Phylogenetic Diversity and Symbiotic Effectiveness of Bradyrhizobium Strains Nodulating Glycine max in Côte d’Ivoire
Abstract
1. Introduction
2. Materials and Methods
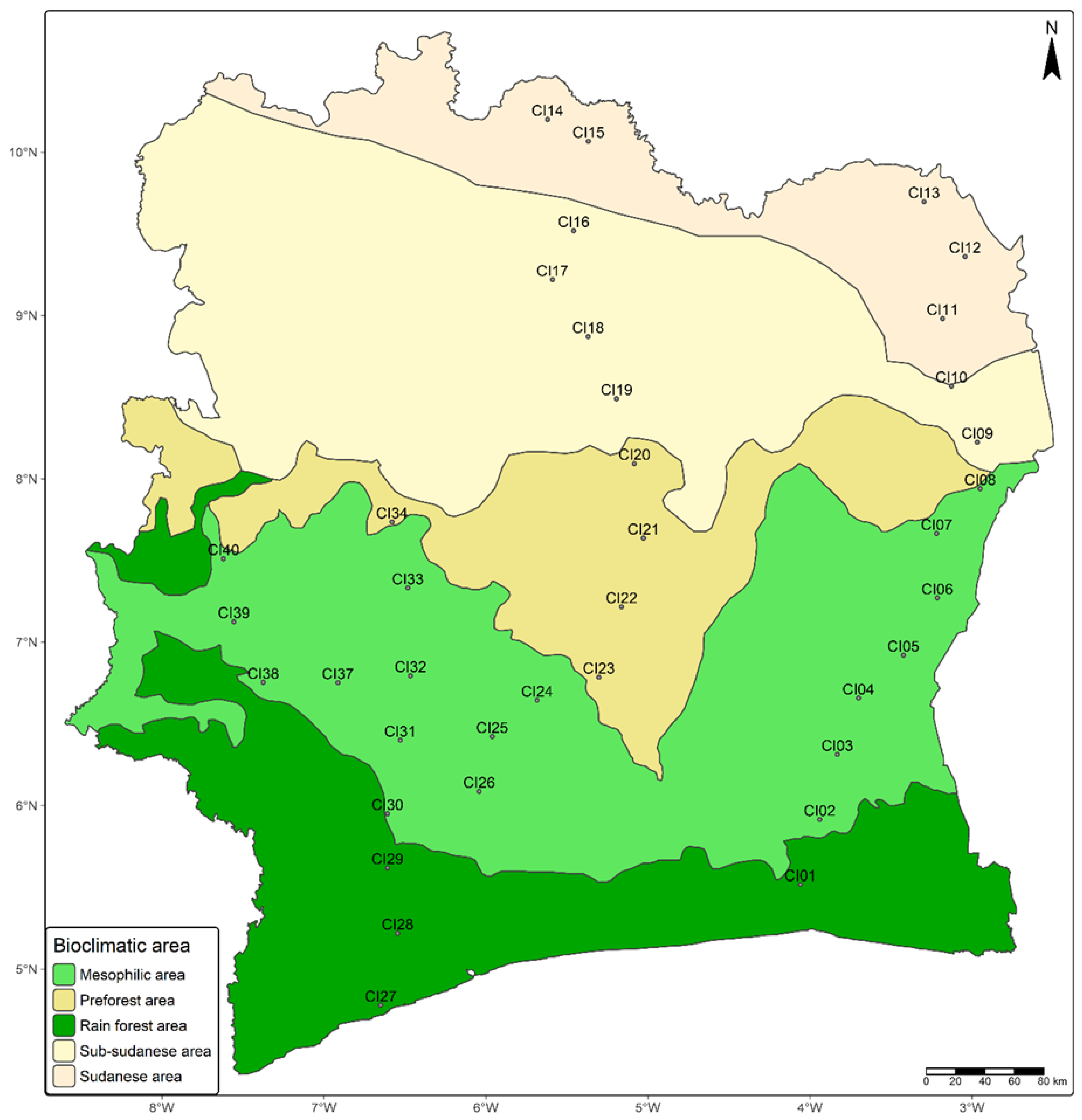
2.1. Soil Sampling
2.2. Soil Physico-Chemical Analyses
2.3. Extraction of Bioclimatic Variables
2.4. Bacterial Trapping and Isolation
2.5. Authentication Experiment and Storage of Isolates
2.6. PCR Amplification and Phylogenetic Analyses
2.7. Bacterial Symbiotic Efficiency Test
2.8. Determination of Viable and Infective Indigenous Rhizobia
2.9. Statistical Analyses and Diversity Estimation
3. Results
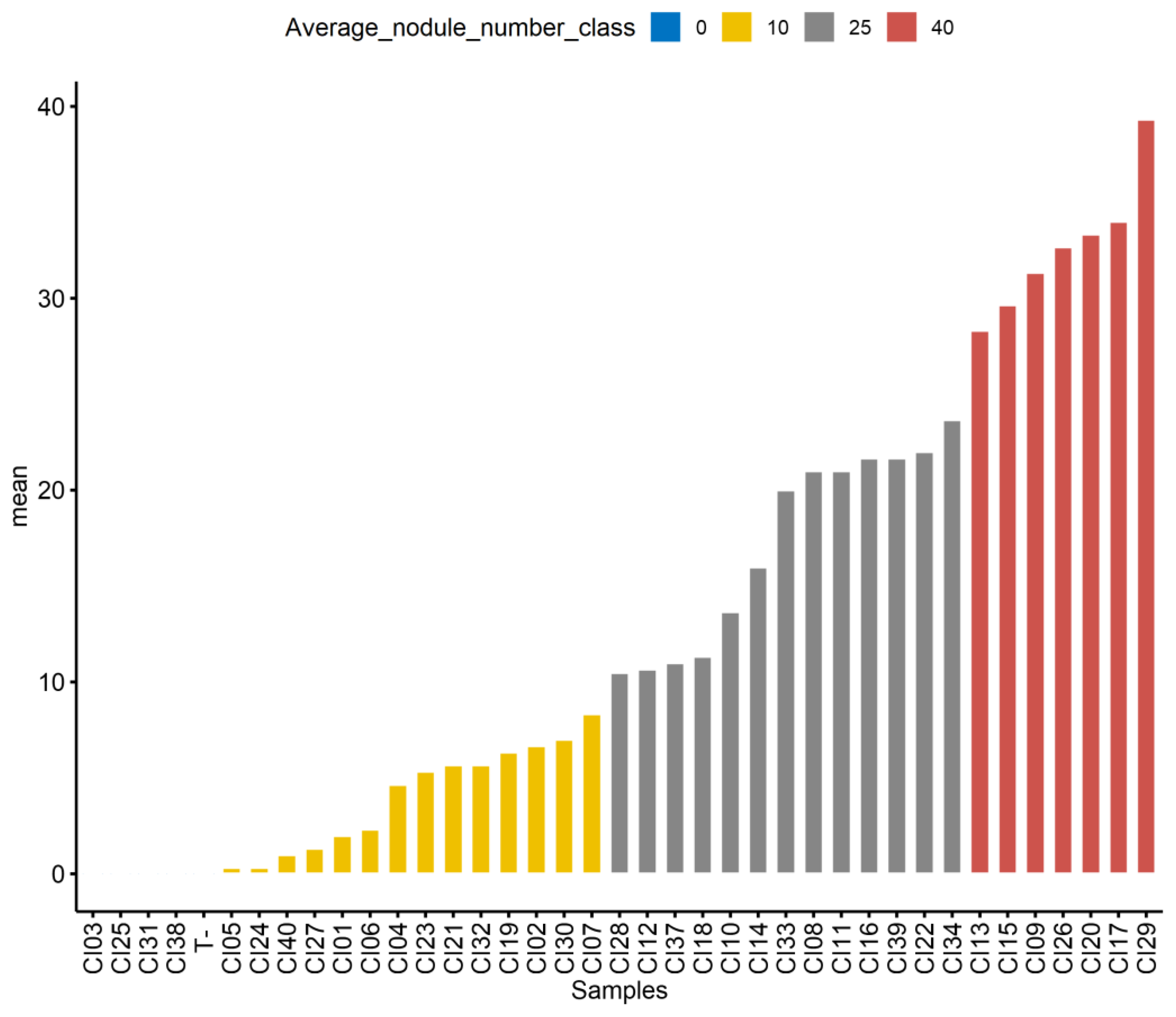
3.1. Côte d’Ivoire Harbor Diverse Soil Types Exhibiting Different Nodulating Potential
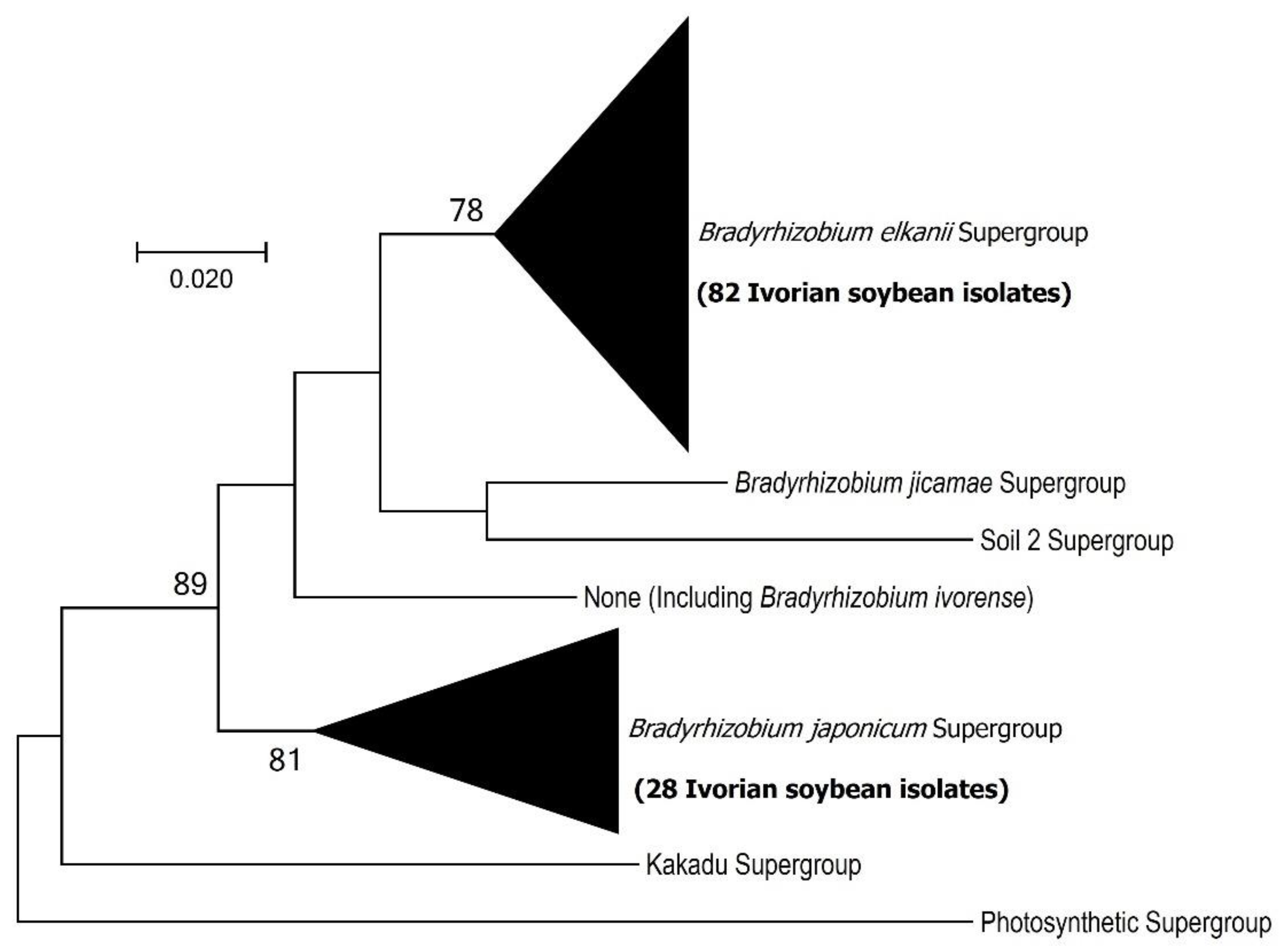
