Machine Learning-Assisted NIR Spectroscopy for Dynamic Monitoring of Leaf Potassium in Korla Fragrant Pear
Abstract
1. Introduction
1.1. The Core Regulatory Role of Potassium Ions (K+) in Korla Pear Quality and the Advantages of Near-Infrared Detection
1.2. Research Status
1.2.1. Research on Plant Nutritional Physiology
1.2.2. Application of Near-Infrared Spectroscopy Technology
1.3. Research Content and Value
2. Materials and Methods
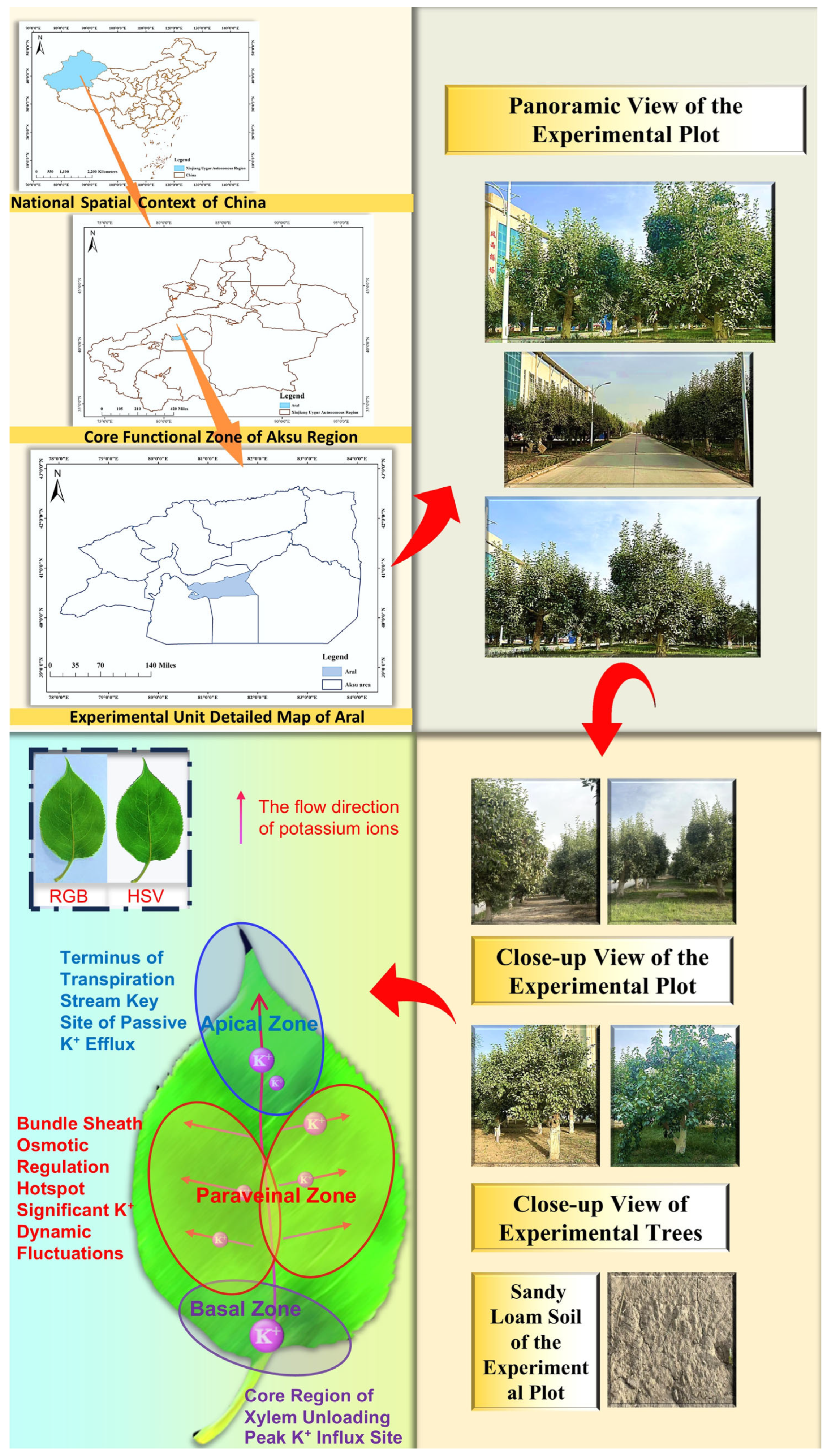
2.1. Survey of Test Sites and Materials
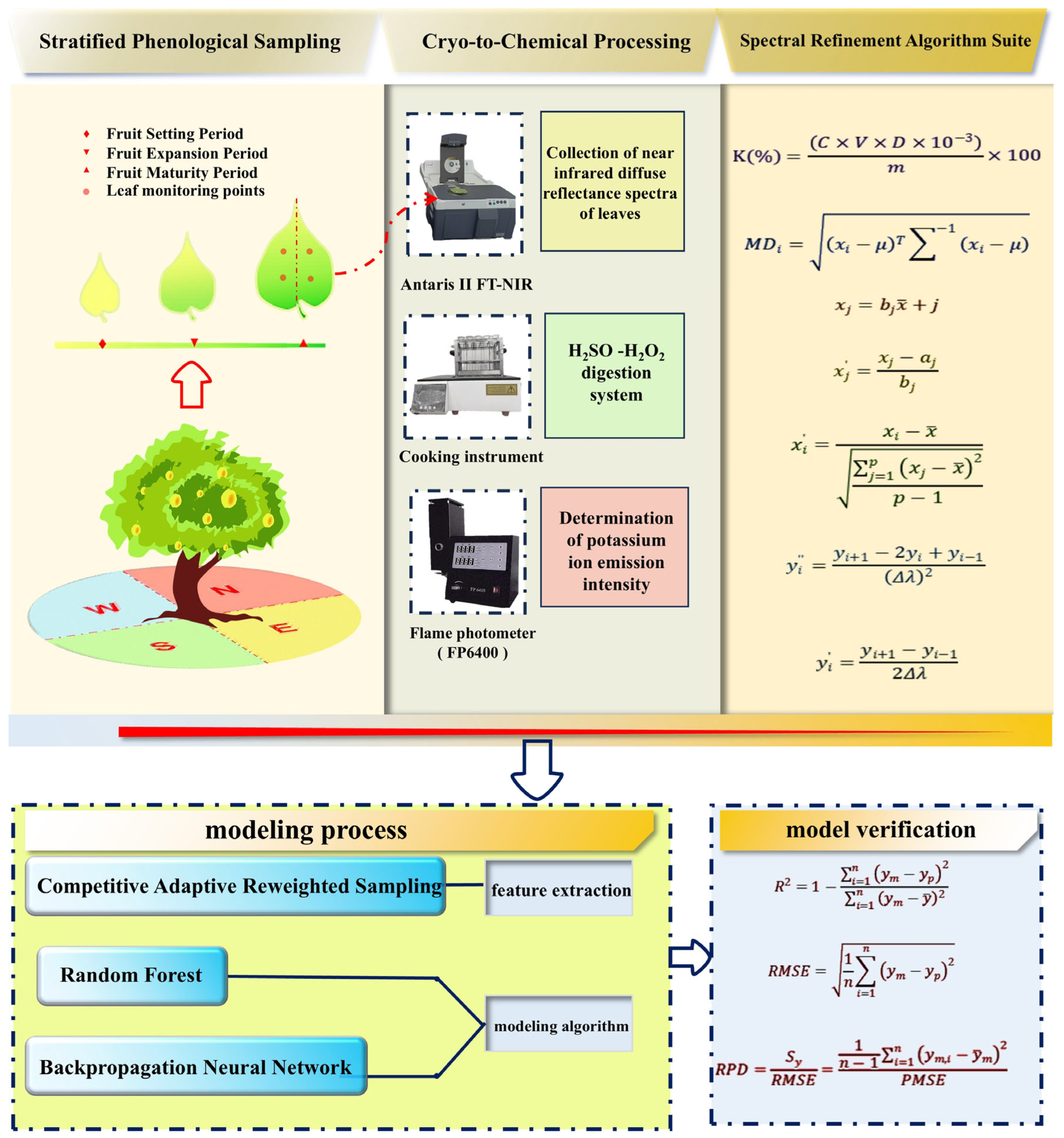
2.2. Sample Collection
2.3. Original Spectral Data Acquisition
2.4. Determination of Total Potassium in Korla Pear Leaves
2.5. A Method for Eliminating Spectral Outliers
2.6. Spectral Pretreatment Methods
2.7. Feature Extraction
2.8. Dataset Partitioning
2.9. Modeling Algorithm
2.10. Model Evaluation Method
3. Results and Analysis
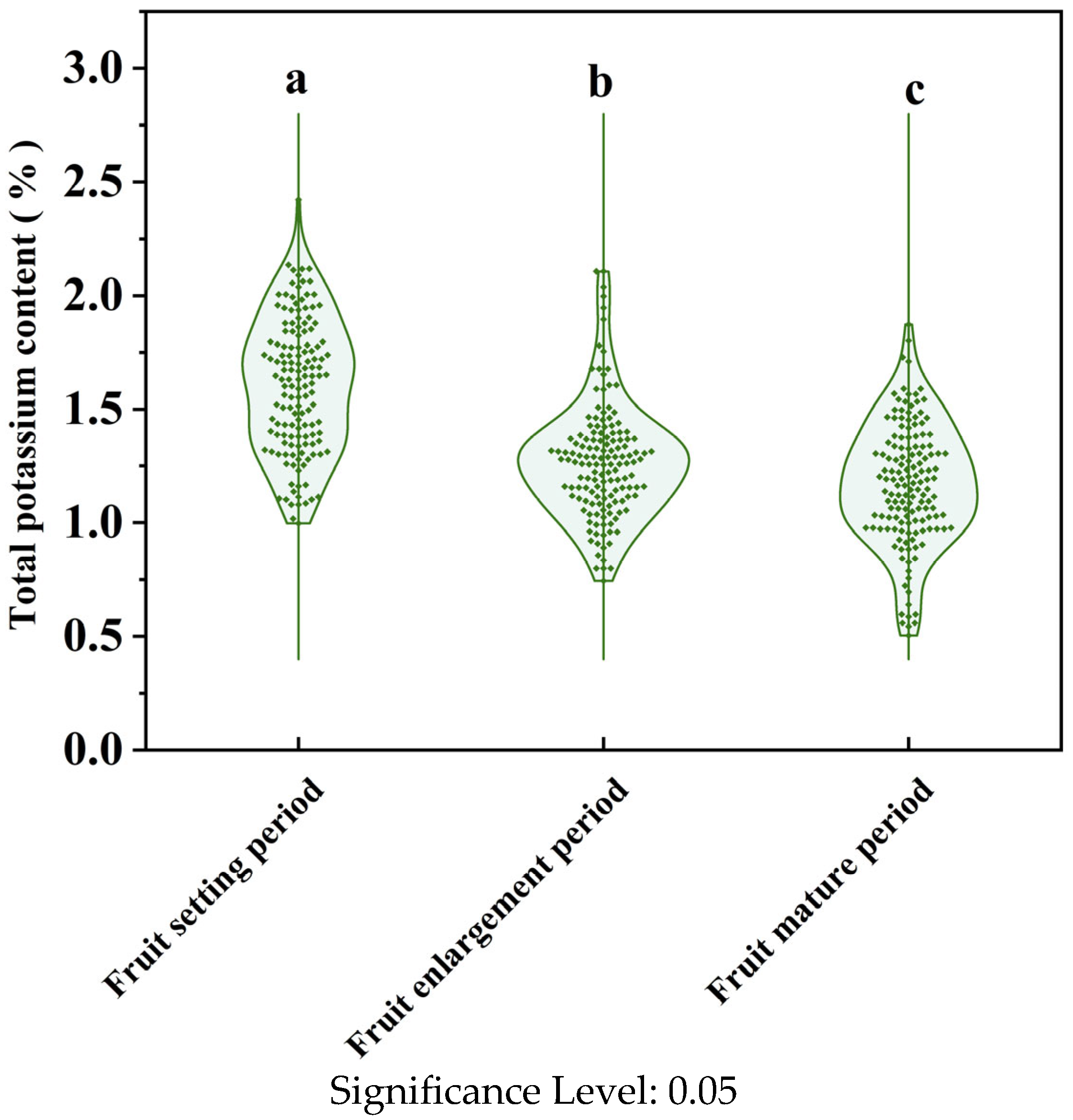
3.1. Analysis of Total Potassium Content in Leaves of Korla Pears at Different Periods
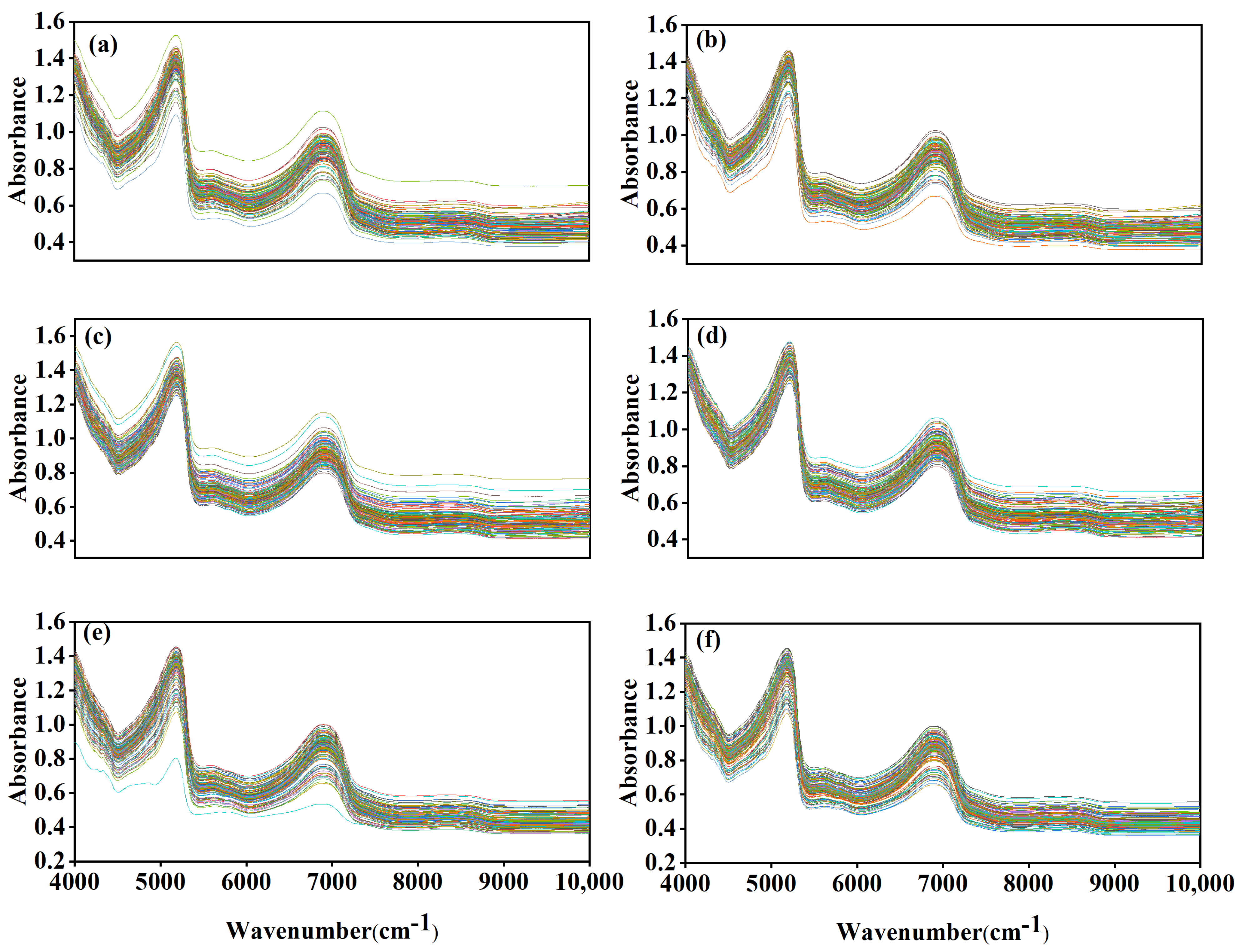
3.2. Spectral Analysis of Korla Fragrant Pear Leaves
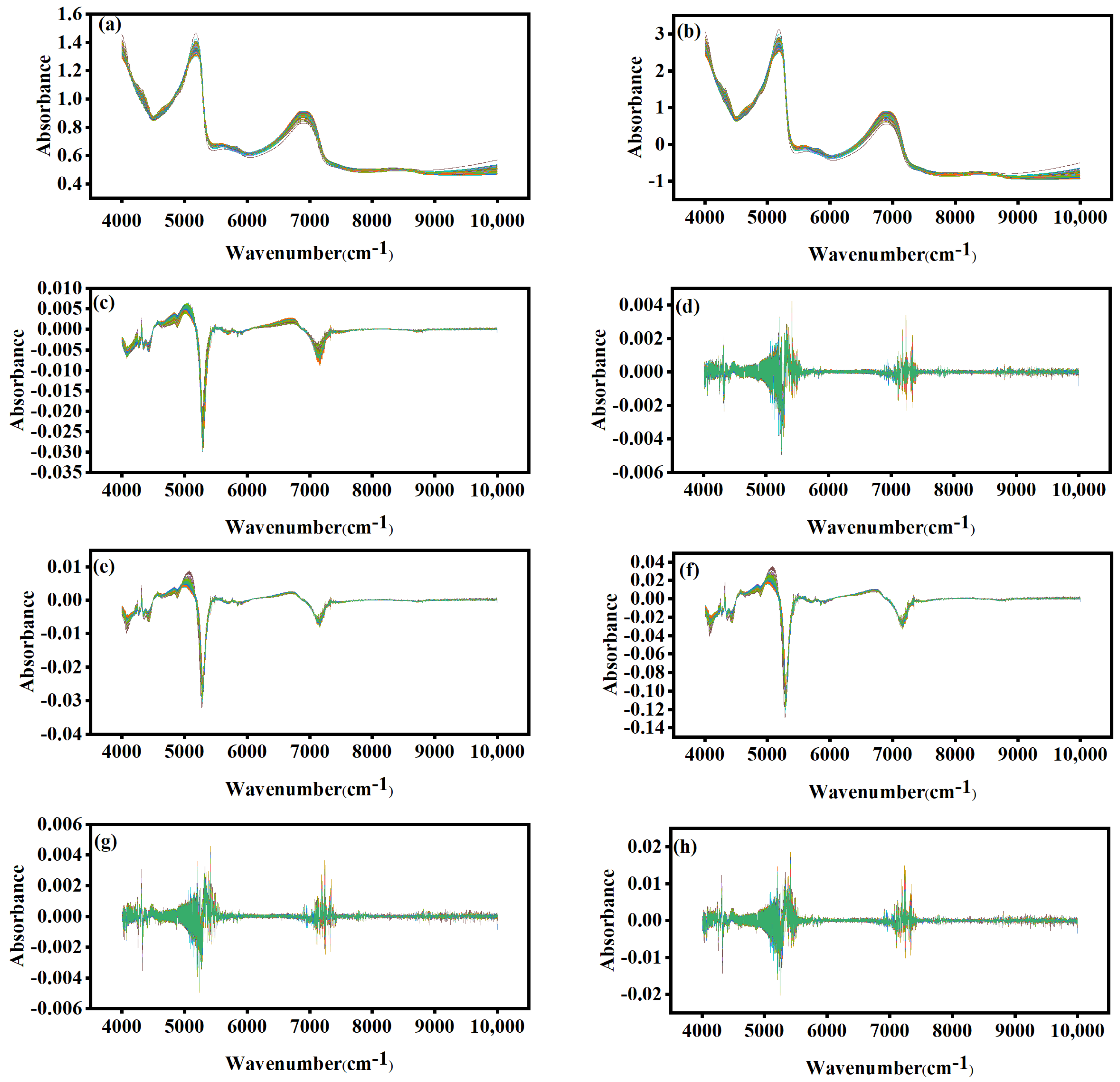
3.3. Spectral Pretreatment
3.4. Correlation Between Leaf Spectrum and Total Potassium Content of Korla Fragrant Pear
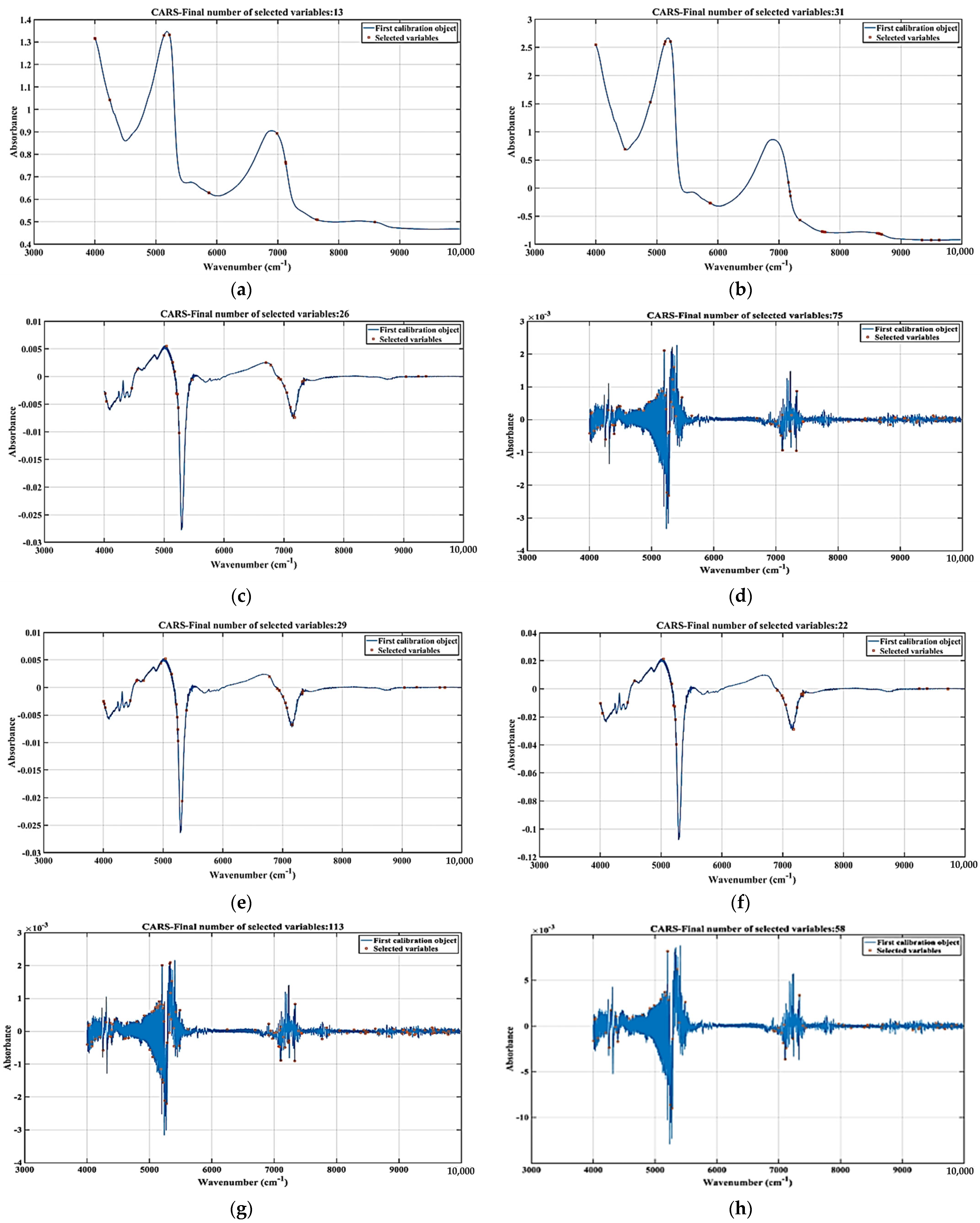
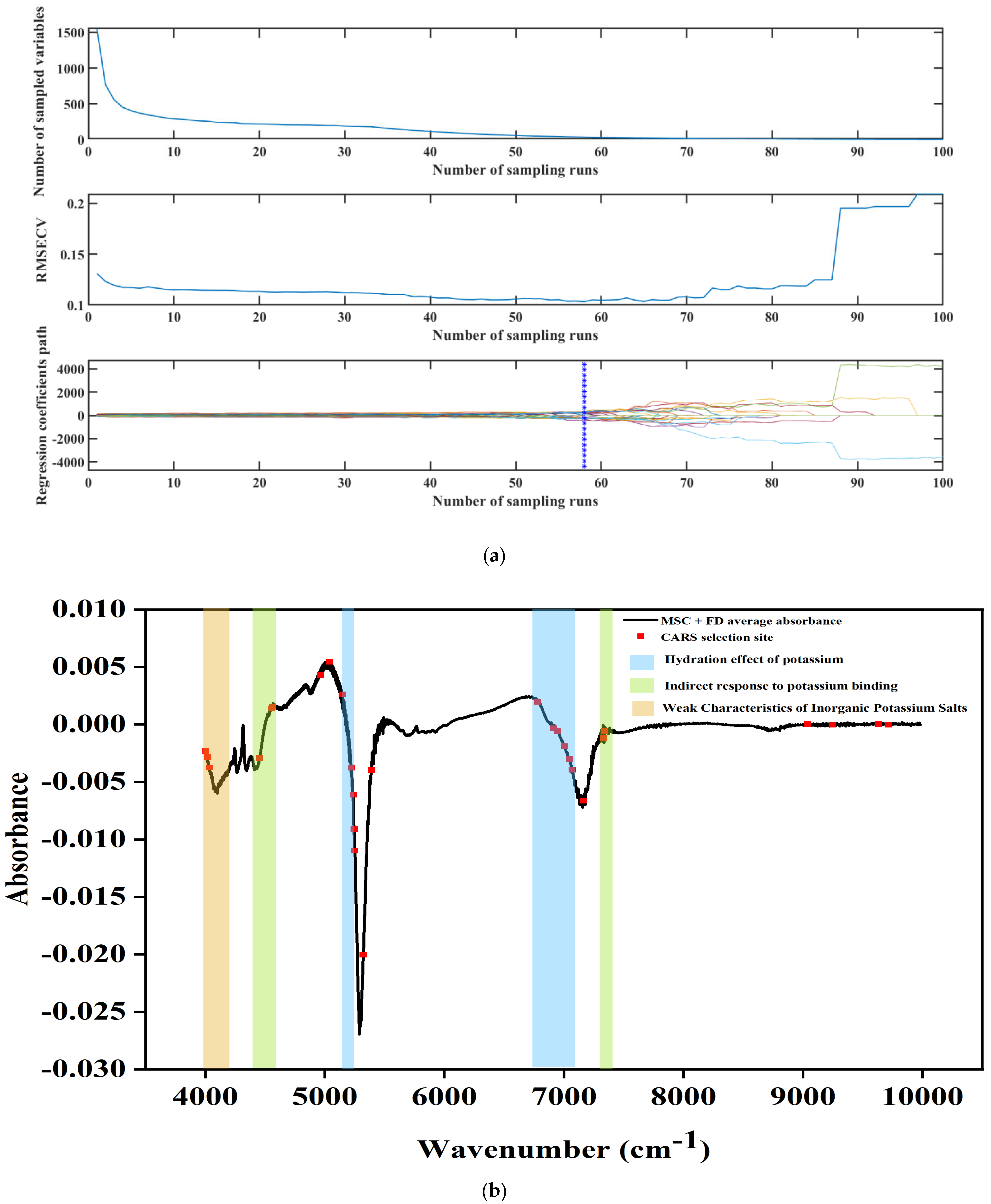
3.5. Spectral Feature Extraction
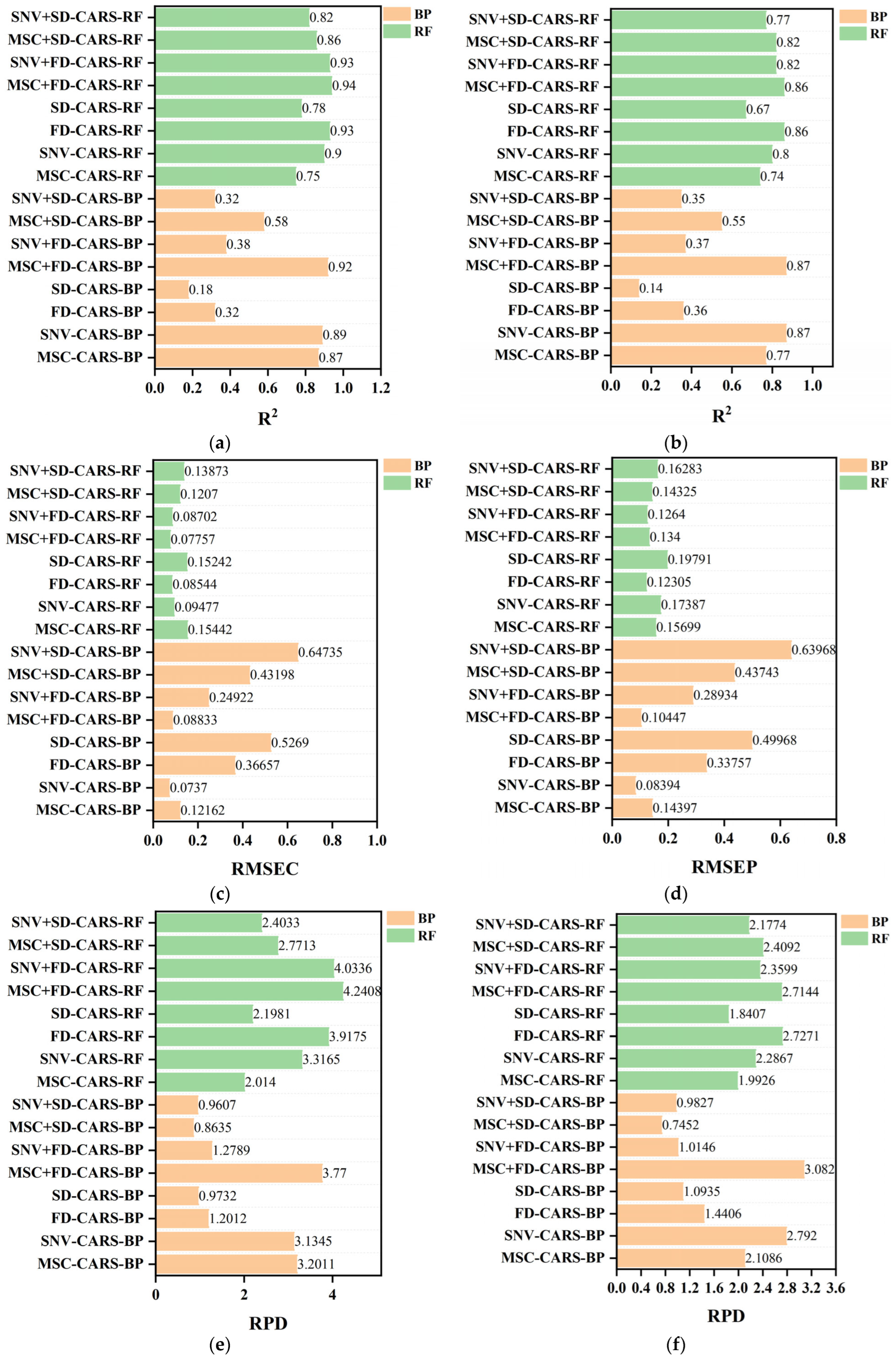
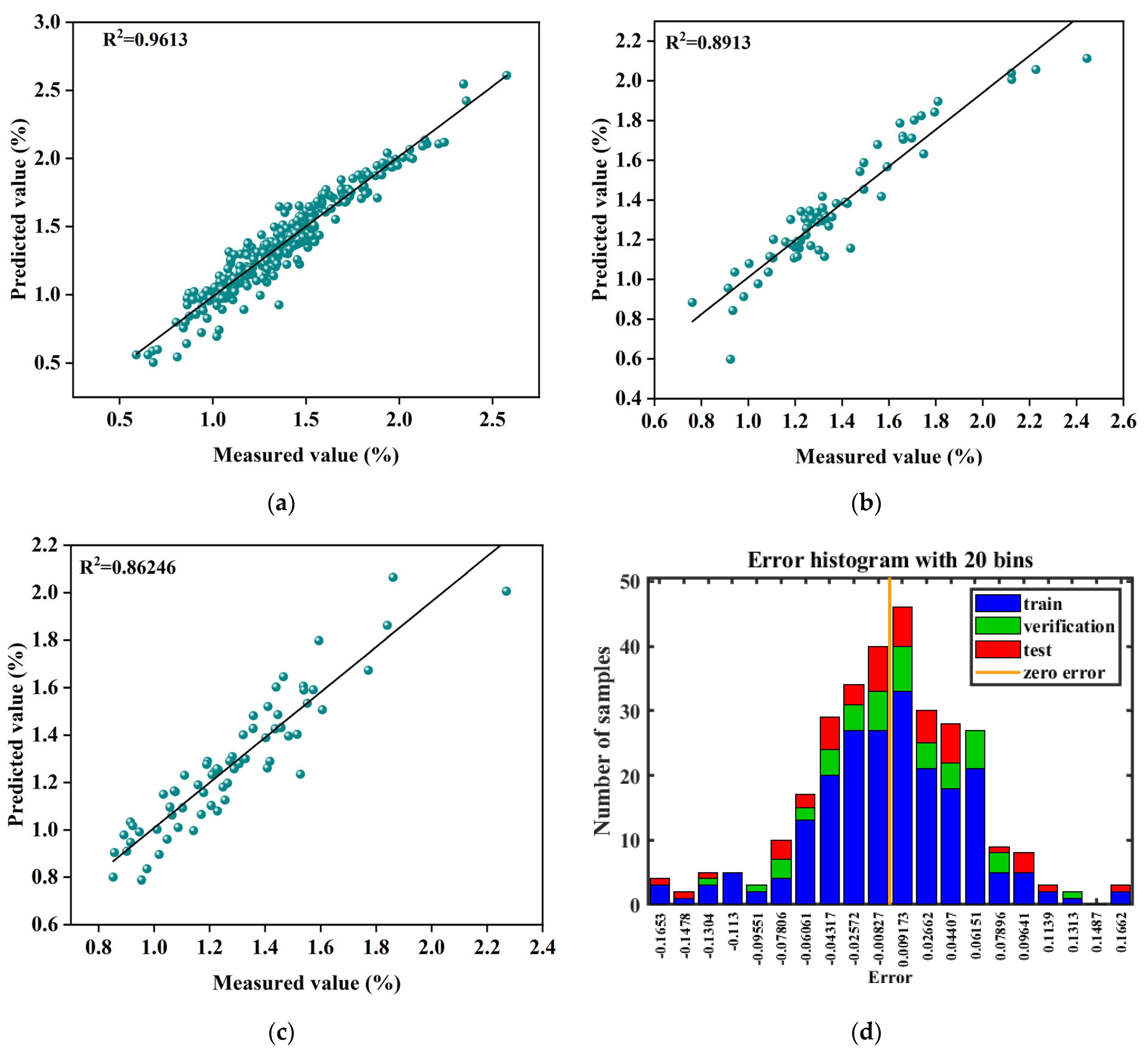
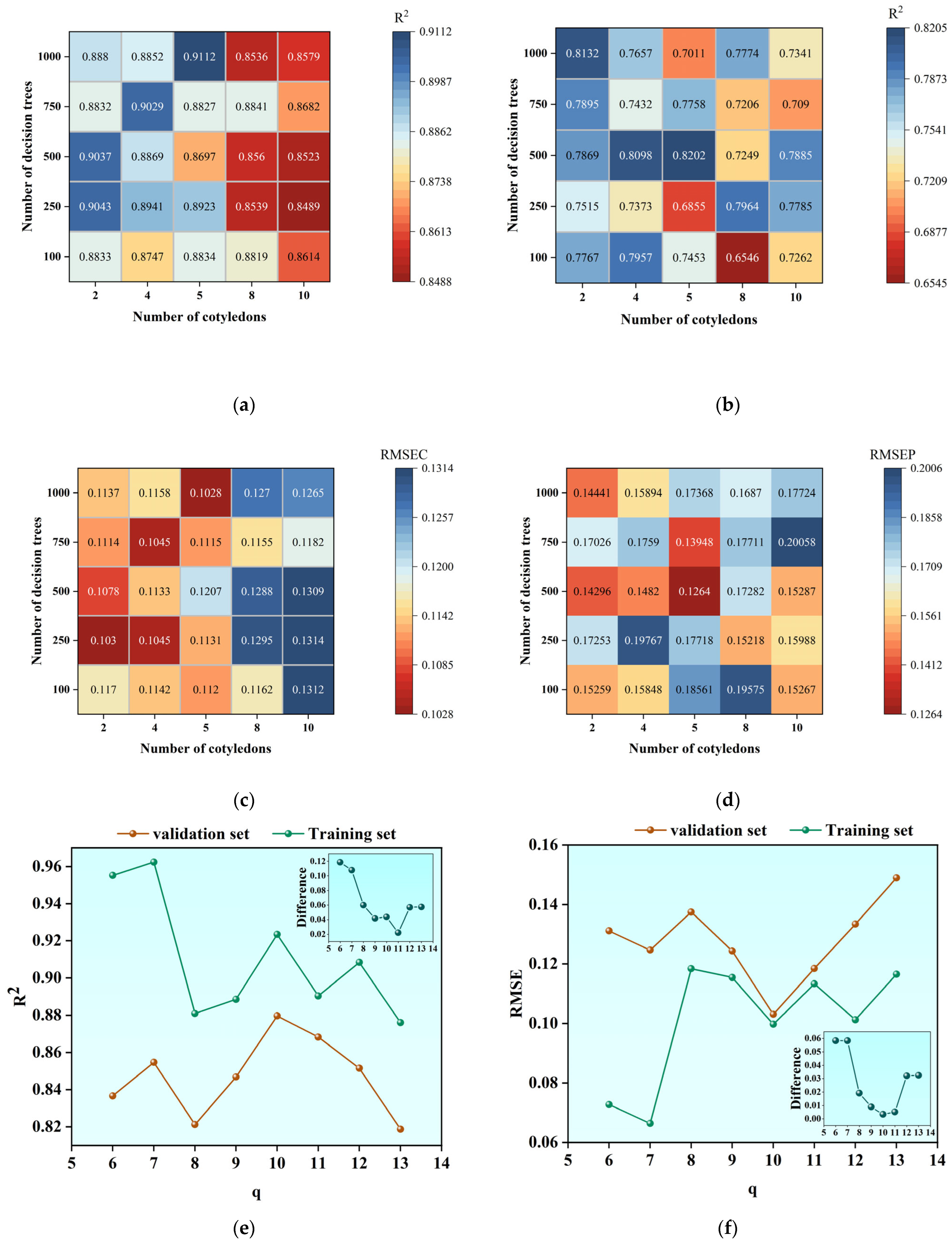
3.6. Model Comparison
3.7. Training Function Selection
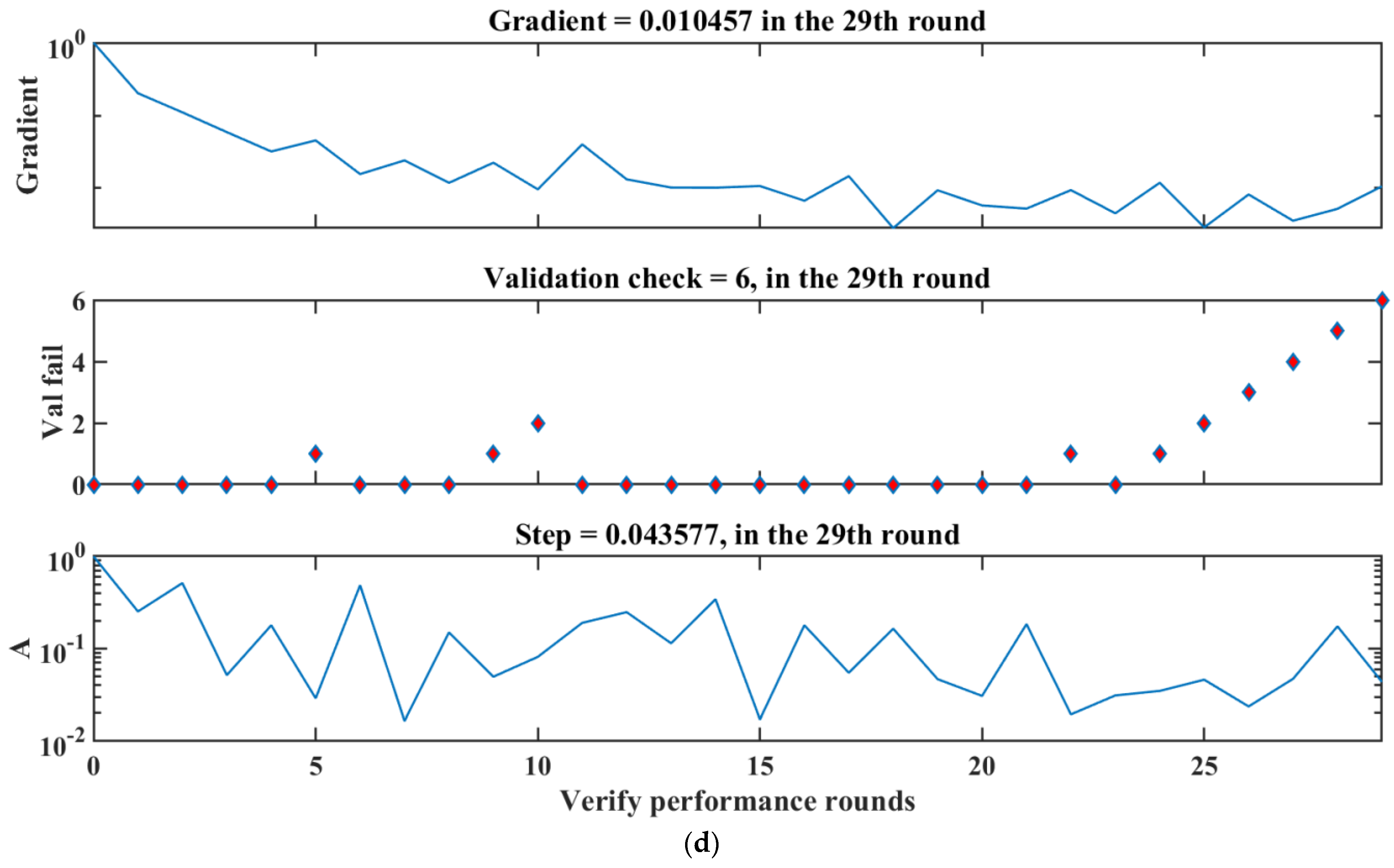
3.8. Model Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| R2 | Coefficient of Determination |
| RMSE | Root Mean Square Error |
| RPD | Ratio of Performance to Deviation |
| BP | Backpropagation |
| CARS | Competitive Adaptive Reweighted Sampling |
| SPA | Successive Projections Algorithm |
| SG | Savitzky-Golay |
| MSC | Multiplicative Scatter Correction |
| SD | Second Derivative |
| FD | First Derivative |
References
- Zhuang, Y.; Wang, X.; Gong, X.; Bao, J. Effects of Different Foliar Fertilizer Treatments on Fruit Quality of the Korla Fragrant Pear. Horticulturae 2024, 10, 51. [Google Scholar] [CrossRef]
- Appiah, E.A.; Balla-Kovács, A.; Ocwa, A.; Csajbók, J.; Kutasy, E. Enhancing Alfalfa (Medicago sativa L.) Productivity: Exploring the Significance of Potassium Nutrition. Agronomy 2024, 14, 1806. [Google Scholar] [CrossRef]
