Abstract
The Iridaceae family comprises approximately 1800 species, including Iris pallida Lam., which is widely recognized for its ornamental and aromatic properties and particularly adopted in the perfume industry. In this study, we evaluated the effects of planting density and maturity age on biomass production, morphological traits, rhizome biomass, and orris concrete yield in Iris pallida grown in Tuscany (Italy). The experiment consisted of four agricultural parcels, each one containing six plots arranged to test combinations of two planting densities (low density [LD], 8 plants/m2 and high density [HD], 15 plants/m2) and harvesting age (2, 3, and 4 years). Results indicated that planting density significantly influenced biomass variables—including rhizome, bud, and stem biomass—with the low planting density (LD) exhibiting higher total biomass (5.48 ± 0.59 kg/m2) compared to that observed under high planting density (HD) (1.82 ± 0.54 kg/m2). Orris concrete yield varied significantly across planting densities and harvesting age, consistently favoring LD (0.055 ± 0.01%) over HD (0.045 ± 0.01%). Also, orris concrete yield showed a positive correlation with floral stem number (r = 0.73, p < 0.001), root biomass (r = 0.66, p < 0.01) and floral stem biomass (r = 0.63, p < 0.01), while no significant correlations were found between orris concrete yield and total biomass or rhizome biomass. A shorter production cycle under low-density planting may improve orris concrete yield without compromising biomass productivity.
1. Introduction
The Iridaceae family comprises approximately 1800 species, with the genus Iris Lam. Tourn being the largest, encompassing over 260 species distributed worldwide, mainly in temperate zones [1]. Irises are perennial herbaceous plants cultivated primarily for their ornamental value due to their attractive flowers and low water requirements, making them suitable for home gardens [2]. Among these species, Iris germanica L. and Iris pallida are known for their aromatic properties, often adopted in the perfume industry [3]. These species are specifically employed to produce iris butter, also known as orris concrete, a solid compound extracted from iris rhizomes as an essential oil [4]. Orris concrete contains ketonic compounds providing a distinct violet-like fragrance, characterized as sweetly floral, warm, tenacious, and with a fruity undertone [5,6,7,8]. However, the high production costs of orris oil restricts its widespread use.
In Italy, orris rhizome cultivation is mainly performed for Iris pallida in Tuscany, with the main cultivation areas located in two distinct areas named Pratomagno and Chianti Fiorentino [9,10]. In this region, the utilization of rhizomes began in the mid-1800s, reaching significant production quantities due to the constant demand for rhizomes from French and Northern European companies for use in perfumery and distillation. Since the mid-1900s, there has been a progressive reduction in cultivated areas due to the crop’s high labor requirements, stone-rich terrain, damage inflicted by predators on crops, challenges in negotiating prices for dried rhizomes, and competition from synthetic products. Nowadays, the cultivation of Iris pallida is usually associated with main crop systems such as olive, grape and fruit trees, or at the edge of crop fields over dry-stone walls to create a green barrier for separating different cropping systems [11], reducing edge erosion processes and improving soil water retention [10,12]. Iris pallida is planted in late summer; the rhizome life cycle can last for several years but is usually harvested in a range of 2–4 years [10,12]. Harvesting occurs in summertime, so that the rhizomes can be sun-dried and then left to mature in burlap sacks for at least 36 months within dry and dark rooms [11]. This method is essential to convert the triterpenoid iridals—also known as cycloiridals—present in freshly harvested rhizomes into irones, through an oxidative degradation process. [13]. Thus, the whole production process of orris last between 6 and 8 years, resulting in a relatively high price of orris concrete which can reach several thousand euros per kg [11].
Over the years, several studies including biochemical, technological and agronomical approaches have been carried out to accelerate the production process of orris rhizome. For instance, different types of bacteria such as Rahnella aquatilis, Serratia liquefaciens and Pseudomonas maltophilia, have been tested to enhance rhizome maturation and promote the oxidative degradation of iridal precursors into irones [14,15,16]. However, the potential pathogenicity of these bacteria for human health limited their adoption in the process of orris concrete production as well as the related perfume industries [17,18,19]. Ieri et al. [20] investigated the chemical and pharmaceutical properties of Iris pallida, particularly focusing on approaches capable of improving iron extraction and orris concrete yield; Lucchesini et al. [2] explored the potential of micropropagation as an alternative to traditional rhizome division, while Meucci et al. [21] refined micropropagation techniques through somatic embryogenesis, enabling large-scale production of genetically uniform plants while preserving their phytochemical traits. Furthermore, Meucci et al. [21] suggested innovative acclimatization methods to improve Iris pallida seedlings’ resilience to salt stress and harsh environmental conditions.
Whilst in recent years the effect of agronomic techniques has been explored for different species of iris worldwide [22,23,24], only limited literature can be found for Iris pallida cultivation in Tuscany. For instance, Landi and Nicolelli [12] evaluated the effects of fertilization, weed control, and plant density on rhizome yield and plant development, while a more recent study by Pezzarossa et al. [10] analyzed the influence of soil and climatic conditions on rhizome quality. On these bases, this study aimed to evaluate the effects of two planting densities (low and high) and three maturity ages (2, 3 and 4 years old) on total biomass production, morphological traits, rhizome biomass and orris concrete yield of Iris pallida in the Chianti Fiorentino area (Tuscany, Italy).
2. Materials and Methods
2.1. Study Area
The experiment was conducted in a hilly area (i.e., 300 m a.s.l) at Greve in Chianti (43.58519° N, 11.31667° E, Italy), located within the historical Iris spp. cultivation area of Chianti. The test site consisted of four parcels, each approximately 60 m2, with homogeneous soil and climate characteristics (Figure 1).
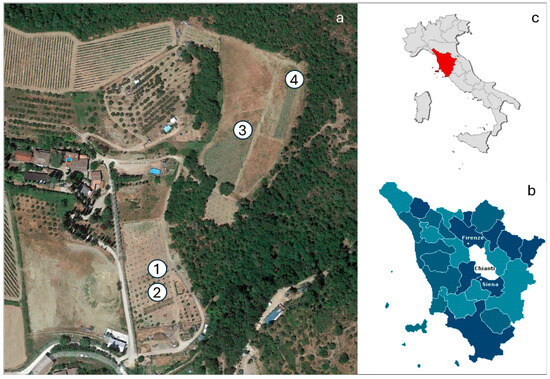
Figure 1.
