Foliar Nitrogen Application Enhances Nitrogen Assimilation and Modulates Gene Expression in Spring Wheat Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Determination of N Concentrations
2.3. Determination of Key Nitrogen Assimilation Enzyme Activities and Total Amino Acid Concentrations
2.4. Transcriptomic Profiling Analysis
2.5. Quantitative Real-Time PCR (qPCR)
2.6. Determination of Grain Protein Concentration and Grain Physical Parameters
2.7. Data Analysis and Visualization
3. Results
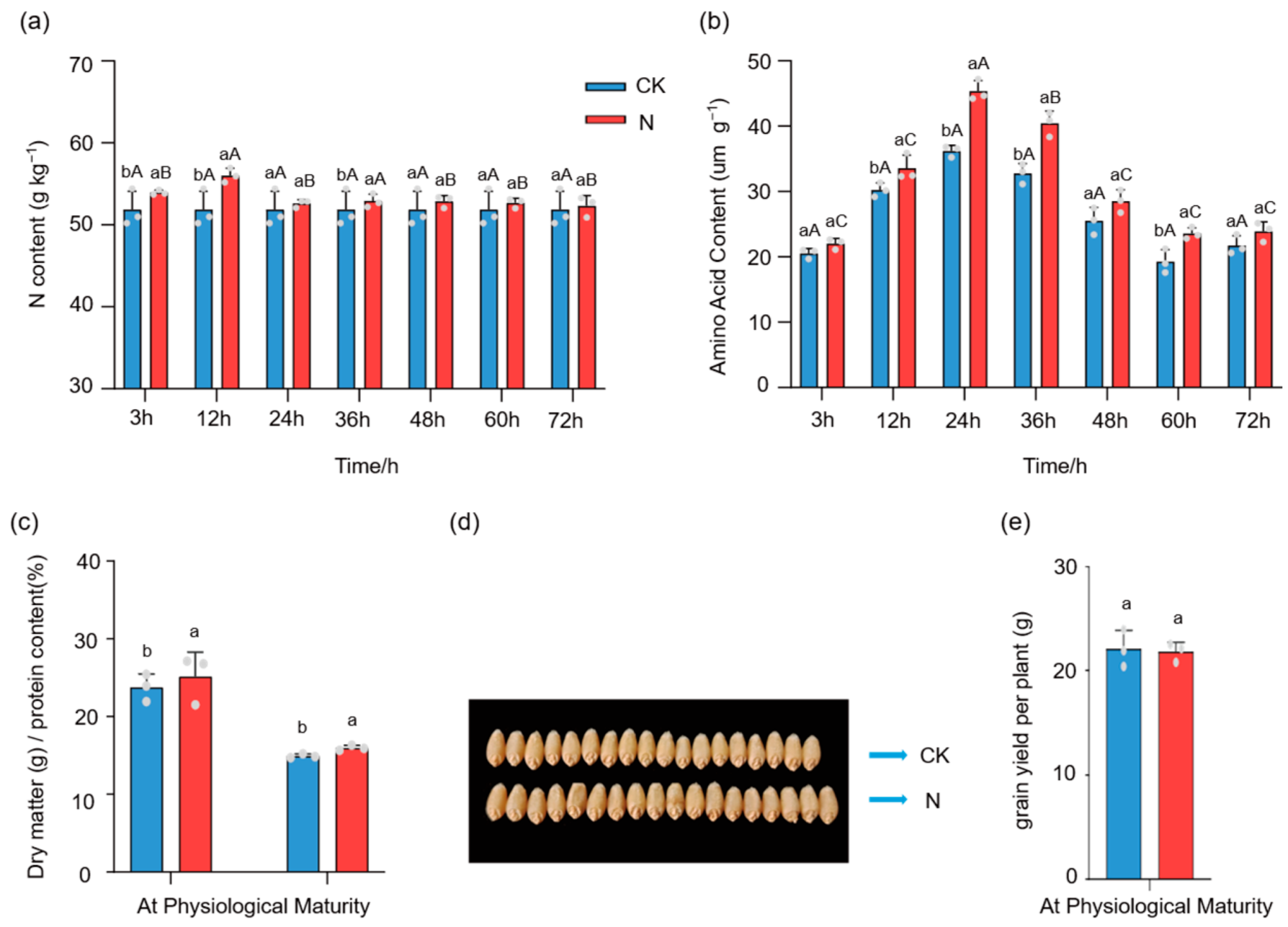
3.1. Nitrogen Assimilation Following Foliar Urea Application to Spring Wheat
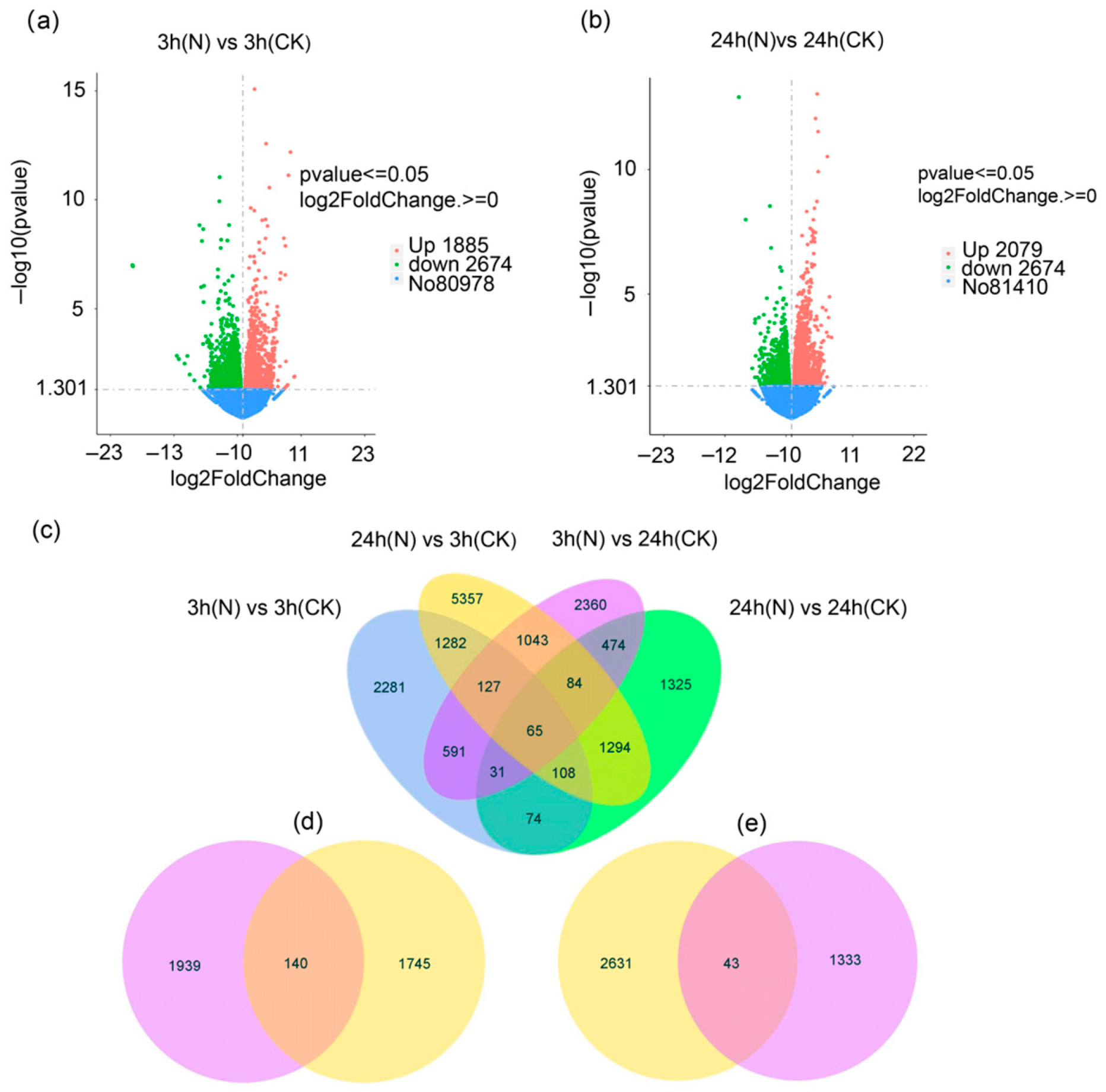
3.2. Transcriptomics Overview
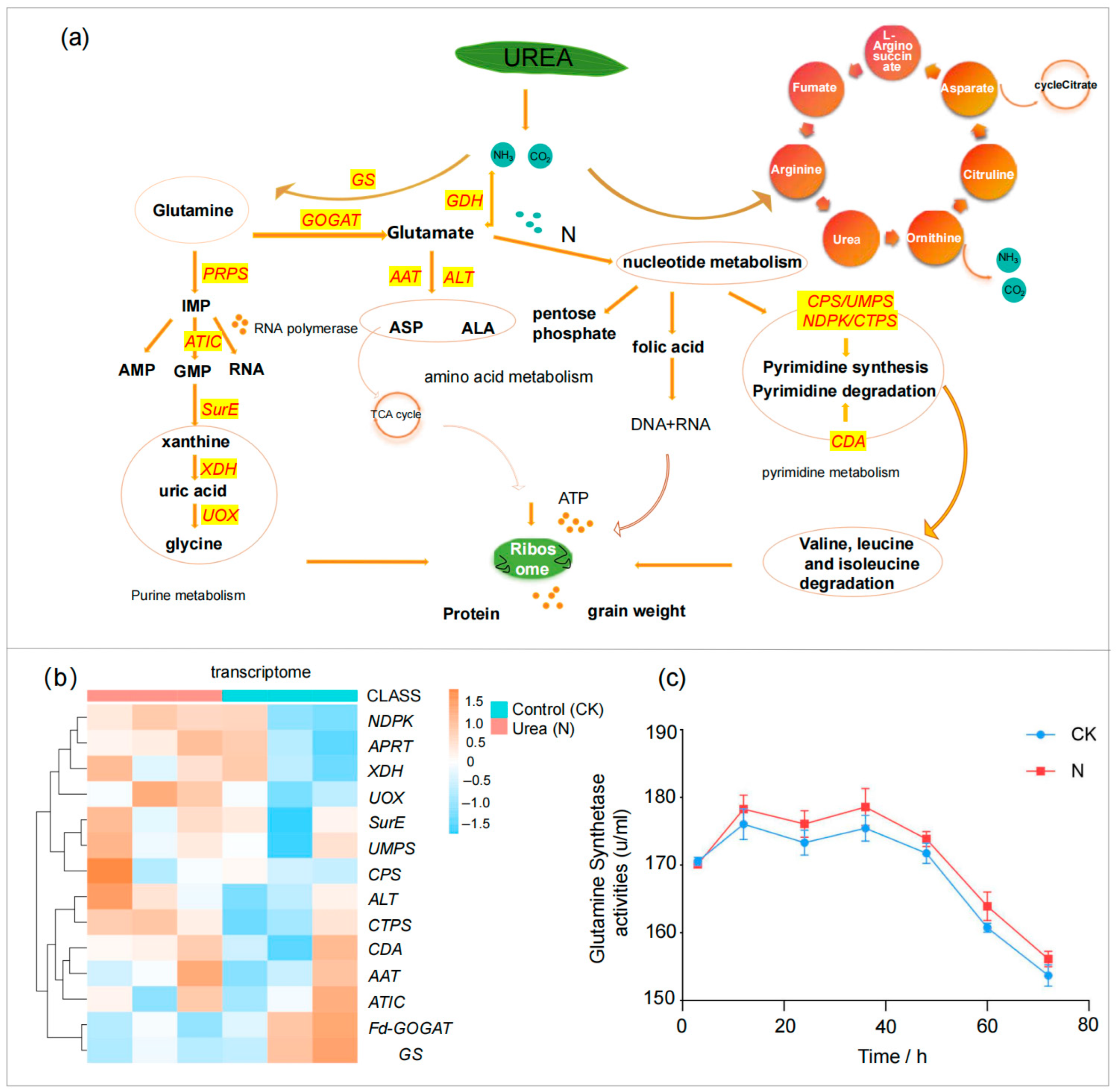
3.3. Effects of Foliar Urea Application on Metabolic Pathways and Associated DEGs
3.4. Dynamic Model of Nitrogen Metabolism in Response to Foliar Application of Urea+
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, J.; Pu, F.; Li, Y.; Xu, J.; Li, N.; Zhang, Y.; Guo, J.; Pan, Z. Assessing the combined effects of climatic factors on spring wheat phenophase and grain yield in Inner Mongolia, China. PLoS ONE 2017, 12, e0185690. [Google Scholar] [CrossRef] [PubMed]
- Mitura, K.; Cacak-Pietrzak, G.; Feledyn-Szewczyk, B.; Szablewski, T.; Studnicki, M. Yield and Grain Quality of Common Wheat (Triticum aestivum L.) Depending on the Different Farming Systems (Organic vs. Integrated vs. Conventional). Plants 2023, 12, 1022. [Google Scholar] [CrossRef]
- Krouk, G.; Kiba, T. Nitrogen and Phosphorus interactions in plants: From agronomic to physiological and molecular insights. Curr. Opin. Plant Biol. 2020, 57, 104–109. [Google Scholar] [CrossRef]
