Effect of Energycane Integration on Ground-Dwelling Arthropod Biodiversity in a Sugarcane-Sweet Corn Cropping System
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Design
2.2. Soil Biodiversity Sampling Using Pitfall Traps
2.3. Soil Biodiversity Sample Processing
2.4. Statistical Analysis
3. Results
3.1. Daily Traps Catch Across Crop Types and Sampling Times
3.2. Soil Arthropods Distribution in Taxonomic Orders
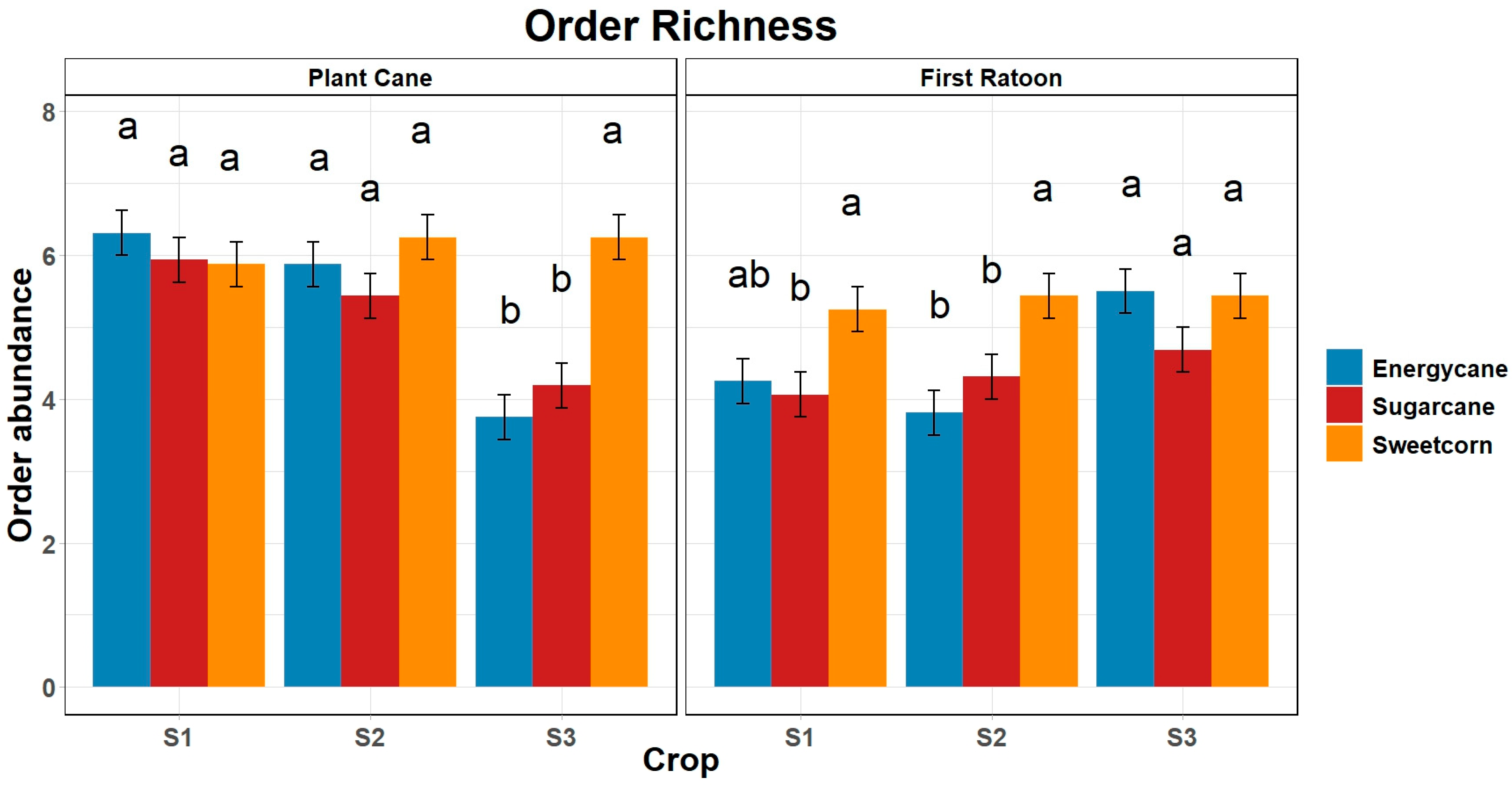
3.3. Order Richness
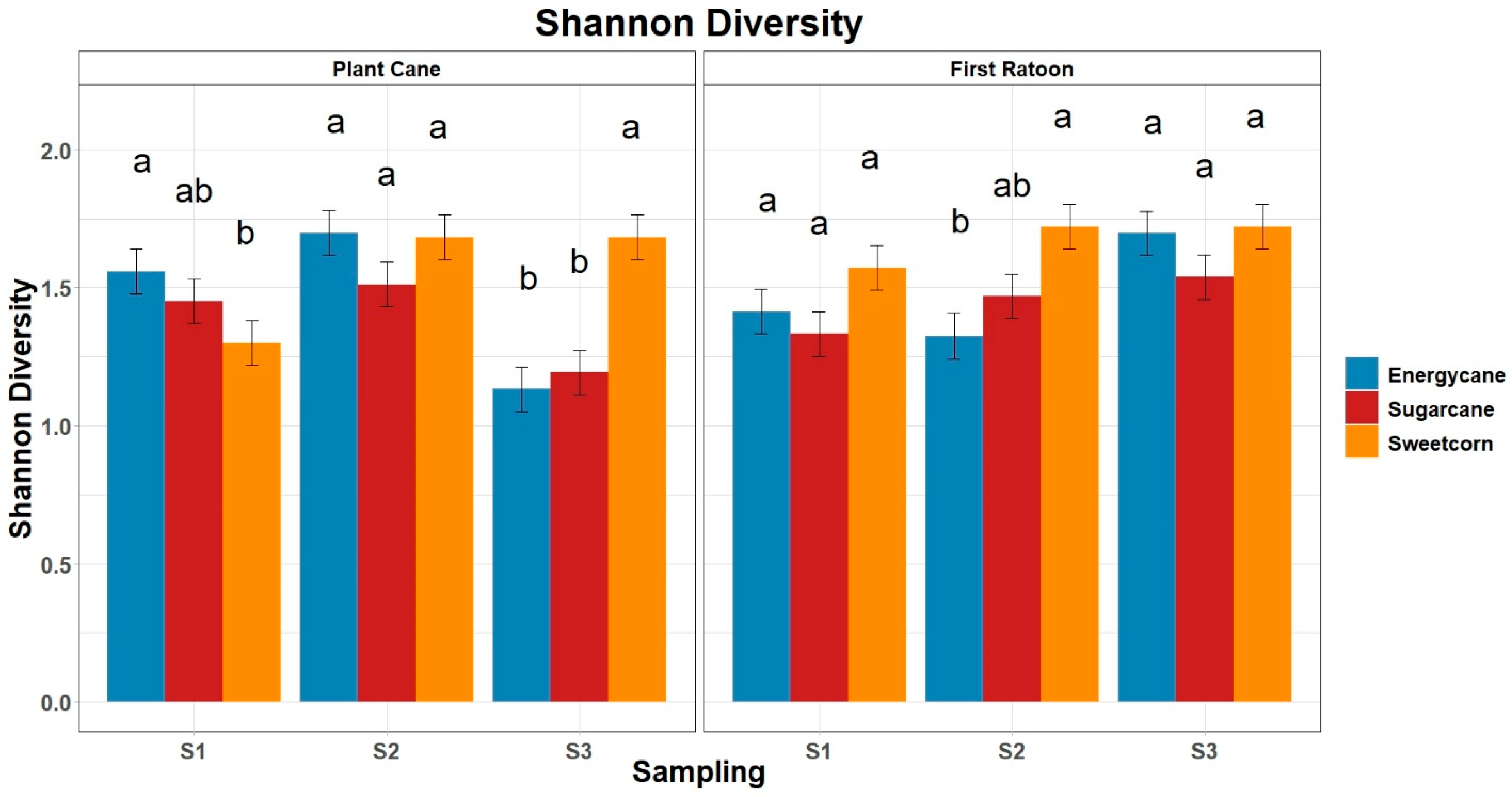
3.4. Shannon Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EC | Energycane |
| SC | Sugarcane |
| SW | Sweetcorn was grown |
| SWF | Sweetcorn Fallow plots |
| SWRC | Sweetcorn as Rotation Crop |
| EAA | Everglades Agricultural Area |
| ILM | Integrated Landscape Management |
References
- McDonald, R.I.; Fargione, J.; Kiesecker, J.; Miller, W.M.; Powell, J. Energy sprawl or energy efficiency: Climate policy impacts on natural habitat for the United States of America. PLoS ONE 2009, 4, e6802. [Google Scholar] [CrossRef] [PubMed]
- Edwards, F.A.; Edwards, D.P.; Larsen, T.H.; Hsu, W.W.; Benedick, S.; Chung, A.; Vun Khen, C.; Wilcove, D.S.; Hamer, K.C. Does logging and forest conversion to oil palm agriculture alter functional diversity in a biodiversity hotspot? Anim. Conserv. 2014, 17, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Immerzeel, D.J.; Verweij, P.A.; Van Der Hilst, F.; Faaij, A.P. Biodiversity impacts of bioenergy crop production: A state-of-the-art review. GCB Bioenergy 2014, 6, 183–209. [Google Scholar] [CrossRef]
- Dornburg, V.; van Vuuren, D.; van de Ven, G.; Langeveld, H.; Meeusen, M.; Banse, M.; van Oorschot, M.; Ros, J.; van den Born, G.J.; Aiking, H. Bioenergy revisited: Key factors in global potentials of bioenergy. Energy Environ. Sci. 2010, 3, 258–267. [Google Scholar] [CrossRef]
- MEa, M.E.A. Ecosystems and Human Well-Being: Wetlands and Water Synthesis; World Resources Institute: Washington, DC, USA, 2005. [Google Scholar]
- Hudson, L.N.; Newbold, T.; Contu, S.; Hill, S.L.; Lysenko, I.; De Palma, A.; Phillips, H.R.; Alhusseini, T.I.; Bedford, F.E.; Bennett, D.J. The database of the PREDICTS (projecting responses of ecological diversity in changing terrestrial systems) project. Ecol. Evol. 2017, 7, 145–188. [Google Scholar] [CrossRef]
