Quantification of Microplastics in Urban Compost-Amended Farmland Soil Using an Elutriation Device
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Sampling
2.2. Chemical and Physical Analysis of Soil
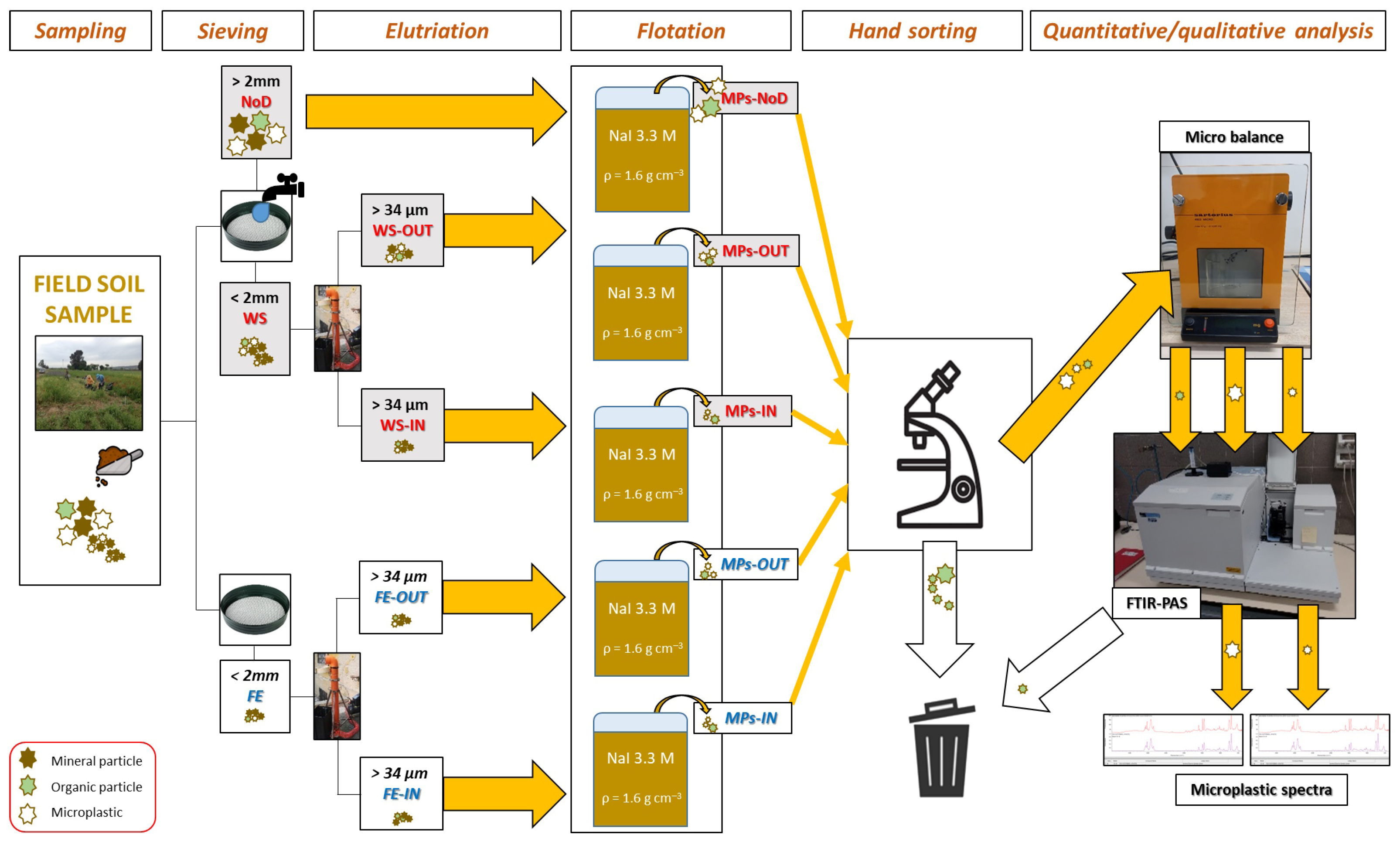
2.3. Microplastics Extraction and Characterization
2.4. Extraction Efficiency