- Kuzin, A.; Solovchenko, A. Essential Role of Potassium in Apple and Its Implications for Management of Orchard Fertilization. Plants 2021, 10, 2624. [Google Scholar] [CrossRef]
- Naz, T.; Mazhar Iqbal, M.; Tahir, M.; Hassan, M.M.; Rehmani, M.I.A.; Zafar, M.I.; Ghafoor, U.; Qazi, M.A.; EL Sabagh, A.; Sakran, M.I. Foliar Application of Potassium Mitigates Salinity Stress Conditions in Spinach (Spinacia oleracea L.) through Reducing NaCl Toxicity and Enhancing the Activity of Antioxidant Enzymes. Horticulturae 2021, 7, 566. [Google Scholar] [CrossRef]
- Cui, J.; Davanture, M.; Zivy, M.; Lamade, E.; Tcherkez, G. Metabolic responses to potassium availability and waterlogging reshape respiration and carbon use efficiency in oil palm. New Phytol. 2019, 223, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zheng, L.; Zhao, J.; Ma, J.; Li, X. Biochar Application Maintains Photosynthesis of Cabbage by Regulating Stomatal Parameters in Salt-Stressed Soil. Sustainability 2023, 15, 4206. [Google Scholar] [CrossRef]
- Yabiku, T.; Ueno, O. Variations in physiological, biochemical, and structural traits of photosynthesis and resource use efficiency in maize and teosintes (NADP-ME-type C 4). Plant Prod. Sci. 2017, 20, 448–458. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Wang, C.; Shangguan, Z. Leaf photosynthetic function duration during yield formation of large-spike wheat in rainfed cropping systems. PeerJ 2018, 6, e5532. [Google Scholar] [CrossRef]
- Lu, L.; Chen, S.; Yang, W.; Wu, Y.; Liu, Y.; Yin, X.; Yang, Y.; Yang, Y. Integrated transcriptomic and metabolomic analyses reveal key metabolic pathways in response to potassium deficiency in coconut (Cocos nucifera L.) seedlings. Front. Plant Sci. 2023, 14, 1112264. [Google Scholar] [CrossRef]
- Woldemariam, S.; Lal, S.; Zeru Zelelew, D.; Solomon, M. Effect of Potassium Levels on Productivity and Fruit Quality of Tomato (Lycopersicon esculentum L.). J. Agric. Stud. 2018, 6, 104. [Google Scholar] [CrossRef]
- Shen, C.; Wang, J.; Shi, X.; Kang, Y.; Xie, C.; Peng, L.; Dong, C.; Shen, Q.; Xu, Y. Transcriptome Analysis of Differentially Expressed Genes Induced by Low and High Potassium Levels Provides Insight into Fruit Sugar Metabolism of Pear. Front. Plant Sci. 2017, 8, 938. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Mukherjee, S.; Al-Huqail, A.A.; Basahi, R.A.; Ali, H.M.; Al-Munqedhi, B.M.A.; Siddiqui, M.H.; Kalaji, H.M. Exogenous Potassium (K+) Positively Regulates Na+/H+ Antiport System, Carbohydrate Metabolism, and Ascorbate–Glutathione Cycle in H2S-Dependent Manner in NaCl-Stressed Tomato Seedling Roots. Plants 2021, 10, 948. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Guo, X.; Du, J.; Yu, M. ALA Promotes Sucrose Accumulation in Early Peach Fruit by Regulating SPS Activity. Curr. Issues Mol. Biol. 2024, 46, 7944–7954. [Google Scholar] [CrossRef]