- Smeets, E.M.; Bouwman, L.F.; Stehfest, E.; Van Vuuren, D.P.; Posthuma, A. Contribution of N2O to the greenhouse gas balance of first-generation biofuels. Glob. Change Biol. 2009, 15, 1–23. [Google Scholar] [CrossRef]
- Wicke, B.; Van Der Hilst, F.; Daioglou, V.; Banse, M.; Beringer, T.; Gerssen-Gondelach, S.; Heijnen, S.; Karssenberg, D.; Laborde, D.; Lippe, M. Model collaboration for the improved assessment of biomass supply, demand, and impacts. GCB Bioenergy 2015, 7, 422–437. [Google Scholar] [CrossRef]
- Beringer, T.; Lucht, W.; Schaphoff, S. Bioenergy production potential of global biomass plantations under environmental and agricultural constraints. GCB Bioenergy 2011, 3, 299–312. [Google Scholar] [CrossRef]
- Chum, H.L.; Nigro, F.; McCormick, R.; Beckham, G.; Seabra, J.; Saddler, J.; Tao, L.; Warner, E.; Overend, R. Conversion technologies for biofuels and their use. Bioenergy Sustain. Bridg. Gaps 2015, 72, 374–467. [Google Scholar]
- Van Dam, J.; Faaij, A.; Hilbert, J.; Petruzzi, H.; Turkenburg, W. Large-scale bioenergy production from soybeans and switchgrass in Argentina: Part B. Environmental and socio-economic impacts on a regional level. Renew. Sustain. Energy Rev. 2009, 13, 1679–1709. [Google Scholar] [CrossRef]
- Dauber, J.; Jones, M.B.; Stout, J.C. The impact of biomass crop cultivation on temperate biodiversity. GCB Bioenergy 2010, 2, 289–309. [Google Scholar] [CrossRef]
- Fitzherbert, E.B.; Struebig, M.J.; Morel, A.; Danielsen, F.; Brühl, C.A.; Donald, P.F.; Phalan, B. How will oil palm expansion affect biodiversity? Trends Ecol. Evol. 2008, 23, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.; Daoust, G.; Bigué, B. Negative or positive effects of plantation and intensive forestry on biodiversity: A matter of scale and perspective. For. Chron. 2010, 86, 354–364. [Google Scholar] [CrossRef]
- Berndes, G.; Hansson, J.; Egeskog, A.; Johnsson, F. Strategies for 2nd generation biofuels in EU–Co-firing to stimulate feedstock supply development and process integration to improve energy efficiency and economic competitiveness. Biomass Bioenergy 2010, 34, 227–236. [Google Scholar] [CrossRef]