2.5. Data Treatment
3. Results
3.1. Chemical and Physical Characteristics of Soil
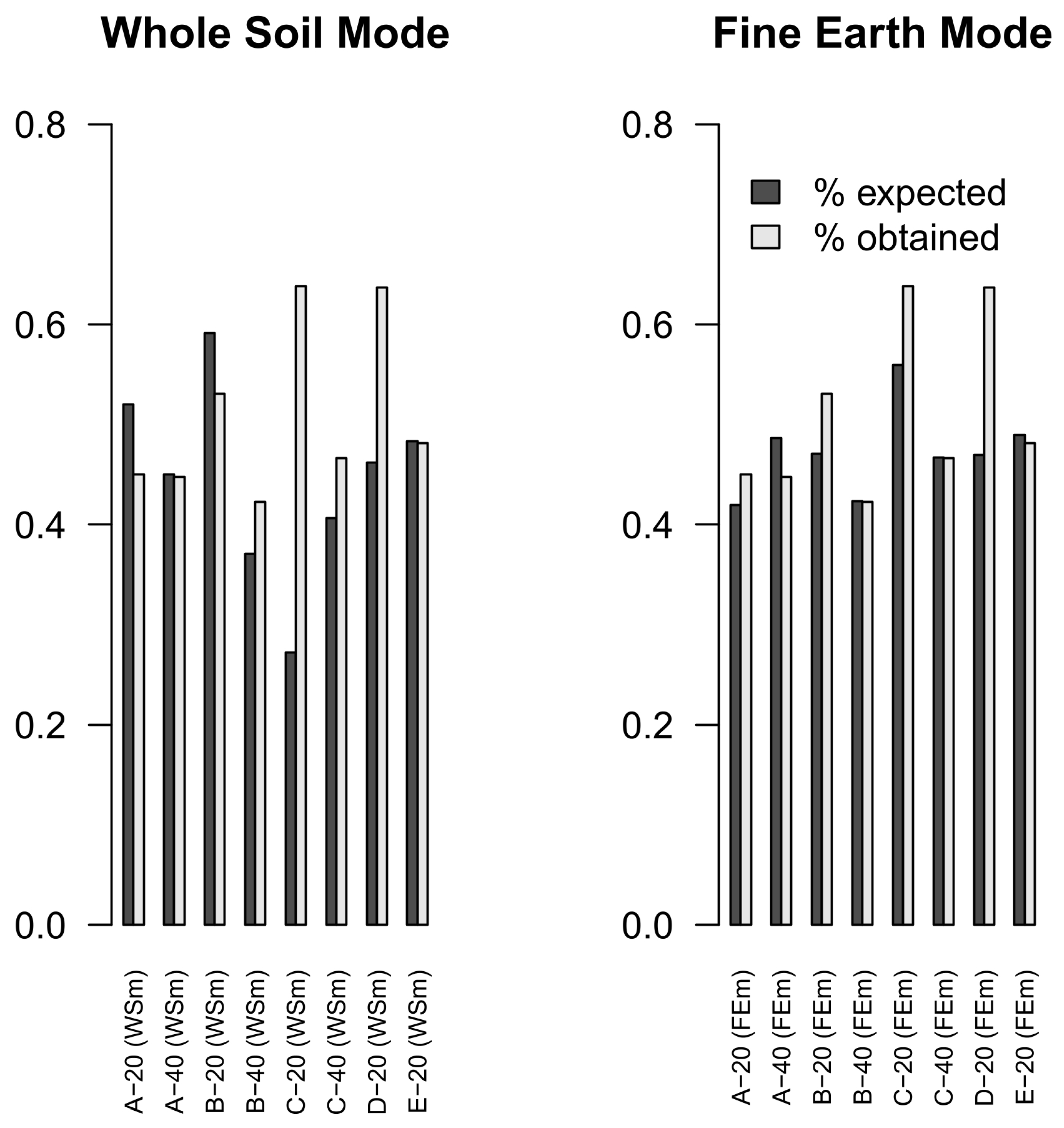
3.2. Extraction Efficiency
3.3. Repartition of Soil Particles in and out of the Device
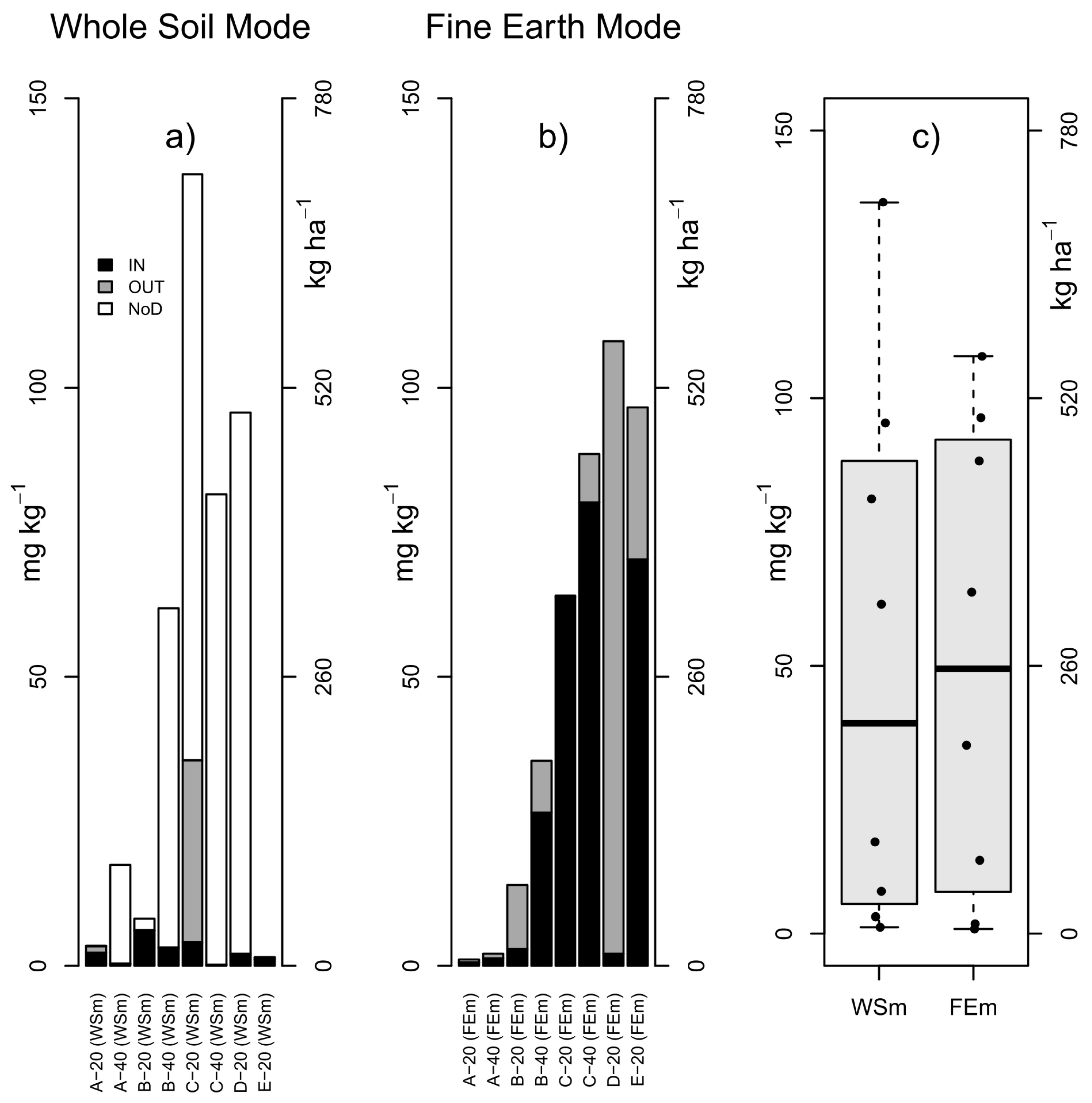
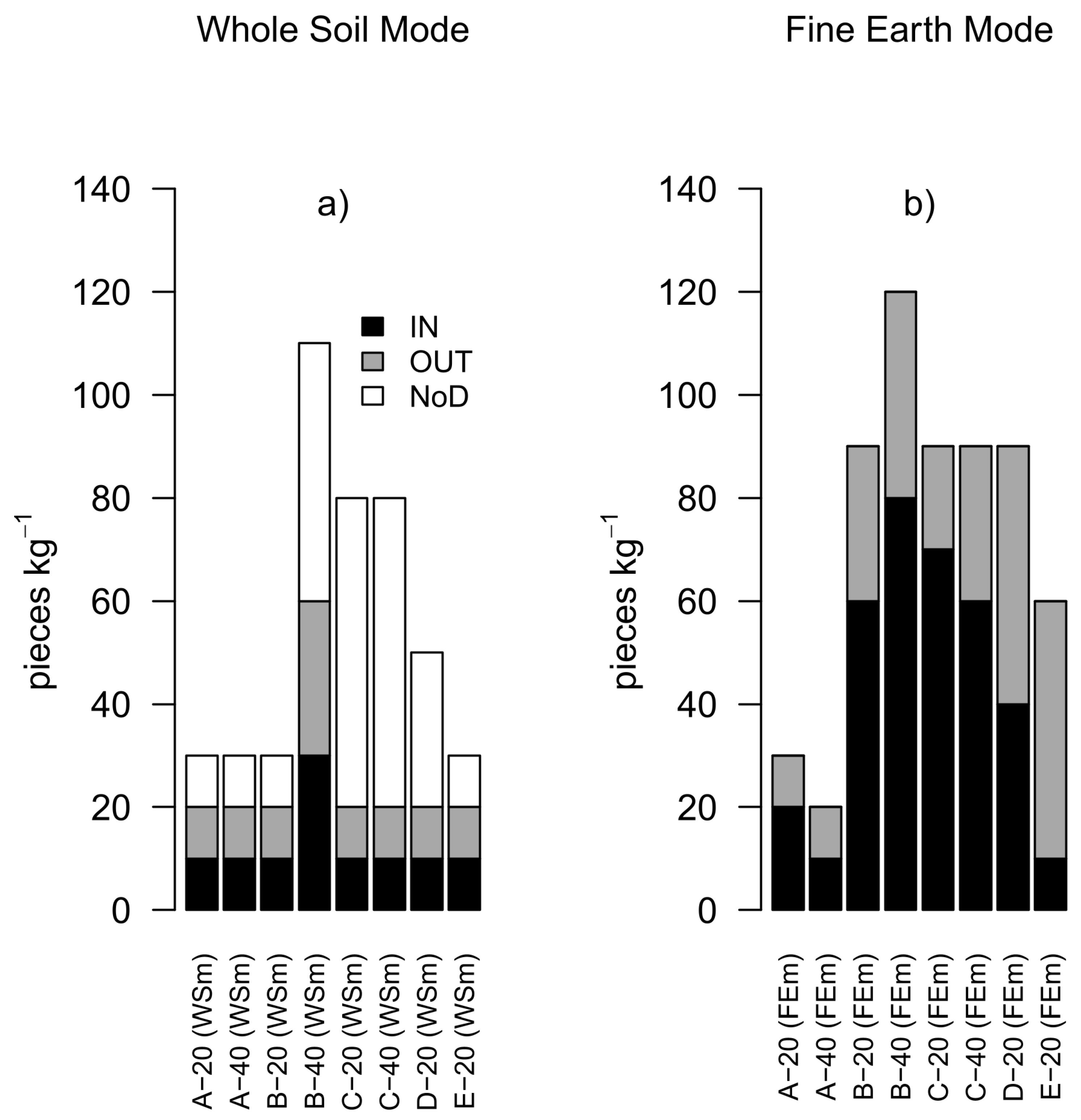
3.4. Quantification of MPs: Masses and Counts
3.5. Microplastic Characterization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COP21. Paris Agreement. 2015. Available online: https://unfccc.int/sites/default/files/english_paris_agreement.pdf (accessed on 20 May 2025).
- Initiative. 4x1000 Initiative. 2015. Available online: https://www.4p1000.org/ (accessed on 20 May 2025).