Localization of: (a) aerial view of the test site located in Greve in Chianti (Tuscany, Italy), showing the four experimental parcels (1–4) used for testing different planting densities and harvesting ages of Iris pallida.; (b) the Chianti area in the Tuscany region; (c) position of Tuscany in Italy.
The soil, classified as Central Appenian Combisol, is mainly composed of alternating layers of sandstone and marl [25]. Soil analysis indicated a sandy texture (48.5% sand; 30.2% silt; 21.3% clay), a slightly acid soil (pH; 6.9) and electrical conductivity (EC) of 0.673 mS cm−1, medium cation exchange capacity (CEC; 10.1 meq 100 g−1), and a low organic matter content (OM; 1.15%). Further details of soil properties are reported in Table S1.
The meteorological conditions during the study years (2015–2019) showed a typical Mediterranean trend (Figure 2), with monthly minimum air temperatures recorded in winter (January, 2.7 ± 2.3 °C) and maximum in summer (July, 29.1 ± 0.9 °C). Total annual precipitation was mainly recorded in February (140.5 ± 64.1 mm) and November (160.1 ± 115.2 mm), with considerable variations among seasons and years (Figure S1; Table S2). Specifically, the annual cumulative precipitation was, on average, 964.4 mm, with the maximum (1218.4) and minimum (756) observed in 2019 and 2017, respectively (Table S2).
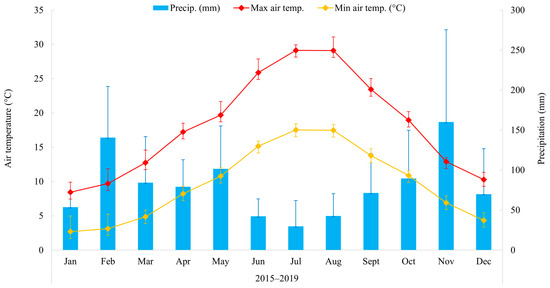
Figure 2.
Monthly average maximum (red line) and minimum (orange line) air temperatures, and precipitation (blue histograms) in the study area during for the period 2015–2019. Vertical bars represent monthly standard deviation between years.
2.2. Experimental Design
The experiment started in summer 2015 and consisted of four agricultural parcels (Figure 1). Each parcel contained six plots of 8 m2 (2 m × 4 m) (Figure 3), for a total of 24 plots. These plots were arranged to test combinations of two planting densities and three planting ages. The planting densities were categorized as low density (LD) with 8 plants/m2 and high density with 15 plants/m2, while the planting ages ranged from 2 to 4 years. Soil preparation involved ploughing to a depth of 20 cm followed by light harrowing at the beginning of August 2015, with orris planting taking place at the end of the same month. During the experiment, no fertilization or controlled water stress treatments were applied, aiming to replicate the traditional low-input and rain-fed cultivation practices historically associated with Iris pallida in the Chianti Fiorentino region. Plots were manually weeded in autumn (October) and spring (April) of the following year (2016) and were harvested in late July at the end of the second (2017), third (2018), and fourth (2019) years of cultivation. No irrigation was applied throughout the experiment. Biomass produced in each plot was collected and transported to the laboratory on the same day it was harvested.
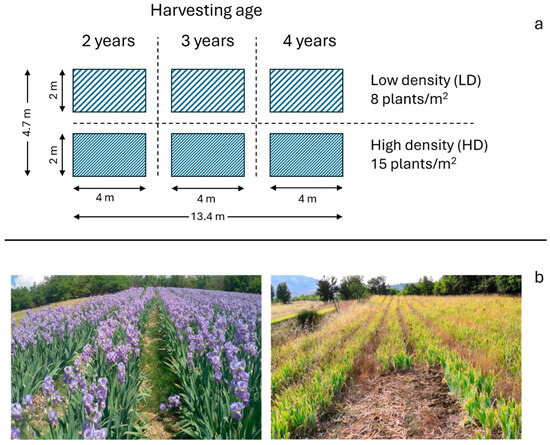
Figure 3.
Representation of (a) the experimental parcel with six plots, including two different planting densities and three harvesting ages; (b) a close-up of the experimental parcel during flowering (left) and harvesting (right).
2.3. Biomass Data
The sampled plants were first washed to remove any residuals, then weighed and divided into four parts: buds, rhizomes, floral stems, and roots. Each part was individually weighed to determine its fresh biomass. Additionally, measurements were taken for central leaf width and length, the number of buds and flowers per plant, dry rhizome biomass, and orris concrete yield. The rhizome biomass, which is the primary product of Iris cultivation, was then sliced and dried under shade conditions, resulting in approximately 70% loss of the fresh biomass weight over a period of 9 days. Then, the rhizomes collected from each plot were stored in a cool, dark, and dry environment (T° 15–20 °C) for 36 months to ensure maturation and slow oxidation of the iridial compounds to iron derivatives [22,26]. After the three-year storage period, the rhizomes from each plot were subjected to hydrodistillation using stainless steel distiller (Spring 12 L, Albrigi Luigi S.r.l., Grezzana, Italy). Three independent distillation replicates were performed for each plot, using approximately 100 g of dried rhizome per replicate. The amount of orris concrete obtained was measured and expressed as milligrams per 100 g of dry rhizome.
2.4. Orris Concrete Extraction
The dried rhizomes were ground into powder using a stainless steel electric spice grinder (Model 46074, LR Forniture, Italy), ensuring a uniform particle size suitable for hydrodistillation. For each plot, 1 kg of rhizome powder was collected and placed in seven liters (L) of deionized water for 24 h at room temperature. The resulting mixture was then hydrodistilled for six hours (h) using a commercial stainless steel essential oil distiller (Spring 12 L, Albrigi Luigi S.r.l., Grezzana, Italy) equipped with a 1600-watt (W) induction plate (model HA-INDUC-11, Konig, Ruhr area, Germany). The cooling circuit was built using two consecutive condensers. Instruments and methodological approach are described in Figure S2. The water temperature was set to 10 °C in the first condenser, while the temperature of the second condenser was maintained at 46 °C, the melting point of orris concrete [27], to keep the orris concrete in a liquid state. The orris concrete was finally recovered from the apparatus without the addition of any solvent. The yield of orris concrete (OC) was calculated using the following formula:
where is the yield of orris concrete (%); is the rhizome powder biomass, expressed in grams; is the specific gravity of orris concrete at 25 °C [27]; and is the volume of orris concrete at 25 °C, expressed in milliliters.