- Krauspe, V.; Timm, S.; Hagemann, M.; Hess, W.R. Phycobilisome breakdown effector NblD is required to maintain cellular amino acid composition during nitrogen starvation. J. Bacteriol. 2022, 204, e00158-21. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhao, J.; Bi, C.; Li, L.; Wang, Z. Transcriptome and Proteomics Analysis of Wheat Seedling Roots Reveals that Increasing NH4+/NO3− Ratio Induced Root Lignification and Reduced Nitrogen Utilization. Front Plant Sci. 2022, 12, 797260. [Google Scholar] [CrossRef] [PubMed]
- Vraný, J.; Vančura, V.; Staněk, M. Control of microorganisms in the rhizosphere of wheat by inoculation of seeds with Pseudomonas putida and by foliar application of urea. Folia Microbiol. 1981, 26, 45–51. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, S.; Wei, Y.; Meng, X.; Wang, X.; Ma, X. The role of glutamine synthetase isozymes in enhancing nitrogen use efficiency of N-efficient winter wheat. Sci. Rep. 2017, 7, 1000. [Google Scholar] [CrossRef]
- Kaya, C.; Ugurlar, F.; Farooq, S.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Combined application of asparagine and thiourea improves tolerance to leadstress in wheat by modulating AsA-GSH cycle, lead detoxification and nitrogen metabolism. Plant Physiol. Biochem. 2022, 190, 119–132. [Google Scholar] [CrossRef]
- Mataffo, A.; Scognamiglio, P.; Dente, A.; Strollo, D.; Colla, G.; Rouphael, Y.; Basile, B. Foliar Application of an Amino Acid-Enriched Urea Fertilizer on ‘Greco’ Grapevines at Full Veraison Increases Berry Yeast-Assimilable Nitrogen Content. Plants 2020, 9, 619. [Google Scholar] [CrossRef]
- Roy, S.; Verma, O. Seed Quality and Storage of Wheat (Triticum aestivum L.) as Influenced by Basal and Foliar Application of Nitrogen. Natl. Acad. Sci. Lett. 2018, 41, 337–340. [Google Scholar] [CrossRef]