- Aggelides, S.; Londra, P. Effects of compost produced from town wastes and sewage sludge on the physical properties of a loamy and a clay soil. Bioresour. Technol. 2000, 71, 253–259. [Google Scholar] [CrossRef]
- Weber, J.; Karczewska, A.; Drozd, J.; Licznar, M.; Licznar, S.; Jamroz, E.; Kocowicz, A. Agricultural and ecological aspects of a sandy soil as affected by the application of municipal solid waste composts. Soil Biol. Biochem. 2007, 39, 1294–1302. [Google Scholar] [CrossRef]
- Watteau, F.; Dignac, M.F.; Bouchard, A.; Revallier, A.; Houot, S. Microplastic detection in soil amended with municipal solid waste composts as revealed by transmission electronic microscopy and pyrolysis/GC/MS. Front. Sustain. Food Syst. 2018, 2, 81. [Google Scholar] [CrossRef]
- Weithmann, N.; Möller, J.N.; Löder, M.G.J.; Piehl, S.; Laforsch, C.; Freitag, R. Organic fertilizer as a vehicle for the entry of microplastic into the environment. Sci. Adv. 2018, 4, eaap8060. [Google Scholar] [CrossRef] [PubMed]
- Brinton, W.F. Characterization of man-made foreign matter and its presence in multiple size fractions from mixed waste composting. Compost Sci. Util. 2005, 13, 274–280. [Google Scholar] [CrossRef]
- Zubris, K.A.V.; Richards, B.K. Synthetic fibers as an indicator of land application of sludge. Environ. Pollut. 2005, 138, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Phuong, N.N.; Zalouk-Vergnoux, A.; Poirier, L.; Kamari, A.; Châtel, A.; Mouneyrac, C.; Lagarde, F. Is there any consistency between the microplastics found in the field and those used in laboratory experiments? Environ. Pollut. 2016, 211, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Barth, J.; Amlinger, F.; Favoino, E.; Siebert, S.; Kehres, B.; Gottschall, R.; Bieker, M.; Löbig, A.; Bidlingmaier, W. Compost Production and Use in the EU. 2008. Available online: http://www.organics-recycling.org.uk/dmdocuments/compostproduction_and_usein_EU.pdf (accessed on 20 May 2025).
- Zhang, J.; Liu, J.; Ding, W.; Zhang, B.; Zhao, M.; Zou, G.; Chen, Y. Composting treatment increases the risk of microplastics pollution in process and compost products. J. Hazard. Mater. 2025, 486, 137084. [Google Scholar] [CrossRef] [PubMed]
- de Souza Machado, A.A.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of microplastics on the soil biophysical environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef] [PubMed]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics can change soil properties and affect plant performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [PubMed]
- Wang FWang, X.; Song, N. Polyethylene microplastics increase cadmium uptake in lettuce (Lactuca sativa L.) by altering the soil microenvironment. Sci. Total Environ. 2021, 784, 147133. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, Z.; Zhu, C.; Wang, C.; Gu, C. Effect of polyvinyl chloride microplastics ion bacterial community and nutrient status in two agricultural soils. Environ. Contam. Toxicol. 2021, 107, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Han, B.; Sun, Y.; Wang, F. Microplastics influence the adsorption and desorption characteristics of Cd in an agricultural soil. J. Hazard. Mater. 2020, 388, 121775. [Google Scholar] [CrossRef] [PubMed]
- Lozano, Y.M.; Lehnert, T.; Linck, L.T.; Lehmann, A.; Rillig, M.C. Microplastic shape, polymer type, and concentration affect soil properties and plant biomass. Front. Plant Sci. 2021, 12, 616645. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, P.; Yang, X.; Wang, Z.; Lu, B.; Chen, W.; Wu, Y.; Li, G.W.; Zhao, Z.W.; Liu, G.B.; et al. Soil texture is an important factor determining how microplastics affect soil hydraulic characteristics. Environ. Int. 2022, 165, 107293. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Q.; Adams, C.A.; Sun, Y.; Zhang, S. Effects of microplastics on soil properties. Current knowledge and future perspectives. J. Hazard. Mater. 2022, 424, 127531. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, J.; Qu, Z.; Ayurzana, B.; Zhao, G.; Li, W. Effects of microplastics on the pore structure and connectivity with different soil textures: Based on CT scanning. Environ. Technol. Innov. 2024, 36, 103791. [Google Scholar] [CrossRef]
- Hasan, M.M.; Tarannum, M.N. Adverse impacts of microplastics on soil physicochemical properties and crop health in agricultural systems. J. Hazard. Mater. Adv. 2025, 17, 100528. [Google Scholar] [CrossRef]
- Zang, H.; Marshall, M.P.; Chadwick, D.R.; Wen, Y.; Jones, D.J. Microplastics in the agroecosystems. Are they an emerging threat to the plant-soil system? Soil Biol. Biochem. 2020, 148, 107926. [Google Scholar] [CrossRef]
- Esposito, G.; Prearo, M.; Renzi, M.; Anselmi, S.; Cesarani, A.; Barcelò, D.; Dondo, A.; Pastorino, P. Occurrence of microplastics in the gastrointestinal tract of benthic by-catches from an eastern mediterranean deep-sea environment. Mar. Pollut. Bull. 2022, 174, 113231. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, P.; Prearo, M.; Di Blasio, A.; Barcelò, D.; Anselmi, S.; Colussi, S.; Alberti, S.; Tedde, G.; Dondo, A.; Ottino, M.; et al. Microplastics occurrence in the European common frog (Rana temporaria) from Cottian Alps (Northwest Italy). Diversity 2022, 14, 66. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Nizzetto, L.; Futter, M.; Langaas, S. Are agricultural soils dumps for microplastics of urban origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, Y. The distribution of microplastics in soil aggregate fractions in South Western China. Sci. Total Environ. 2018, 642, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Pita, F.; Castilho, A. Separation of plastics by froth flotation. the role of size, shape and density of the particles. Waste Manag. 2017, 60, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Claessens, M.; Meester, S.D.; Landuyt, L.V.; Clerck, K.D.; Janssen, C.R. Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar. Pollut. Bull. 2011, 62, 2199–2204. [Google Scholar] [CrossRef] [PubMed]