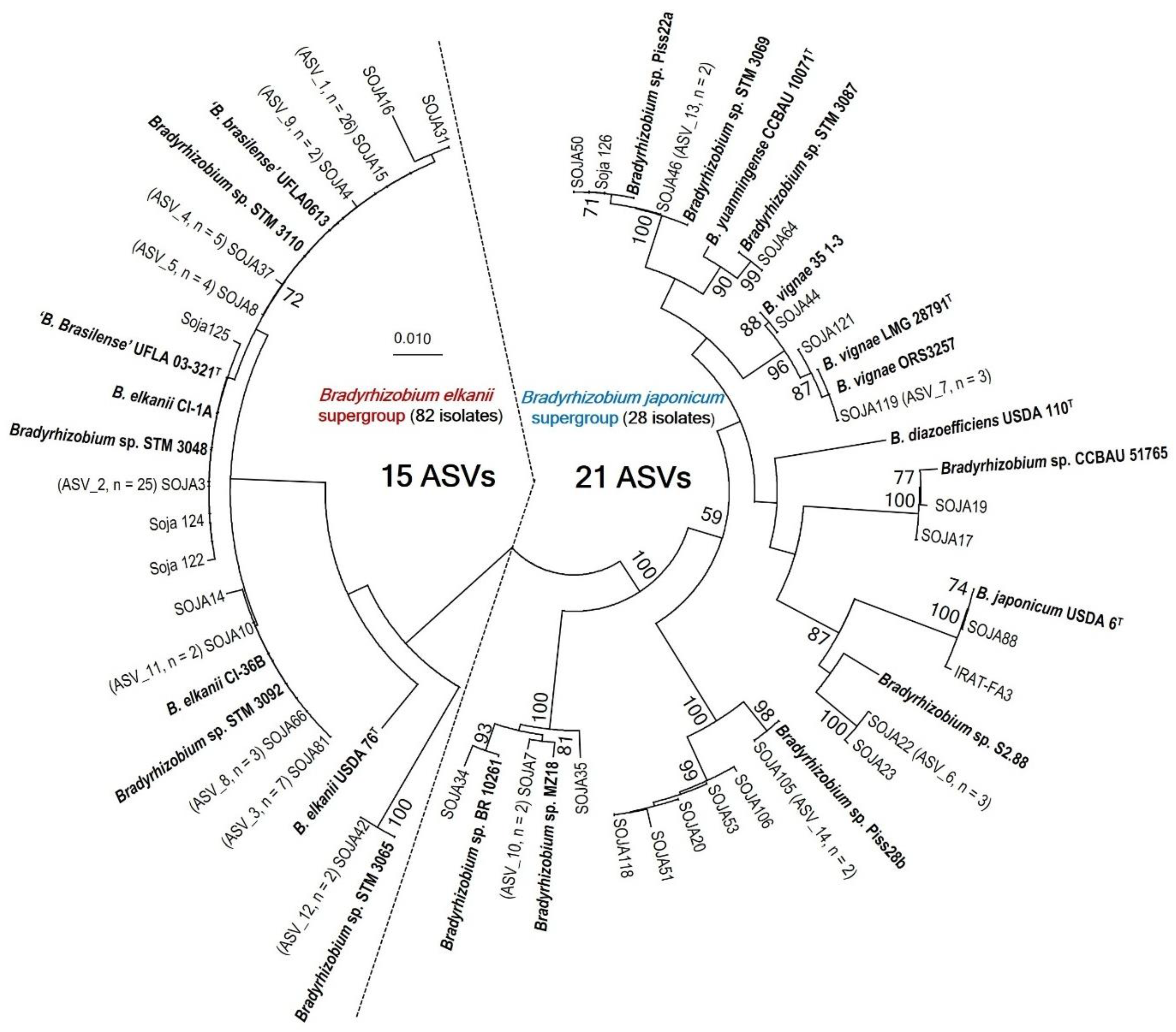
3.2. Bradyrhizobium elkani Supergroup Dominates Soybean Nodulating Communities in Côte d’Ivoire
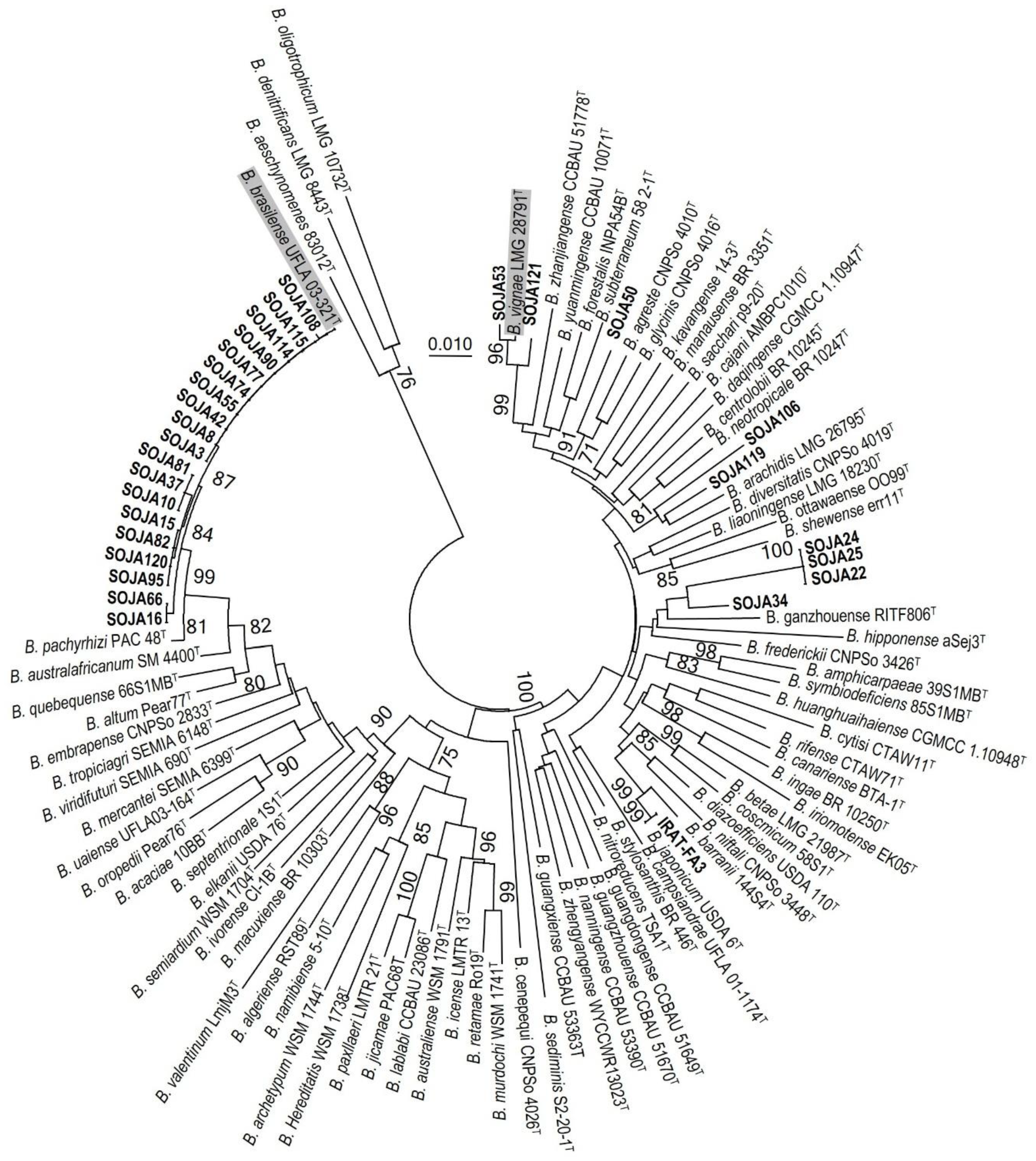
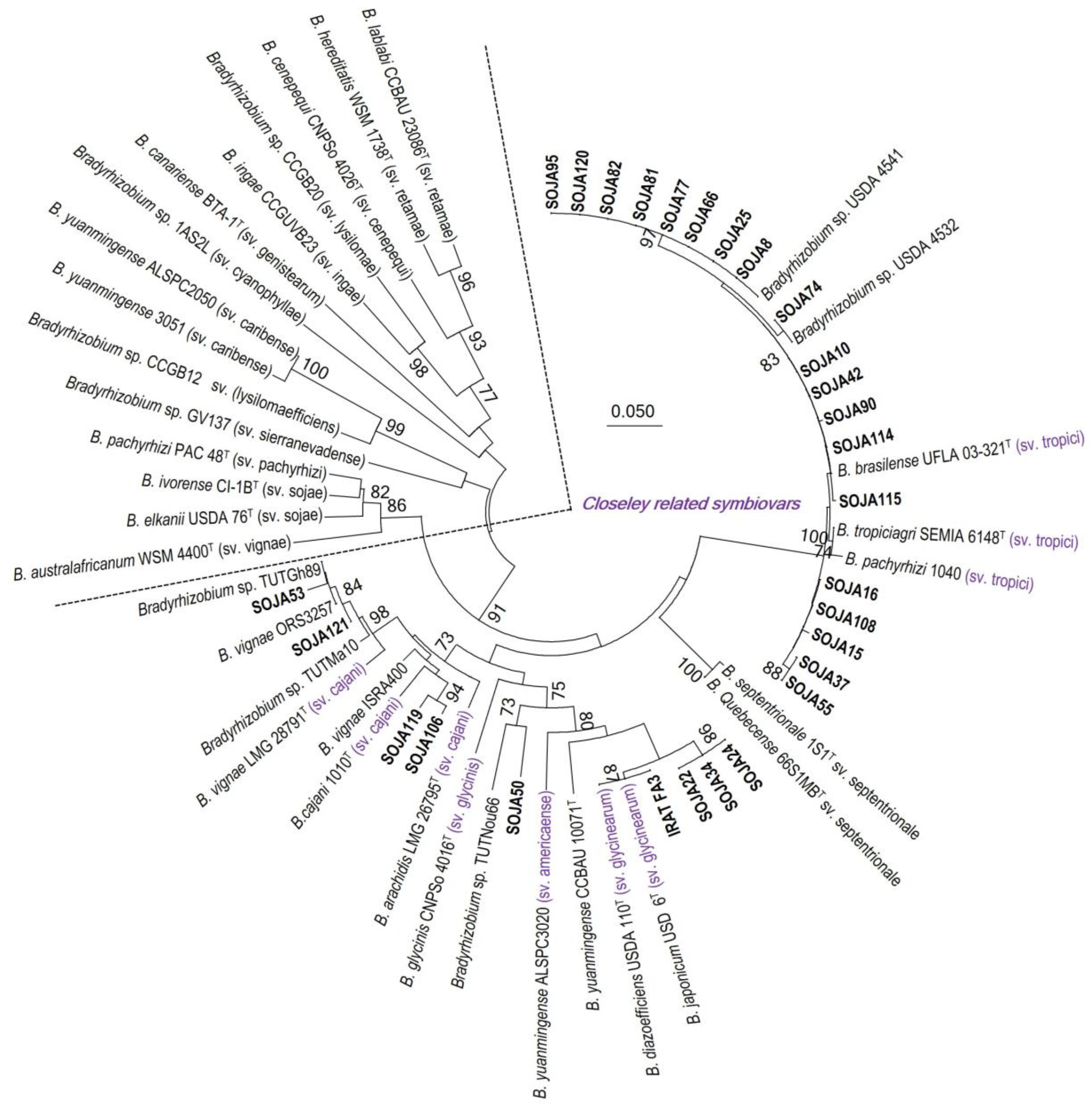
3.3. MLSA and Symbiotic Gene Phylogenies
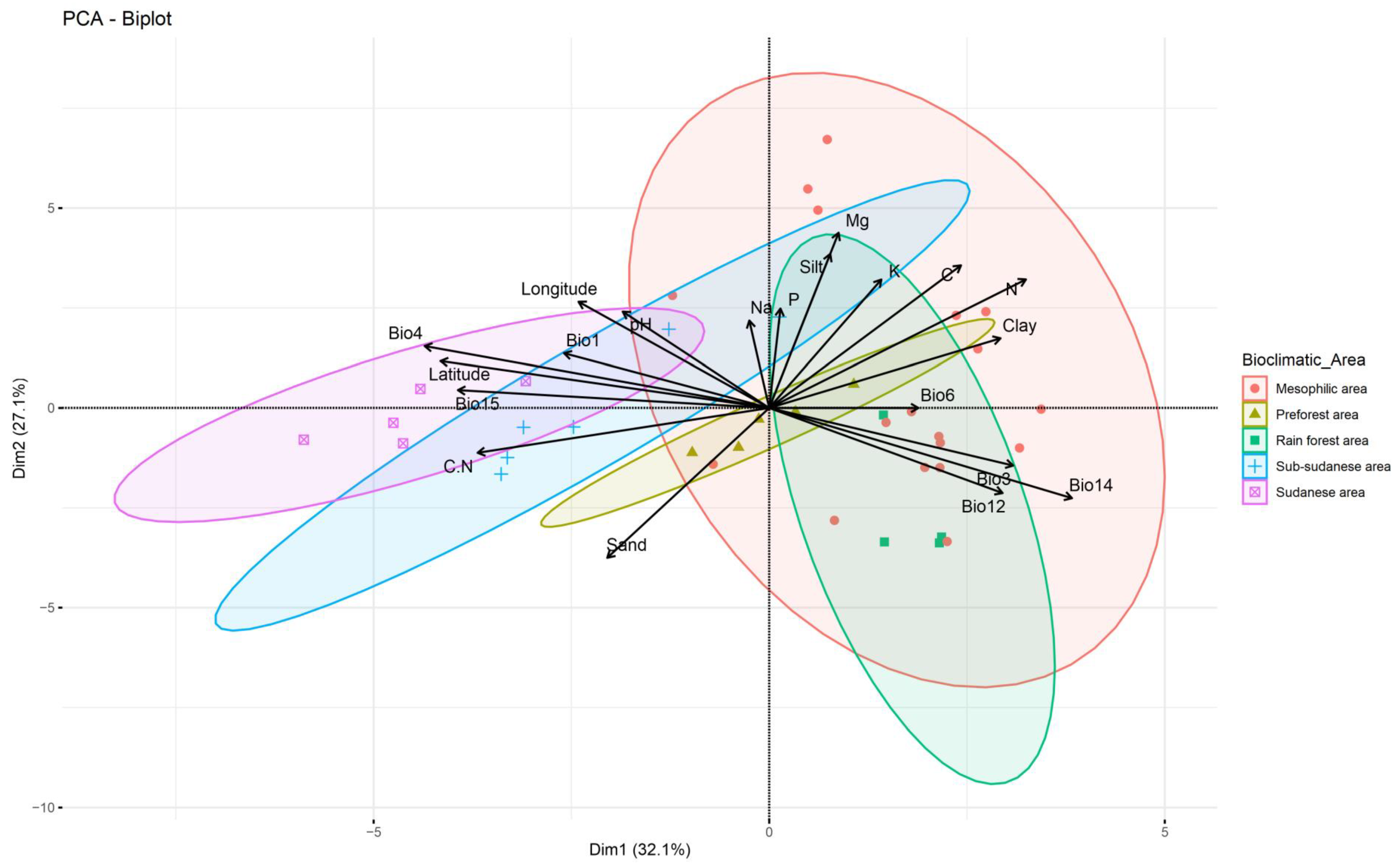
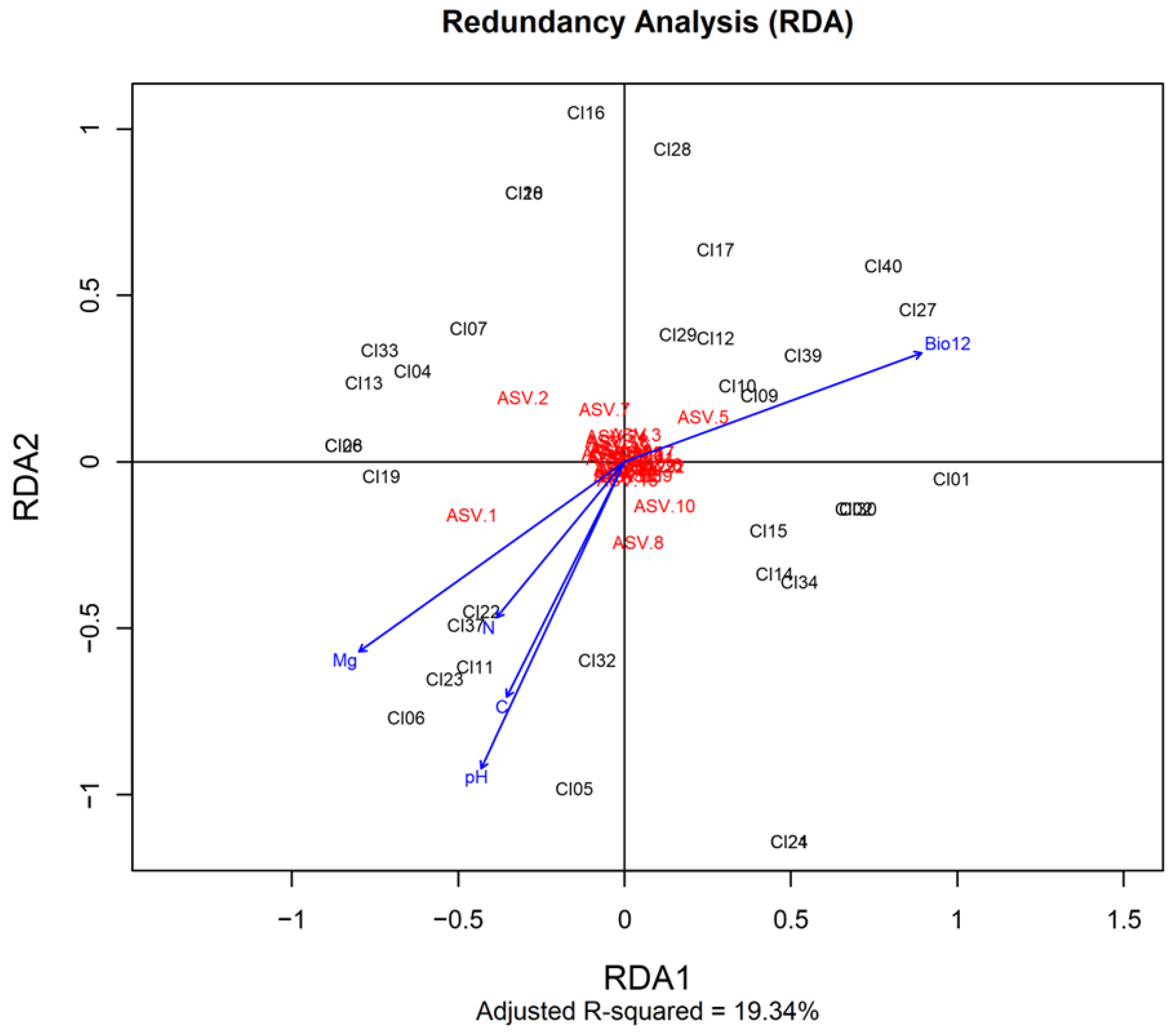
3.4. Soil Factors That Shape the Distribution of Soybean Nodulating Bradyrhizobium Isolates in Côte d’Ivoire
3.5. Diversity and Distribution of Soybean Nodulating Bradyrhizobium Isolates Within Côte d’Ivoire Soils
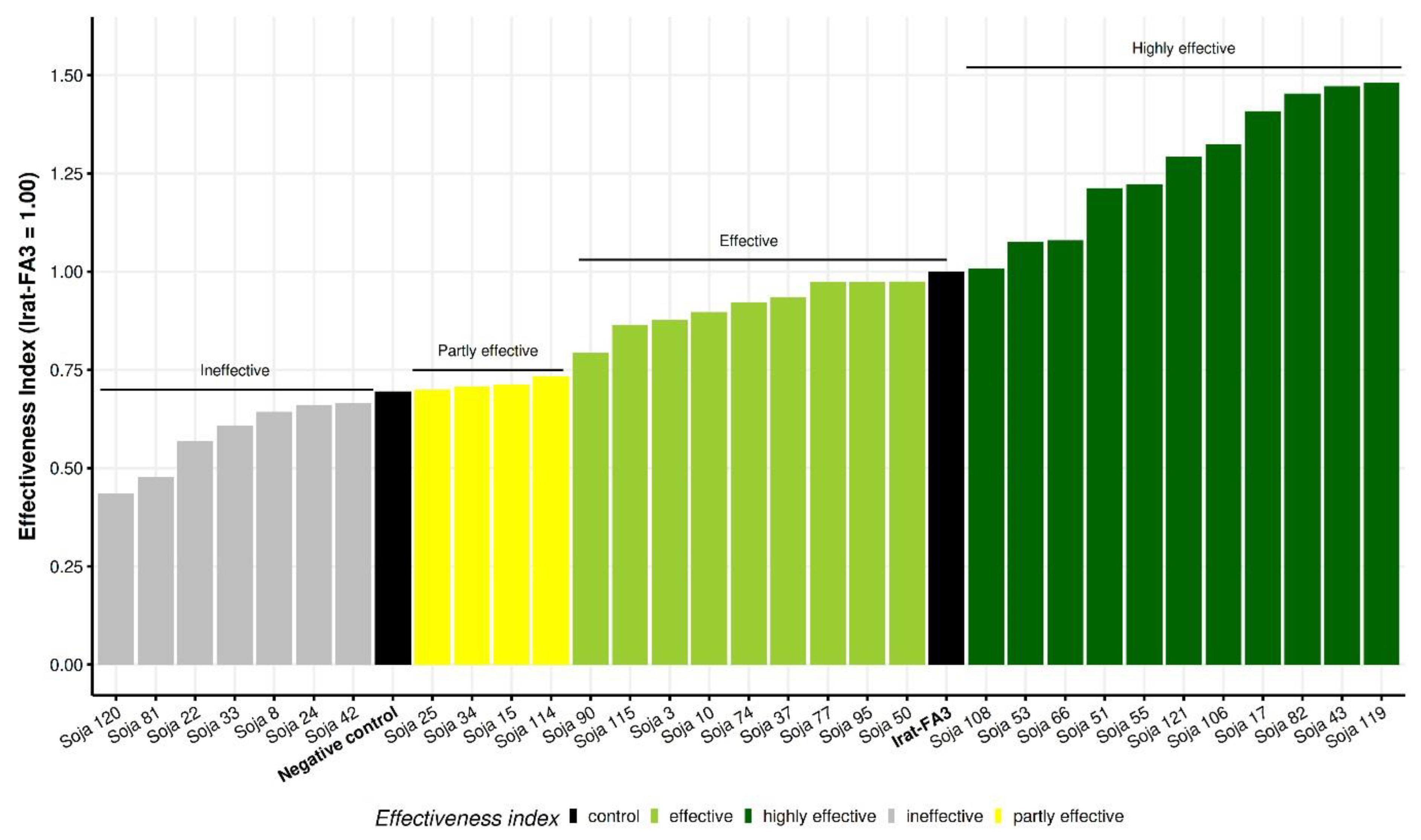
3.6. Highly Effective Indigenous Bradyrhizobium Strains from Côte d’Ivoire Outcompeted the Commercial Inoculant Strain IRAT-FA3 on Soybean
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APC | Principal component analysis |