2.5. Statistical Analysis
Statistical analyses were performed using R Studio software (version 4.2.1) [28]. Prior to statistical analysis, data were tested for normality using the Shapiro-Wilk test [29] and for homogeneity of variances using Levene’s test [30]. Only datasets that met these assumptions were subjected to ANOVA or mixed linear models. Statistical significance was set at p < 0.05 for all analyses. A linear mixed model (LMM) was fitted using the lme4 package (version 1.1-37) [31]. Plant density (D) and planting age (H) were treated as fixed factors, while the four blocks (i.e., parcels) were considered as a random factor. Both main effects and interaction effects were examined. A post-hoc Tukey test with Bonferroni correction was applied for multiple comparisons among the levels of each factor, using the multcompView package (version 0.1.10) [32]. Pearson’s correlation test (p ≤ 0.05) was used to assess the relationships between all considered parameters, employing the bruceR package (version 2024.6) [33].
3. Results
3.1. Biomass
The LMM revealed that planting density had a significant effect on most biomass variables, with the exception of root biomass, where the effect was not statistically significant (p > 0.05; Table 1). Additionally, the harvesting age (H), for all biomass-related variables (p < 0.001) except for total biomass, had a moderately significant impact (p < 0.05). Concerning the interaction effect between planting density and harvesting age (D A), significant effects were observed for rhizome, flower, and root biomass (p < 0.05), whereas the interaction effect was not significant for total biomass and bud biomass (p > 0.05) (Table 1). Treatment effects were consistent and not influenced by spatial variability. In fact, the variance associated with the experimental plot (block), as shown in the last column of Table 1, was negligible or low for most traits, with only a moderate influence observed for total biomass and rhizome biomass.

Table 1.
Linear mixed model (LMM) results summarized for all considered parameters. Significance code: * p < 0.05. ** p < 0.01. *** p < 0.001, ns = not significant. The last column reports the variance associated with the plot (block) as a random effect, classified as negligible (≤0.05), low (≤0.15), moderate (≤0.30), or high (>0.30), based on variance estimates from the mixed-effects models.
Globally, LD provided highest values for all biomass variables compared to HD (Table 2). On average, total biomass was found to be more than three times higher under LD (5.48 ± 0.59 kg/m2) compared to HD (1.82 ± 0.54 kg/m2) (Table 2). Similarly, the biomasses of rhizomes and stems were more than two times higher under LD (1.27 ± 0.06 kg/m2 and 0.2 ± 0.04 kg/m2) than HD (0.60 ± 0.19 kg/m2 and 0.09 ± 0.04 kg/m2) (Table 2). A greater difference was observed for bud biomass, which was almost four times higher under LD (3.74 ± 0.62 kg/m2) than HD (0.95 ± 0.3 kg/m2), while the lowest difference was observed in root biomass (0.26 ± 0.05 under LD and 0.16 ± 0.02 under HD) (Table 2).

Table 2.
Total fresh biomass and compartments (kg/m2) in response to different plant densities (D) and harvest age (H). All values were reported as the average value of three replicates ± standard deviation. Different letters (a–d) indicate significant differences between treatments according to Tukey’s multiple-comparison test (p < 0.05).
Concerning inter-year changes, the highest values of total biomass were observed in the third and fourth year both under LD (6.26 ± 1.07 kg/m2 and 6.17 ± 0.34 kg/m2, respectively) and HD (2.4 ± 0.92 kg/m2) (Table 2). By contrast, lower values were observed in both planting density in the second year (3.99 ± 0.36 kg/m2 under LD and 1.09 ± 0.27 kg/m2 under HD) (Table 2). In both plant density conditions, the total biomass significantly increased over the years, reaching higher values in the fourth year compared to the second year (+58% in LD and +137% in HD). This pattern was observed also for the rhizome’s biomass under both planting densities, and for buds and floral stems under HD (Table 2). Specifically, rhizome biomass showed higher values in the fourth year both for LD (1.70 ± 0.10 kg/m2) and HD (0.82 ± 0.32 kg/m2), increasing by +122% and +155% compared to that observed in the second year (0.76 ± 0.05 kg/m2 under LD and 0.34 ± 0.08 kg/m2 under HD). The biomass of buds and floral stems showed higher values in the fourth year under HD (1.28 ± 0.56 kg/m2 and 0.14 ± 0.05 kg/m2, respectively, thus increasing by +129% and +109%) compared to the values observed in the second year (Table 2). By contrast, only the biomass of floral stems growth under LD showed an opposite trend, with the highest value recorded in the second year (0.26 ± 0.03 kg/m2) and the lowest in the fourth year (0.12 ± 0.03 kg/m2), resulting in a decrease of −54.5% (Table 2). Finally, the root biomass was globally lower under HD (0.16 ± 0.02 kg/m2) than LD (0.26 ± 0.05 kg/m2), with the highest values observed in the third year under both planting densities (i.e., LD = 0.32 ± 0.07 kg/m2; HD = 0.22 ± 0.02 kg/m2).
3.2. Morphological Traits
The LMM (Table 1) revealed significant effect of the planting density (D) on all the morphological and productive traits (p < 0.01) as well as the planting age (H). Concerning the interaction effect between age and planting density (D H), highly significant effects were observed for the number of floral stems and number of buds per plant (p < 0.001), whereas the interaction effect was not significant for dry rhizome biomass and the leaves length/width ratio (p > 0.05).
Concerning rhizome dry biomass, higher production was found on average under LD (0.38 ± 0.02 kg/m2) than HD (0.18 ± 0.05 kg/m2) (Table 3). The highest rhizome dry biomass production occurred in the fourth year under both LD (0.51 ± 0.03 kg/m2) and HD (0.24 ± 0.09 kg/m2), while the lowest occurred in the second year for both planting densities (Table 3).

Table 3.
Morphological traits and rhizome dry biomass in response to plant density and harvesting age. All values are reported as the average value of three replicates ± standard deviation. Different letters (a–d) indicate significant differences between treatments according to Tukey’s multiple-comparison test (p < 0.05).).
The floral stem number per plant followed a similar trend to floral biomass, with the highest value observed under low density (LD) in the second year (1.82 ± 0.09), while the lowest was observed in high density (HD) in the second year (0.15 ± 0.09) (Table 3). The trend of floral stem biomass varied over time, showing an opposite pattern between LD and HD from the second to the fourth year (−51% in LD; +150% in HD). Similarly, the number of buds per plant reflected the same pattern observed for bud biomass, with the highest bud count recorded under LD in the third year (61.42 ± 2.47), while the lowest in HD plots was during the second year (12.33 ± 2.78) (Table 3). Globally, bud number per plant showed a consistent increase over time, rising by 56% in LD and 129% in HD between the second and third years (Table 3).