- Bläsing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Scopetani, C.; Chelazzi, D.; Mikola, J.; Leiniö, V.; Heikkinen, R.; Cincinelli, A.; Pellinen, J. Olive oil-based method for the extraction, quantification and identification of microplastics in soil and compost samples. Sci. Total Environ. 2020, 733, 139338. [Google Scholar] [CrossRef] [PubMed]
- Junhao, C.; Xining, Z.; Xiaodong, G.; Li, Z.; Qi, H.; Siddique, K.H. Extraction and identification methods of microplastics and nanoplastics in agricultural soil: A review. J. Envrion. Manag. 2021, 294, 112997. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Chevali, V.; Lai, Y.; Zhou, Z.; Chen, G.; Burey, P.; Wang, S.; Song, P. Microplastics in soils: A comparative review on extraction, identification and quantification methods. J. Envrion. Manag. 2025, 377, 124556. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, D. Use of pyrolysis-gas chromatography/mass spectrometry to study environmental pollution caused by synthetic polymers: A case study: The Ravenna lagoon. J. Anal. Appl. Pyrol. 2001, 58–59, 361–370. [Google Scholar] [CrossRef]
- Dümichen, E.; Eisentraut, P.; Bannick, C.G.; Barthel, A.K.; Senz, R.; Braun, U. Fast identification of microplastics in complex environmental samples by a thermal degradation method. Chemosphere 2017, 174, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Scholz-Böttcher, B.M. Simultaneous trace identification and quantification of common types of microplastics in environmental samples by pyrolysis-gas chromatography–mass spectrometry. Environ. Sci. Technol. 2017, 51, 5052–5060. [Google Scholar] [CrossRef] [PubMed]
- Elert, A.M.; Becker, R.; Duemichen, E.; Eisentraut, P.; Falkenhagen, J.; Sturm, H.; Braun, U. Comparison of different methods for MP detection: What can we learn from them, and why asking the right question before measurements matters? Environ. Pollut. 2017, 231, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Käppler, A.; Fischer, D.; Oberbeckmann, S.; Schernewski, G.; Labrenz, M.; Eichhorn, K.J.; Voit, B. Analysis of environmental microplastics by vibrational microspectroscopy: FTIR, Raman or both? Anal. Bioanal. Chem. 2016, 408, 8377–8391. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiang, L.; Amelung, W.; Elsner, M.; Gan, J.; Kueppers, S.; Christian, L.; Jiang, X.; Adu-Gyamfi, J.; Heng, L.; et al. Micro- and nanoplastics in soil ecosystems: Analytical methods, fate, and effects. Trends Anal. Chem. 2023, 169, 117309. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analyses of soils 1. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Santi, C.; Certini, G.; D’Acqui, L.P. Direct determination of organic carbon by dry combustion in soils with carbonates. Commun. Soil Sci. Plant Anal. 2006, 37, 155–162. [Google Scholar] [CrossRef]
- Li, C.; Du, C.; Ma, F.; Zhou, J. Diagnosis of nitrogen status in Chinese cabbage (Brassica rapa chinensis) using the ratio of amide II to amide in leaves based on mid-infrared photoacoustic spectroscopy. J. Plant. Nutr. Soil Sci. 2015, 178, 888–895. [Google Scholar] [CrossRef]
- Löder, M.G.J.; Kuczera, M.; Mintenig, S.; Lorenz, C.; Gerdts, G. Focal plane array detector-based micro-Fourier-Transform infrared imaging for the analysis of microplastics in environmental samples. Environ. Chem. 2015, 12, 563. [Google Scholar] [CrossRef]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lu, S.; Song, Y.; Lei, L.; Hu, J.; Lv, W.; Zhou, W.; Cao, C.; Shi, H.; Yang, X.; et al. Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environ. Pollut. 2018, 242, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Piehl, S.; Leibner, A.; Löder, M.G.J.; Dris, R.; Bogner, C.; Laforsch, C. Identification and quantification of macro- and microplastics on an agricultural farmland. Sci. Rep. 2018, 8, 17950. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Zhao, J.; Liu, L.; Zhang, Y.; Wang, X.; Wu, F. A novel way to rapidly monitor microplastics in soil by hyperspectral imaging technology and chemometrics. Environ. Pollut. 2018, 238, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Benzecri, J.P. Statistical analysis as a tool to make patterns emerge from data. In Methodologies of Pattern Recognition; Watanabe, S., Ed.; Academic Press: Cambridge, MA, USA, 1969; pp. 35–74. Available online: https://www.sciencedirect.com/science/article/pii/B9781483230931500092 (accessed on 20 May 2025). [CrossRef]
- Roux, B.; Rouanet, H. Multiple Correspondence Analysis. Quantitative Applications in the Social Sciences; SAGE Publications: Los Angeles, CA, USA, 2010; Available online: https://books.google.it/books?id=GWsHakQGEHsC (accessed on 20 May 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 20 May 2025).
- Wickham, H. The split-apply-combine strategy for data analysis. J. Stat. Softw. 2011, 40, 1–29. Available online: http://www.jstatsoft.org/v40/i01/ (accessed on 20 May 2025). [CrossRef]
- Dahl, D.B.; Scott, D.; Roosen, C.; Magnusson, A.; Swinton, J. xtable: Export Tables to LaTeX or HTML. 2019. Available online: https://cran.r-project.org/web/packages/xtable/xtable.pdf (accessed on 20 May 2025).
