Metabolomic Profiling of Desiccation Response in Recalcitrant Quercus acutissima Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Desiccation Design
2.3. Determination of Seed MC
2.4. Determination of Seed Germination Percentage, Proline Content, and Soluble Protein Content
2.4.1. Seed Germination Percentage (GP)
2.4.2. Soluble Protein Content
2.4.3. Proline Content
2.5. Metabolome Sample Preparation
2.6. Liquid Chromatography and Mass Spectrometry Analysis Conditions
2.7. Data Preprocessing and Metabolite Identification
2.8. Statistical Analysis, Differential Metabolite Screening and Metabolic Pathway Enrichment Analysis
3. Results
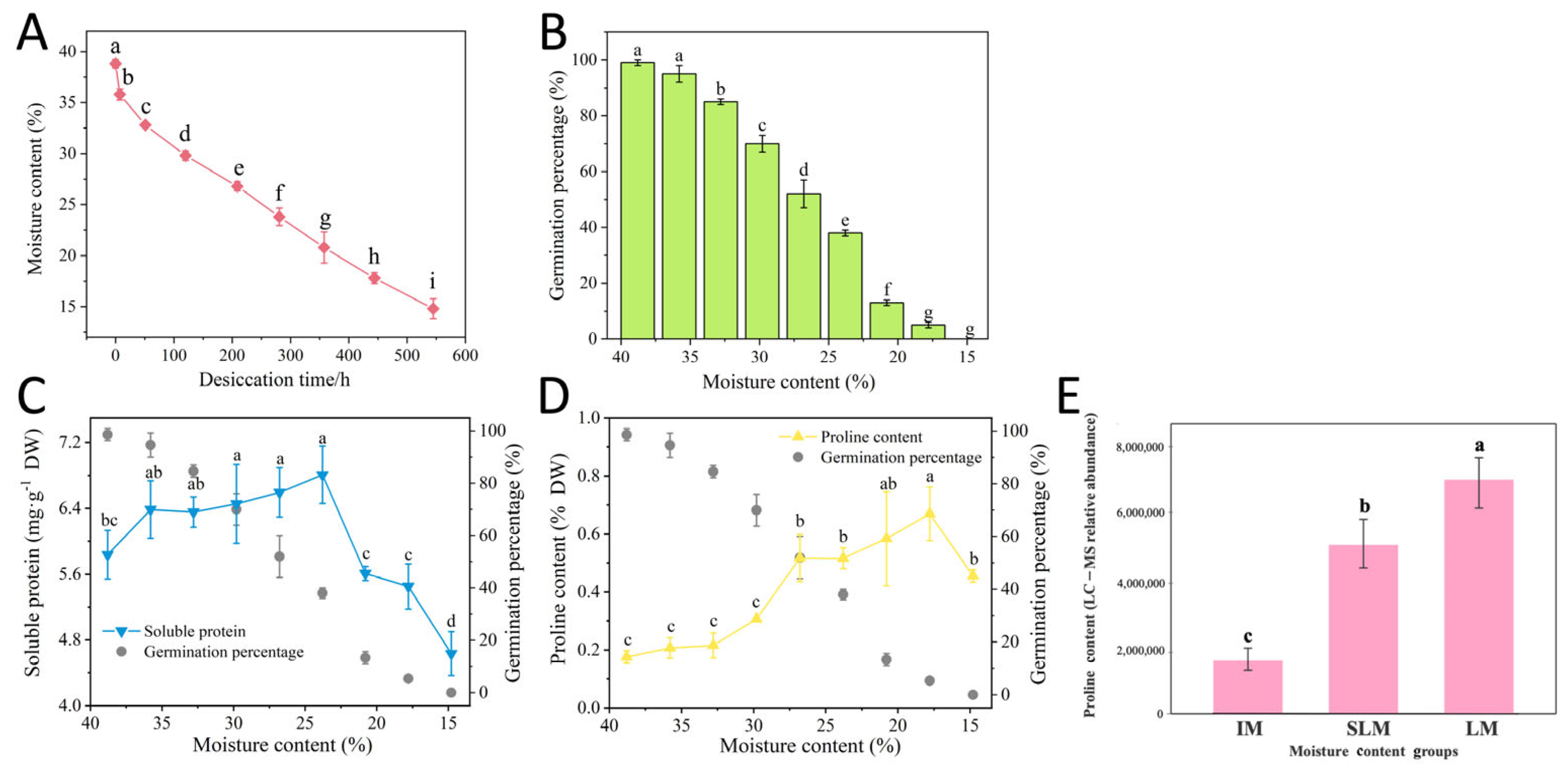
3.1. Effects of Desiccation on Seed MC and GP
3.2. Effects of Desiccation on Seed Osmotic Regulation
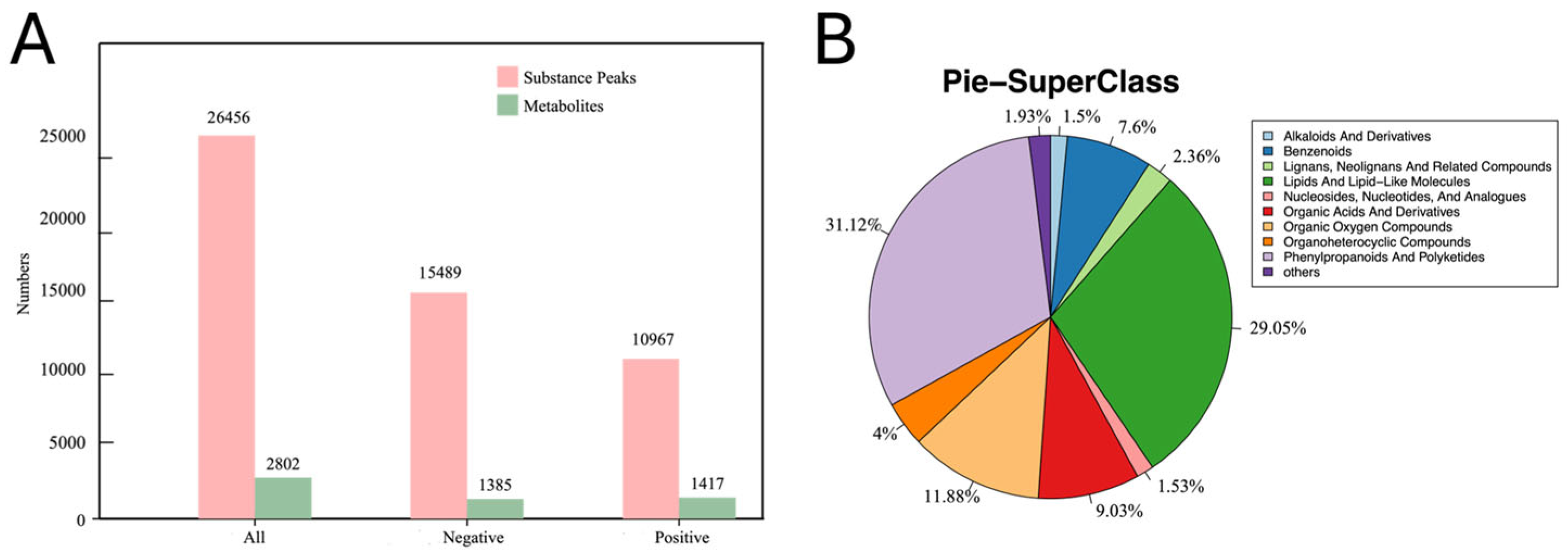
3.3. Metabolite Analysis of Q. acutissima Seeds During Desiccation
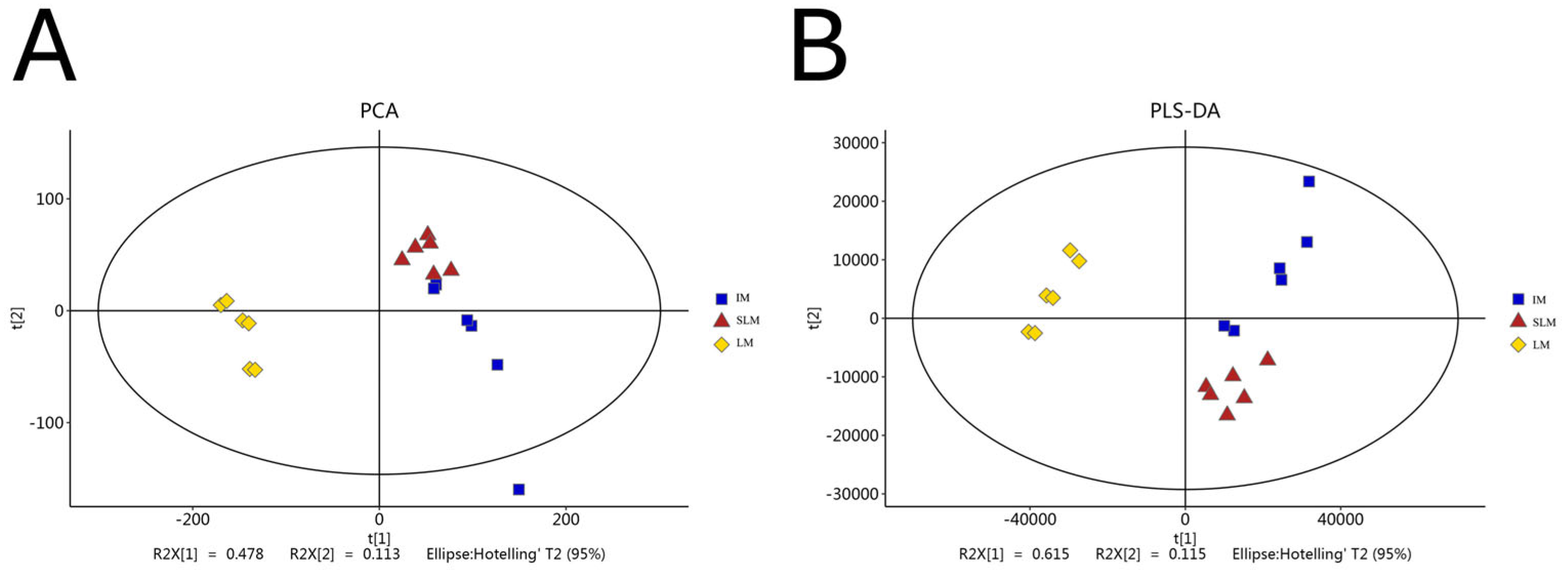
3.4. Multivariate Statistical Analysis of Metabolic Profiles During Desiccation in Q. acutissima Seeds
3.5. Differentially Expressed Metabolites (DEMs) Screening and Analysis
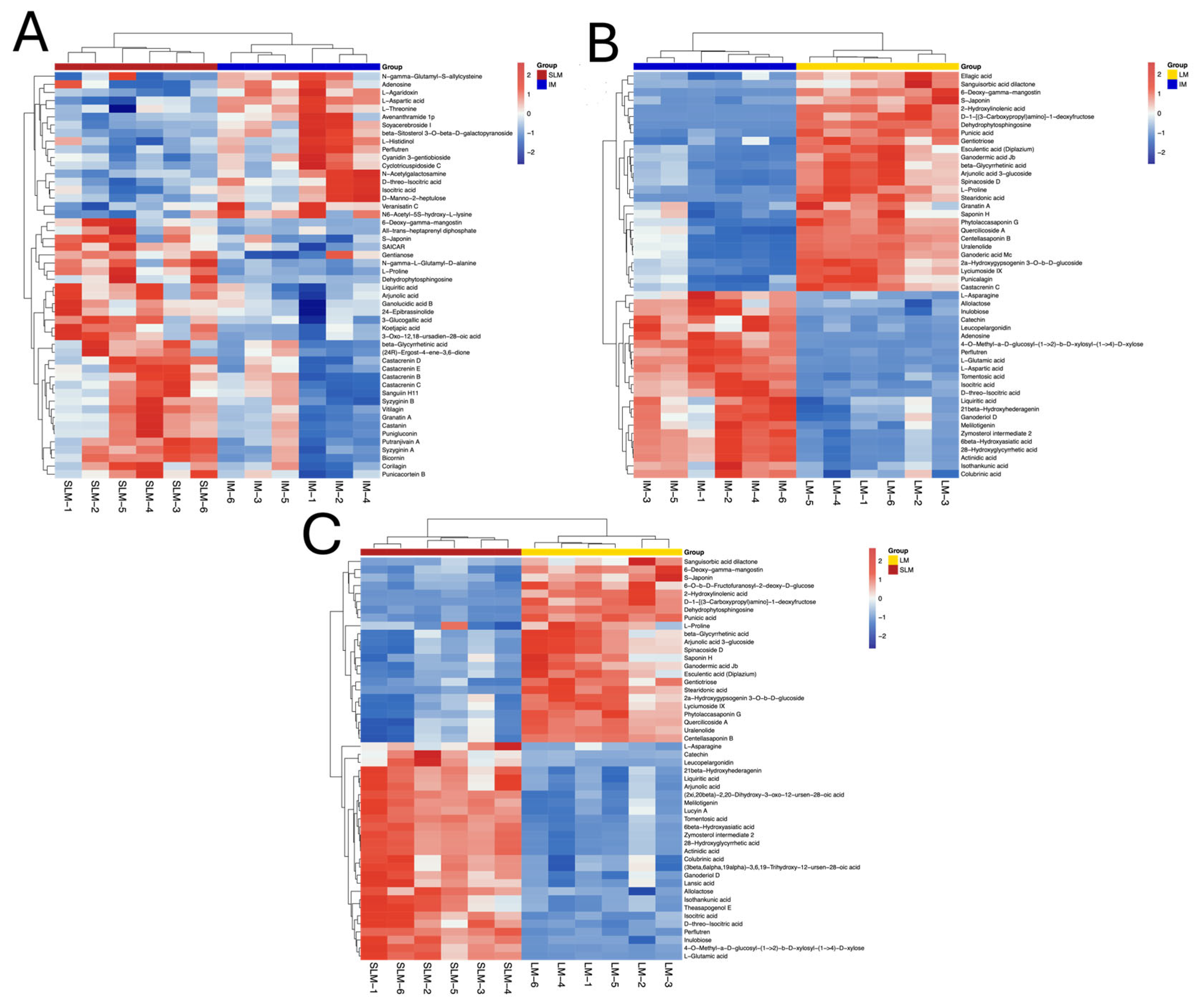
3.6. DEMs Clustering Analysis
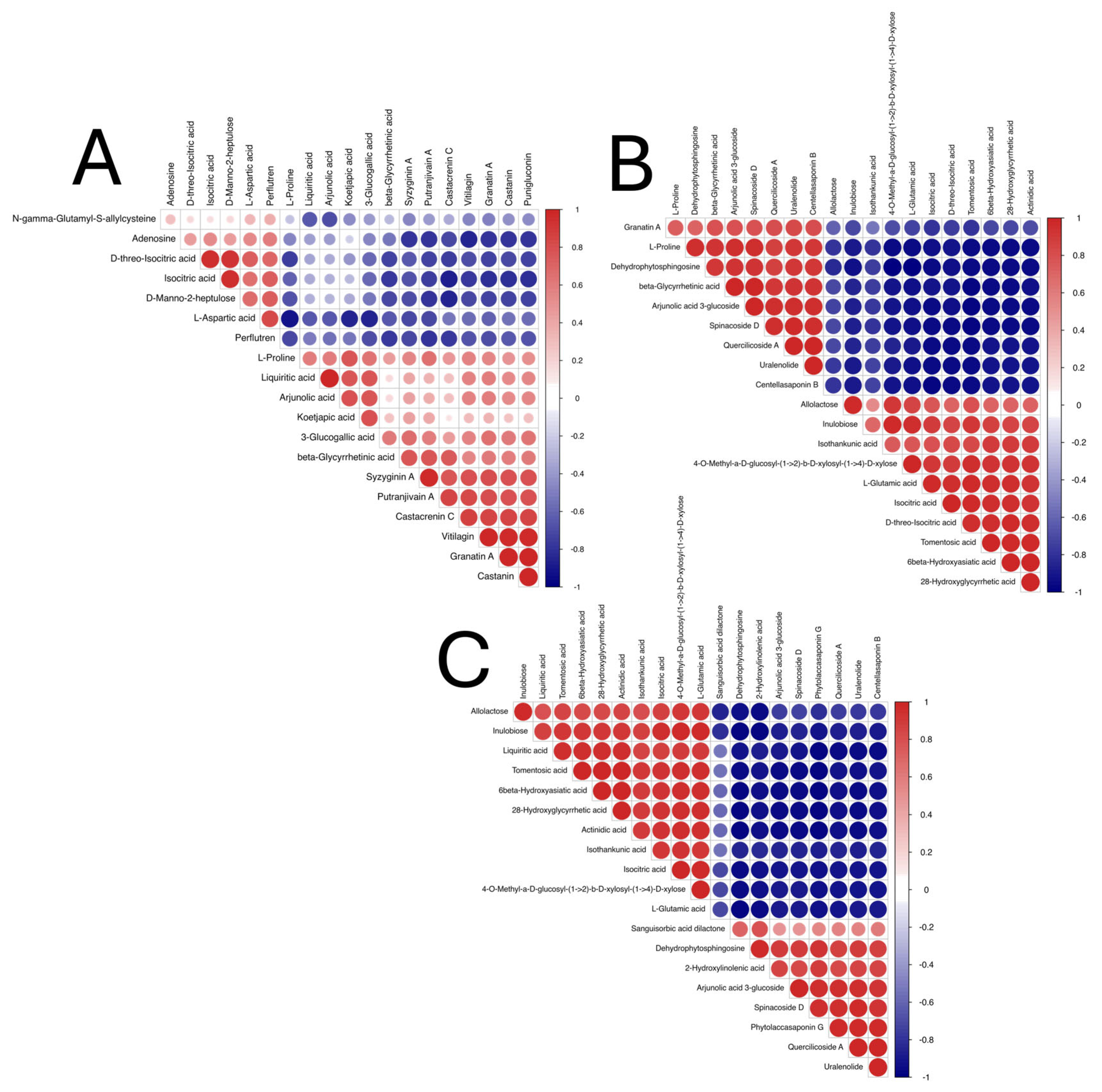
3.7. DEMs Correlation Analysis
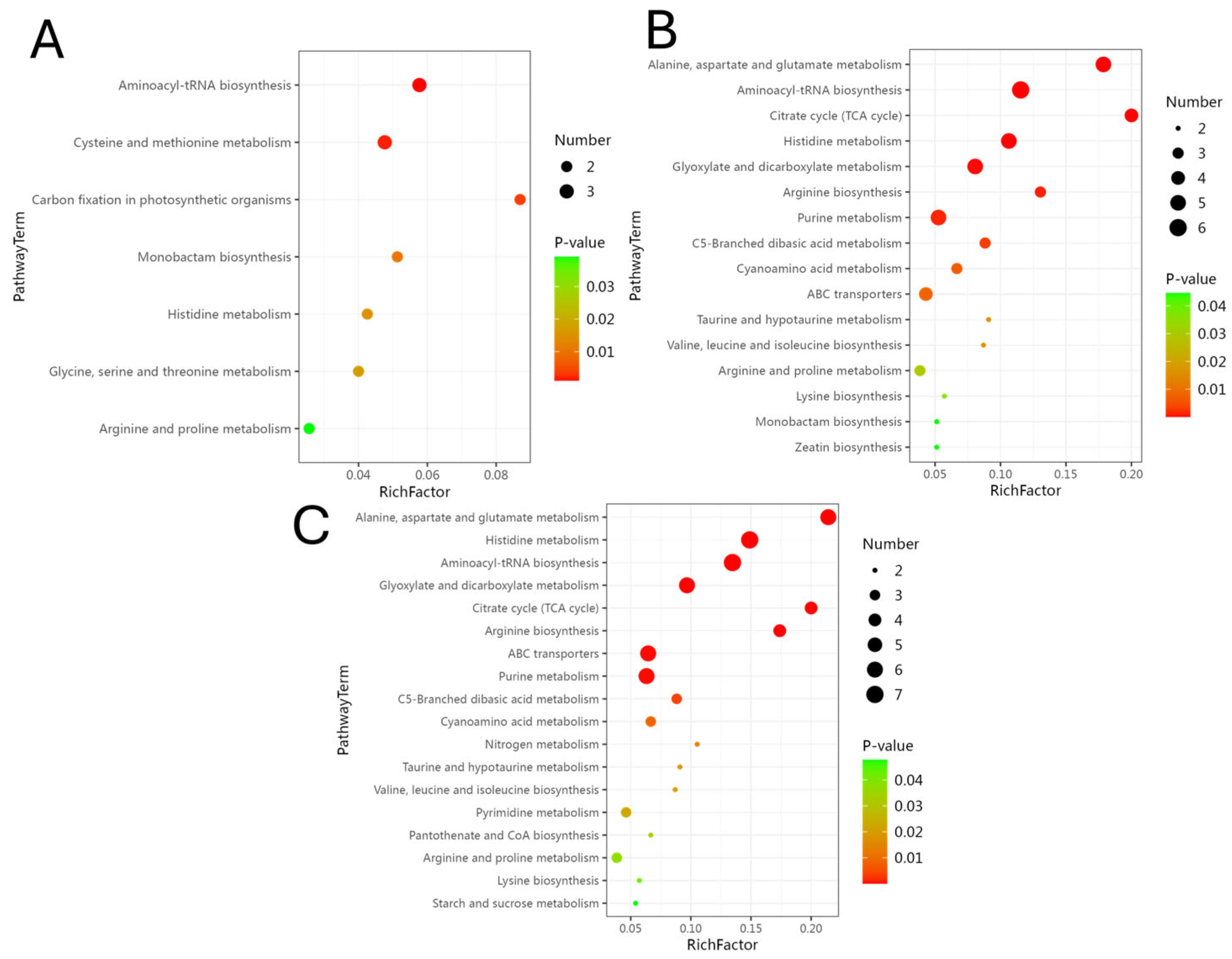
3.8. KEGG Pathway Enrichment Analysis of DEMs
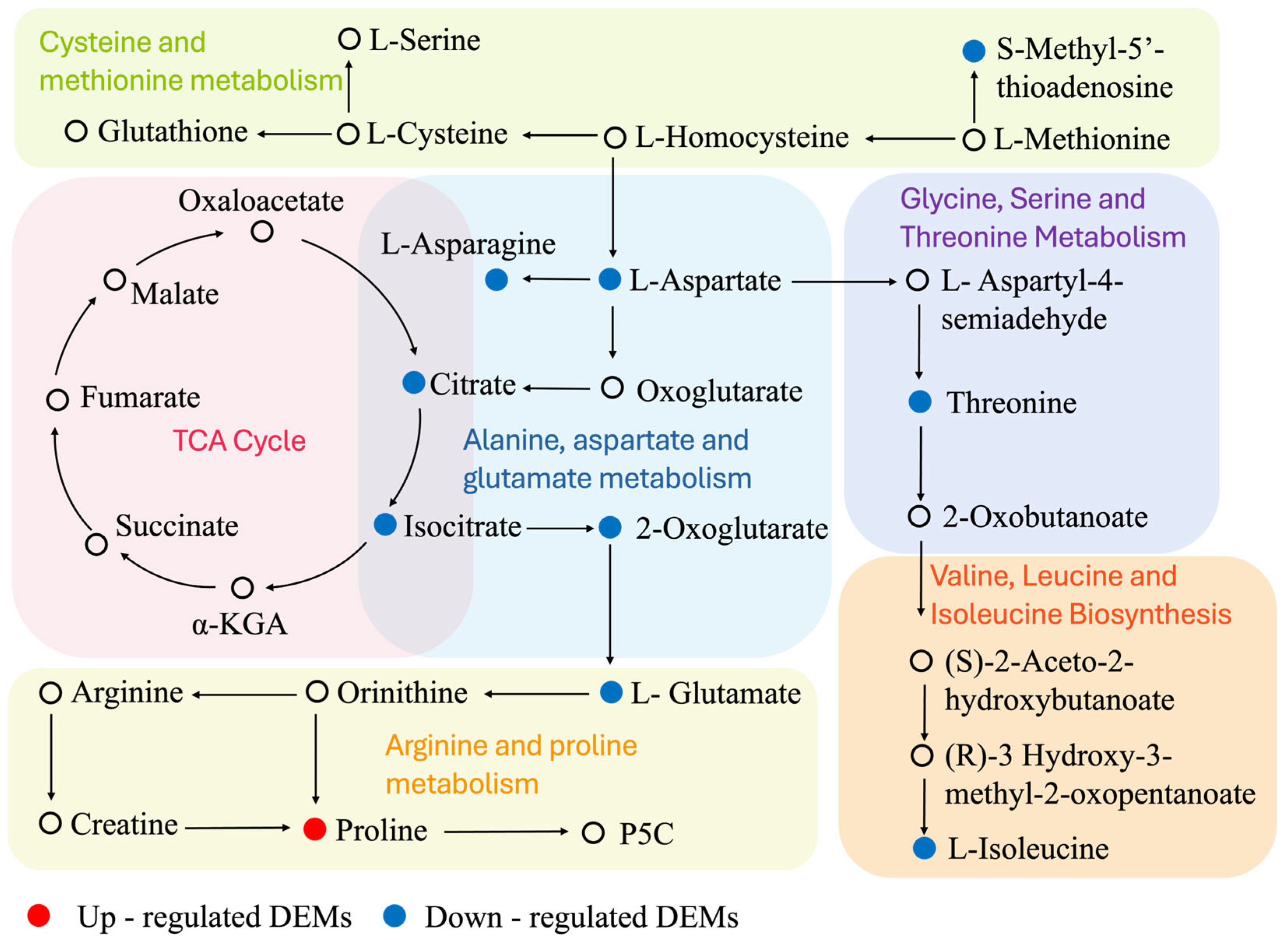
3.9. DEMs Associated with Amino Acid and Energy Metabolism Changes
4. Discussion
4.1. Critical Moisture Thresholds and Osmotic Regulation Response
4.2. Metabolic Disturbances During Desiccation
4.3. Amino Acid Metabolism Reorganization
4.4. Energy Metabolism Imbalance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berjak, P.; Pammenter, N.W. From Avicennia to Zizania: Seed recalcitrance in perspective. Ann. Bot. 2008, 101, 213–228. [Google Scholar] [CrossRef]
- Roberts, E.H. Predicting the storage life of seeds. Seed Sci. Technol. 1973, 1, 499–514. [Google Scholar]
- Walters, C. Orthodoxy, recalcitrance and in-between: Describing variation in seed storage characteristics using threshold responses to water loss. Planta 2015, 242, 397–406. [Google Scholar] [CrossRef]
