthesis-light Response Curve-fitting Models of the Karst Plant. J. Wuhan Bot. Res. 2009, 27, 340–344. [Google Scholar]
- Berry, J.A.; Downton, W. Environmental Regulation of Photosynthesis. Photosynthesis 1982, 2, 263–343. [Google Scholar]
- Li, X.; Guo, L.; Lei, X.; Zhao, S.; Huang, S.; Liu, Y.; Zhong, L. Nutritional Analysis and Evaluation of Chrysanthemum. Mod. Food Sci. Technol. 2019, 35, 237–241+260. (In Chinese) [Google Scholar] [CrossRef]
- Li, H.; Qi, J.; Weiming, Q. Determination of Three Active Components in Hanyuan Dendranthema morifolium ‘Jinsi Huang’ by HPLC. Guide China Med. 2019, 17, 20–21. (In Chinese) [Google Scholar] [CrossRef]
- Florentín, M.; Dia, C.E.D.C.; Conservación, P.; Moriya, K.; Deag, C.C. The Laws of Diminishing Yields in the Tropics; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2006.
- Duan, A.; Lei, J.; Hu, X.; Zhang, J.; Du, H.; Zhang, X.; Guo, W.; Sun, J. Effects of planting density on soil bulk density, pH and nutrients of unthinned Chinese fir mature stands in south subtropical region of China. Forests 2019, 10, 351. [Google Scholar] [CrossRef]
- Huang, L.; Liu, X.; Wang, Z.; Liang, Z.; Wang, M.; Liu, M.; Suarez, D.L. Interactive effects of pH, EC and nitrogen on yields and nutrient absorption of rice (Oryza sativa L.). Agric. Water Manag. 2017, 194, 48–57. [Google Scholar] [CrossRef]
- Laishram, N.; Dhiman, S.; Gupta, Y.; Bhardwaj, S.; Singh, A. Microbial dynamics and physico-chemical properties of soil in the rhizosphere of chrysanthemum (Dendranthema grandiflora) as influenced by integrated nutrient management. Indian J. Agric. Sci. 2013, 83, 447–455. [Google Scholar]
- Morra, L.; Bilotto, M.; Baldantoni, D.; Alfani, A.; Baiano, S. A seven-year experiment in a vegetable crops sequence: Effects of replacing mineral fertilizers with Biowaste compost on crop productivity, soil organic carbon and nitrates concentrations. Sci. Hortic. 2021, 290, 110534. [Google Scholar] [CrossRef]
- Su, J.-Y.; Liu, C.-H.; Tampus, K.; Lin, Y.-C.; Huang, C.-H. Organic amendment types influence soil properties, the soil bacterial microbiome, and tomato growth. Agronomy 2022, 12, 1236. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Wu, W.; Jin, Q.; Wang, R.; Zhang, R.; Gao, F.; Zhao, Y.; Wang, W. Effects of bio-organic fertilizer and microbial agent on the growth of tea chrysanthemum and soil fertility under continuous cropping cultivation system in the mountainous area of Beijing. Soil Fertil. Sci. China 2023, 12, 107–113. (In Chinese) [Google Scholar]
- Akalin, G.O.; Pulat, M. Controlled release behavior of zinc-loaded carboxymethyl cellulose and carrageenan hydrogels and their effects on wheatgrass growth. J. Polym. Res. 2020, 27, 6. [Google Scholar] [CrossRef]
- Si, J.; Yang, C.; Ma, W.; Chen, Y.; Xie, J.; Qin, X.; Hu, X.; Yu, Q. Screen of high efficiency cellulose degrading strains and effects on tea residues dietary fiber modification: Structural properties and adsorption capacities. Int. J. Biol. Macromol. 2022, 220, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, P.; Safique, S.; Jahan, A. Humic substrates extracted by recycling factory tea waste improved soil properties and tea productivity: An innovative approach. Int. J. Environ. Sci. Technol. 2019, 16, 3761–3770. [Google Scholar] [CrossRef]
