Fine Mapping and Genetic Effect Analysis of Rf21(t) for the Fertility Restoration of Chinsurah-Boro-II-Type Cytoplasmic Male Sterile Oryza sativa (ssp. japonica) Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Fertility Scoring and Genetic Analysis
2.3. Specific-Locus Amplified Fragment (SLAF) Library Construction and Sequencing
2.4. Sequence Data and Linkage Analyses
2.5. Development of the Knockout Transgenic Lines
2.6. DNA Extraction, Polymerase Chain Reaction and Sequencing
2.7. Quantitative Real-Time (qRT) PCR
2.8. Data Analysis
3. Results
3.1. Evaluation of the Fertility Restoration Capability of ‘02428’
3.2. Genetic Analysis of Fertility Restoration
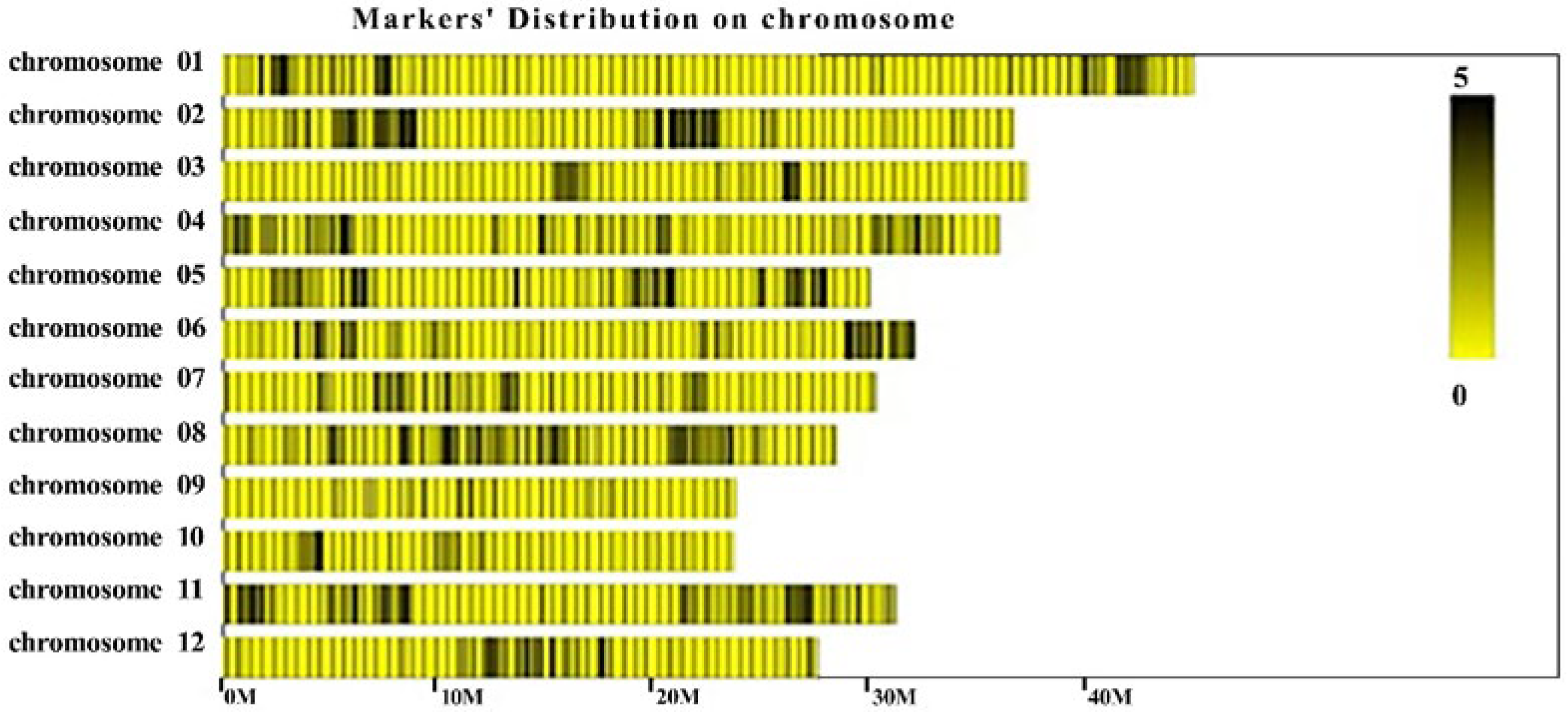
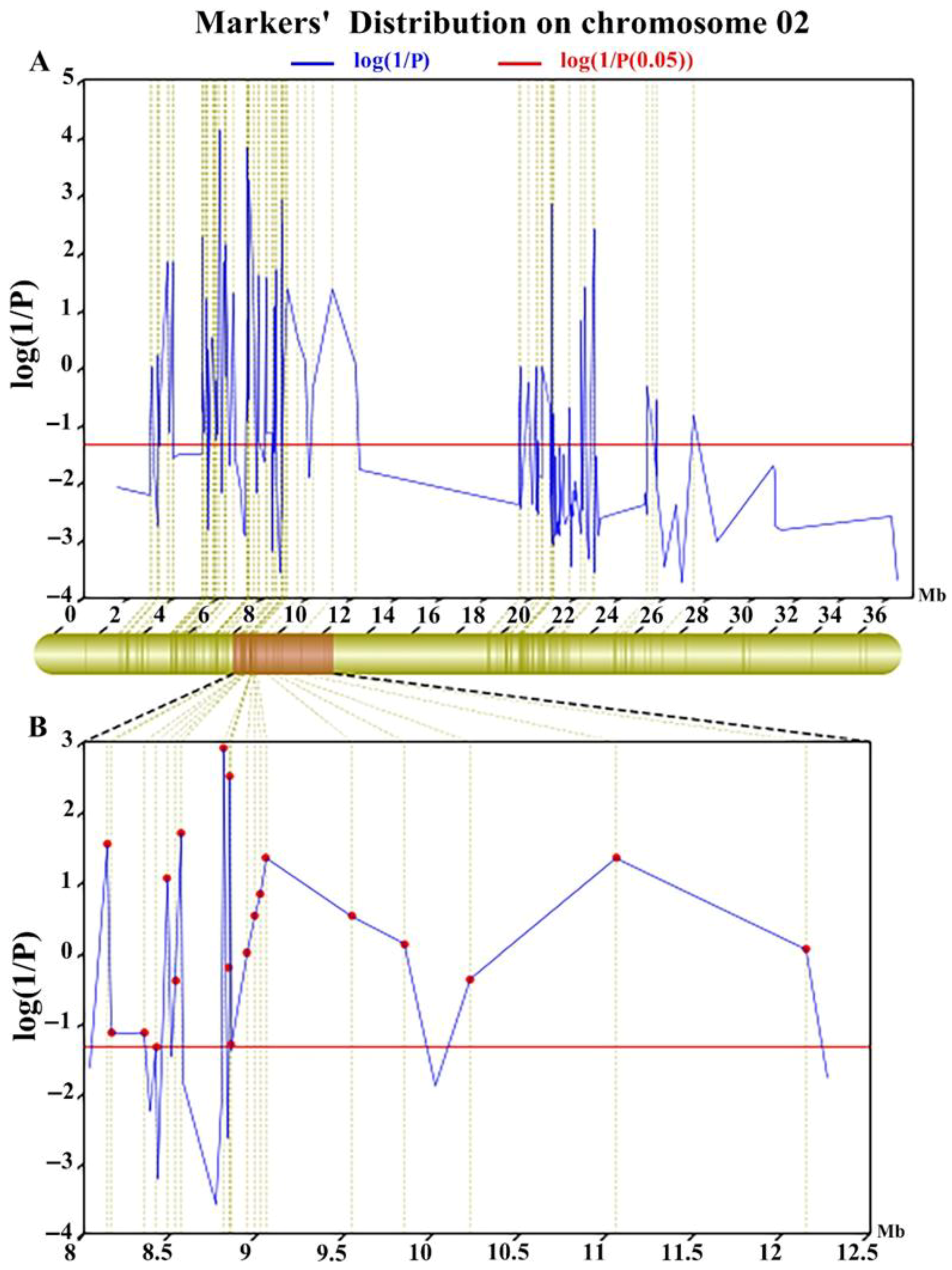
3.3. Mapping of Rf21(t) by BSA Combined with the SLAF Sequence
3.4. Fine Mapping of Rf21(t)
3.5. Candidate Gene Analysis of Rf21(t)
3.6. Genetic Effects of Rf21(t) Using CRISPR/Cas9-Based Mutagenesis
3.7. Ability of Rf21(t) to Restore Fertility
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CMS | Cytoplasmic male sterility |
| Rf | Fertility restorer gene |
| PPR | Pentatricopeptide repeat |
| BSA | Bulk segregant analysis |
| I2-KI | Iodine/potassium iodide |
| χ2 | Chi-square test |
| SLAF | Specific-Locus Amplified Fragment |
| SLAF-seq | SLAF sequencing |
| SSR | Simple sequence repeat markers |
| InDel | Insertion/deletion markers |
| PCR | Polymerase chain reaction |
| qRT-PCR | Quantitative real-time PCR |
| ORFs | Open reading frames |
Appendix A

| Marker | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| RM12906 | CATGCTCTTCGTCACACCATCG | GTGTGATGTGGATAATTGGCATCC |
| RM12914 | CGGAGGGAGTAGTGGTGTATTGG | CACAATGGAGGAGCAGATGAGG |
| RM6639 | ACCGGAAGGGATACTTATCAGC | CTTCCTGTGAAATAGTAGAGGTAGGC |
| RM12921 | TCGTATTTCCCGGTGTCTCAGG | ACTAGTACTCGGTGCAGGGAATCG |
| RM6375 | CGAATGGAACGACAAGAGATGC | ATAGATCGACAACGTAGATCCACAGG |
| RM12941 | TTATGCCATGTGGTCCAATCAGC | ATTTGAACCATTTGGGCCTTGG |
| RM12938 | CCATCTGTCTCTCCTCCTTATCTCC | CTCCCACCAAATCTAGTGAATGG |
| STS-2-39.6 | TGCTGATGTTAATCTGTGGGTG | TGGCGGCTTGAGAGTGTTTGTAG |
| STS-2-55.4 | TGACTATGTAAGGTTGCTGCTG | ACAGCTCCACAGCAAAGAACC |
| RM1347 | AACAAATTAAACTGCCAAG | GTCTTATCATCAGAACTGGA |
| STS2-31 | TTAACTGGTAACGTGAAT | CAGCAAGGCAACAACAAT |
| STS2-39 | TGCTGATGTTAATCTGTGGGTG | TGGCGGCTTGAGAGTGTTTGTAG |
| STS2-101 | ATGGGTTCAGACCTTAGGCG | AGGGAAGGGTTTATGTTGTTCT |
| STS2-12 | AAGTCAAGCTCCTGTAAGATTC | TGTTGATGTGATGAAAAAAAGT |