- Li, Z.; Duan, S.; Lu, B.; Yang, C.; Ding, H.; Shen, H. Spraying alginate oligosaccharide improves photosynthetic performance and sugar accumulation in citrus by regulating antioxidant system and related gene expression. Front. Plant Sci. 2023, 13, 1108848. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, K.; Wen, S.; Yang, D.; Gao, J.; Wang, Z.; Zhu, P.; Bie, Z.; Cheng, J. Phloem unloading in cultivated melon fruits follows an apoplasmic pathway during enlargement and ripening. Hortic. Res. 2023, 10, uhad123. [Google Scholar] [CrossRef]
- Kumar, K.; Madhumala, K.; Sahay, S.; Ahmad, M. Response of Different Sources of Potassium on Biochemical Quality of Litchi cv. Deshi. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2281–2290. [Google Scholar] [CrossRef]
- Garcia, K.; Chasman, D.; Roy, S.; Ané, J.M. Physiological Responses and Gene Co-Expression Network of Mycorrhizal Roots under K(+) Deprivation. Plant Physiol. 2017, 173, 1811–1823. [Google Scholar] [CrossRef]
- Shin, R.; Schachtman, D.P. Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc. Natl. Acad. Sci. USA 2004, 101, 8827–8832. [Google Scholar] [CrossRef]
- Adams, E.; Shin, R. Transport, signaling, and homeostasis of potassium and sodium in plants. J. Integr. Plant Biol. 2014, 56, 231–249. [Google Scholar] [CrossRef]
- Wu, K.; Hu, C.; Liao, P.; Hu, Y.; Sun, X.; Tan, Q.; Pan, Z.; Xu, S.; Dong, Z.; Wu, S. Potassium stimulates fruit sugar accumulation by increasing carbon flow in Citrus sinensis. Hortic. Res. 2024, 11, uhae240, Erratum in Hortic. Res. 2025, 12, uhaf057. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, T.; Singh, S.; Tuteja, N.; Prasad, R.; Singh, J. Potassium: A key modulator for cell homeostasis. J. Biotechnol. 2020, 324, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Etienne, A. Which Physiological Processes Control Banana Acidity (sp. Musa) During Pre And Post-Harvest Stages? Ecophysiological Modeling and Experimental Analysis of the Effects of Genotype and Fruit Growth Conditions [Quels Processus Physiologiques Pilotent l’Acidité de la Banane Dessert (sp. Musa) en Pré et Post Récolte? Modélisation Ecophysiologique et Analyse Expérimentale de l’Effet du Génotype et des Conditions de Croissance du Fruit]; Université des Antilles-Guyane: Pointe-à-Pitre, France, 2014. [Google Scholar]