- Shanthi, N.; Al-Huqail, A.A.; Perveen, K.; Vaidya, G.; Bhaskar, K.; Khan, F.; Alfagham, A. Drought stress alleviation through nutrient management in Cyamopsis tetrogonoloba L. J. King Saud Univ.-Sci. 2023, 35, 102842. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Y.; Zhang, Z.; Wei, Y.; Wang, H.; Lu, Q.; Li, Y.; Wei, Z. Effect of thermo-tolerant actinomycetes inoculation on cellulose degradation and the formation of humic substances during composting. Waste Manag. 2017, 68, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Hisamatsu, T.; Sumitomo, K.; Shibata, M.; Koshioka, M. Seasonal variability in dormancy and flowering competence in Chrysanthemum: Chilling impacts on shoot extension growth and flowering capacity. Jpn. Agric. Res. Q. JARQ 2017, 51, 343–350. [Google Scholar] [CrossRef]
- Barro, F.; González-Fontes, A.; Maldonado, J.M. Relation between photosynthesis and dark respiration in cereal leaves. J. Plant Physiol. 1996, 149, 64–68. [Google Scholar] [CrossRef]
- Shahsavandi, F.; Eshghi, S.; Gharaghani, A.; Ghasemi-Fasaei, R.; Jafarinia, M. Effects of bicarbonate induced iron chlorosis on photosynthesis apparatus in grapevine. Sci. Hortic. 2020, 270, 109427. [Google Scholar] [CrossRef]
- Iqbal, A.; He, L.; Ali, I.; Ullah, S.; Khan, A.; Akhtar, K.; Wei, S.; Fahad, S.; Khan, R.; Jiang, L. Co-incorporation of manure and inorganic fertilizer improves leaf physiological traits, rice production and soil functionality in a paddy field. Sci. Rep. 2021, 11, 10048. [Google Scholar] [CrossRef] [PubMed]
- Pilla, N.; Tranchida-Lombardo, V.; Gabrielli, P.; Aguzzi, A.; Caputo, M.; Lucarini, M.; Durazzo, A.; Zaccardelli, M. Effect of compost tea in horticulture. Horticulturae 2023, 9, 984. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Y.; Peng, Z.; Gou, L.; Liu, D.-H. Effects of chemical fertilizer reduction combined with organic fertilizer on the yield, quality, and pharmacological activity of Chrysanthemum morifolium. Chin. J. Appl. Ecol. 2021, 32, 2800–2808. (In Chinese) [Google Scholar] [CrossRef]
- Li, Z.; Jiang, H.; Yan, H.; Jiang, X.; Ma, Y.; Qin, Y. Carbon and nitrogen metabolism under nitrogen variation affects flavonoid accumulation in the leaves of Coreopsis tinctoria. PeerJ 2021, 9, e12152. [Google Scholar] [CrossRef] [PubMed]
- Sałata, A.; Nurzyńska-Wierdak, R.; Lombardo, S.; Pandino, G.; Mauromicale, G.; Ibáñez-Asensio, S.; Moreno-Ramón, H.; Kalisz, A. Polyphenol Profile, Antioxidant Activity and Yield of Cynara cardunculus altilis in Response to Nitrogen Fertilisation. Agronomy 2024, 14, 739. [Google Scholar] [CrossRef]
- Deng, B.; Li, Y.; Xu, D.; Ye, Q.; Liu, G. Nitrogen availability alters flavonoid accumulation in Cyclocarya paliurus via the effects on the internal carbon/nitrogen balance. Sci. Rep. 2019, 9, 2370. [Google Scholar] [CrossRef] [PubMed]