| STS2-13 | TAGCGTTTTACGGAGAGATTTT | GATTAGGAGTGTGACGTGGACT |
| STS2-20 | CATGTATGACAAAAACAGAGCC | TTTAGTAGTTTGAAAAGCGTGC |
| STS2-21 | CTTCTTCTTCGTTGCGATT | CGTGTGGAGGTTAGGCTGT |
| RM1358 | GATCGATGCAGCAGCATATG | ACGTGTGGCTGCTTTTGC |
| 17360ce-1 | CGGTGTTCAATGTTCACTTGTT | ATGCCTCCTCCTTGCTCAG |
| 17360ce-2 | TGAGCAAGGAGGAGGCATT | AGTTCTTACAGCGTGTGAATCA |
| 17360ce-3 | CCAGTGAGATGTCGGTTATGC | AGACCTGAAGAGCCTGAGTTG |
| 17360ce-4 | TCTCCAACTCAGGCTCTTCAG | TTCGTTCCTCCTCCTCATCTC |
| OsActin | GGAAGTACAGTGTCTGGATTGGAG | TCTTGGCTTAGCATTCTTGGGT |
| qRT-02g17360 | AATGCCTCCTCCTTGCTCAG | GTCCTTCTCTAGTGAACCTTCCT |
| KO-Identified 12159 | TCGGCAAAAGAAACGAAAAG | GGGGTGGAGGAGGAGATGGG |
| GP15924-12323 | TACTCTGGCATCTCCCCGAA | CAGCCACCACCTCATCATCC |
| Chromosome | Number of Polymorphic Markers | Chromosome | Number of Polymorphic Markers |
|---|---|---|---|
| Chromosome 1 | 261 | Chromosome 7 | 188 |
| Chromosome 2 | 326 | Chromosome 8 | 346 |
| Chromosome 3 | 132 | Chromosome 9 | 77 |
| Chromosome 4 | 296 | chromosome10 | 120 |
| Chromosome 5 | 273 | chromosome11 | 325 |
| Chromosome 6 | 249 | chromosome12 | 182 |
| Chromosome | No. of Different Markers | Chromosome | No. of Different
Markers |
|---|---|---|---|
| Chromosome 2 | 45 | Chromosome 6 | 2 |
| Chromosome 4 | 1 | Chromosome 8 | 1 |
| Chromosome 5 | 2 | Chromosome11 | 1 |
| Total | 52 |
| Locus Name | Gene Product Name |
|---|---|
| LOC_Os02g17360 | PPR-repeat-domain-containing protein, putative, expressed |
| LOC_Os02g17370 | Expressed protein |
| LOC_Os02g17380 | EMB1303, putative, expressed |
| LOC_Os02g17390 | 3-hydroxy acyl-CoA dehydrogenase, putative, expressed |
| LOC_Os02g17400 | Leucine-rich repeat protein, putative, expressed |
| LOC_Os02g17410 | Hypothetical protein |
| LOC_Os02g17420 | Retrotransposon protein, putative, unclassified, expressed |
| LOC_Os02g17430 | Retrotransposon protein, putative, unclassified, expressed |
| LOC_Os02g17440 | Retrotransposon protein, putative, unclassified, expressed |
| LOC_Os02g17450 | Retrotransposon protein, putative, unclassified, expressed |
| LOC_Os02g17460 | Tesmin/TSO1-like CXC domain-containing protein, expressed |
| LOC_Os02g17470 | RNA-binding-protein-related, putative, expressed |
References