- Mota, M.; Martins, M.J.; Policarpo, G.; Sprey, L.; Pastaneira, M.; Almeida, P.; Maurício, A.; Rosa, C.; Faria, J.; Martins, M.B.; et al. Nutrient Content with Different Fertilizer Management and Influence on Yield and Fruit Quality in Apple cv. Gala. Horticulturae 2022, 8, 713. [Google Scholar] [CrossRef]
- Ksouri, N.; Sánchez, G.; Forcada, C.; Contreras-Moreira, B.; Gogorcena, Y. ddRAD-seq-derived SNPs reveal novel association signatures for fruit-related traits in peach. bioXriv 2023. [Google Scholar] [CrossRef]
- Singh, S.; Bahadur, V.; Mishra, S. Effect of Different Spacing and NPK Combination on Plant Growth, Fruit Yield and Fruit Quality of Strawberry (Fragaria ananassa Duch.) cv. Winter Dawn. J. Adv. Biol. Biotechnol. 2024, 27, 415–427. [Google Scholar] [CrossRef]
- Azam, M.; Qadri, R.; Aslam, A.; Khan, M.I.; Khan, A.S.; Anwar, R.; Ghani, M.A.; Ejaz, S.; Hussain, Z.; Iqbal, M.A.; et al. Effects of different combinations of N, P and K at different time interval on vegetative, reproductive, yield and quality traits of mango (Mangifera indica L.) cv. Dusehri. Braz. J. Biol. 2021, 82, e235612. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Xiao, H.; Li, R.; Zeng, Y.; Gu, M.; Moran, N.; Yu, L.; Xu, G. Potassium transporter OsHAK18 mediates potassium and sodium circulation and sugar translocation in rice. Plant Physiol. 2023, 193, 2003–2020. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, J.; Zhang, Q.; Li, X.; Li, M.; Yang, Y.; Zhou, J.; Wei, Q.; Zhou, B. Transcriptional and metabolic responses of apple to different potassium environments. Front. Plant Sci. 2023, 14, 1131708. [Google Scholar] [CrossRef]
- Gorji, R.; Skvaril, J.; Odlare, M. Determining Moisture Content of Basil Using Handheld Near-Infrared Spectroscopy. Horticulturae 2024, 10, 336. [Google Scholar] [CrossRef]
- Guarçoni, M.A.; Ventura, J. Nitrogen, P and K fertilization and the development, yield and fruit quality of pineapple ‘Gold’ (MD-2). Rev. Bras. Ciência Solo 2011, 35, 1367–1376. [Google Scholar] [CrossRef]
- Wang, J.; He, X.; Gong, P.; Zhao, D.; Zhang, Y.; Wang, Z.; Zhang, J. Optimization of a Water-Saving and Fertilizer-Saving Model for Enhancing Xinjiang Korla Fragrant Pear Yield, Quality, and Net Profits under Water and Fertilizer Coupling. Sustainability 2022, 14, 8495. [Google Scholar] [CrossRef]