- Sarkar, D. Lattice: Multivariate Data Visualization with R; Springer: New York, NY, USA, 2008; ISBN 978-0-387-75968-5. Available online: http://lmdvr.r-forge.r-project.org (accessed on 20 May 2025).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: A package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Leisch, F. Sweave: Dynamic generation of statistical reports using literate data analysis. In Compstat 2002—Proceedings in Computational Statistics; Härdle, W., Rönz, B., Eds.; Physica Verlag: Berlin/Heidelberg, Germany, 2002; pp. 575–580. ISBN 3-7908-1517-9. Available online: https://link.springer.com/chapter/10.1007/978-3-642-57489-4_89 (accessed on 20 May 2025). [CrossRef]
- Xu, B.; Liu, F.; Cryder, Z.; Huang, D.; Lu, Z.; He, Y.; Wang, H.; Lu, Z.; Brookes, P.C.; Tang, C.; et al. Microplastics in the soil environment: Occurrence, risks, interactions and fate—A review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2175–2222. [Google Scholar] [CrossRef]
- Hurley, R.R.; Nizzetto, L. Fate and occurrence of micro(nano)plastics in soils: Knowledge gaps and possible risks. Curr. Opin. Environ. Sci. Health 2018, 1, 6–11. [Google Scholar] [CrossRef]
- Claessens, M.; Van Cauwenberghe, L.; Vandegehuchte, M.B.; Janssen, C.R. New techniques for the detection of microplastics in sediments and field collected organisms. Mar. Pollut. Bull. 2013, 70, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Binda, G.; Kalčíková, G.; Allan, I.J.; Hurley, R.; Rødland, E.; Spanu, D.; Nizzetto, L. Microplastic aging processes: Environmental relevance and analytical implications. Trends Anal. Chem. 2024, 172, 117566. [Google Scholar] [CrossRef]
- Shafea, L.; Yoffre, R.C.A.; Woche, S.K.; Sauheitl, L.; Göbel, M.O.; Peth, S. Effects of microplastic aging on its detectability and physico-chemical properties in loess and sandy soil. In Proceedings of the EGU General Assembly 2023, Vienna, Austria, 22–28 April 2023; p. EGU-4371. [Google Scholar] [CrossRef]