- Hanson, M.R.; Bentolila, S. Interactions of mitochondrial and nuclear genes that affect male gametophytic development. Plant Cell 2004, 16, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Chase, C.D. Cytoplasmic male sterility: A window to the world of plant mitochondri-al–nuclear interactions. Trends Genet. 2007, 23, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Havey, M.J. The use of cytoplasmic male sterility for hybrid seed production. In Molecular Biology and Biotechnology of Plant Organelles; Daniell, H., Chase, C., Eds.; Springer Publishers: Berlin, Germany, 2004; pp. 617–628. [Google Scholar]
- Chikh-Rouhou, H.; Singh, S.; Priyadarsini, S. Onion Male Sterility: Genetics, Genomics and Breeding. Horticulturae 2025, 11, 539. [Google Scholar] [CrossRef]
- Fujimura, T.; Akagi, H.; Oka, M. Establishment of a rice protoplast culture and application of an asymmetric protoplast fusion technique to hybrid rice breeding. Plant Tissue Cult. Lett. 1996, 13, 243–247. [Google Scholar] [CrossRef]
- Yuan, L.P. Increasing yield potential in rice by exploitation of heterosis. In Hybrid Rice Technology; Virmanni, S.S., Ed.; New Developments and Future Prospects; IRRI: Manila, Philippines, 1994; pp. 1–6. [Google Scholar]
- Deng, H. Japonica Hybrid Rice in China; China Agric Press: Beijing, China, 2008. [Google Scholar]
- Zeng, Q.C.; Zhou, K.D.; Zhu, Z. Current Status in the Use of Hybrid Rice Heterosis in China. Chin. J. Rice Sci. 2000, 14, 243–246. (In Chinese) [Google Scholar]
- Jiang, H.C.; Lu, Q.; Qiu, S.Q. Fujian cytoplasmic male sterility and the fertility restorer gene OsRf19 provide a promising breeding system for hybrid rice. Proc. Natl. Acad. Sci. USA 2022, 119, e2208759119. [Google Scholar] [CrossRef]
- Chen, L.T.; Liu, Y.G. Male sterility and fertility restoration in crops. Annu. Rev. Plant Biol. 2014, 65, 579–606. [Google Scholar] [CrossRef]
- Huang, J.Z.; E, Z.G.; Zhang, H.L. Workable male sterility systems for hybrid rice: Genetics, biochemistry, molecular biology, and utilization. Rice 2014, 7, 13. [Google Scholar] [CrossRef]
- Li, S.Q.; Yang, D.C.; Zhu, Y.G. Characterization and use of male sterility in hybrid rice breeding. J. Integr. Plant Biol. 2007, 49, 791–804. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zou, Y.J.; Li, X.Y. Cytoplasmic male sterility of rice with boro II cytoplasm is caused by a cytotoxic peptide and is restored by two related PPR motif genes via distinct modes of mRNA silencing. Plant Cell 2006, 18, 676–687. [Google Scholar] [CrossRef]