- Mudau, F.N.; Soundy, P.; Toit, E.S.D. Effects of nitrogen, phosphorus, and potassium nutrition on total polyphenol content of bush tea (Athrixia phylicoides L.) leaves in shaded nursery environment. HortScience 2007, 42, 334–338. [Google Scholar] [CrossRef]
- Li, L.; Guo, X.; Zhao, T.; Li, T. Green waste composting with bean dregs, tea residue, and biochar: Effects on organic matter degradation, humification and compost maturity. Environ. Technol. Innov. 2021, 24, 101887. [Google Scholar] [CrossRef]
- Najar, B.; Demasi, S.; Caser, M.; Gaino, W.; Cioni, P.L.; Pistelli, L.; Scariot, V. Cultivation Substrate composition influences morphology, volatilome and essential oil of Lavandula angustifolia Mill. Agronomy 2019, 9, 411. [Google Scholar] [CrossRef]
- Fan, H.-M.; Wang, X.-W.; Sun, X.; Li, Y.-Y.; Sun, X.-Z.; Zheng, C.-S. Effects of humic acid derived from sediments on growth, photosynthesis and chloroplast ultrastructure in chrysanthemum. Sci. Hortic. 2014, 177, 118–123. [Google Scholar] [CrossRef]
- Yusuf, R.; Syakur, A.; Kalaba, Y.; Rostiati, R. The flowering of chrysanthemum (Chrysanthemum sp.) growing under various concentrations of liquid organic fertilizer. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; Volume 1075, p. 012001. [Google Scholar]







| Month | Jan. | Feb. | Mar. | Apr. | May | Jun. | Jul. | Aug. | Sep. | Oct. | Nov. | Dec. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average daily high temperature | 10 °C | 15 °C | 15 °C | 18 °C | 25 °C | 31 °C | 34 °C | 32 °C | 33 °C | 22 °C | 18 °C | 13 °C |
| Monthly extreme high temperature | 20 °C | 26 °C | 29 °C | 32 °C | 34 °C | 37 °C | 37 °C | 40 °C | 39 °C | 37 °C | 23 °C | 21 °C |
| Nutrients | Methods | Reference |
|---|---|---|
| Total nitrogen (N) | Elemental analyzer | [37] |
| Total potassium (K) | The flame photometry method after melting with sodium hydroxide | [38] |
| Total phosphorus (TP) | The molybdate colorimetric method after perchloric acid digestion | [39] |
| Available phosphorus (AP) | Ammonium fluoride extraction molybdenum antimony colorimetry | [39] |
| Available potassium (AK) | Ammonium acetate extraction atomic absorption spectrometry | [38] |
| Alkali hydrolyzed nitrogen (AHN) | Alkali hydrolysis diffusion method | [38] |
| Organic carbon (C) | Potassium dichromate volumetric method and external heating method | [38] |
| Organic matter | Soil organic matter (g·kg−1) = soil organic carbon (g·kg−1) × 1.724 | [40] |
| Treatment Groups | Fv/Fm | ABS/RC | TRo/RC | DIO/RC | ETo/RC |
|---|---|---|---|---|---|
| CK | 0.788 ± 0.024 bc | 2.42 ± 0.25 abc | 1.90 ± 0.16 ab | 0.51 ± 0.10 ab | 1.19 ± 0.07 a |
| 0.5 × DT | 0.784 ± 0.017 c | 2.54 ± 0.10 a | 1.99 ± 0.10 a | 0.54 ± 0.04 a | 1.19 ± 0.06 a |
| 1 × DT | 0.803 ± 0.014 abc | 2.46 ± 0.16 ab | 1.97 ± 0.10 a | 0.48 ± 0.06 abc | 1.24 ± 0.07 a |
| 1.5 × DT | 0.818 ± 0.003 ab | 2.14 ± 0.04 c | 1.75 ± 0.03 b | 0.38 ± 0.01 c | 1.16 ± 0.04 a |
| 2 × DT | 0.823 ± 0.008 a | 2.24 ± 0.09 bc | 1.84 ± 0.09 ab | 0.39 ± 0.01 c | 1.22 ± 0.07 a |