- Thomas, D.; Schütze, B.; Heinze, W.M.; Steinmetz, Z. Sample preparation techniques for the analysis of microplastics in soil—A review. Sustainability 2020, 12, 9074. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Gertsen, H.; Peters, P.; Salánki, T.; Geissen, V. A simple method for the extraction and identification of light density microplastics from soil. Sci. Total Environ. 2018, 616–617, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- GESAMP. Sources, fate and effects of MP in the marine environment. J. Ser. GESAMP Rep. Stud. 2015, 90, 98. [Google Scholar]
- Vollertsen, J.; Hansen, A. Microplastic in Danish Wastewater: Sources, Occurrences and Fate; Environmental Project ed. 2017; The Danish Environmental Protection Agency: Odense, Denmark, 2017; Volume 1906. Available online: https://vbn.aau.dk/en/publications/microplastic-in-danish-wastewater-sources-occurrences-and-fate (accessed on 20 May 2025).
- Lv, W.; Zhou, W.; Lu, S.; Huang, W.; Yuan, Q.; Tian, M.; Lv, W.; He, D. Microplastic pollution in rice-fish co-culture system: A report of three farmland stations in Shanghai, China. Sci. Total Environ. 2019, 652, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. B 2009, 364, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
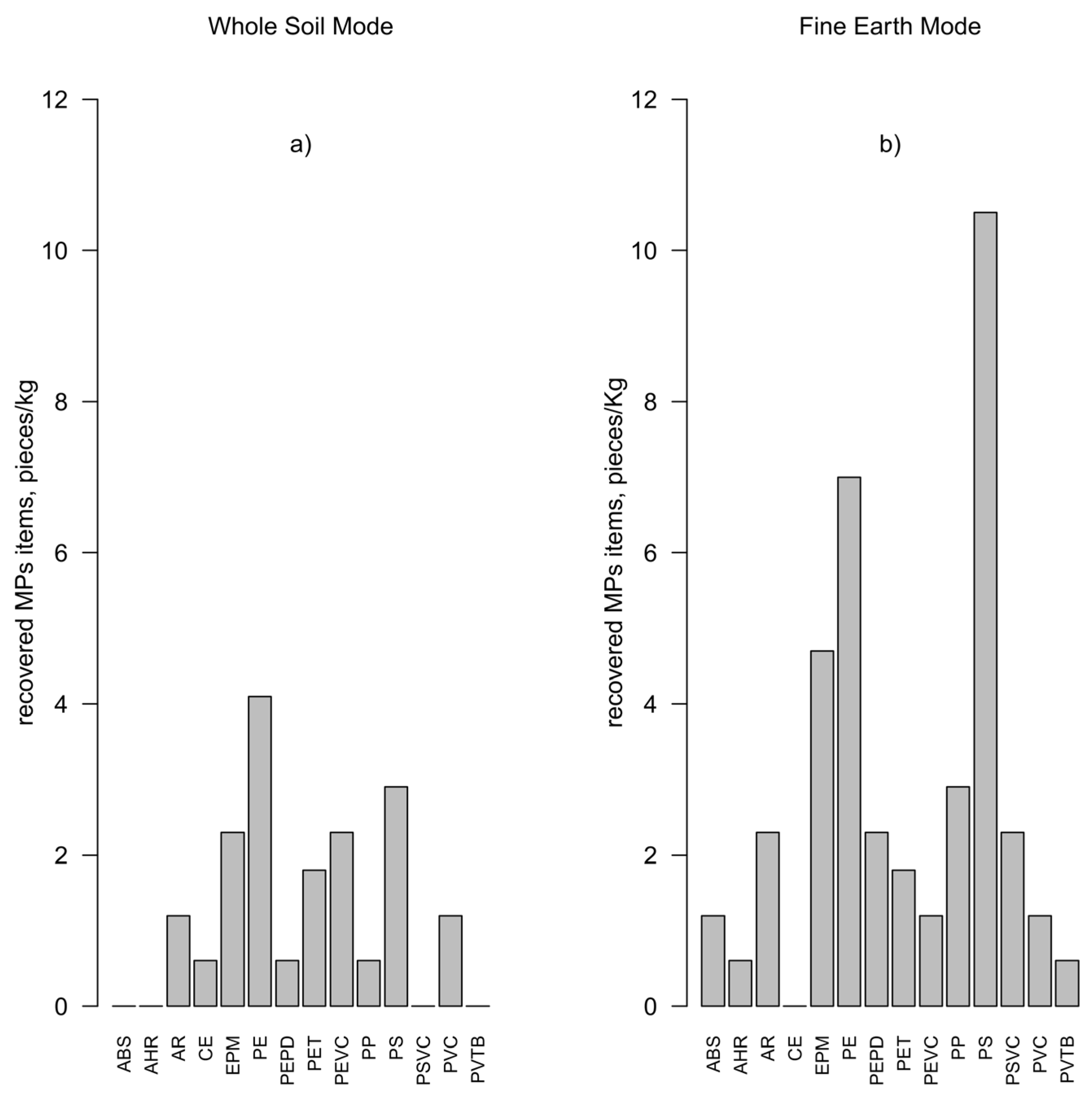
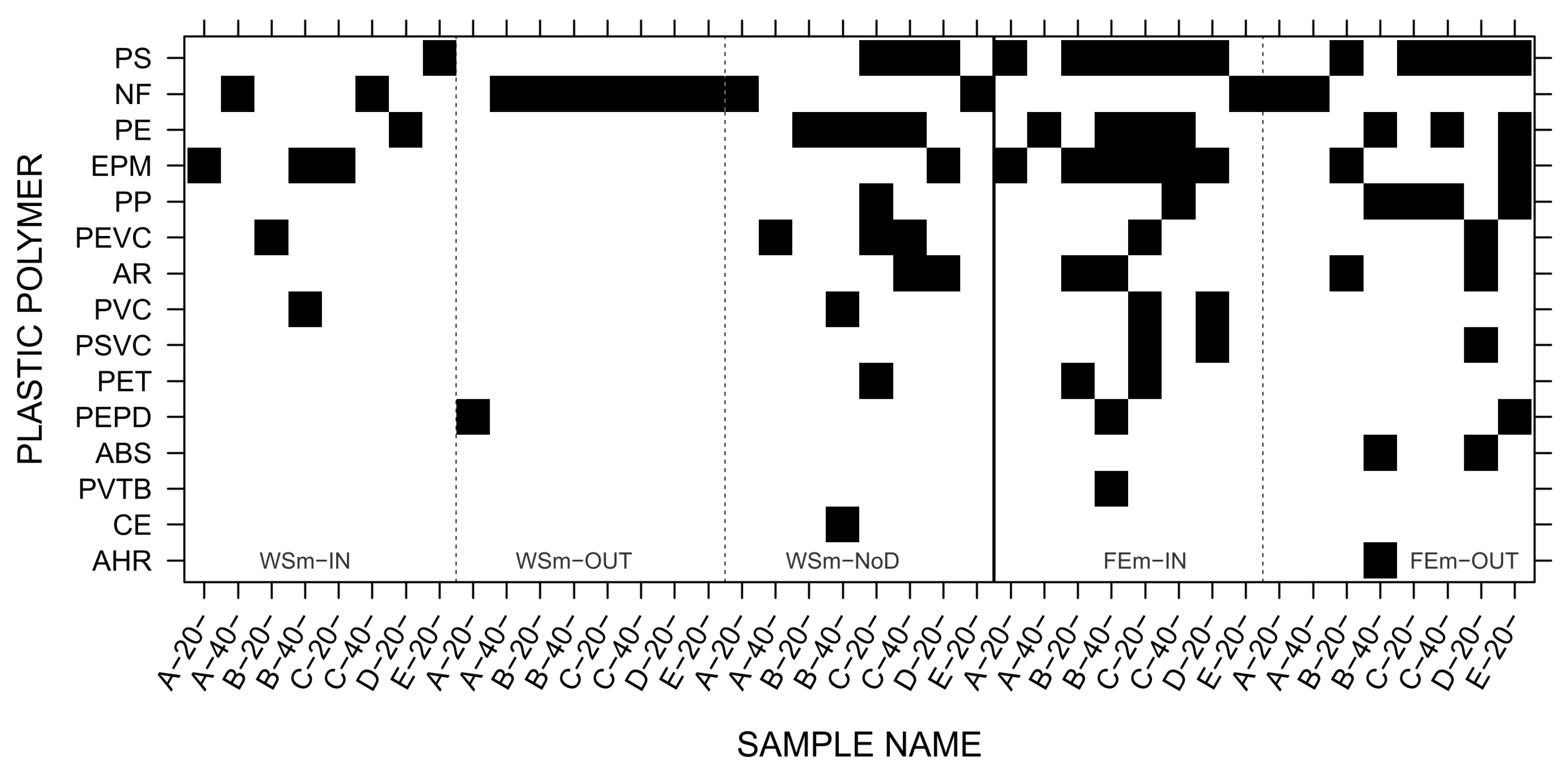
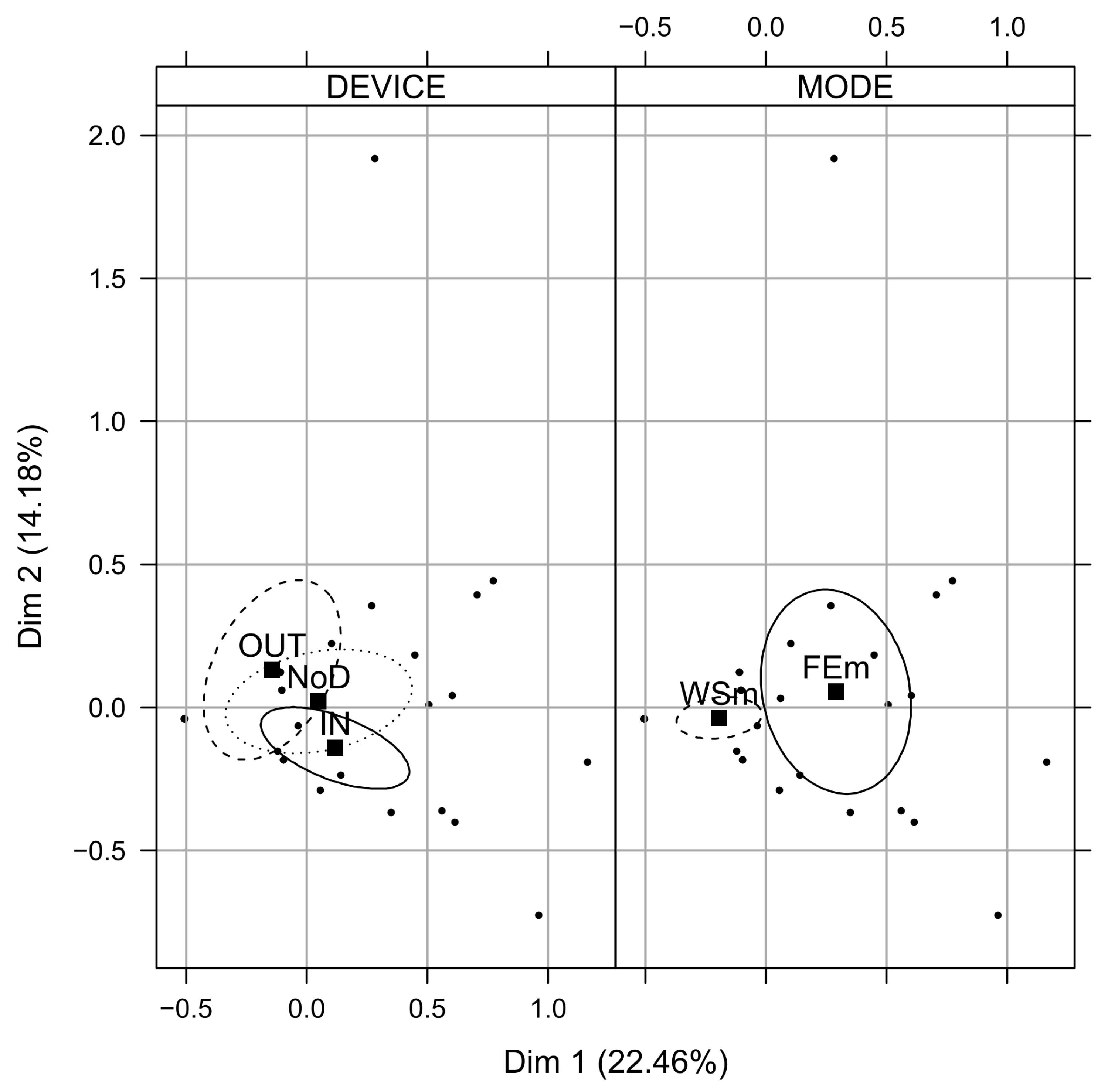
| Sample Name | Sampling Depth cm | Surface Area ha | Sand | Silt | Clay | >34 µm | OCTot | NTot | pH |
|---|---|---|---|---|---|---|---|---|---|
| % | |||||||||
| A-20 | 0–20 | ~2 | 48.2 | 37.0 | 14.8 | 45.0 | 3.3 | 0.79 | 7.85 |
| A-40 | 20–40 | ~2 | 47.2 | 36.8 | 16.0 | 44.8 | 3.2 | 0.79 | 7.60 |
| B-20 | 0–20 | ~2 | 41.8 | 36.0 | 22.2 | 53.1 | 3.1 | 0.76 | 7.59 |