- Gleiser, G.; Picher, M.C.; Veintimilla, P.; Martinez, J.; Verdu, M. Seed dormancy in relation to seed storage behaviour in Acer. Bot. J. Linn. Soc. 2004, 145, 203–208. [Google Scholar] [CrossRef]
- Berjak, P.; Pammenter, N.W. Implications of the lack of desiccation tolerance in recalcitrant seeds. Front. Plant Sci. 2013, 4, 478. [Google Scholar] [CrossRef]
- Wyse, S.V.; Dickie, J.B. Taxonomic affinity, habitat and seed mass strongly predict seed desiccation response: A boosted regression trees analysis based on 17 539 species. Ann. Bot. 2018, 121, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Walters, C.; Berjak, P.; Pammenter, N.; Kennedy, K.; Raven, P. Preservation of recalcitrant seeds. Science 2013, 339, 915–916. [Google Scholar] [CrossRef]
- Ganatsas, P.; Tsakaldimi, M.A. Comparative study of desiccation responses of seeds of three drought-resistant mediterranean oaks. Forest Ecol. Manag. 2013, 305, 189–194. [Google Scholar] [CrossRef]
- Li, D.-Z.; Pritchard, H.W. The science and economics of ex situ plant conservation. Trends Plant Sci. 2009, 14, 614–621. [Google Scholar] [CrossRef]
- Kijowska-Oberc, J.; Staszak, A.M.; Wawrzyniak, M.K.; Ratajczak, E. Changes in proline levels during seed development of orthodox and recalcitrant seeds of genus Acer in a climate change scenario. Forests 2020, 11, 1362. [Google Scholar] [CrossRef]
- Delahaie, J.; Hundertmark, M.; Bove, J.; Leprince, O.; Rogniaux, H.; Buitink, J. LEA polypeptide profiling of recalcitrant and orthodox legume seeds reveals ABI3-regulated LEA protein abundance linked to desiccation tolerance. J. Plant Physiol. 2013, 64, 4559–4573. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, L.; Ahmad, P.; Wan, X.; Hu, X. Proteomic profiling and redox status alteration of recalcitrant tea (Camellia sinensis) seed in response to desiccation. Planta 2011, 233, 583–592. [Google Scholar] [CrossRef]
- Lima Lawrance, S.; Sunil Kesava Deth, G.; Ajith Kumar, K.G.; Raju, P.; John, S.; Nair, S.P. Status of moisture and sugar during embryogenesis and embryo desiccation in the desiccation-intolerant seeds of Humboldtia vahliana Wight. Russ. J. Plant Physiol. 2022, 69, 124. [Google Scholar] [CrossRef]