- Shinjyo, C. Genetical studies of cytoplasmic male sterility and fertility restoration in rice (Oryza sativa L.). Sci. Bull. Coll. Agric. Univ. Ryukyus 1975, 22, 1–57. [Google Scholar]
- Yang, Z.Y. Retrospects and prospect on the development of japonica hybrid rice in the north of China. Acta. Agron. Sin. 1998, 24, 7. (In Chinese) [Google Scholar]
- Huang, W.C.; Hu, J.; Yu, C.C. Two non-allelic nuclear genes restore fertility in a gametophytic pattern and enhance abiotic stress tolerance in the hybrid rice plant. Theor. Appl. Genet. 2012, 124, 799–807. [Google Scholar] [CrossRef]
- Komori, T.; Imaseki, H. Transgenic rice hybrids that carry the Rf-1 gene at multiple loci show improved fertility at low temperature. Plant Cell Environ. 2005, 28, 425–431. [Google Scholar] [CrossRef]
- Ahmadikhah, A.; Karlov, G.I. Molecular mapping of the fertility-restoration gene Rf4 for WA-cytoplasmic male sterility in rice. Plant Breed. 2006, 125, 363–367. [Google Scholar] [CrossRef]
- Tang, H.W.; Luo, D.P.; Zhou, D.G. The rice restorer Rf4 for wild-abortive cytoplasmic male sterility encodes a mitochondrial-localized PPR protein that functions in reduction of WA352 transcripts. Mol. Plant 2014, 7, 1497–1500. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, Y.; Bharaj, T.S. Mapping of the Rf3 nuclear fertility-restoring gene for WA cytoplasmic male sterility in rice using RAPD and RFLP markers. Theor. Appl. Genet. 1997, 94, 27–33. [Google Scholar] [CrossRef]
- Hu, J.; Wang, K.; Huang, W.C. The rice pentatricopeptide repeat protein Rf5 restores fertility in Hong-Lian cytoplasmic male-sterile lines via a complex with the glycine-rich protein GRP162. Plant Cell 2012, 24, 109–122. [Google Scholar] [CrossRef]
- Huang, W.C.; Yu, C.C.; Hu, J. Pentatricopeptide-repeat family protein RF6 functions with hexokinase 6 to rescue rice cytoplasmic male sterility. Proc. Natl. Acad. Sci. USA 2015, 112, 14984–14989. [Google Scholar] [CrossRef]
- Fujii, S.; Toriyama, K. Suppressed expression of retrograde-regulated male sterility restores pollen fertility in cytoplasmic male sterile rice plants. Proc. Natl. Acad. Sci. USA 2009, 106, 9513–9518. [Google Scholar] [CrossRef]