- Haughton, A.J.; Bohan, D.A.; Clark, S.J.; Mallott, M.D.; Mallott, V.; Sage, R.; Karp, A. Dedicated biomass crops can enhance biodiversity in the arable landscape. GCB Bioenergy 2016, 8, 1071–1081. [Google Scholar] [CrossRef]
- Werling, B.P.; Dickson, T.L.; Isaacs, R.; Gaines, H.; Gratton, C.; Gross, K.L.; Liere, H.; Malmstrom, C.M.; Meehan, T.D.; Ruan, L. Perennial grasslands enhance biodiversity and multiple ecosystem services in bioenergy landscapes. Proc. Natl. Acad. Sci. USA 2014, 111, 1652–1657. [Google Scholar] [CrossRef]
- Helms IV, J.A.; Ijelu, S.E.; Wills, B.D.; Landis, D.A.; Haddad, N.M. Ant biodiversity and ecosystem services in bioenergy landscapes. Agric. Ecosyst. Environ. 2020, 290, 106780. [Google Scholar] [CrossRef]
- Haan, N.L.; Benucci, G.N.; Fiser, C.M.; Bonito, G.; Landis, D.A. Contrasting effects of bioenergy crops on biodiversity. Sci. Adv. 2023, 9, eadh7960. [Google Scholar] [CrossRef] [PubMed]
- Manning, P.; Gossner, M.M.; Bossdorf, O.; Allan, E.; Zhang, Y.-Y.; Prati, D.; Blüthgen, N.; Boch, S.; Böhm, S.; Börschig, C. Grassland management intensification weakens the associations among the diversities of multiple plant and animal taxa. Ecology 2015, 96, 1492–1501. [Google Scholar] [CrossRef]
- Sang, T.; Zhu, W. China’s bioenergy potential. GCB Bioenergy 2011, 3, 79–90. [Google Scholar] [CrossRef]
- Nunes, D.H.; Pasini, A.; Benito, N.P.; Brown, G.G. Earthworm diversity in four land use systems in the region of Jaguapitã, Paraná State, Brazil. Caribb. J. Sci. 2006, 42, 331. [Google Scholar]
- Gordon, V.S.; Comstock, J.C.; Sandhu, H.S.; Gilbert, R.A.; Sood, S.; Korndorfer, P.; El-Hout, N.; Arundale, R. Registration of ‘UFCP 84-1047’ Sugarcane for Use as a Biofuel Feedstock. J. Plant Regist. 2016, 10, 251–257. [Google Scholar] [CrossRef]
- Gordon, V.S.; Comstock, J.C.; Sandhu, H.S.; Gilbert, R.A.; Sood, S.G.; Korndorfer, P.; El-Hout, N.; Arundale, R. Registration of ‘UFCP 87-0053’ sugarcane for use as a biofuel feedstock. J. Plant Regist. 2016, 10, 258–264. [Google Scholar] [CrossRef]
- Sandhu, H.S.; Gilbert, R.A.; Comstock, J.C.; Gordon, V.S.; Korndörfer, P.; Arundale, R.A.; El-Hout, N. Registration of ‘UFCP 82-1655’ sugarcane. J. Plant Regist. 2016, 10, 22–27. [Google Scholar] [CrossRef]