- Chandra, J.; Dubey, M.; Varghese, B.; Sershen; Keshavkant, S. Towards understanding the basis of desiccation-induced oxidative stress in recalcitrant seeds: The case of Madhuca latifolia Roxb. S. Afr. J. Bot. 2021, 142, 100–105. [Google Scholar] [CrossRef]
- Lah, N.H.C.; El Enshasy, H.A.; Mediani, A.; Azizan, K.A.; Aizat, W.M.; Tan, J.K.; Afzan, A.; Noor, N.M.; Rohani, E.R. An insight into the behaviour of recalcitrant seeds by understanding their molecular changes upon desiccation and low temperature. Agronomy 2023, 13, 2099. [Google Scholar] [CrossRef]
- Fukushima, A.; Kusano, M.; Mejia, R.F.; Iwasa, M.; Kobayashi, M.; Hayashi, N.; Watanabe-Takahashi, A.; Narisawa, T.; Tohge, T.; Hur, M.; et al. Metabolomic characterization of knockout mutants in Arabidopsis: Development of a metabolite profiling database for knockout mutants in Arabidopsis. Plant Physiol. 2014, 165, 948–961. [Google Scholar] [CrossRef]
- Mazlan, O.; Aizat, W.M.; Baharum, S.N.; Azizan, K.A.; Noor, N.M. Metabolomics analysis of developing Garcinia mangostana seed reveals modulated levels of sugars, organic acids and phenylpropanoid compounds. Sci. Hortic. 2018, 233, 323–330. [Google Scholar] [CrossRef]
- Szuba, A.; Kalemba, E.M.; Wawrzyniak, M.K.; Suszka, J.; Chmielarz, P. Deterioration in the quality of recalcitrant Quercus robur seeds during six months of storage at subzero temperatures: Ineffective activation of prosurvival mechanisms and evidence of freezing stress from an untargeted metabolomic study. Metabolites 2022, 12, 756. [Google Scholar] [CrossRef]
- Rodrigues, G.A.G.; Mauve, C.; Gakiere, B.; Bailly, C.; Steiner, N. The metabolic profiles of Eugenia astringens and E. uniflora (Myrtaceae) sensitive seeds affect desiccation. Physiol. Plant. 2024, 176, e14220. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zang, M.; Li, M.; Fang, Y. Complete chloroplast genome sequence and phylogenetic analysis of Quercus acutissima. Int. J. Mol. Sci. 2018, 19, 2443. [Google Scholar] [CrossRef]