Concerning leaf morphology, the leaf length-to-width ratio under LD conditions reached its peak in the third year (19.74 ± 0.25), whereas all HD treatments exhibited significantly lower values (ranging from 13.87 ± 0.76 to 14.59 ± 0.36). Notably, under LD, this ratio varied significantly across harvesting age, whereas no substantial differences were detected among HD treatments (Table 3).
3.3. Orris Concrete Productivity
According to LMM (Table 2), orris concrete showed a significant response to both planting density (D) and harvesting age (H), as well as their interaction (p < 0.001).
Orris concrete yield varied significantly across planting densities and harvesting age (Figure 4). Under low density (LD), the highest orris concrete yield was observed in the second year (0.062 ± 0.001%), while the lowest yield occurred in the fourth year (0.044 ± 0.002%). Under high density (HD), the highest yield was recorded in the third year (0.05 ± 0.002%), while the lowest was observed in the second year (0.038 ± 0.008%). Over time, LD showed a decreasing trend (−29%), whereas HD exhibited a gradual increase from the second to the third year (+25%) and a slight reduction from the third to the fourth year (−5%). On average, orris concrete yield under LD (0.055 ± 0.01%) consistently outperformed that under HD (0.045 ± 0.01%).
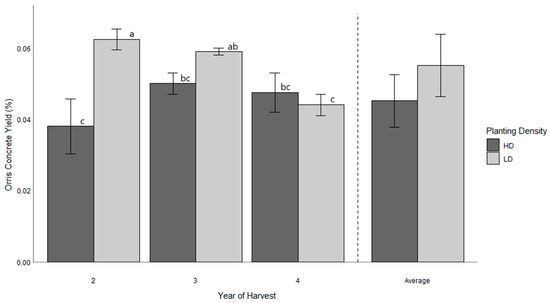
Figure 4.
Histograms of orris concrete yield in response to plant density (LD and HD) and harvesting age (2, 3, 4 and average). The vertical dashed line separates yearly data (left) from the overall average (right). Different letters (a–c) indicate significant differences between treatments according to Tukey’s multiple-comparison test (p < 0.05).
Correlation analysis between orris concrete yield and the measured variables (Table S3) revealed significant associations with several biomass and morphological traits. Orris concrete yield showed a strong positive correlation with floral stem number (r = 0.73, p < 0.001) and moderate correlations with root biomass (r = 0.66, p < 0.01) and floral stem biomass (r = 0.63, p < 0.01). A weaker correlation was found with the leaf length/width ratio (r = 0.47, p < 0.05), suggesting a potential link between leaf morphology and essential oil accumulation. Conversely, orris concrete yield did not show significant correlations with total biomass or rhizome biomass.
4. Discussion
Iris spp. have been recognized since ancient times for the beneficial properties of their dried rhizomes, which have been utilized in both the medical and cosmetic industries for various purposes, including the treatment of snake bites, depression, and the production of perfumes, powders, soaps, and pigments [34]. In Italy, the cultivation of Iris pallida has steadily declined, mainly due to labor-intensive cultivation practices and decreased market prices for dry rhizomes in the global perfume industry [20]. However, the high multifunctional value of the crop could drive and enhance its valorization on a territorial scale, as Iris cultivation can play a significant role in the income of farmers or producing companies. It can serve both as a strategy to support income from primary crops, such as olives, and as a direct accumulation strategy that involves a higher propensity for agronomic investment. Despite the potential key role this crop could play, especially in marginal and rural areas, either as a supplement or directly as farm income, very few studies have explored its production dynamics and their relationship with agronomic and environmental factors (climate, soil).
The combined effects of planting density (D) and harvesting age (H) under specific soil–climate conditions result in significant changes in the biomass parameters of Iris pallida. Specifically, total biomass, rhizome and bud biomass, leaf dimensions, and bud number exhibited a clear trend characterized by an increase from high-density (HD) to low-density (LD) plantings, and from the second to the fourth year (Figure S3). The significance of harvesting age (H) is likely related to the gradual maturation of Iris plants, leading to an increase in the associated morphological traits, while the impact of planting density (D) influences both biophysical (i.e., space, shading, weed control) and biochemical (i.e., nutrient and water use efficiency) properties of plant biomass, thereby enhancing rhizome production. These findings are consistent with the existing literature on the effects of plant density on crop growth, showing that lower planting densities generally promote biomass accumulation and rhizome development. This pattern is primarily attributed to improved access to key resources such as space, light, and nutrients [35,36,37]. This effect has also been reported in other rhizomatous species, such as Zingiber officinale Roscoe, where closer spacing increases total yield per hectare but reduces individual rhizome size due to increased competition for nutrients and space [38].
Concerning the interaction between plant density and planting age (D × H), this combined effect was highly significant (p < 0.001) for the number of floral stems and buds, as well as for orris concrete yield. The increased number of floral stems observed under low density (LD) can be attributed to the potential for greater flowering production after cutting, as low seeding density has been shown to enhance both the number and size of flowers [39]. This effect was further amplified by reduced competition for nutrients and water, which promoted optimal growth and development across all Iris vegetation compartments (i.e., rhizomes, upper biomass, flowers, etc.). These findings are consistent with those observed in other vegetative species, including aromatic plants, where planting density and harvesting age significantly influence floral biomass, stem number, and overall yield. For example, studies on Origanum vulgare L. and Calendula officinalis L. indicated that while higher planting densities initially promote floral stem production, they ultimately reduce biomass at maturity due to increased competition over time [40,41].
A noteworthy finding of this study is the exceptionally high number of buds per plant, a key parameter for crop renewal via rhizome division [36] and a potential income stream, like the commercial use of hop rhizomes [42]. Specifically, the number of buds per plant exceeded the traditionally reported benchmark of 10 propagules per rhizome annually for Iris pallida [9], both under low-density (LD) and high-density (HD) conditions. This enhanced bud production may be attributed to the optimization of environmental and agronomic conditions. First, the favorable soil conditions at the experimental site, including near-neutral pH (6.9), sandy texture, and moderate cation exchange capacity (10.1 meq 100 g−1), likely optimized the rooting environment, thereby promoting active rhizogenesis and lateral bud differentiation. Also, the reduced plant competition due to manual weed removal may have increased nutrient and water availability, which, in turn, may have improved carbohydrate storage and hormonal balance, widely recognized to be critical factors for axillary bud development [43,44]. In geophytes, particularly those with rhizomatous propagation such as Curcuma longa and Zingiber officinale Roscoe, several studies have indicated enhanced vegetative propagation, especially under low-stress conditions [38,45]. This pattern is further supported by the root biomass dynamics, which showed a significant increase, particularly during the third year under LD. From a climatic perspective, the third year (2017–2018) also experienced the lowest level of accumulated spring rainfall (111.2 mm), compared to other seasons (271.8 mm in 2016–2017 and 273 mm in 2018–2019; Table S2). Lower rainfall during the spring, widely recognized as a critical period for root expansion, may have stimulated deeper and more branched root systems as a compensatory mechanism to enhance water uptake. This adaptive strategy is well-documented in tuberous species such as Solanum tuberosum L. [46] and similar geophyte species such as Iris germanica [47], which enhance their root architecture under drought-like conditions to maintain physiological functionality.