- Duersch, B.G.; Bhadha, J.H.; Root, T.L.; Louda, J.W. The role of rice (Oryza sativa L.) in sequestering phosphorus compounds and trace elements: Speciation and dynamics. Sci. Total Environ. 2020, 725, 138366. [Google Scholar] [CrossRef]
- Sandhu, H.; Davidson, W. Sugarcane Cultivars Descriptive Fact Sheet: CP 96-1252, CP 01-1372 and CP 00-1101: SS-AGR-410/SC102, 12/2016. EDIS 2017, 2017, 1–4. [Google Scholar] [CrossRef]
- Brown, G.R.; Matthews, I.M. A review of extensive variation in the design of pitfall traps and a proposal for a standard pitfall trap design for monitoring ground-active arthropod biodiversity. Ecol. Evol. 2016, 6, 3953–3964. [Google Scholar] [CrossRef]
- Borror, D.; Triplehorn, C.; Johnson, N. An Introduction to the Study of Insects, 6th ed.; Saunders College: Philadelphia, PA, USA, 1989. [Google Scholar]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Williams, M.A.; Feest, A. The effect of Miscanthus cultivation on the biodiversity of ground beetles (Coleoptera: Carabidae), spiders and harvestmen (Arachnida: Araneae and Opiliones). Agric. Sci. 2019, 10, 903–917. [Google Scholar] [CrossRef]
- The R Core Team. R: A Language and Environment for Statistical Computing, version 4.1.2; R Foundation for Statistical Computing: Vienna, Austria, 2024.
- Pinheiro, H.P.; Rodrigues-Motta, M.; Franco, G. Modelling performance of students with generalized linear mixed models. In Proceedings of the 29th International Workshop on Statistical Modelling, Göttingen, Germany, 14–18 July 2014; pp. 133–136. [Google Scholar]
- Lenth, R.V. Emmeans: Estimated Marginal Means, aka Least-Squares Means. 2021. Available online: https://rdrr.io/cran/emmeans/ (accessed on 7 July 2025).
- Mohr, A.; Raman, S. Lessons from first generation biofuels and implications for the sustainability appraisal of second generation biofuels. Energy Policy 2013, 63, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Tudge, S.J.; Purvis, A.; De Palma, A. The impacts of biofuel crops on local biodiversity: A global synthesis. Biodivers. Conserv. 2021, 30, 2863–2883. [Google Scholar] [CrossRef]
- Núñez-Regueiro, M.M.; Siddiqui, S.F.; Fletcher, R.J., Jr. Effects of bioenergy on biodiversity arising from land-use change and crop type. Conserv. Biol. 2021, 35, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Hasibuan, R.; Cindowarni, O.; Lumbanraja, J.; Lumbanraja, F.R. Impact of soil fertilization on arthropod abundance and diversity on soybean agroecosystem. J. Biodiversitas 2022, 23, 1828–1835. [Google Scholar] [CrossRef]