- Tamburini, E.; Ferrari, G.; Marchetti, M.G.; Pedrini, P.; Ferro, S. Development of FT-NIR Models for the Simultaneous Estimation of Chlorophyll and Nitrogen Content in Fresh Apple (Malus domestica) Leaves. Sensors 2015, 15, 2662–2679. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, H.; Li, X.; Zhang, L.; Sui, Y. Prediction of Potassium Content in Rice Leaves Based on Spectral Features and Random Forests. Agronomy 2023, 13, 2337. [Google Scholar] [CrossRef]
- Liu, Y.; Niu, X.; Tang, Y.; Li, S.; Lan, H.; Niu, H. Internal Quality Prediction Method of Damaged Korla Fragrant Pears during Storage. Horticulturae 2023, 9, 666. [Google Scholar] [CrossRef]
- Su, T.; Wang, C.; Zhao, G.; Fan, S.; Yang, G.; Xu, C.; Su, H. Investigations on Spectra of Terahertz and Raman of L-Alabinose at Fingerprint Region. Spectrosc. Spectr. Anal. 2018, 38, 2713–2719. [Google Scholar]
- Lei, Y.; Kesu, W.; Delun, L.; Fugui, Z.; Xuemei, W. Analysis of hyperspectral characteristics of flue—Cured tobacco oil based on improved RF feature selection strategy. J. Chin. Agric. Mech. 2021, 42, 196–202. [Google Scholar] [CrossRef]
- Hong, Y.; Xue, T.; Huang, M. PSO-BP Based Prediction Study for Nonlinear Regression Problems. In Proceedings of the 2024 IEEE 2nd International Conference on Image Processing and Computer Applications (ICIPCA), Shenyang, China, 28–30 June 2024; pp. 903–910. [Google Scholar] [CrossRef]
- Lang, J.; Ramos, S.E.; Smohunova, M.; Bigler, L.; Schuman, M.C. Screening of leaf extraction and storage conditions for eco-metabolomics studies. Plant Direct 2024, 8, e578. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, P.; Zhang, Y.; Zhou, D.; Dong, Q.; Jia, P.; Liu, Z.; Zhao, X.; Yu, H. Physiological Mechanism of Photosynthetic, Nutrient, and Yield Responses of Peanut Cultivars with Different Tolerances under Low K Stress. Agronomy 2023, 13, 185. [Google Scholar] [CrossRef]
- Zhou, X.; Su, F.; Tian, Y.; Youngbull, C.; Johnson, R.H.; Meldrum, D.R. A New Highly Selective Fluorescent K+ Sensor. J. Am. Chem. Soc. 2011, 133, 18530–18533. [Google Scholar] [CrossRef]
- Yaron, J.R.; Gangaraju, S.; Rao, M.Y.; Kong, X.; Zhang, L.; Su, F.; Tian, Y.; Glenn, H.L.; Meldrum, D.R. K(+) regulates Ca(2+) to drive inflammasome signaling: Dynamic visualization of ion flux in live cells. Cell Death Dis. 2015, 6, e1954. [Google Scholar] [CrossRef]
- Yu, M.; Bai, X.; Bao, J.; Wang, Z.; Tang, Z.; Zheng, Q.; Zhi, J. The Prediction Model of Total Nitrogen Content in Leaves of Korla Fragrant Pear Was Established Based on Near Infrared Spectroscopy. Agronomy 2024, 14, 1284. [Google Scholar] [CrossRef]