- Negi, M.; Rawal, R.S. Desiccation response of seeds of Himalayan oak, Quercus floribunda Lindl. Ex A. Camus. Natl. Acad. Sci. Lett. 2019, 42, 291–294. [Google Scholar] [CrossRef]
- Ballesteros, D.; Pritchard, H.W. The cryobiotechnology of oaks: An integration of approaches for the long-term ex situ conservation of Quercus species. Forests 2020, 11, 1281. [Google Scholar] [CrossRef]
- Xia, K.; Daws, M.; Peng, L.-L. Climate drives patterns of seed traits in Quercus species across China. New Phytol. 2022, 234, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shen, Y. Investigation of water distribution and mobility dynamics in recalcitrant Quercus acutissima seeds during desiccation using magnetic resonance methods. Forests 2023, 14, 738. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Qian, J.; Liu, X.; Xu, H.; Zhang, G.; Ren, J.; Wang, L.; Zhang, L.; Yu, H. Comparative transcriptome analysis revealed candidate genes potentially related to desiccation sensitivity of recalcitrant Quercus variabilis seeds. Front. Plant Sci. 2021, 12, 717563. [Google Scholar] [CrossRef]
- International Seed Testing Association (ISTA). Chapter 9—Determination of Moisture Content. In International Rules for Seed Testing 2021 Edition; ISTA: Bassersdorf, Switzerland, 2020. [Google Scholar]
- Xia, K.; Daws, M.I.; Hay, F.R.; Chen, W.-Y.; Zhou, Z.-K.; Pritchard, H.W. A comparative study of desiccation responses of seeds of Asian evergreen oaks, Quercus subgenus Cyclobalanopsis and Quercus subgenus Quercus. S. Afr. J. Bot. 2012, 78, 47–54. [Google Scholar] [CrossRef]
- Wen, B. Cytological and physiological changes related to cryotolerance in recalcitrant Livistona chinensis embryos during seed development. Protoplasma 2011, 248, 483–491. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, S.; Thakur, P.; Malik, J.A.; Bhandhari, K.; Sharma, K.D.; Nayyar, H. Involvement of proline in response of chickpea (Cicer arietinum L.) to chilling stress at reproductive stage. Sci. Hortic. 2011, 128, 174–181. [Google Scholar] [CrossRef]