- Moradi, L.; Siosemardeh, A. Combination of seed priming and nutrient foliar application improved physiological attributes, grain yield, and biofortification of rainfed wheat. Front. Plant Sci. 2023, 14, 1287677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H. Effects of Late Foliar Nitrogen Application on Wheat Yield, Quality, and Nitrogen Absorption and Utilization. Master’s thesis, Hebei Agricultural University, Baoding, China, 2022. [Google Scholar]
- Zhao, X.; Zhang, W.; Li, S. Molecular mechanisms of wheat response to foliar urea: A transcriptomic approach. Front. Plant Sci. 2021, 12, 691567. [Google Scholar]
- Zhang, Y.; Li, Y.; Jiang, J. Transcriptomic analysis of wheat under nitrogen fertilization: The role of urea in nitrogen metabolism. Sci. Rep. 2020, 10, 12345. [Google Scholar]
- Balotf, S.; Kavoosi, G.; Kholdebarin, B. Nitrate reductase, nitrite reductase, glutamine synthetase, and glutamate synthase expression and activity in response to different nitrogen sources in nitrogen-starved wheat seedlings. Biotechnol. Appl. Biochem. 2016, 63, 220–229. [Google Scholar] [CrossRef]
- Heath-Pagliuso, S.; Huffaker, R.C.; Allard, R.W. Inheritance of nitrite reductase and regulation of nitrate reductase, nitrite reductase, and glutamine synthetase isozymes. Plant Physiol. 1984, 76, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, Z.; Sun, J.; Cui, X.; Liu, Y. Research Progress and Future Development Trends in Medicinal Plant Transcriptomics. Front Plant Sci. 2021, 12, 691838. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Hao, D.-L.; Song, Z.-Z.; Yang, G.-Z.; Wang, L.; Su, Y.-H. RNA-Seq analysis of differentially expressed genes in rice under varied nitrogen supplies. Gene 2015, 555, 305–317. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Quan, X.; Zeng, J.; Ye, L.; Chen, G.; Han, Z.; Shah, J.M.; Zhang, G. Transcriptome profiling analysis for two Tibetan wild barley genotypes in responses to low nitrogen. BMC Plant Biol. 2016, 16, 30. [Google Scholar] [CrossRef]
- Wang, P.; Fu, C.; Wang, L.; Yan, T. Delayed autumnal leaf senescence following nutrient fertilization results in altered nitrogen resorption. Tree Physiol. 2022, 42, 1549–1559. [Google Scholar] [CrossRef]
- Leite, J.M.; Pitumpe Arachchige, P.S.; Ciampitti, I.A.; Hettiarachchi, G.M.; Maurmann, L.; Trivelin, P.C.O.; Prasad, P.V.V.; Sunoj, S.V.J. Co-addition of humic substances and humic acids with urea enhances foliar nitrogen use efficiency in sugarcane (Saccharum officinarum L.). Heliyon 2020, 6, e05100. [Google Scholar] [CrossRef] [PubMed]
- Škarpa, P.; Antošovský, J.; Ryant, P.; Hammerschmiedt, T.; Kintl, A.; Brtnický, M. Using Waste Sulfur from Biogas Production in Combination with Nitrogen Fertilization of Maize (Zea mays L.) by Foliar Application. Plants 2021, 10, 2188. [Google Scholar] [CrossRef]
- Kumar, N.; Tripathi, S.C.; Yadav, D.B.; Samota, S.R.; Venkatesh, K.; Sareen, S.; Singh, G. Boosting wheat yield, profitability and NUE with prilled and nano urea in conservation tillage. Sci Rep. 2023, 13, 18073. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.L.; Toscano, N.C.; Madore, M.A. Effect of urea fertilizer application on soluble protein and free amino acid content of cotton petioles in relation to silverleaf whitefly (Bemisia argentifolii) populations. J. Chem. Ecol. 2003, 29, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Moreno, N.; Moler, J.A.; Palacios, M.B.; Esparza, I.; Nieto-Rojo, R.; Ancín-Azpilicueta, C. Foliar application of urea to Tempranillo vines increased the amino acid concentration of the must. Food Addit. Contam. Part A 2019, 37, 216–227. [Google Scholar] [CrossRef]