- Xu, K.; Sun, L.-L.; Wang, J.; Liu, S.-X.; Yang, H.-W.; Xu, N.; Zhang, H.-J.; Wang, J.-X. Potassium deficiency diagnosis method of apple leaves based on MLR-LDA-SVM. Front. Plant Sci. 2023, 14, 1271933. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Ren, G.; Wang, J. Using Fourier Transform Near-Infrared Spectroscopy for the Evaluation and Regional Analysis of Pea (Pisum sativum L.). J. Plant Genet. Resour. 2014, 15, 779–787. [Google Scholar] [CrossRef]
- Li, X.; Fu, X.; Li, H. A CARS-SPA-GA Feature Wavelength Selection Method Based on Hyperspectral Imaging with Potato Leaf Disease Classification. Sensors 2024, 24, 6566. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Munir, M.T.; Yu, W.; Young, B. Wavelength Selection FOR Rapid Identification of Different Particle Size Fractions of Milk Powder Using Hyperspectral Imaging. Sensors 2020, 20, 4645. [Google Scholar] [CrossRef]
- Speiser, J.L.; Miller, M.E.; Tooze, J.; Ip, E. A comparison of random forest variable selection methods for classification prediction modeling. Expert Syst. Appl. 2019, 134, 93–101. [Google Scholar] [CrossRef]
- Li, P.; Cai, M.; Miao, S.; Li, Y.; Sun, L.; Wang, J.; Gorjian, M. Accurate measurement techniques and prediction approaches for the in-situ rock stress. Sci. Rep. 2024, 14, 13226. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Yasmin, N.S.; Abdul Wahab, N.; Ismail, F.S.; Musa, M.a.J.; Halim, M.H.A.; Anuar, A.N. Support Vector Regression Modelling of an Aerobic Granular Sludge in Sequential Batch Reactor. Membranes 2021, 11, 554. [Google Scholar] [CrossRef]
- Li, S.; Jin, N.; Dogani, A.; Yang, Y.; Zhang, M.; Gu, X. Enhancing LightGBM for Industrial Fault Warning: An Innovative Hybrid Algorithm. Processes 2024, 12, 221. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, X.; Yan, C.; Chen, S.; Wang, S.; Zhang, J.; Xu, Z.; Ju, X.; Ding, N.; Dong, Y.; et al. Wavelength Selection for Estimating Soil Organic Matter Contents Through the Radiative Transfer Model. IEEE Access 2020, 8, 176286–176293. [Google Scholar] [CrossRef]
- Chu, C.; Wang, H.; Luo, X.; Wen, P.; Nan, L.; Du, C.; Fan, Y.; Gao, D.; Wang, D.; Yang, Z.; et al. Possible Alternatives: Identifying and Quantifying Adulteration in Buffalo, Goat, and Camel Milk Using Mid-Infrared Spectroscopy Combined with Modern Statistical Machine Learning Methods. Foods 2023, 12, 3856. [Google Scholar] [CrossRef]