- Sandhu, H.S.; Cherry, R.H. Effects of tillage practices on arthropod ground predators in Florida sugarcane. J. Entomol. Sci. 2014, 49, 415–419. [Google Scholar] [CrossRef]
- Platen, R.; Konrad, J.; Glemnitz, M. Novel energy crops: An opportunity to enhance the biodiversity of arthropod assemblages in biomass feedstock cultures? Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2017, 13, 162–171. [Google Scholar] [CrossRef]
- Brooks, D.R.; Clark, S.J.; Perry, J.N.; Bohan, D.A.; Champion, G.T.; Firbank, L.G.; Haughton, A.J.; Hawes, C.; Heard, M.S.; Woiwod, I.P. Invertebrate biodiversity in maize following withdrawal of triazine herbicides. Proc. R. Soc. B Biol. Sci. 2005, 272, 1497–1502. [Google Scholar] [CrossRef]
- Ebeling, A.; Hines, J.; Hertzog, L.R.; Lange, M.; Meyer, S.T.; Simons, N.K.; Weisser, W.W. Plant diversity effects on arthropods and arthropod-dependent ecosystem functions in a biodiversity experiment. Basic. Appl. Ecol. 2018, 26, 50–63. [Google Scholar] [CrossRef]
- Semere, T.; Slater, F.M. Ground flora, small mammal and bird species diversity in miscanthus (Miscanthus × giganteus) and reed canary-grass (Phalaris arundinacea) fields. Biomass Bioenergy 2007, 31, 20–29. [Google Scholar] [CrossRef]
- Batáry, P.; Dicks, L.V.; Kleijn, D.; Sutherland, W.J. The role of agri-environment schemes in conservation and environmental management. Conserv. Biol. 2015, 29, 1006–1016. [Google Scholar] [CrossRef]
- Evans, J.R. Improving photosynthesis. Plant Physiol. 2013, 162, 1780–1793. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, J.; Panassitm, B.; Heine, F.; Wolfrum, S.; Moriniere, J. Perennial alternative crops for biogas production increase arthropod abundance and diversity after harvest–results of suction sampling and metabarcoding. Eur. J. Entomol. 2023, 120, 59–69. [Google Scholar] [CrossRef]
- Landis, D.A.; Werling, B.P. Arthropods and biofuel production systems in North America. Insect Sci. 2010, 17, 220–236. [Google Scholar] [CrossRef]