- Itabashi, E.; Iwata, N.; Fujii, S. The fertility restorer gene, Rf2, for Lead Rice-type cytoplasmic male sterility of rice encodes a mitochondrial glycine-rich protein. Plant J. 2011, 65, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Ohta, S.; Murai, N.Y. Map-based cloning of a fertility restorer gene, Rf1, in rice (Oryza sativa L.). Plant J. Cell Mol. Biol. 2004, 37, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Kazama, T.; Toriyama, K. A pentatricopeptide repeat-containing gene that promotes the processing of aberrant atp6 RNA of cytoplasmic male-sterile rice. FEBS Lett. 2003, 544, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Akagi, H.; Nakamura, A.; Yokozeki-Misono, Y. Positional cloning of the rice Rf-1 gene, a restorer of BT-type cytoplasmic male sterility that encodes a mitochondria-targeting PPR protein. Theor. Appl. Genet. 2004, 108, 1449–1457. [Google Scholar] [CrossRef]
- Sun, X.W.; Liu, D.Y.; Zhang, X.F. SLAF-seq: An efficient method of large-scale de novo SNP discovery and genotyping using high-throughput sequencing. PLoS ONE 2013, 8, e58700. [Google Scholar] [CrossRef]
- Abe, A.; Kosugi, S.; Yoshida, K. Genome sequencing reveals agronomically important loci in rice using MutMap. Nat. Biotechnol. 2012, 30, 174–178. [Google Scholar] [CrossRef]
- Hill, J.T.; Demarest, B.L.; Bisgrove, B.W. MMAPPR: Mutation mapping analysis pipeline for pooled RNA-seq. Genome Res. 2013, 23, 687–697. [Google Scholar] [CrossRef]
- Rogers, S.O.; Bendich, A.J. Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Mol. Biol. 1985, 5, 69–76. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Itabashi, E.; Kazama, T.; Toriyama, K. Characterization of cytoplasmic male sterility of rice with Lead Rice cytoplasm in comparison with that with Chinsurah Boro II cytoplasm. Plant Cell Rep. 2009, 28, 233–239. [Google Scholar] [CrossRef]
- Akagi, H.; Yokozeki, Y.; Inagaki, A. A codominant DNA marker closely linked to the rice nuclear restorer gene, Rf1, identified with inter-SSR fingerprinting. Genome 1996, 39, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Liao, Q.P.; Dai, Z.J. Allelic differentiations and effects of the Rf3 and Rf4 genes on fertility restoration in rice with wild abortive cytoplasmic male sterility. Biol. Plant. 2013, 57, 274–280. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, H.; Zhu, H. Rice Rotation System Affects the Spatial Dynamics of the Diazotrophic Community in Paddy Soil of the Yangtze Delta, China. Eurasian Soil. Sci. 2019, 52, 696–706. [Google Scholar] [CrossRef]
- Shinjyo, C.; Nishime, R.; Watanabe, Y. Inheritance of fertility restoring gene Rfx and Rf in male-sterile cytoplasm derived from Lead Rice. Jpn. J. Breed. 1974, 24 (Suppl. S1), 130–131. (In Japanese) [Google Scholar]