The peak leaf L/W ratio observed under low-density conditions in Year 3 may reflect an adaptive morphological adjustment aimed at enhancing light interception efficiency. Under reduced inter-plant competition, elongated leaves with higher L/W ratios might increase the vertical light capture and reduce self-shading, contributing to improved photosynthetic potential [48]. This trend has been documented across perennial species with varying canopy architecture. Additionally, such traits may align with conservative resource use strategies under low-stress environments [49], further supporting the role of density-driven morphological plasticity in Iris pallida. Finally, the interaction between planting density and harvesting age (D × H) emerged as a critical determinant of orris concrete yield, emphasizing how agronomic choices can significantly influence the efficiency and viability of essential oil production in this species. Results indicated that orris concrete yields were generally lower (0.038–0.062%) compared to those reported in other studies. Traditional orris manufacturing involves steam distillation with water for 30 or more hours, which can be reduced to 8 h by operating under pressure [50]. Depending on the storage duration of orris rhizomes (2–3 years), however, Garnero et al. [25] reported orris concrete yields ranging from 0.28–0.33%. These values were slightly above those indicated by Almaarri et al. [47], which reported orris concrete yields ranging from 0.14 to 0.24% for four different wild Iris species in Syria (i.e., I. aurantiaca Baker, I. barnumae Foster, I. bostrensis Mouterde, I. germanica L.) using methanol and ultrasound-assisted extraction techniques. Again, Wollinger et al. [50] reported an average orris concrete yield of about 0.25% from traditional steam distillation of dried rhizomes.
The lower orris concrete yields observed in this study (0.038–0.062%) could be likely attributable to the methodological approach that environmental factors. Firstly, hydrodistillation was performed using a stainless-steel distiller equipped with a two-stage condenser system (Figure S2). The first condenser was maintained at 10 °C, while the second was kept at 46 °C to match the melting point of orris concrete and ensure its recovery in liquid form.Although this apparatus was calibrated and previously used in essential oil research [51], its efficiency may be lower than that of conventional Clevenger-type setups, which typically operate for longer periods (≥8 h) or under pressure-assisted conditions, thus resulting in reduced extraction efficiency. Moreover, since the chemical composition of the extracted orris concrete was not analyzed, irone content was not quantified. This partially hindered the interpretation of the results, particularly in understanding whether low yield corresponded to low concentration or incomplete plant recovery.
On the other hand, local climate and environmental conditions can affect plant growth and development, thereby influencing final yields. Precipitation and temperature, for instance, play a crucial role in the biosynthesis and accumulation of essential oils (EOs). Several studies have shown that reduced precipitation can lead to an increase in EO concentration [52], likely due to adaptive physiological responses that favor root development. This enhanced root system is directly associated with greater rhizome biomass, which in turn correlates with higher essential oil yields. Similar effects have been observed in other EO-producing species, such as Lavandula angustifolia Mill. [53] and Salvia officinalis L. [54], where water stress or dry growing conditions were linked to increased EO concentration and biomass allocation to storage organs.
Furthermore, orris concrete yield was found to be strongly positively correlated with the number of floral stems (r = 0.73; p < 0.001), likely due to a more developed root–rhizome system, which is the primary source of orris concrete. In low-density plots, orris concrete yield was consistently higher, likely due to increased total biomass per plant, reduced intraspecific competition, and greater resource allocation to underground organs. In addition, orris concrete yield under LD conditions declined progressively with increasing harvesting age, decreasing from 0.062% in Year 2 to 0.044% in Year 4 (–29%). Prolonging the cultivation cycle beyond three years therefore appears to reduce yield efficiency rather than enhance essential oil output. For instance, Garnero et al. [25] reported yields ranging from 0.28% to 0.33%, which are approximately 4.5 to 7 times higher than the highest value recorded in our study. Similarly, Wollinger [49] documented an average yield of 0.25%, corresponding to a relative difference of about 75–85% compared to our results. However, it is important to note that higher biomass does not always correspond to higher EO yield, as the concentration of aromatic compounds per unit mass may vary significantly depending on the plant’s developmental stage and environmental stress conditions [54]. Comparable findings have been reported in other rhizomatous aromatic species. For instance, in Atractylodes lancea (Thunb.) DC., the content of major EO compounds such as β-eudesmol and atractylon exhibited high genetic determinism, independent of rhizome biomass, although environmental conditions (e.g., growing location) still significantly impacted oil composition [55]. Environmental variables such as altitude, soil type, and climate were also shown to affect the chemical profile of EOs in Hedychium spicatum Sm., with notable variation among populations from different habitats [56].
5. Conclusions
This study offers valuable insights into the agronomic and morphological factors that influence Iris pallida cultivation, particularly concerning orris concrete yield, a key product in the perfume industry. The results highlighted the crucial roles of both planting density and maturity age in biomass production, morphological traits, and overall orris concrete yield. Specifically, low-density planting (LD) consistently outperformed high-density planting (HD) in terms of total biomass, rhizome, floral stem, and bud biomass, as well as orris concrete yield. The interaction between planting density and harvesting age (D × H) was a significant factor in determining floral stem and bud numbers, with the highest values observed in the third and fourth years under LD, indicating that a longer growth period may benefit these plant components.
Orris concrete yield varied significantly across planting densities and harvesting age, showing highest and lowest yields in the second year under LD (0.062 ± 0.001%) and HD (0.038 ± 0.008%), respectively.
On average, low-density conditions consistently outperformed high-density (0.055 ± 0.01% vs. 0.045 ± 0.01%), suggesting that lower plant competition may create a more favorable environment for rhizome development and essential oil accumulation. While higher biomass does not necessarily correlate with higher essential oil yield, reduced plant density likely favored both biomass quality and oil concentration.