| ASV | Amplicon sequence variants |
| BLAST | Basic local alignment |
| BNM | Buffered nodulation medium |
| CNRA | National Agronomic Research Center |
| DNA | Deoxyribonucleic acid |
| EI | Effectiveness index |
| FAO | Food and Agriculture Organization of the United Nations |
| GPS | Global Positioning System |
| IITA | International Institute for Tropical Agriculture |
| IRAT | Institut de Recherche d’Agronomie Tropicale |
| ITS | Internal transcribed spacer |
| LPSN | List of prokaryotic names with standing in nomenclature |
| MEGA | Molecular evolutionary genetic analysis |
| MLSA | Multi-locus sequence analysis |
| MPN | Most probable number |
| OGRI | Overall general relatedness index |
| PCR | Polymerase chain reaction |
| RDA | Redundancy analysis |
| RNA | Ribonucleic acid |
| SDW | Shoot biomass |
| SPAD | Chlorophyll meter |
| TGx | Tropical glycine cross |
| USA | United States of America |
| YEM | Yeast-extract-mannitol |
| YMA | Yeast mannitol agar |
References
- Meghvansi, M.K.; Prasad, K.; Mahna, S.K. Symbiotic Potential, Competitiveness and Compatibility of Indigenous Bradyrhizobium japonicum Isolates to Three Soybean Genotypes of Two Distinct Agro-Climatic Regions of Rajasthan, India. Saudi. J. Biol. Sci. 2010, 17, 303–310. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qiu, L.-J.; Chang, R.-Z. The Origin and History of Soybean. In The Soybean Botany, Production and Uses; Singh, G., Ed.; CABI: London, UK, 2010; pp. 1–23. [Google Scholar]
- FAO. 2020. Available online: https://www.fao.org (accessed on 16 June 2025).
