Transcriptomic Insights into Salt Stress Tolerance Mechanisms in Melia azedarach: 24-Epibrassinolide-Mediated Modulation of Auxin and ABA Signaling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experiment Design
2.2. RNA Extraction, cDNA Library Construction, Sequencing, and Functional Annotation
2.3. Transcriptome Analysis, Enrichment Analysis, and Quantitative Real-Time PCR
3. Results
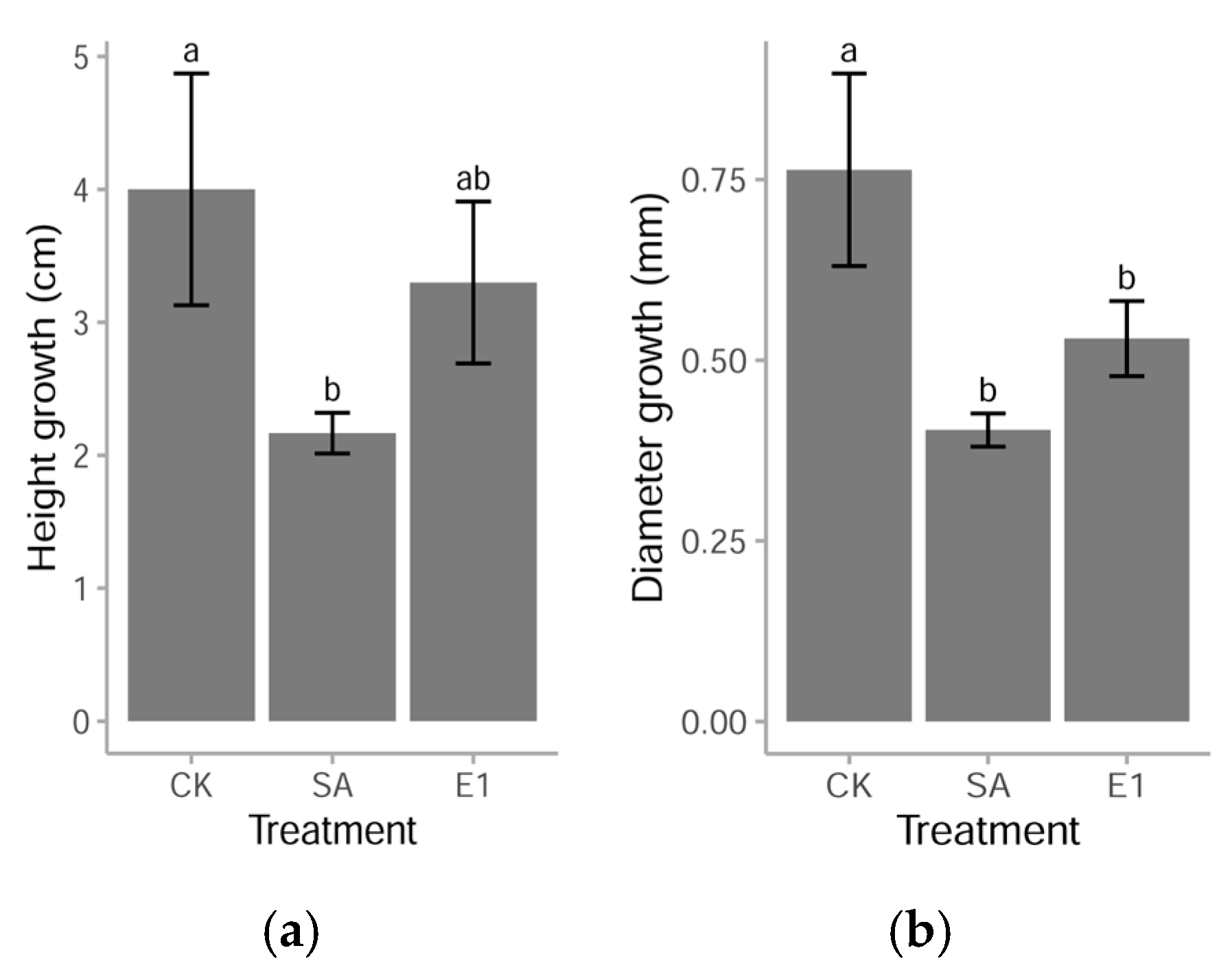
3.1. Growth Changes