Environmental conditions also played a role: notably, low spring precipitation in the second year appeared to stimulate root and rhizome development, potentially enhancing orris concrete production. This points to a degree of resilience in Iris pallida under drought-like conditions, which may favor its cultivation in drier Mediterranean environments.
Importantly, when orris yield was expressed on an annual basis per hectare, the low-density treatment harvested after three years emerged as the most productive strategy (6.30 kg/ha/year), outperforming both shorter and longer cycles. These results highlight that a 3-year cycle under low planting density maximizes yield efficiency and may offer the most sustainable return for commercial growers.
Although these results provide valuable guidelines for improving orris concrete production efficiency, some limitations are still present. First, the chemical composition of orris, particularly the content of irones, was not analyzed, which limited a full understanding of the biomass-yield relationship on a mechanistic level. Additionally, the semi-operational stainless steel distiller (Spring 12 L) used in this study operated with a reduced distillation time (6 h) and controlled condenser temperatures, which may have decreased extraction efficiency compared to conventional Clevenger-type systems (which typically operate for 8 h). This could have potentially affected the magnitude of the orris yield.
Future research should include chemical profiling of the extracts, comparisons of different methodological approaches, evaluation of alternative genotypes or ecotypes, and testing of agronomic performance across various climatic years and regions. These efforts would help validate the reproducibility and scalability of the findings, specifically contributing to the revitalization of Iris pallida cultivation in Tuscany and other regions. This could ensure the continued relevance of Iris pallida in supporting the income of farmers and producing companies, as well as its importance to the global perfume industry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15071719/s1. Figure S1. Monthly maximum (red line) and minimum (orange line) air temperatures, and precipitation (blue histograms) in the study area during the period 2015-2019. Figure S2. Hydrodistillation approach for iris concrete extraction: induction plates (1); boiler (5) containing bottom cavity (2) and grate (3); biomass allocation for distillation (4); top grate (6), boiler top cover (7) including silicone safety valve (8); thermometer (9); and a hole with a steel ring nut (10) where the stainless-steel collector (11) is screwed, and complete with the first condenser (12) and a steel valve (15). The stainless-steel collector (11) ends with a plastic ring (16) connected to the separation column, or second condenser (19), complete with a Florentine vessel (17) and a tap (18). The separation column, via a Clevenger collector (20), allows the distillation water to flow back into the boiler (5). Both the first (12) and second (19) condenser are formed by a cavity where the cooling water flows in reverse flow. In the first condenser, the cooling water was kept at 10 °C while the cooling water in the second condenser was kept at 45 °C, to keep the iris concrete in a liquid state and facilitate its separation from hydrosol. Figure S3. Picture of the four-year harvested plants from the experimental field. The plant biomass in the low-density conditions (A) is bigger and heavier than the plants harvested in the high-density conditions (B). Table S1. Physical and chemical soil properties of the test site. Table S2. Seasonal air temperatures (maximum and minimum) and cumulated precipitation over the study area during the period 2015-2019. Table S3. Correlation Matrix. Significance code: * p < 0.05. ** p < 0.01. *** p < 0.001.
Author Contributions
Conceptualization, E.P., L.M., L.B. and M.M.; methodology, E.P., L.M. and M.M.; software, L.M. and M.M.; validation, E.P., L.B. and M.M.; formal analysis, E.P. and M.M.; investigation, E.P., G.M. and G.P.; resources, E.P.; data curation, E.P., L.M., G.P. and M.M.; writing—original draft preparation, E.P., L.M., G.P., L.B. and M.M.; writing—review and editing, E.P., L.M., L.B. and M.M.; visualization, L.B. and M.M.; supervision, E.P. and M.M.; project administration, E.P.; funding acquisition, E.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data available on request from the authors.
Acknowledgments
We gratefully acknowledge the support of the farms of Chianti Fiorentino for their collaboration on the experimentation setup. We also sincerely express our gratitude to Gionni Pruneti and the Mariani family for their technical and practical support.
Conflicts of Interest
Author Lorenzo Marini was employed by the company Azienda Agraria Sperimentale Stuard SCRL, Azienda Sperimentale per l’Innovazione Agricola. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Kamenetsky, R.; Okubo, H. Ornamental Geophytes: From Basic Science to Sustainable Production; Kamenetsky, R., Okubo, H., Eds.; Taylor & Francis: Boca Raton, Florida, 2012; p. 597, eBook; ISBN 9780429061776. [Google Scholar] [CrossRef]
- Lucchesini, M.; Bedini, L.; Florio, E.F.; Maggini, R.; Malorgio, F.; Pezzarossa, B.; Mensuali-Sodi, A. The improvement of Iris pallida propagation by somatic embryogenesis. Acta Hortic. 2017, 1155, 127–134. [Google Scholar] [CrossRef]
- Belletti, G.; Fani, E.; Marescotti, A.; Scaramuzzi, S. The role of traditional products in the valorisation of marginal rural areas: The case of Iris pallida. In Proceedings of the 10th European IFSA Symposium, Aarhus, Danmark, 1–4 July 2012. [Google Scholar]
- Roger, B.; Jeannot, V.; Fernandez, X.; Cerantola, S.; Chahboun, J. Characterisation and quantification of flavonoids in Iris Germanica L. and Iris Pallida Lam. resinoids from Morocco. Phytochem. Anal. 2012, 23, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Kaššák, P. Secondary metabolites of the choosen genus Iris species. Acta Univ. Agric. Silvic. Mendel. Brun. 2013, 60, 269–280. [Google Scholar] [CrossRef]