- Herridge, D.F.; Giller, K.E.; Jensen, E.S.; Peoples, M.B. Quantifying Country-to-Global Scale Nitrogen Fixation for Grain Legumes II. Coefficients, Templates and Estimates for Soybean, Groundnut and Pulses. Plant Soil. 2022, 474, 1–15. [Google Scholar] [CrossRef]
- Graham, P.H.; Vance, C.P. Legumes: Importance and Constraints to Greater Use. Plant Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Perret, X.; Staehelin, C.; Broughton, W.J. Molecular Basis of Symbiotic Promiscuity. Microbiol. Mol. Biol. Rev. 2000, 64, 180–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, S.; Tang, F.; Zhu, H. Symbiosis Specificity in the Legume—Rhizobial Mutualism. Cell. Microbiol. 2012, 14, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.M.; Lum, M.R.; Downie, J.A. What Makes the Rhizobia-Legume Symbiosis so Special? Plant Physiol. 2001, 127, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Simon, Z.; Mtei, K.; Gessesse, A.; Ndakidemi, P.A. Isolation and Characterization of Nitrogen Fixing Rhizobia from Cultivated and Uncultivated Soils of Northern Tanzania. Am. J. Plant Sci. 2014, 5, 4050–4067. [Google Scholar] [CrossRef]
- Snapp, S.; Rahmanian, M.; Batello, C.; Calles, T. Légumes Secs et Exploitations Durables en Afrique Subsaharienne; FAO: Rome, Italy, 2018. [Google Scholar]
- Fahde, S.; Boughribil, S.; Sijilmassi, B.; Amri, A. Rhizobia: A Promising Source of Plant Growth-Promoting Molecules and Their Non-Legume Interactions: Examining Applications and Mechanisms. Agriculture 2023, 13, 1279. [Google Scholar] [CrossRef]
- Velázquez, E.; Carro, L.; Flores-Félix, J.D.; Martínez-Hidalgo, P.; Menéndez, E.; Ramírez-Bahena, M.-H.; Mulas, R.; González-Andrés, F.; Martínez-Molina, E.; Peix, A. The Legume Nodule Microbiome: A Source of Plant Growth-Promoting Bacteria. In Probiotics and Plant Health; Kumar, V., Kumar, M., Sharma, S., Prasad, R., Eds.; Springer: Singapore, 2017; pp. 41–70. [Google Scholar] [CrossRef]
- Xavier, G.R.; Jesus Ede, C.; Dias, A.; Coelho, M.R.R.; Molina, Y.C.; Rumjanek, N.G. Contribution of Biofertilizers to Pulse Crops: From Single-Strain Inoculants to New Technologies Based on Microbiomes Strategies. Plants 2023, 12, 954. [Google Scholar] [CrossRef] [PubMed]
- Hungria, M.; Mendes, I.C. Nitrogen Fixation with Soybean: The Perfect Symbiosis? John Wiley & Sons: Hoboken, NJ, USA, 2015; Volume 2. [Google Scholar] [CrossRef]
- Thilakarathna, M.S.; Raizada, M.N. A Meta-Analysis of the Effectiveness of Diverse Rhizobia Inoculants on Soybean Traits under Field Conditions. Soil. Biol. Biochem. 2017, 105, 177–196. [Google Scholar] [CrossRef]
- Thuita, M.; Pypers, P.; Herrmann, L.; Okalebo, R.J.; Othieno, C.; Muema, E.; Lesueur, D. Commercial Rhizobial Inoculants Significantly Enhance Growth and Nitrogen Fixation of a Promiscuous Soybean Variety in Kenyan Soils. Biol. Fertil. Soils 2012, 48, 87–96. [Google Scholar] [CrossRef]
- Desta, M.; Akuma, A.; Minay, M.; Yusuf, Z.; Baye, K. Effects of Indigenous and Commercial Rhizobia on Growth and Nodulation of Soybean (Glycine max L.) under Greenhouse Condition. Open Biotechnol. J. 2023, 17, 1–8. [Google Scholar] [CrossRef]
- Checcucci, A.; DiCenzo, G.C.; Bazzicalupo, M.; Mengoni, A. Trade, Diplomacy, and Warfare: The Quest for Elite Rhizobia Inoculant Strains. Front. Microbiol. 2017, 8, 2207. [Google Scholar] [CrossRef] [PubMed]
- Onishchuk, O.P.; Vorobyov, N.I.; Provorov, N.A. Nodulation Competitiveness of Nodule Bacteria: Genetic Control and Adaptive Significance: Review. Appl. Biochem. Microbiol. 2017, 53, 131–139. [Google Scholar] [CrossRef]
- Alves, B.J.R.; Boddey, R.M.; Urquiaga, S. The Success of BNF in Soybean in Brazil. Plant Soil. 2003, 252, 1–9. [Google Scholar] [CrossRef]
- Vinuesa, P.; Rojas-Jiménez, K.; Contreras-Moreira, B.; Mahna, S.K.; Prasad, B.N.; Moe, H.; Selvaraju, S.B.; Thierfelder, H.; Werner, D. Multilocus Sequence Analysis for Assessment of the Biogeography and Evolutionary Genetics of Four Bradyrhizobium Species That Nodulate Soybeans on the Asiatic Continent. Appl. Environ. Microbiol. 2008, 74, 6987–6996. [Google Scholar] [CrossRef] [PubMed]
- Nakei, M.D.; Venkataramana, P.B.; Ndakidemi, P.A. Soybean-Nodulating Rhizobia: Ecology, Characterization, Diversity, and Growth Promoting Functions. Front. Sustain. Food Syst. 2022, 6, 824444. [Google Scholar] [CrossRef]
- Parte, A.C.; Carbasse, J.S.; Meier-Kolthoff, J.P.; Reimer, L.C.; Göker, M. List of Prokaryotic Names with Standing in Nomenclature (LPSN) Moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef] [PubMed]
- Avontuur, J.R.; Palmer, M.; Beukes, C.W.; Chan, W.Y.; Coetzee, M.P.A.; Blom, J.; Stępkowski, T.; Kyrpides, N.C.; Woyke, T.; Shapiro, N.; et al. Genome-Informed Bradyrhizobium Taxonomy: Where to from Here? Syst. Appl. Microbiol. 2019, 42, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Ormeño-Orrillo, E.; Martínez-Romero, E. A Genomotaxonomy View of the Bradyrhizobium Genus. Front. Microbiol. 2019, 10, 1334. [Google Scholar] [CrossRef] [PubMed]
- Menna, P.; Barcellos, F.G.; Hungria, M. Phylogeny and Taxonomy of a Diverse Collection of Bradyrhizobium Strains Based on Multilocus Sequence Analysis of the 16S RRNA Gene, ITS Region and GlnII, RecA, AtpD and DnaK Genes. Int. J. Syst. Evol. Microbiol. 2009, 59, 2934–2950. [Google Scholar] [CrossRef] [PubMed]
- Fossou, R.K.; Ziegler, D.; Zézé, A.; Barja, F.; Perret, X. Two Major Clades of Bradyrhizobia Dominate Symbiotic Interactions with Pigeonpea in Fields of Côte d’Ivoire. Front. Microbiol. 2016, 7, 01793. [Google Scholar] [CrossRef] [PubMed]
- Martens, M.; Dawyndt, P.; Coopman, R.; Gillis, M.; De Vos, P.; Willems, A. Advantages of Multilocus Sequence Analysis for Taxonomic Studies: A Case Study Using 10 Housekeeping Genes in the Genus Ensifer (Including Former Sinorhizobium). Int. J. Syst. Evol. Microbiol. 2008, 58, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Klepa, M.S.; Helene, L.C.F.; O’hara, G.; Hungria, M. Bradyrhizobium cenepequi sp. nov., Bradyrhizobium semiaridum sp. nov., Bradyrhizobium hereditatis sp. nov. and Bradyrhizobium australafricanum sp. nov., Symbionts of Different Leguminous Plants of Western Australia and South Africa and Definition of Three. Int. J. Syst. Evol. Microbiol. 2022, 72, 5446. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Romero, E.; Peix, A.; Hungria, M.; Mousavi, S.A.; Martinez-Romero, J.; Young, P. Guidelines for the Description of Rhizobial Symbiovars. Int. J. Syst. Evol. Microbiol. 2024, 74, 006373. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Rainey, F.A. Integrating Genomics into the Taxonomy and Systematics of the Bacteria and Archaea. Int. J. Syst. Evol. Microbiol. 2014, 64 Pt 2, 316–324. [Google Scholar] [CrossRef] [PubMed]
- FAO. FAO.STAT. Available online: https://www.fao.org/statistics/en/ (accessed on 6 June 2025).