| B-40 | 20–40 | ~2 | 51.0 | 30.1 | 18.9 | 42.3 | 3.5 | 0.74 | 7.84 |
| C-20 | 0–20 | ~6 | 24.4 | 44.7 | 30.9 | 63.8 | 6.0 | 0.76 | 7.39 |
| C-40 | 20–40 | ~6 | 29.4 | 47.2 | 23.4 | 46.6 | 6.9 | 0.73 | 7.62 |
| D-20 | 0–20 | ~2 | 24.4 | 55.1 | 20.5 | 63.7 | 3.4 | 0.75 | 7.82 |
| E-20 | 0–20 | ~2 | 42.7 | 38.0 | 19.3 | 48.2 | 4.0 | 0.79 | 7.42 |
| Acronym | Name |
|---|---|
| ABS | acrylonitrile butadiene styrene |
| AHR | aromatic hydrocarburic resin |
| AR | alkydic resin |
| CE | cellophane |
| EPM | polyethylenpropylene |
| PE | polyethylene |
| PEPD | polyethylene propylene diene |
| PET | polyethylene terephthalate |
| PEVC | polyethylene-vinyl chloride |
| PP | polypropylene |
| PS | polystyrene |
| PSVC | polystyrene vinyliden chloride |
| PVC | polyvinyl chloride |
| PVTB | polyvinyl toluene butadiene |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Acqui, L.P.; Di Lonardo, S.; Grattacaso, M.; Bonetti, A.; Pantani, O.-L. Quantification of Microplastics in Urban Compost-Amended Farmland Soil Using an Elutriation Device. Agronomy 2025, 15, 1736. https://doi.org/10.3390/agronomy15071736
D’Acqui LP, Di Lonardo S, Grattacaso M, Bonetti A, Pantani O-L. Quantification of Microplastics in Urban Compost-Amended Farmland Soil Using an Elutriation Device. Agronomy. 2025; 15(7):1736. https://doi.org/10.3390/agronomy15071736
Chicago/Turabian StyleD’Acqui, Luigi Paolo, Sara Di Lonardo, Martina Grattacaso, Alessandra Bonetti, and Ottorino-Luca Pantani. 2025. "Quantification of Microplastics in Urban Compost-Amended Farmland Soil Using an Elutriation Device" Agronomy 15, no. 7: 1736. https://doi.org/10.3390/agronomy15071736
APA StyleD’Acqui, L. P., Di Lonardo, S., Grattacaso, M., Bonetti, A., & Pantani, O.-L. (2025). Quantification of Microplastics in Urban Compost-Amended Farmland Soil Using an Elutriation Device. Agronomy, 15(7), 1736. https://doi.org/10.3390/agronomy15071736