- Masson, J.; Liberto, E.; Brevard, H.; Bicchi, C.; Rubiolo, P. A metabolomic approach to quality determination and authentication of raw plant material in the fragrance field. J. Chromatogr. 2014, 14, 143–154. [Google Scholar] [CrossRef]
- Kukula-Koch, W.; Sieniawska, E.; Widelski, J.; Urjin, O.; Głowniak, P.; Skalicka-Woźniak, K. Major secondary metabolites of Iris spp. Phytochem. Rev. 2015, 14, 51–80. [Google Scholar] [CrossRef]
- Mykhailenko, O. Composition of volatile oil of Iris pallida Lam. from Ukraine. Turk. J. Pharm. Sci. 2018, 15, 85–90. [Google Scholar] [CrossRef]
- Jthan, H.; Courtois, D.; Ehret, C.; Lerch, K.; Pttiard, V. Plant regeneration of Iris pallida Lam. and Iris germanica L. via somatic embryogenesis from leaves, apices and young flowers. Plant Cell Rep. 1994, 13, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Pezzarossa, B.; Borghesi, E.; Pini, R.; Bretzel, F.; Maggini, R.; Malorgio, F. Influence of pedo-climatic conditions on the quality of Iris pallida rhizomes. Eur. J. Hortic. Sci. 2020, 85, 100–109. [Google Scholar] [CrossRef]
- Pezzarossa, B.; Bretzel, F.; Malorgio, F.; Borghesi, E.; Maggini, R.; Scaramuzzi, S. La coltivazione del giaggiolo in Toscana: Dalla marginalità alla valorizzazione. Colt. Protette 2016, 7/8, 62–66. [Google Scholar]
- Landi, R.; Nicolelli, M. Tecnica colturale del giaggiolo (otto anni di sperimentazione a Caspri). In Estratto dal Bollettino Della Società Italiana Dell’iris; Società Italiana Dell’iris: Firenze, Italy, 1977; p. 28. [Google Scholar]
- Marner, F.J.; Kerp, B. Composition of iridals, unusual triterpenoids from sword-Lilies, and the seasonal dependence of their content in various parts of different iris species. Z. Naturforschung C 1992, 47, 21–25. [Google Scholar] [CrossRef]
- Belcour, B.; Courtois, D.; Petiard, V.; Ehret, C. Rapid production of irones by maturation of orris rhizomes with two bacterial strains. Phytochemistry 1993, 34, 1313–1315. [Google Scholar] [CrossRef]
- Firmin, L.; Courtois, D.; Pétiard, V.; Ehret, C.; Lerch, K. Evaluation of the natural variability in irone content and selection of Iris sp. for perfume production. HortScience 1998, 33, 1046–1047. [Google Scholar] [CrossRef]
- Brenna, E.; Fuganti, C.; Ronzani, S.; Serra, S. Enzyme-mediated syntheses of the enantiomers of γ-irones. Helv. Chim. Acta 2001, 84, 3650–3666. [Google Scholar] [CrossRef]
- Konig, W.; Faltin, Y.; Scheffer, J.; Schoffler, H.; Braun, V. Role of Cell-Bound Hemolysin as a Pathogenicity Factor for Serratia Infections. Infect. Immun. 1987, 55, 2554–2561. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tash, K. Rahnella aquatilis bacteremia from a suspected urinary source. J. Clin. Microbiol. 2005, 43, 2526–2528. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.W.; Khan, A.U. Updates on the pathogenicity status of Pseudomonas aeruginosa. Drug Discov. Today 2019, 24, 350–359. [Google Scholar] [CrossRef]
- Ieri, F.; Vignolini, P.; Urciuoli, S.; Pinelli, P.; Romani, A. The Cultivation of Iris pallida as an Opportunity for the Enhancement of Tuscan Agro-Biodiversity and a Resource for the Local Economy. In Innovation, Quality and Sustainability for a Resilient Circular Economy; Lagioia, G., Paiano, A., Amicarelli, V., Gallucci, T., Ingrao, C., Eds.; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Meucci, A.; Ghelardi, C.; Maggini, R.; Malorgio, F.; Pezzarossa, B.; Rosellini, I.; Mensuali, A. Insights into orris (Iris pallida Lam.) in vivo acclimatization and response to salt stress via exogenous melatonin application. Agriculture 2024, 14, 2353. [Google Scholar] [CrossRef]
- Gürbüzer, G.; Kara, N. Effect of Harvest Times on Rhizoma Yield, Essential Oil Content and Composition in Iris germanica L. Species. Turk. J. Agric. Food Sci. Technol. 2019, 7, 707–713. [Google Scholar] [CrossRef]
- Alsheikly, A.A. Effect of growing media on growth, flowering and bulbs production of Iris hollandica. Diyala Agric. Sci. J. 2010, 5, 581–592. [Google Scholar]
- Mahgoub, H.J.; Eid, R.A.; Abou Leila, B.H. Response of Iris bulbs grown in sandy soil to nitrogen and potassium fertilization. J. Appl. Sci. Res. 2006, 2, 899–903. [Google Scholar]
- Garnero, J.; Joulain, D.; Buil, P. De l’influence du stockage des rhizomes d’iris sur la composition de l’huile essentielle ou beurre d’iris et quelques constituants inédits. Riv. Ital. EPPOS 1978, 10, 568–590. [Google Scholar]
- TGSC Information System, 2022. Orris Rhizome Concrete Butter (Iris pallida). CAS Number: 8002-73-1. Available online: http://www.thegoodscentscompany.com/data/co1001091.html (accessed on 4 January 2025).
- RStudio Team. RStudio: Integrated Development Environment for R. 2021. Available online: http://www.rstudio.com/ (accessed on 17 January 2025).
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Razali, N.M.; Wah, Y.B. Power comparisons of Shapiro–Wilk, Kolmogorov–Smirnov, Lilliefors and Anderson–Darling tests. J. Stat. Model. Anal. 2011, 2, 21–33. [Google Scholar]
- Gastwirth, J.L.; Gel, Y.R.; Miao, W. The impact of Levene’s test of equality of variances on statistical theory and practice. Stat. Sci. 2009, 24, 343–360. [Google Scholar] [CrossRef]
- Graves, S.; Piepho, H.P.; Selzer, L.; Dorai-Raj, S. multcompView: Visualizations of Paired Comparisons. 2023. Available online: https://CRAN.R-project.org/package=multcompView (accessed on 20 February 2025).
- Han-Wu-Shuang, B. bruceR: Broadly Useful Convenient and Efficient R Functions. 2023. Available online: https://CRAN.R-project.org/package=bruceR (accessed on 28 March 2025).
- Kratky, B.; Bernabe, C.I. Outdoor growing of clean edible ginger seed by a pot-in-pot-in-pot sub-irrigation method. In Proceedings of the 35th National Agricultural Plastics Congress, American Society for Plasticulture, Bellafonte, PA, USA, 13–16 July 2009; Available online: https://www.ctahr.hawaii.edu/hawaii/downloads/outdoor_growing_of_clean_edible_ginger_seed_by_a_pot-in-pot-in-pot_sub-irrigation_method.pdf (accessed on 13 February 2023).