- Ferrer, J.; San-Fabián, E. Energy calculations for sodium vs. potassium on a prokaryotic voltage-gated sodium channel: A quantum-chemical study. Theor. Chem. Acc. 2024, 143, 57. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, W.; Zhang, X.; Adamowski, J.F.; Biswas, A. Leaf Stoichiometry of Potentilla fruticosa Across Elevations in China’s Qilian Mountains. Front. Plant Sci. 2022, 13, 814059. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhu, X.; Li, C.; Wei, Y.; Yu, X.; Zhao, G.; Sun, H. Hyperspectral Inversion of Potassium Content in Apple Leaves Based on Vegetation Index. Agric. Sci. 2017, 8, 825–836. [Google Scholar] [CrossRef][Green Version]
- Galvez-Sola, L.; García-Sánchez, F.; Pérez-Pérez, J.G.; Gimeno, V.; Navarro, J.M.; Moral, R.; Martínez-Nicolás, J.J.; Nieves, M. Rapid estimation of nutritional elements on citrus leaves by near infrared reflectance spectroscopy. Front. Plant Sci. 2015, 6, 571. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Yu, M.; Li, J.; Wang, G.; Tang, Z.; Zhi, J. Determination of leaf nitrogen content in apple and jujube by near-infrared spectroscopy. Sci. Rep. 2024, 14, 20884. [Google Scholar] [CrossRef]
- Sarkar, S.; Basak, J.K.; Moon, B.E.; Kim, H.T. A Comparative Study of PLSR and SVM-R with Various Preprocessing Techniques for the Quantitative Determination of Soluble Solids Content of Hardy Kiwi Fruit by a Portable Vis/NIR Spectrometer. Foods 2020, 9, 1078. [Google Scholar] [CrossRef]
- Yue, J.; Feng, H.; Yang, G.; Li, Z. A Comparison of Regression Techniques for Estimation of Above-Ground Winter Wheat Biomass Using Near-Surface Spectroscopy. Remote Sens. 2018, 10, 66. [Google Scholar] [CrossRef]
- Teleszko, M.; Wojdyło, A. Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves. J. Funct. Foods 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Wiens, R.; Findlay, C.R.; Baldwin, S.G.; Kreplak, L.; Lee, J.M.; Veres, S.P.; Gough, K.M. High spatial resolution (1.1 μm and 20 nm) FTIR polarization contrast imaging reveals pre-rupture disorder in damaged tendon. Faraday Discuss. 2016, 187, 555–573. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y. Fourier Transform Infrared Spectroscopy in Oral Cancer Diagnosis. Int. J. Mol. Sci. 2021, 22, 1206. [Google Scholar] [CrossRef]
- Nallala, J.; Lloyd, G.R.; Hermes, M.; Shepherd, N.; Stone, N. Enhanced spectral histology in the colon using high-magnification benchtop FTIR imaging. Vib. Spectrosc. 2017, 91, 83–91. [Google Scholar] [CrossRef]
- Hammer, S.; Griffith, D.W.T.; Konrad, G.; Vardag, S.; Caldow, C.; Levin, I. Assessment of a multi-species in situ FTIR for precise atmospheric greenhouse gas observations. Atmos. Meas. Tech. 2013, 6, 1153–1170. [Google Scholar] [CrossRef]