- Cao, M.; Liu, Y.; Jiang, W.; Meng, X.; Zhang, W.; Chen, W.; Peng, D.; Xing, S. UPLC/MS-based untargeted metabolomics reveals the changes of metabolites profile of Salvia miltiorrhiza Bunge during sweating processing. Sci. Rep. 2020, 10, 19524. [Google Scholar] [CrossRef]
- Rischer, H.; Orešič, M.; Seppänen-Laakso, T.; Katajamaa, M.; Lammertyn, F.; Ardiles-Diaz, W.; Van Montagu, M.C.E.; Inzé, D.; Oksman-Caldentey, K.-M.; Goossens, A. Gene-to-metabolite networks for terpenoid indole alkaloid biosynthesis in Catharanthus roseus cells. Proc. Natl. Acad. Sci. USA 2006, 103, 5614–5619. [Google Scholar] [CrossRef]
- Xie, C.; Yan, S.; Zhang, Z.; Gong, W.; Zhu, Z.; Zhou, Y.; Yan, L.; Hu, Z.; Ai, L.; Peng, Y. Mapping the metabolic signatures of fermentation broth, mycelium, fruiting body and spores powder from Ganoderma lucidum by untargeted metabolomics. LWT 2020, 129, 109494. [Google Scholar] [CrossRef]
- Chen, H.; Shen, Y. Comparative proteomic analysis of desiccation responses in recalcitrant Quercus acutissima seeds. Plant Mol. Biol. 2025, 115, 68. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Shen, Y. Comparison of seed desiccation sensitivity between Quercus chenii and Q. acutissima. Trees 2025, 39, 28. [Google Scholar] [CrossRef]
- Farrant, J.M.; Moore, J.P. Programming desiccation-tolerance: From plants to seeds to resurrection plants. Curr. Opin. Plant Biol. 2011, 14, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.; Buijs, G.; Ligterink, W.; Hilhorst, H. Evolutionary ecophysiology of seed desiccation sensitivity. Funct. Plant Biol. 2018, 45, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, B.G.; Ribeiro, L.M.; Dias, D.S.; Mazzottini-dos-Santos, H.C.; Santos Martins, C.d.P.; Nascimento Lopes, P.S.; Mercadante-Simoes, M.O. Embryo responses to extreme water events provide insights into the behavior of Butia capitata (Arecaceae) seed banks during hydration cycles. Environ. Exp. Bot. 2020, 169, 103904. [Google Scholar] [CrossRef]
- Fàbregas, N.; Fernie, A.R. The metabolic response to drought. J. Exp. Bot. 2019, 70, 1077–1085. [Google Scholar] [CrossRef]