- Osunde, A.O.; Gwam, S.; Bala, A.; Sanginga, N.; Okogun, J.A. Responses to Rhizobial Inoculation by Two Promiscuous Soybean Cultivars in Soils of the Southern Guinea Savanna Zone of Nigeria. Biol. Fertil. Soils 2003, 37, 274–279. [Google Scholar] [CrossRef]
- Pule-Meulenberg, F.; Gyogluu, C.; Naab, J.; Dakora, F.D. Symbiotic N Nutrition, Bradyrhizobial Biodiversity and Photosynthetic Functioning of Six Inoculated Promiscuous-Nodulating Soybean Genotypes. J. Plant Physiol. 2011, 168, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Abaidoo, R.C.; Keyser, H.H.; Singleton, P.W.; Dashiell, K.E.; Sanginga, N. Population Size, Distribution, and Symbiotic Characteristics of Indigenous Bradyrhizobium spp. That Nodulate TGx Soybean Genotypes in Africa. Appl. Soil Ecol. 2007, 35, 57–67. [Google Scholar] [CrossRef]
- Tefera, H. Breeding for Promiscuous Soybeans at IITA. In Soybean—Molecular Aspects of Breeding; IITA: Ibadan, Nigeria, 2011. [Google Scholar] [CrossRef]
- Klogo, P.; Ofori, J.K.; Klogo, P.Y.; Amaglo, H. Soybean (Glycine max (L.) Merill) Promiscuity Reaction to Indigenous Bradyrhizobia Inoculation in Some Ghanaian Soils. Int. J. Sci. Technol. Res. 2015, 4, 306–313. [Google Scholar]
- Giller, K.E. Nitrogen Fixation in Tropical Cropping Systems; CABI Publishing: Wallingford, UK, 2001. [Google Scholar] [CrossRef]
- N’gbesso, M.F.; N’guetta, A.S.P.; Kouamé, N.; Bi, K.F. Evaluation de l’efficience de l’inoculation Des Semences Chez 11 Génotypes de Soja (Glycine max L. Merril) En Zone de Savane de Côte d’Ivoire. Sci. Nat. 2010, 7. [Google Scholar] [CrossRef]
- Kouamé, N.C.; Fondio, L.; Djidji, A.H.; N’Gbesso, M.F.P. Rapport d’activités de Recherche Pour Le Développement de La Culture Du Soja Dans Les Zones Centre et Centre-Nord de La Côte d’Ivoire. In Convention CNRA-Projet Pacil; African Development Bank Group: Abidjan, Côte d’Ivoire, 2002; 105p. [Google Scholar]
- Niste, M.; Vidican, R.; Pop, R.; Rotar, I. Stress Factors Affecting Symbiosis Activity and Nitrogen Fixation by Rhizobium Cultured In Vitro. ProEnvironment 2013, 6, 42–45. [Google Scholar]
- Singleton, P.W.; Bohlool, B.B.; Nakao, P.L. Legume Response to Rhizobial Inoculation in the Tropics: Myths and Realities. Myth. Sci. Soils Trop. 2015, 29, 135–155. [Google Scholar] [CrossRef]
- Amani, K.; Fondio, L.; Ibrahim, K.; DPN’Gbesso, M.F.; AMaxwell, B.G.; Abiba Sanogo, T.; Filali-Maltouf, A. Response of Indigenous Rhizobia to the Inoculation of Soybean [Glycine max (L.) Merrill] Varieties Cultivated under Controlled Conditions in Côte d’Ivoire. Adv. Microbiol. 2020, 10, 110–122. [Google Scholar] [CrossRef]
- Amani, K.; Yao, G.F.; Fondio, L.; N’Gbesso, M.F.D.P.; Zro, F.B.; Kouamé, C.; Konaté, I. Effects of Native Rhizobia on Soybean [Glycine max (L.) Merrill] Production and Soil Properties in Daloa, Center-West of Côte d’Ivoire. Int. J. Plant Soil Sci. 2024, 36, 239–247. [Google Scholar] [CrossRef]
- Ducroquet, H.; Tillie, P.; Louhichi, K.; Gomez-y-Paloma, S. L’agriculture de La Côte d’Ivoire à La Loupe: Etats Des Lieux Des Filières de Production Végétales et Animales et Revue Des Politiques Agricoles; European Commissions: Brussels, Belgium, 2017; ISBN 978-92-79-73180-8. [Google Scholar] [CrossRef]
- FAO. État Des Ressources Phytogénétiques Pour l’alimentation et l’agriculture: Second Rapport National; FAO: Rome, Italy, 2009; p. 64.
- Gautier, L. Contact Forêt-Savane En Côte d’ivoire Centrale: Évolution de La Surface Forestière de La Réserve de Lamto (Sud Du V-Baoulé). Bull. La Soc. Bot. France. Actual. Bot. 1989, 136, 85–92. [Google Scholar] [CrossRef]
- Amon, C.E.R.; Fossou, R.K.; Ebou, A.E.T.; Koua, D.K.; Kouadjo, C.G.; Brou, Y.C.; Voko Bi, D.R.R.; Cowan, D.A.; Zézé, A. The Core Bacteriobiome of Côte d’Ivoire Soils across Three Vegetation Zones. Front. Microbiol. 2023, 14, 1220655. [Google Scholar] [CrossRef] [PubMed]
- Gnangui, S.L.E.; Fossou, R.K.; Ebou, A.; Amon, C.E.R.; Koua, D.K.; Kouadjo, C.G.Z.; Cowan, D.A.; Zézé, A. The Rhizobial Microbiome from the Tropical Savannah Zones in Northern Côte d’Ivoire. Microorganisms 2021, 9, 1842. [Google Scholar] [CrossRef] [PubMed]
- Cowan, D.; Lebre, P.; Amon, C.; Becker, R.; Boga, H.; Boulangé, A.; Chiyaka, T.; Coetzee, T.; de Jager, P.; Dikinya, O.; et al. Biogeographical Survey of Soil Microbiomes across Sub-Saharan Africa: Structure, Drivers, and Predicted Climate-Driven Changes. Microbiome 2022, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Mehlich, A. Mehlich 3 Soil Test Extractant: A Modification of Mehlich 2 Extractant, Communications in Soil Science and Plant Analysis. Commun. Soil. Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer Method Improved for Making Particle Size Analyses of Soils 1. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Bremner, M. Chapter 37: Nitrogen-Total. In Methods of Soil Analysis Part 3; Chemical Methods-SSSA Book Series 5; John Wiley & Sons: Hoboken, NJ, USA, 1996; Number 5; pp. 1085–1121. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Lewin, A.; Cervantes, E.; Wong, C.H.; Broughton, W.J. nodSU, Two New nod Genes of the Broad Host Range Rhizobium Strain NGR234 Encode Host-Specific Nodulation of the Tropical Tree Leucaena leucocephala. Mol. Plant-Microbe Interact. 1990, 3, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, D.W.; Atkinson, E.M.; Long, S.R. Depolarization of Alfalfa Root Hair Membrane Potential by Rhizobium meliloti Nod Factors. Science 1992, 256, 998–1000. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.M. A Manual for the Practical Study of Root Nodule Bacteria. In International Biological Program Handbook; John Wiley & Sons: Hoboken, NJ, USA, 1970; Volume 15. [Google Scholar]
- Sy, A.; Giraud, E.; Jourand, P.; Garcia, N.; Willems, A.; de Lajudie, P.; Prin, Y.; Neyra, M.; Gillis, M.; Boivin-Masson, C.; et al. Methylotrophic Methylobacterium Bacteria Nodulate and Fix Nitrogen in Symbiosis with Legumes. J. Bacteriol. 2001, 183, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Willems, A.; Coopman, R.; Gillis, M. Phylogenetic and DNA-DNA Hybridization Analyses of Bradyrhizobium Species. Int. J. Syst. Evol. Microbiol. 2001, 51, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Vinuesa, P.; Silva, C.; Werner, D.; Martínez-Romero, E. Population Genetics and Phylogenetic Inference in Bacterial Molecular Systematics: The Roles of Migration and Recombination in Bradyrhizobium Species Cohesion and Delineation. Mol. Phylogenet. Evol. 2005, 34, 29–54. [Google Scholar] [CrossRef] [PubMed]
- Nzoué, A.; Miché, L.; Klonowska, A.; Laguerre, G.; de Lajudie, P.; Moulin, L. Multilocus Sequence Analysis of Bradyrhizobia Isolated from Aeschynomene Species in Senegal. Syst. Appl. Microbiol. 2009, 32, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Sterner, J.P.; Parker, M.A. Diversity and Relationships of Bradyrhizobia from Amphicarpaea bracteata Based on Partial Nod and Ribosomal Sequences. Syst. Appl. Microbiol. 1999, 22, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, G. Estimating the Dimension of a Model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Eren, A.M.; Maignien, L.; Sul, W.J.; Murphy, L.G.; Grim, S.L.; Morrison, H.G.; Sogin, M.L. Oligotyping: Differentiating between Closely Related Microbial Taxa Using 16S RRNA Gene Data. Methods Ecol. Evol. 2013, 4, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Bromfield, E.S.P.; Cloutier, S.; Wasai-Hara, S.; Minamisawa, K. Strains of Bradyrhizobium barranii sp. nov. Associated with Legumes Native to Canada Are Symbionts of Soybeans and Belong to Different Subspecies (subsp. Barranii subsp. nov. and subsp. Apii subsp. nov.) and Symbiovars (sv. Glycinearum and sv. Septentrion). Int. J. Syst. Evol. Microbiol. 2022, 72, 5549. [Google Scholar] [CrossRef] [PubMed]
- Waswa, M.N.; Karanja, N.K.; Woomer, P.L.; Mwenda, G.M. Identifying Elite Rhizobia for Soybean (Glycine max) in Kenya 1. Afr. J. Crop Sci. 2014, 2, 60–66. [Google Scholar]
- Woomer, P.L. Most Probable Number Counts. In Methods of Soil Analysis, Part 2: Microbiological and Biochemical Properties; Bottomley, P.J., Angle, J.S., Weaver, R.W., Eds.; Wiley: Hoboken, NJ, USA, USA, 1994. [Google Scholar]
- Tabaro, A.R. Evaluation of Effectiveness of Rhizobia Isolates from Rwandan. Ph.D. Dissertation, University of Nairobi, Nairobi, Kenya, 2014. [Google Scholar]
- Woomer, P.L. A Ranking System for Legume Root Nodules. N2Africa Training Report. Technical Training. Available online: https://n2africa.org/ranking-system-legume-root-nodules-n2africa-training-report (accessed on 3 April 2014).