- dos Santos, L.A.O.; Naranjo-Guevara, N.; Fernandes, O.A. Diversity and abundance of edaphic arthropods associated with conventional and organic sugarcane crops in Brazil. Fla. Entomol. 2017, 100, 134–144. [Google Scholar] [CrossRef]
- Silva, A.B.D.; Batista, J.D.L.; Brito, C.H.D. Capacidade predatória de Euborellia annulipes (Lucas, 1847) sobre Spodoptera frugiperda (Smith, 1797). Acta Sci. Agron. 2009, 31, 7–11. [Google Scholar] [CrossRef]
- Schatz, B.; Kjellberg, F.; Nyawa, S.; Hossaert-McKey, M. Fig wasps: A staple food for ants on Ficus. Biotropica 2008, 40, 190–195. [Google Scholar] [CrossRef]
- Li, S.; Barreiro, A.; Almeida, J.P.; Prade, T.; Mårtensson, L.-M.D. Perennial crops shape the soil microbial community and increase the soil carbon in the upper soil layer. Soil. Biol. Biochem. 2025, 200, 109621. [Google Scholar] [CrossRef]
- Burmeister, J. Promotion of ground beetles by integrating perennial energy crops into existing agricultural landscapes. Biomass Bioenergy 2021, 146, 105973. [Google Scholar] [CrossRef]
- Radzikowski, P.; Matyka, M.; Berbeć, A.K. Biodiversity of weeds and arthropods in five different perennial industrial crops in eastern Poland. Agriculture 2020, 10, 636. [Google Scholar] [CrossRef]
- Lask, J.; Magenau, E.; Ferrarini, A.; Kiesel, A.; Wagner, M.; Lewandowski, I. Perennial rhizomatous grasses: Can they really increase species richness and abundance in arable land?—A meta-analysis. GCB Bioenergy 2020, 12, 968–978. [Google Scholar] [CrossRef]
- Altieri, M.; Whitcomb, W. Manipulation of insect populations through seasonal disturbance of weed communities. Prot. Ecol. 1979, 1, 185–202. [Google Scholar]
- Chao, A.; Chiu, C.-H.; Jost, L. Unifying species diversity, phylogenetic diversity, functional diversity, and related similarity and differentiation measures through Hill numbers. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 297–324. [Google Scholar] [CrossRef]



| Time of Traps Deployment | Duration of Deployment (Days) | EC | SC | SW | EC | SC | SW | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PC | FR | PC | FR | PC | FR | PC | FR | Total | PC | FR | PC | FR | PC | FR | Total | |
| After Planting / After Regrowth (S1) | 30 | 15 | 16 | 16 | 16 | 16 | 16 | 16 | 96 | 1271 | 518 | 1107 | 508 | 1460 | 538 | 5402 |