- Moothoo-Padayachie, A.; Varghese, B.; Pammenter, N.W.; Govender, P.; Sershen. A comparison of partial desiccation and hydrated storage-induced changes in viability, reactive oxygen species production, and glutathione metabolism in two contrasting recalcitrant-seeded species. Acta Physiol. Plant. 2018, 40, 14. [Google Scholar] [CrossRef]
- Chandrakar, V.; Dubey, A.; Keshavkant, S. Modulation of arsenic-induced oxidative stress and protein metabolism by diphenyleneiodonium, 24-epibrassinolide and proline in Glycine max L. Acta Bot. Croat. 2018, 77, 51–61. [Google Scholar] [CrossRef]
- Jin, X.; Liu, D.; Ma, L.; Gong, Z.; Cao, D.; Liu, Y.; Li, Y.; Jiang, C. Transcriptome and expression profiling analysis of recalcitrant tea (Camellia sinensis L.) seeds sensitive to desiccation. Int. J. Genomics 2018, 2018, e5963797. [Google Scholar] [CrossRef]
- Lv, H.-X.; Xu, H.; Yang, K.; Yan, M. Comparative metabolomic analyses reveal metabolites associated with seed deterioration in Chinese cabbage. Sci. Hortic. 2024, 331, 113170. [Google Scholar] [CrossRef]
- Somi, C. Induction of Desiccation Tolerance in Araucaria angustifolia Seeds. Master’s Thesis, Wageningen University, Wageningen, The Netherlands, 2015. [Google Scholar]
- Azevedo, R.A.; Lancien, M.; Lea, P.J. The aspartic acid metabolic pathway, an exciting and essential pathway in plants. Amino Acids 2006, 30, 143–162. [Google Scholar] [CrossRef]
- Suprasanna, P.; Nikalje, G.C.; Rai, A.N. Osmolyte accumulation and implications in plant abiotic stress tolerance. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Iqbal, N., Nazar, R., Khan, N.A., Eds.; Springer: New Delhi, India, 2016; pp. 1–12. ISBN 978-81-322-2616-1. [Google Scholar]
- Dinakar, C.; Bartels, D. Desiccation tolerance in resurrection plants: New insights from transcriptome, proteome and metabolome analysis. Front. Plant Sci. 2013, 4, 482. [Google Scholar] [CrossRef]
- Goeten, D.; Elias, R.A.; Polesi, L.G.; Walters, C.; Guerra, M.P.; Steiner, N. Effect of water content and biochemical cell state on the germination percentage of cryopreserved Butia eriospatha embryos (Arecaceae). Plant Cell Tissue Organ Cult. 2023, 152, 339–356. [Google Scholar] [CrossRef]