- Pérez-Álvarez, E.P.; Garde-Cerdán, T.; García-Escudero, E.; Martínez-Vidaurre, J.M. Effect of two doses of urea foliar application on leaves and grape nitrogen composition during two vintages. J. Sci. Food Agric. 2016, 97, 2524–2532. [Google Scholar] [CrossRef]
- Chen, J.J.; Lin, X.; Gu, S.B.; Wang, W.Y.; Zhang, B.J.; Zhu, J.K.; Wang, D. Effects of foliar urea application post-anthesis on nitrogen uptake, utilization, and yield of winter wheat. Acta Agron. Sin. 2023, 49, 277–285. [Google Scholar]
- Chen, X.; Wang, M.; Kroeze, C.; Chen, X.; Ma, L.; Chen, X.; Shi, X.; Strokal, M. Nitrogen in the Yangtze River Basin: Pollution Reduction through Coupling Crop and Livestock Production. Environ. Sci. Technol. 2022, 56, 17591–17603. [Google Scholar] [CrossRef]
- Whittington, S.J.; Chellgren, B.W.; Hermann, V.M.; Creamer, T.P. Urea Promotes Polyproline II Helix Formation: Implications for Protein Denatured States. Biochemistry 2005, 44, 6269–6275. [Google Scholar] [CrossRef]
- Mugita, Y.; Nakagami, G.; Minematsu, T.; Kitamura, A.; Sanada, H. Combination of urease inhibitor and antiseptic inhibits urea decomposition-induced ammonia production by Proteus mirabilis. Int. Wound J. 2020, 17, 1558–1565. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.-C. Nitrogen Journey in Plants: From Uptake to Metabolism, Stress Response, and Microbe Interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef] [PubMed]