- Shinjyo, C.; Watanabe, Y. Allelism test of two genes showing fertility restoring effect for male sterile cytoplasm of Lead Rice and their inheritance. Jpn. J. Breed. 1977, 27 (Suppl. S2), 70–71. (In Japanese) [Google Scholar]
- Kazama, T.; Toriyama, K. A fertility restorer gene, Rf4, widely used for hybrid rice breeding encodes a pentatricopeptide repeat protein. Rice 2014, 7, 28. [Google Scholar] [CrossRef]




| Lines | Cytoplasm | Phenotype | Bagged Spikelet Fertility (%) | Natural Spikelet Fertility (%) |
|---|---|---|---|---|
| BT-9201A | BT | Sterile | 0 | 3.15 |
| 9201B | Normal | Fertile | 54.74 | 89.18 |
| BT-9201A/02428 F1 | BT | Fertile | 12.24 | 41.15 |
| 9201B/02428 F1 | Normal | Fertile | 34.76 | 65.33 |
| BT-LiuqianxinA | BT | Sterile | 0 | 2.27 |
| LiuqianxinB | Normal | Fertile | 63.22 | 96.12 |
| BT-LiuqianxinA/02428 F1 | BT | Fertile | 28.65 | 61.94 |
| LiuqianxinB/02428 F1 | Normal | Fertile | 50.96 | 92.33 |
| 02428 | Normal | Fertile | 57.81 | 94.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Fan, L.; Gu, Y.; Wang, C.; Shi, K.; Qin, Y.; Li, Z.; Liu, Q.; Tang, S.; Zhang, H.; et al. Fine Mapping and Genetic Effect Analysis of Rf21(t) for the Fertility Restoration of Chinsurah-Boro-II-Type Cytoplasmic Male Sterile Oryza sativa (ssp. japonica) Lines. Agronomy 2025, 15, 1690. https://doi.org/10.3390/agronomy15071690
Du Y, Fan L, Gu Y, Wang C, Shi K, Qin Y, Li Z, Liu Q, Tang S, Zhang H, et al. Fine Mapping and Genetic Effect Analysis of Rf21(t) for the Fertility Restoration of Chinsurah-Boro-II-Type Cytoplasmic Male Sterile Oryza sativa (ssp. japonica) Lines. Agronomy. 2025; 15(7):1690. https://doi.org/10.3390/agronomy15071690
Chicago/Turabian StyleDu, Yuanyue, Liying Fan, Yunhua Gu, Chen Wang, Kai Shi, Yebin Qin, Zhejun Li, Qiaoquan Liu, Shuzhu Tang, Honggen Zhang, and et al. 2025. "Fine Mapping and Genetic Effect Analysis of Rf21(t) for the Fertility Restoration of Chinsurah-Boro-II-Type Cytoplasmic Male Sterile Oryza sativa (ssp. japonica) Lines" Agronomy 15, no. 7: 1690. https://doi.org/10.3390/agronomy15071690
APA StyleDu, Y., Fan, L., Gu, Y., Wang, C., Shi, K., Qin, Y., Li, Z., Liu, Q., Tang, S., Zhang, H., & Xu, Z. (2025). Fine Mapping and Genetic Effect Analysis of Rf21(t) for the Fertility Restoration of Chinsurah-Boro-II-Type Cytoplasmic Male Sterile Oryza sativa (ssp. japonica) Lines. Agronomy, 15(7), 1690. https://doi.org/10.3390/agronomy15071690