- Yobi, A.; Wone, B.W.M.; Xu, W.; Alexander, D.C.; Guo, L.; Ryals, J.A.; Oliver, M.J.; Cushman, J.C. Metabolomic profiling in Selaginella lepidophylla at various hydration states provides new insights into the mechanistic basis of desiccation tolerance. Mol. Plant 2013, 6, 369–385. [Google Scholar] [CrossRef]
- Martinelli, T. In situ localization of glucose and sucrose in dehydrating leaves of Sporobolus stapfianus. J. Plant Physiol. 2008, 165, 580–587. [Google Scholar] [CrossRef]
- Oliver, M.J.; Guo, L.; Alexander, D.C.; Ryals, J.A.; Wone, B.W.M.; Cushman, J.C. A sister group contrast using untargeted global metabolomic analysis delineates the biochemical regulation underlying desiccation tolerance in Sporobolus stapfianus. Plant Cell 2011, 23, 1231–1248. [Google Scholar] [CrossRef]
- Oliveira, I.C.; Brenner, E.; Chiu, J.; Hsieh, M.-H.; Kouranov, A.; Lam, H.-M.; Shin, M.J.; Coruzzi, G. Metabolite and light regulation of metabolism in plants: Lessons from the study of a single biochemical pathway. Braz. J. Med. Biol. Res. 2001, 34, 567–575. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Ma, X.; Guo, L.; He, Y.; Ren, Z.; Kuang, Z.; Zhang, X.; Zhang, Z. Analysis of potential strategies for cadmium stress tolerance revealed by transcriptome analysis of upland cotton. Sci. Rep. 2019, 9, 86. [Google Scholar] [CrossRef]
- Hoekstra, F.A.; Golovina, E.A.; Buitink, J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001, 6, 431–438. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018, 162, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Bragante, R.B.; Hell, A.F.; Silva, J.P.N.; Centeno, D.d.C.; Figueiredo-Ribeiro, R.d.C.L.; Barbedo, C.J. Physiological and metabolic responses of immature and mature seeds of Libidibia ferrea ((Mart. Ex Tul.) L.P. Queiroz) under contrasting storage temperatures. Braz. J. Bot. 2018, 41, 43–55. [Google Scholar] [CrossRef]
- Wang, L.; Fu, J.; Li, M.; Fragner, L.; Weckwerth, W.; Yang, P. Metabolomic and proteomic profiles reveal the dynamics of primary metabolism during seed development of lotus (Nelumbo nucifera). Front. Plant Sci. 2016, 7, 750. [Google Scholar] [CrossRef] [PubMed]