- Travis, B.A.; Peck, J.V.; Salinas, R.; Dopkins, B.; Lent, N.; Nguyen, V.D.; Borgnia, M.J.; Brennan, R.G.; Schumacher, M.A. Molecular dissection of the glutamine synthetase-GlnR nitrogen regulatory circuitry in Gram-positive bacteria. Nat. Commun. 2022, 13, 3793. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-Y.; Jeong, J.S.; Lim, J.Y.; Kim, T.; Park, J.H.; Kim, J.-K.; Shin, C. Transcriptomic analyses of rice (Oryza sativa) genes and non-coding RNAs under nitrogen starvation using multiple omics technologies. BMC Genom. 2018, 19, 1–20. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Yao, L.; Li, B.; Ma, X.; Si, E.; Yang, K.; Li, C.; Shang, X.; Meng, Y.; et al. Transcriptome and Metabolome Reveal the Molecular Mechanism of Barley Genotypes Underlying the Response to Low Nitrogen and Resupply. Int. J. Mol. Sci. 2023, 24, 4706. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Ma, W.; Jin, X.; Liu, G.; Li, Y.; Liu, B.; Cao, D. Foliar Nitrogen Application Enhances Nitrogen Assimilation and Modulates Gene Expression in Spring Wheat Leaves. Agronomy 2025, 15, 1688. https://doi.org/10.3390/agronomy15071688
Yao Y, Ma W, Jin X, Liu G, Li Y, Liu B, Cao D. Foliar Nitrogen Application Enhances Nitrogen Assimilation and Modulates Gene Expression in Spring Wheat Leaves. Agronomy. 2025; 15(7):1688. https://doi.org/10.3390/agronomy15071688
Chicago/Turabian StyleYao, Yanlin, Wenyan Ma, Xin Jin, Guangrui Liu, Yun Li, Baolong Liu, and Dong Cao. 2025. "Foliar Nitrogen Application Enhances Nitrogen Assimilation and Modulates Gene Expression in Spring Wheat Leaves" Agronomy 15, no. 7: 1688. https://doi.org/10.3390/agronomy15071688
APA StyleYao, Y., Ma, W., Jin, X., Liu, G., Li, Y., Liu, B., & Cao, D. (2025). Foliar Nitrogen Application Enhances Nitrogen Assimilation and Modulates Gene Expression in Spring Wheat Leaves. Agronomy, 15(7), 1688. https://doi.org/10.3390/agronomy15071688