- Sebastien Le Julie Josse, F.H. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.M.F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses, R Package Version 1.0.7; GESIS: Mannheim, Germany, 2020.
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package, R Package Version 2.6-6.1; GESIS: Mannheim, Germany, 2024.
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Royston, J.P. An Extension of Shapiro and Wilk’s W Test for Normality to Large Samples. Appl. Stat. 1982, 31, 115. [Google Scholar] [CrossRef]
- Hollander, M.; Wolfe, D.A. Nonparametric Statistical Methods; Wiley: New York, NY, USA, 1973. [Google Scholar]
- Jordan, D.C. Transfer of Rhizobium japonicum Buchanan 1980 to Bradyrhizobium gen. nov., a Genus of Slow-Growing, Root Nodule Bacteria from Leguminous Plants. Int. J. Syst. Bacteriol. 1982, 32, 136–139. [Google Scholar] [CrossRef]
- Jordan, D.C. Genus I. Rhizobium. In Bergey’s Manual of Systematic Bacteriology, 1st ed.; Krieg, N.R., Holt, J.G., Eds.; The Williams & Wilkins Co.: Baltimore, MD, USA, 1984; Volume 1, pp. 235–242. [Google Scholar]
- Chibeba, A.M.; Kyei-Boahen, S.; de Guimarães, M.F.; Nogueira, M.A.; Hungria, M. Isolation, Characterization and Selection of Indigenous Bradyrhizobium Strains with Outstanding Symbiotic Performance to Increase Soybean Yields in Mozambique. Agric. Ecosyst. Environ. 2017, 246, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Brockwell, J.; Bottomley, P.J.; Thies, J.E. Manipulation of Rhizobia Microflora for Improving Legume Productivity and Soil Fertility: A Critical Assessment. In Management of Biological Nitrogen Fixation for the Development of More Productive and Sustainable Agricultural System; Ladha, J.K., Peoples, M.B., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 143–180. [Google Scholar]
- Okogun, J.A.; Sanginga, N. Can Introduced and Indigenous Rhizobial Strains Compete for Nodule Formation by Promiscuous Soybean in the Moist Savanna Agroecological Zone of Nigeria? Biol. Fertil. Soils 2003, 38, 26–31. [Google Scholar] [CrossRef]
- Kyei-Boahen, S.; Savala, C.E.N.; Muananamuale, C.P.; Malita, C.; Wiredu, A.N.; Chibeba, A.M.; Elia, P.; Chikoye, D. Symbiotic Effectiveness of Bradyrhizobium Strains on Soybean Growth and Productivity in Northern Mozambique. Front. Sustain. Food Syst. 2023, 6, 1084745. [Google Scholar] [CrossRef]
- Amani, K.; Konate, I.; N’gbesso, D.P.; François, M.; Attien, Y.P.; Fondio, L.; Abdelkarim Filali, M.; Tidou Abiba, S. Phenotypic and Symbiotic Diversity of Rhizobia Isolated from Root Nodules of Soybean [Glycine max (L.) Merrill] in Côte d’Ivoire. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 766–774. [Google Scholar] [CrossRef]
- Fossou, R.K.; Pothier, J.F.; Zézé, A.; Perret, X. Bradyrhizobium ivorense sp. nov. as a Potential Local Bioinoculant for Cajanus cajan Cultures in Côte d’ivoire. Int. J. Syst. Evol. Microbiol. 2020, 70, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- N’Zoué, A. Diversité Génétique et Fonctionnelle Des Souches de Bradyrhizobium Impliquées Dans Les Cultures Mixtes Niébé-Soja-Arachide/Céréales (Maïs) En Côte d’Ivoire. Ph.D. Dissertation, Université des Sciences et Techniques de Montpellier 2, Montpellier, France, 2008. [Google Scholar]
- Gnangui, S.; Kouagdo, C.; Nat, A.Z.-M. First Report of Rhizobium Pusense within Voandzou (Vigna subterranea (L.) Verdc.) Rhizosphere in Côte d’Ivoire. Microbiol. Nat. 2019, 1, 55–65. [Google Scholar]
- Raissa, G.N.K.; Ibrahim, K.; Kaoutar, T.; Issouf, B.; Maxwell, B.G.A.; Sélastique, A.D.; Maltouf Abdelkarim, F. Molecular and Symbiotic Efficiency Characterization of Rhizobia Nodulating Bambara Groundnut (Vigna subterranea L.) from Agricultural Soils of Daloa Localities in Côte D’Ivoire. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 507–519. [Google Scholar] [CrossRef]
- Atse, M.-P.A.; N’gbesso, M.F.D.P.; Abe, A.I.; Coulibaly, N.D.; Yeo, K.T.; Butare, L.; Konate, I. Diversity and Phylogeny of Symbiotic Bacteria Nodulating Common Bean (Phaseolus vulgaris L.) in Côte d’Ivoire. Microbiol. Res. J. Int. 2024, 34, 45–53. [Google Scholar] [CrossRef]
- Willems, A.; Munive, A.; De Lajudie, P.; Gillis, M. In Most Bradyrhizobium Groups Sequence Comparison of 16S-23S RDNA Internal Transcribed Spacer Regions Corroborates DNA-DNA Hybridizations. Syst. Appl. Microbiol. 2003, 26, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Saeki, Y.; Kaneko, A.; Hara, T.; Suzuki, K.; Yamakawa, T.; Minh, T.N.; Nagatomo, Y.; Akao, S. Phylogenetic Analysis of Soybean-Nodulating Rhizobia Isolated from Alkaline Soils in Vietnam. Soil Sci. Plant Nutr. 2005, 51, 1043–1052. [Google Scholar] [CrossRef][Green Version]
- Wang, M.; Cao, B.; Yu, Q.; Liu, L.; Gao, Q.; Wang, L.; Feng, L. Analysis of the 16S-23S RRNA Gene Internal Transcribed Spacer Region in Klebsiella Species. J. Clin. Microbiol. 2008, 46, 3555–3563. [Google Scholar] [CrossRef] [PubMed]
- Puozaa, D.K.; Jaiswal, S.K.; Dakora, F.D. African Origin of Bradyrhizobium Populations Nodulating Bambara Groundnut (Vigna subterranea L. Verdc) in Ghanaian and South African Soils. PLoS ONE 2017, 12, 184943. [Google Scholar] [CrossRef] [PubMed]
- Asfaw, B.; Aserse, A.A.; Asefa, F.; Yli-Halla, M.; Lindström, K. Genetically Diverse Lentil- and Faba Bean-Nodulating Rhizobia Are Present in Soils across Central and Southern Ethiopia. FEMS Microbiol. Ecol. 2020, 96, fiaa015. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Oguro, H.; Yamakawa, T.; Yamamoto, A.; Akao, S.; Saeki, Y. Diversity and Distribution of Indigenous Soybean-Nodulating Rhizobia in the Okinawa Islands, Japan. Soil Sci. Plant Nutr. 2008, 54, 237–246. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Li, Y.; Chen, W.F.; Wang, E.T.; Tian, C.F.; Li, Q.Q.; Zhang, Y.Z.; Sui, X.H.; Chen, W.X. Biodiversity and Biogeography of Rhizobia Associated with Soybean Plants Grown in the North China Plain. Appl. Environ. Microbiol. 2011, 77, 6331–6342. [Google Scholar] [CrossRef] [PubMed]
- Wasike, V.W.; Lesueur, D.; Wachira, F.N.; Mungai, N.W.; Mumera, L.M.; Sanginga, N.; Mburu, H.N.; Mugadi, D.; Wango, P.; Vanlauwe, B. Genetic Diversity of Indigenous Bradyrhizobium Nodulating Promiscuous Soybean [Glycine max (L.) Merr.] Varieties in Kenya: Impact of Phosphorus and Lime Fertilization in Two Contrasting Sites. Plant Soil 2009, 322, 151–163. [Google Scholar] [CrossRef]
- Gyogluu, C.; Jaiswal, S.K.; Kyei-Boahen, S.; Dakora, F.D. Identification and Distribution of Microsymbionts Associated with Soybean Nodulation in Mozambican Soils. Syst. Appl. Microbiol. 2018, 41, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.S.; Figueredo, A.; Pedrosa, F.O.; Hungria, M. Genetic Characterization of Soybean Rhizobia in Paraguay. Appl. Environ. Microbiol. 2000, 66, 5099–5103. [Google Scholar] [CrossRef] [PubMed]