| CF | 0.815 ± 0.013 ab | 2.22 ± 0.11 bc | 1.81 ± 0.06 ab | 0.41 ± 0.05 bc | 1.14 ± 0.03 a |
| RC | 0.801 ± 0.020 abc | 2.34 ± 0.15 abc | 1.87 ± 0.08 ab | 0.45 ± 0.07 abc | 1.21 ± 0.08 a |
| Treatment Groups | AQY μmol m−2 s−1 | LSP μmol m−2 s−1 | Pnmax μmol m−2 s−1 | LCP μmol m−2 s−1 | Rd μmol m−2 s−1 |
|---|---|---|---|---|---|
| CK | 0.107 ± 0.003 a | 1687.37 ± 12.60 d | 16.29 ± 0.47 c | 41.13 ± 4.58 a | 3.76 ± 0.28 a |
| 0.5 × DT | 0.072 ± 0.014 b | 1765.65 ± 15.75 b | 18.85 ± 0.39 b | 51.09 ± 11.88 a | 3.23 ± 0.21 ab |
| 1 × DT | 0.091 ± 0.015 ab | 1772.71 ± 17.08 b | 20.03 ± 1.35 b | 40.41 ± 5.00 a | 3.28 ± 0.27 ab |
| 1.5 × DT | 0.082 ± 0.009 ab | 1817.24 ± 18.57 a | 22.81 ± 1.29 a | 41.58 ± 12.63 a | 3.10 ± 0.65 ab |
| 2 × DT | 0.086 ± 0.009 ab | 1819.66 ± 4.25 a | 23.04 ± 1.84 a | 35.64 ± 3.27 a | 2.83 ± 0.19 b |
| CF | 0.100 ± 0.021 a | 1805.06 ± 13.36 a | 20.01 ± 0.40 b | 39.56 ± 13.40 a | 3.37 ± 0.51 ab |
| RC | 0.090 ± 0.004 ab | 1739.54 ± 12.65 c | 19.49 ± 0.88 b | 38.62 ± 9.88 a | 3.11 ± 0.74 ab |
| Treatment Groups | Pn μmol m−2 s−1 | Gs μmol m−2 s−1 | Ci μmol mol−1 | Tr μmol m−2 s−1 | WUE μmol mol−1 |
|---|---|---|---|---|---|
| CK | 16.64 ± 1.15 c | 0.13 ± 0.01 b | 241.95 ± 10.10 c | 3.38 ± 0.33 c | 4.69 ± 0.45 a |
| 0.5 × DT | 19.62 ± 0.75 b | 0.26 ± 0.03 a | 261.53 ± 16.61 b | 7.83 ± 0.71 a | 2.01 ± 0.51 c |
| 1 × DT | 20.23 ± 1.66 ab | 0.25 ± 0.03 a | 273.67 ± 13.32 b | 6.79 ± 1.61 ab | 2.97 ± 1.03 abc |
| 1.5 × DT | 22.54 ± 1.21 a | 0.27 ± 0.02 a | 301.66 ± 8.02 a | 6.00 ± 2.65 ab | 4.07 ± 1.35 ab |
| 2 × DT | 22.64 ± 1.71 a | 0.23 ± 0.05 a | 303.77 ± 13.59 a | 5.23 ± 0.45 abc | 4.43 ± 0.57 a |
| CF | 20.37 ± 1.03 ab | 0.21 ± 0.07 a | 271.25 ± 4.76 b | 4.64 ± 1.63 bc | 4.66 ± 1.37 a |
| RC | 19.64 ± 1.77 b | 0.23 ± 0.04 a | 268.96 ± 5.40 b | 7.48 ± 0.29 a | 2.54 ± 0.20 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, J.; Yin, J.; Liu, L.; Xu, L. Optimal Dark Tea Fertilization Enhances the Growth and Flower Quality of Tea Chrysanthemum by Improving the Soil Nutrient Availability in Simultaneous Precipitation and High-Temperature Regions. Agronomy 2025, 15, 1753. https://doi.org/10.3390/agronomy15071753
Hou J, Yin J, Liu L, Xu L. Optimal Dark Tea Fertilization Enhances the Growth and Flower Quality of Tea Chrysanthemum by Improving the Soil Nutrient Availability in Simultaneous Precipitation and High-Temperature Regions. Agronomy. 2025; 15(7):1753. https://doi.org/10.3390/agronomy15071753
Chicago/Turabian StyleHou, Jiayi, Jiayuan Yin, Lei Liu, and Lu Xu. 2025. "Optimal Dark Tea Fertilization Enhances the Growth and Flower Quality of Tea Chrysanthemum by Improving the Soil Nutrient Availability in Simultaneous Precipitation and High-Temperature Regions" Agronomy 15, no. 7: 1753. https://doi.org/10.3390/agronomy15071753
APA StyleHou, J., Yin, J., Liu, L., & Xu, L. (2025). Optimal Dark Tea Fertilization Enhances the Growth and Flower Quality of Tea Chrysanthemum by Improving the Soil Nutrient Availability in Simultaneous Precipitation and High-Temperature Regions. Agronomy, 15(7), 1753. https://doi.org/10.3390/agronomy15071753