| Mid-Seasonal (S2) | 30 | 15 | 16 | 16 | 16 | 16 | 16 | 16 | 96 | 859 | 512 | 1111 | 473 | 1382 | 618 | 4955 |
| Before harvesting (S3) | 30 | 15 | 16 | 16 | 16 | 16 | 16 | 16 | 96 | 675 | 668 | 529 | 474 | 1300 | 602 | 4248 |
| Total | 90 | 45 | 48 | 48 | 48 | 48 | 48 | 48 | 288 | 2805 | 1698 | 2747 | 1455 | 4142 | 1758 | 14,605 |
| Response Variable | Fixed Effect (p-Values) | ||||||
|---|---|---|---|---|---|---|---|
| Crop (C) | Treatment (T) | Sampling Time (S) | C * T | C * S | T * S | C * T * S | |
| Daily Trap Catch | <0.001 | 0.837 | <0.001 | 0.436 | 0.293 | 0.961 | 0.998 |
| Order Richness | <0.001 | 0.238 | <0.001 | 0.63 | <0.0001 | 0.94 | 0.99 |
| Shannon Diversity | 0.046 | 0.935 | <0.001 | 0.804 | <0.001 | 0.938 | 0.989 |
| Response Variable | Fixed Effect (p-Values) | ||||||
|---|---|---|---|---|---|---|---|
| Crop (C) | Treatment (T) | Sampling Time (S) | C * T | C * S | T * S | C * T * S | |
| Daily Trap Catch | 0.024 | 0.244 | 0.785 | 0.267 | 0.591 | 0.426 | 0.804 |
| Order Richness | 0.001 | 0.09 | 0.003 | 0.83 | 0.04 | 0.601 | 0.966 |
| Shannon Diversity | <0.001 | 0.197 | <0.001 | 0.826 | 0.086 | 0.583 | 0.689 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.S.; Lesmes-Vesga, R.A.; Kaur, S.; Singh, H.; Sandhu, H.S. Effect of Energycane Integration on Ground-Dwelling Arthropod Biodiversity in a Sugarcane-Sweet Corn Cropping System. Agronomy 2025, 15, 1685. https://doi.org/10.3390/agronomy15071685
Sharma AS, Lesmes-Vesga RA, Kaur S, Singh H, Sandhu HS. Effect of Energycane Integration on Ground-Dwelling Arthropod Biodiversity in a Sugarcane-Sweet Corn Cropping System. Agronomy. 2025; 15(7):1685. https://doi.org/10.3390/agronomy15071685
Chicago/Turabian StyleSharma, Amandeep Sahil, Ricardo A. Lesmes-Vesga, Simranjot Kaur, Hardeep Singh, and Hardev Singh Sandhu. 2025. "Effect of Energycane Integration on Ground-Dwelling Arthropod Biodiversity in a Sugarcane-Sweet Corn Cropping System" Agronomy 15, no. 7: 1685. https://doi.org/10.3390/agronomy15071685
APA StyleSharma, A. S., Lesmes-Vesga, R. A., Kaur, S., Singh, H., & Sandhu, H. S. (2025). Effect of Energycane Integration on Ground-Dwelling Arthropod Biodiversity in a Sugarcane-Sweet Corn Cropping System. Agronomy, 15(7), 1685. https://doi.org/10.3390/agronomy15071685