- Oztas, F.; Turkmen, A.; Oztas, H.; Turkmen, M. The medical properties of Iris and its usage in pharmaceutical, perfumery and cosmetic industries. In Medical Research and Its Applications; Blue Pen International: Chennai, India, 2024; Volume 4, pp. 114–124. [Google Scholar] [CrossRef]
- Retana-Cordero, M.; Flores, S.J.; Fisher, P.R.; Freyre, R.; Gómez, C. Effect of container volume and planting density on ginger and turmeric growth and yield. HortTechnology 2022, 32, 425–434. [Google Scholar] [CrossRef]
- Landi, R. Effetti della popolazione coltivata, della concimazione e dei fattori ambientali sulla produzione del giaggiolo (Iris pallida Lam.). In Proceedings of the Atti Convegno Internazionale “Coltivazione e Miglioramento di Piante Officinali”, Trento, Italy, 2–3 June 1994; pp. 47–70. [Google Scholar]
- Mahender, B.; Reddy, P.S.S.; Sivaram, G.T.; Balakrishna, M.; Prathap, B. Effect of seed rhizome size and plant spacing on growth, yield and quality of ginger (Zingiber officinale Rosc.) under coconut cropping system. Plant Arch. 2015, 15, 769–774. Available online: https://plantarchives.org/pdf%2015-2/769-774%20(3001).pdf (accessed on 14 March 2025).
- Verma, T.; Sharma, B.P.; Thakur, M. Effect of Different Nitrogen Levels and Spacing on Growth and Flowering of Iris (Iris orientalis Mill.) cv.‘Frigia’. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2101–2109. [Google Scholar] [CrossRef]
- Gerami, F.; Rezvani Moghaddam, P.; Ghorbani, R.; Hassani, A. Influence of planting date and plant density on morphological characteristics, seed yield, and essential oil percentage of oregano (Origanum vulgare L.). J. Appl. Hortic. 2018, 20, 171–176. [Google Scholar] [CrossRef]
- Berimavandi, A.R.; Hashemabadi, D.; Facouri Ghaziani, M.V.; Kaviani, B. Effects of plant density and sowing date on the growth, flowering and quantity of essential oil of Calendula officinalis L. J. Med. Plants Res. 2011, 5, 5110–5115. Available online: http://www.academicjournals.org/JMPR (accessed on 14 March 2025).
- Heuer, B.; Nadler, A. Growth and development of potatoes under salinity and water deficit. Aust. J. Agric. Res. 1995, 46, 1477–1486. [Google Scholar] [CrossRef]
- Häkkinen, S.T.; Oksman-Caldentey, K.M. Progress and prospects of hairy root research. In Hairy Roots: An Effective Tool of Plant Biotechnology; Srivastava, V., Mehrotra, S., Mishra, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; Chapter 1; pp. 3–19. ISBN 978-981-13-2562-5. [Google Scholar]
- Bahl, J.R.; Bansal, R.P.; Garg, S.N.; Gupta, M.M.; Singh, V.; Goel, R.; Kumar, S. Variation in yield of curcumin and yield and quality of leaf and rhizome essential oils among Indian landraces of turmeric Curcuma longa L. Proc. Indian. Natl Sci. Acad. 2014, 80, 143–156. [Google Scholar] [CrossRef]
- Liu, F.; Shahnazari, A.; Andersen, M.N.; Jacobsen, S.E.; Jensen, C.R. Physiological responses of potato (Solanum tuberosum L.) to partial root-zone drying: ABA signalling, leaf gas exchange, and water use efficiency. J. Exp. Bot. 2006, 57, 3727–3735. [Google Scholar] [CrossRef]
- Meyer, C.J.; Seago, J.L.; Peterson, C.A. Environmental effects on the maturation of the endodermis and multiseriate exodermis of Iris germanica roots. Ann. Bot. 2009, 103, 687–702. [Google Scholar] [CrossRef]
- Bicchi, C.; Joulain, D. A comprehensive review on essential oils and extracts from Iris rhizomes. Phytochem. Rev. 2024, 24, 1629–1665. [Google Scholar] [CrossRef]
- Almaarri, K.; Abou Zedan, T.; Albatal, N. Chemical analysis of essential oils of some Syrian wild Iris species. Am. J. Biochem. Mol. Biol. 2013, 3, 38–49. [Google Scholar] [CrossRef]
- Falster, D.S.; Westoby, M. Leaf size and angle vary widely across species: What consequences for light interception? New Phytol. 2003, 158, 509–525. [Google Scholar] [CrossRef]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Functional traits explain variation in plant growth and response to climate across 2000 species from around the globe. Ecol. Res. 2010, 25, 707–723. [Google Scholar] [CrossRef]
- Wollinger, A. Application of a Supercritical Carbon Dioxide Extraction Unit. Ph.D. Thesis, University of Regensburg, Regensburg, Germany, 2016. (In German). Available online: https://epub.uni-regensburg.de/33710/7/Dissertation_Wollinger_18042016.pdf (accessed on 12 February 2025).
- Spadi, A.; Angeloni, G.; Guerrini, L.; Corti, F.; Parenti, A.; Innocenti, M.; Bellumori, M.; Masella, P. Hydrodistillation of Coffee By-Products to Recover Bioactive Compounds: The Spent Coffee Ground and Coffee Silverskin Case-Study. Chem. Eng. Trans. 2021, 87, 313–318. [Google Scholar] [CrossRef]
- Mehalaine, S.; Chenchouni, H. Quantifying how climatic factors influence essential oil yield in wild-growing plants. Arab. J. Geosci. 2021, 14, 1257. [Google Scholar] [CrossRef]
- Zuzarte, M.R.; Dinis, A.M.; Cavaleiro, C.; Salgueiro, L.R.; Canhoto, J.M. Trichomes, essential oils and in vitro propagation of Lavandula pedunculata (Lamiaceae). Ind. Crops Prod. 2010, 32, 580–587. [Google Scholar] [CrossRef]
- Bettaieb, I.; Hamrouni-Sellami, I.; Bourgou, S.; Limam, F.; Marzouk, B. Drought effects on polyphenol composition and antioxidant activities in aerial parts of Salvia officinalis L. Acta Physiol. Plant. 2011, 33, 1103–1111. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y.; Yang, C.; Liu, J.; Li, J.; Chen, S. Genetic and environmental influences on essential oil composition in Atractylodes lancea. PLoS ONE 2019, 14, e0217522. [Google Scholar] [CrossRef]
- Rawat, S.; Bhatt, I.D.; Rawal, R. Variation in essential oil composition in rhizomes of natural populations of Hedychium spicatum in different environmental conditions and habitats. J. Essent. Oil Res. 2020, 32, 348–360. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).