- Smale, D.; Sherlock, V.; Griffith, D.W.T.; Moss, R.; Brailsford, G.; Nichol, S.; Kotkamp, M. A decade of CH4, CO and N2O in situ measurements at Lauder, New Zealand: Assessing the long-term performance of a Fourier transform infrared trace gas and isotope analyser. Atmos. Meas. Tech. 2019, 12, 637–673. [Google Scholar] [CrossRef]
- Leibrandt, D.R.; Thorpe, M.J.; Notcutt, M.; Drullinger, R.E.; Rosenband, T.; Bergquist, J.C. Spherical reference cavities for frequency stabilization of lasers in non-laboratory environments. Opt. Express. 2011, 19, 3471–3482. [Google Scholar] [CrossRef]
- Widyaningrum, W.; Purwanto, Y.A.; Widodo, S.; Supijatno, S.; Iriani, E. Portable/Handheld NIR sebagai Teknologi Evaluasi Mutu Bahan Pertanian secara Non-Destruktif. J. Keteknikan Pertan. 2022, 10, 59–68. [Google Scholar] [CrossRef]
- Gullifa, G.; Barone, L.; Papa, E.; Giuffrida, A.; Materazzi, S.; Risoluti, R. Portable NIR spectroscopy: The route to green analytical chemistry. Front. Chem. 2023, 11, 1214825. [Google Scholar] [CrossRef]



| Soil Indices | Soil Depth (cm) | ||
|---|---|---|---|
| 0–20 cm | 20–40 cm | 40–60 cm | |
| PH (dimensionless) | 8.08 | 8.07 | 8.13 |
| Available potassium (mg/kg) | 16.12 | 20.12 | 13.6 |
| Level | SPAD | Leaf Age (d) | Morphological Characteristics |
|---|---|---|---|
| Mature leaf | 35–45 | 15–30 | Leaves are dark green in color and have a tough texture. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Fan, W.; Zeng, J.; Li, Y.; Wang, L.; Wang, H.; Han, F.; Bao, J. Machine Learning-Assisted NIR Spectroscopy for Dynamic Monitoring of Leaf Potassium in Korla Fragrant Pear. Agronomy 2025, 15, 1672. https://doi.org/10.3390/agronomy15071672
Yu M, Fan W, Zeng J, Li Y, Wang L, Wang H, Han F, Bao J. Machine Learning-Assisted NIR Spectroscopy for Dynamic Monitoring of Leaf Potassium in Korla Fragrant Pear. Agronomy. 2025; 15(7):1672. https://doi.org/10.3390/agronomy15071672
Chicago/Turabian StyleYu, Mingyang, Weifan Fan, Junkai Zeng, Yang Li, Lanfei Wang, Hao Wang, Feng Han, and Jianping Bao. 2025. "Machine Learning-Assisted NIR Spectroscopy for Dynamic Monitoring of Leaf Potassium in Korla Fragrant Pear" Agronomy 15, no. 7: 1672. https://doi.org/10.3390/agronomy15071672
APA StyleYu, M., Fan, W., Zeng, J., Li, Y., Wang, L., Wang, H., Han, F., & Bao, J. (2025). Machine Learning-Assisted NIR Spectroscopy for Dynamic Monitoring of Leaf Potassium in Korla Fragrant Pear. Agronomy, 15(7), 1672. https://doi.org/10.3390/agronomy15071672