- Stavrinides, A.K.; Dussert, S.; Combes, M.-C.; Fock-Bastide, I.; Severac, D.; Minier, J.; Bastos-Siqueira, A.; Demolombe, V.; Hem, S.; Lashermes, P.; et al. Seed comparative genomics in three coffee species identify desiccation tolerance mechanisms in intermediate seeds. J. Exp. Bot. 2020, 71, 1418–1433. [Google Scholar] [CrossRef]
- Fuchs, H.; Plitta-Michalak, B.P.; Małecka, A.; Ciszewska, L.; Sikorski, Ł.; Staszak, A.M.; Michalak, M.; Ratajczak, E. The chances in the redox priming of nondormant recalcitrant seeds by spermidine. Tree Physiol. 2023, 43, 1142–1158. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Y.; Yan, M.; Pu, X.; Lu, D.; Yuan, H.; Wu, C. Transcriptome analyses reveal the mechanism of changes in the sugar constituents of jujube fruits under saline–alkali stress. Agronomy 2023, 13, 2243. [Google Scholar] [CrossRef]

| Elution Time (min) | Mobile Phase A Ratio (%) | Mobile Phase B Ratio (%) |
|---|---|---|
| 0 | 95 | 5 |
| 2 | 95 | 5 |
| 5 | 70 | 30 |
| 8 | 50 | 50 |
| 10 | 20 | 80 |
| 14 | 0 | 100 |
| 15 | 0 | 100 |
| 15.1 | 95 | 5 |
| 16 | 95 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Shi, F.; Tong, B.; Lu, Y.; Shen, Y. Metabolomic Profiling of Desiccation Response in Recalcitrant Quercus acutissima Seeds. Agronomy 2025, 15, 1738. https://doi.org/10.3390/agronomy15071738
Chen H, Shi F, Tong B, Lu Y, Shen Y. Metabolomic Profiling of Desiccation Response in Recalcitrant Quercus acutissima Seeds. Agronomy. 2025; 15(7):1738. https://doi.org/10.3390/agronomy15071738
Chicago/Turabian StyleChen, Haiyan, Fenghou Shi, Boqiang Tong, Yizeng Lu, and Yongbao Shen. 2025. "Metabolomic Profiling of Desiccation Response in Recalcitrant Quercus acutissima Seeds" Agronomy 15, no. 7: 1738. https://doi.org/10.3390/agronomy15071738
APA StyleChen, H., Shi, F., Tong, B., Lu, Y., & Shen, Y. (2025). Metabolomic Profiling of Desiccation Response in Recalcitrant Quercus acutissima Seeds. Agronomy, 15(7), 1738. https://doi.org/10.3390/agronomy15071738