- Shiro, S.; Matsuura, S.; Saiki, R.; Sigua, G.C.; Yamamoto, A.; Umehara, Y.; Hayashi, M.; Saeki, Y. Genetic Diversity and Geographical Distribution of Indigenous Soybean-Nodulating Bradyrhizobia in the United States. Appl. Environ. Microbiol. 2013, 79, 3610–3618. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Wang, E.T.; Zhang, Y.Z.; Zhang, Y.M.; Tian, C.F.; Sui, X.H.; Chen, W.F.; Chen, W.X. Diversity and Biogeography of Rhizobia Isolated from Root Nodules of Glycine Max Grown in Hebei Province, China. Microb. Ecol. 2011, 61, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Maruekarajtinplenga, S.; Homhaulb, W.; Chansa-Ngavej, K. Presence of Natural Variants of Bradyrhizobium elkanii and Bradyrhizobium japonicum and Detection of Bradyrhizobium yuanmingense in Phitsanulok Province, Thailand. Sci. Asia 2012, 38, 24–29. [Google Scholar] [CrossRef]
- Kozieł, M.; Kalita, M.; Janczarek, M. Genetic Diversity of Microsymbionts Nodulating Trifolium Pratense in Subpolar and Temperate Climate Regions. Sci. Rep. 2022, 12, 12144. [Google Scholar] [CrossRef] [PubMed]
- Martens, M.; Delaere, M.; Coopman, R.; de Vos, P.; Gillis, M.; Willems, A. Multilocus Sequence Analysis of Ensifer and Related Taxa. Int. J. Syst. Evol. Microbiol. 2007, 57, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Bahena, M.H.; Flores-Félix, J.D.; Chahboune, R.; Toro, M.; Velázquez, E.; Peix, A. Bradyrhizobium centrosemae (Symbiovar Centrosemae) sp. nov., Bradyrhizobium americanum (Symbiovar Phaseolarum) sp. nov. and a New Symbiovar (Tropici) of Bradyrhizobium viridifuturi Establish Symbiosis with Centrosema Species Native to America. Syst. Appl. Microbiol. 2016, 39, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Delamuta, J.R.M.; Ribeiro, R.A.; Ormeño-Orrillo, E.; Parma, M.M.; Melo, I.S.; Martínez-Romero, E.; Hungria, M. Bradyrhizobium tropiciagri sp. nov. and Bradyrhizobium embrapense sp. nov. Nitrogenfixing Symbionts of Tropical Forage Legumes. Int. J. Syst. Evol. Microbiol. 2015, 65, 4424–4433. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.T.; Patrick, H.N.; Lowther, W.L.; Scott, D.B.; Ronson, C.W. Nodulating Strains of Rhizobium Loti Arise through Chromosomal Symbiotic Gene Transfer in the Environment. Proc. Natl. Acad. Sci. USA 1995, 92, 8985–8989. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.T.; Ronson, C.W. Evolution of Rhizobia by Acquisition of a 500-Kb Symbiosis Island That Integrates into a Phe-TRNA Gene. Proc. Natl. Acad. Sci. USA 1998, 95, 5145–5149. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, J.P.; Sullivan, J.T.; Stuart, G.S.; Lamont, I.L.; Ronson, C.W. Excision and Transfer of the Mesorhizobium loti R7A Symbiosis Island Requires an Integrase IntS, a Novel Recombination Directionality Factor RdfS, and a Putative Relaxase RlxS. Mol. Microbiol. 2006, 62, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Nandasena, K.G.; O’Hara, G.W.; Tiwari, R.P.; Sezmiş, E.; Howieson, J.G. In Situ Lateral Transfer of Symbiosis Islands Results in Rapid Evolution of Diverse Competitive Strains of Mesorhizobia Suboptimal in Symbiotic Nitrogen Fixation on the Pasture Legume Biserrula pelecinus L. Environ. Microbiol. 2007, 9, 2496–2511. [Google Scholar] [CrossRef] [PubMed]
- Barcellos, F.G.; Menna, P.; Batista, J.S.D.S.; Hungria, M. Evidence of Horizontal Transfer of Symbiotic Genes from a Bradyrhizobium japonicum Inoculant Strain to Indigenous Diazotrophs Sinorhizobium (Ensifer) fredii and Bradyrhizobium elkanii in a Brazilian Savannah Soil. Appl. Environ. Microbiol. 2007, 73, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Okubo, T.; Fukushima, S.; Minamisawa, K. Evolution of Bradyrhizobium-Aeschynomene Mutualism: Living Testimony of the Ancient World or Highly Evolved State? Plant Cell Physiol. 2012, 53, 2000–2007. [Google Scholar] [CrossRef] [PubMed]
- Masson-Boivin, C.; Giraud, E.; Perret, X.; Batut, J. Establishing Nitrogen-Fixing Symbiosis with Legumes: How Many Rhizobium Recipes? Trends Microbiol. 2009, 17, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Jean-Claude, N.Z.; Patrice, K.A.; Daouda, K.K.; Jane, K.; Jean-Luc, K.; Christophe, K. Effect of Inoculating Seeds with Bradyrhizobium japonicum on the Agronomic Performance of Five Varieties of Soybean (Glycine max) in Côte d ’Ivoire. Afr. J. Agric. Res. 2015, 10, 3671–3677. [Google Scholar] [CrossRef]
- Cornell, C.; Kokkoris, V.; Richards, A.; Horst, C.; Rosa, D.; Bennett, J.A.; Hart, M.M. Do Bioinoculants Affect Resident Microbial Communities? A Meta-Analysis. Front. Agron. 2021, 3, 753474. [Google Scholar] [CrossRef]
| DNALocus | Primers | Size (bp) | Primer Sequences | Tm (°C) | Amplification Program | Reference |
|---|---|---|---|---|---|---|
| ITS | ITS SM ITS BR3 | 950 | 5′AAGTCGTAACAAGGTAGCC3′ 5′GCTTTTCACCTTTCCCTCAC3′ | 55 | 5 min 96 °C, 35 X (30 s 96 °C, 30 s 55 °C, 2 min), 7 min 72 °C | [59] |
| glnII | glnII 12F glnII 689R | 600 | 5′YAAGCTCGAGTACATYTGGCT3′ 3′TGCATGCCSGAGCCGTTCCA5′ | 63 | 5 min 95 °C, 35 X (45 s 94 °C, 1 min 63 °C, 1 min 30 s 72 °C, 7 min 72° C) | [60] |
| recA | recA 41F recA 640R | 500 | 5′TTCGGCAAGGGMTCGRTSATG3′ 3′ACATSACRCCGATCTTCATGC5′ | 58 | 5 min 95 °C, 35 X (45 s 94 °C, 1 min 58 °C, 1 min 30 s 72 °C), 7 min 72 °C | [60] |
| nifH | nifH-28F nifH-809R | 700 | 5′TACGGNAARGGSGGNATCGGCAA3′ 3′AGCATGTCYTCSAGYYTCNTCCA5′ | 65, 55 | 5 min 94 °C, 20 X (45 s 94 °C, 30 s 65 °C, 1 min 30 s 72 °C), 25 X (45 s 94 °C, 30 s 55 °C, 1 min 30 s 72 °C), 7 min 72 °C | [61] |
| nodC | nodCIf nodCp8 | 243 | 5′GTCGATTGCMRGTCAAGACTACG3′ 3′GCCAGGTCTlGTTGCGATTGCTC5′ | 57 | 5 min 95 °C, 35 X [45 s 94 °C, 1 min 57 °C, 1 min 30 s 72 °C], 7 min 72 °C | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akaffou, M.A.; Fossou, R.K.; Ebou, A.E.T.; Kouadjo-Zézé, Z.G.C.; Amon, C.E.R.-E.; Chaintreuil, C.; Fall, S.; Zézé, A. Phylogenetic Diversity and Symbiotic Effectiveness of Bradyrhizobium Strains Nodulating Glycine max in Côte d’Ivoire. Agronomy 2025, 15, 1720. https://doi.org/10.3390/agronomy15071720
Akaffou MA, Fossou RK, Ebou AET, Kouadjo-Zézé ZGC, Amon CER-E, Chaintreuil C, Fall S, Zézé A. Phylogenetic Diversity and Symbiotic Effectiveness of Bradyrhizobium Strains Nodulating Glycine max in Côte d’Ivoire. Agronomy. 2025; 15(7):1720. https://doi.org/10.3390/agronomy15071720
Chicago/Turabian StyleAkaffou, Marie Ange, Romain Kouakou Fossou, Anicet Ediman Théodore Ebou, Zaka Ghislaine Claude Kouadjo-Zézé, Chiguié Estelle Raïssa-Emma Amon, Clémence Chaintreuil, Saliou Fall, and Adolphe Zézé. 2025. "Phylogenetic Diversity and Symbiotic Effectiveness of Bradyrhizobium Strains Nodulating Glycine max in Côte d’Ivoire" Agronomy 15, no. 7: 1720. https://doi.org/10.3390/agronomy15071720
APA StyleAkaffou, M. A., Fossou, R. K., Ebou, A. E. T., Kouadjo-Zézé, Z. G. C., Amon, C. E. R.-E., Chaintreuil, C., Fall, S., & Zézé, A. (2025). Phylogenetic Diversity and Symbiotic Effectiveness of Bradyrhizobium Strains Nodulating Glycine max in Côte d’Ivoire. Agronomy, 15(7), 1720. https://doi.org/10.3390/agronomy15071720