3.2. Evaluation of Transcriptome Sequencing and De Novo Assembly
3.3. Principal Component Analysis
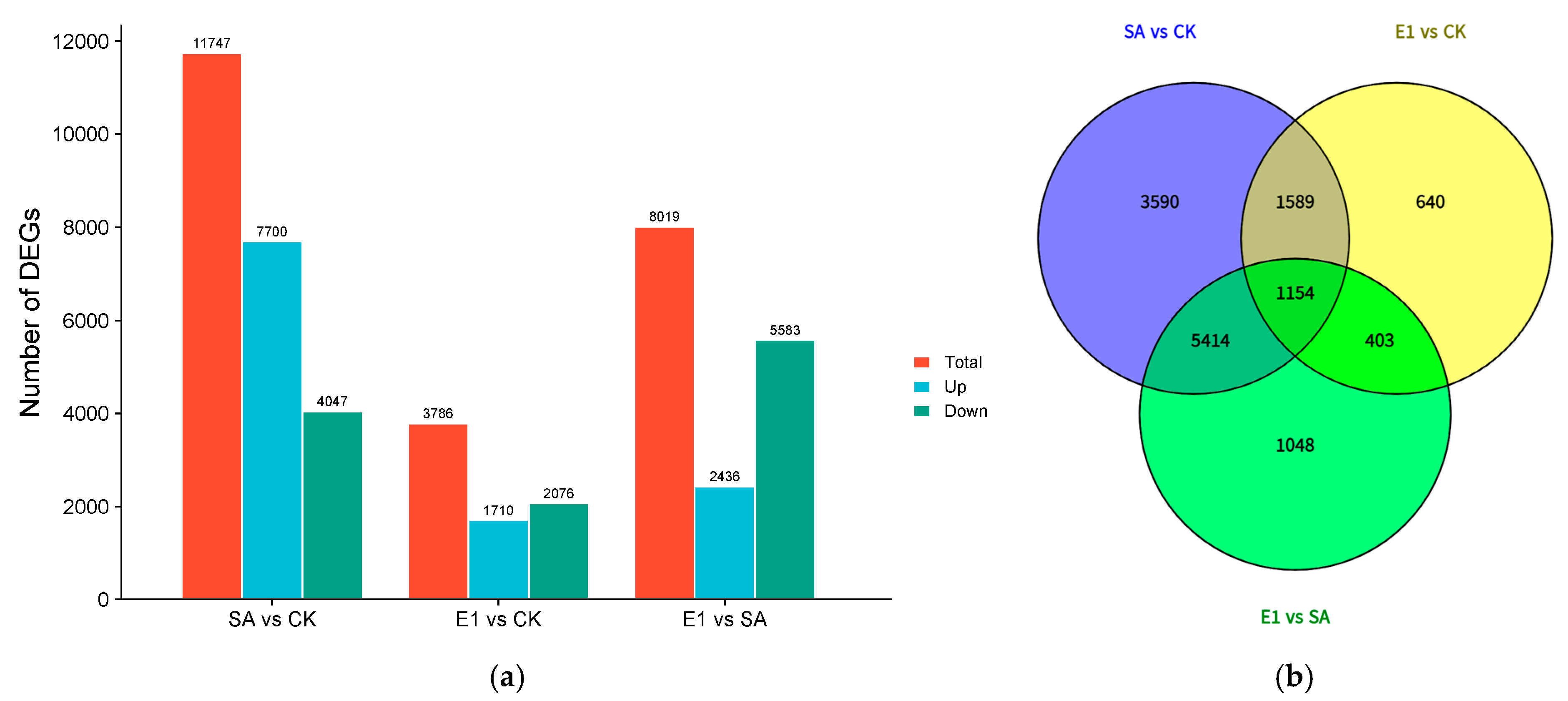
3.4. DEGs Across Different Pairwise Comparisons
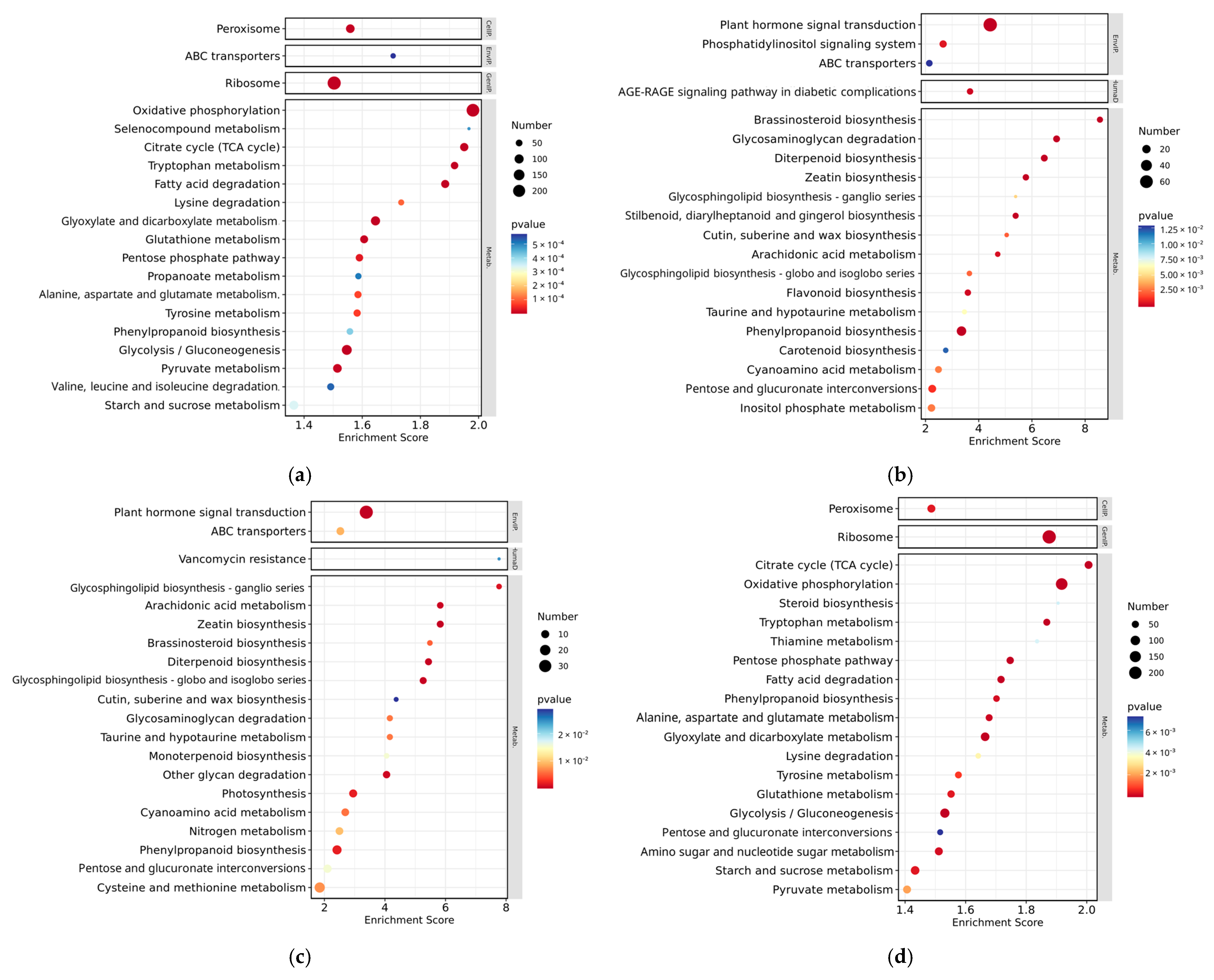
3.5. KEGG Enrichment Analysis of DEGs
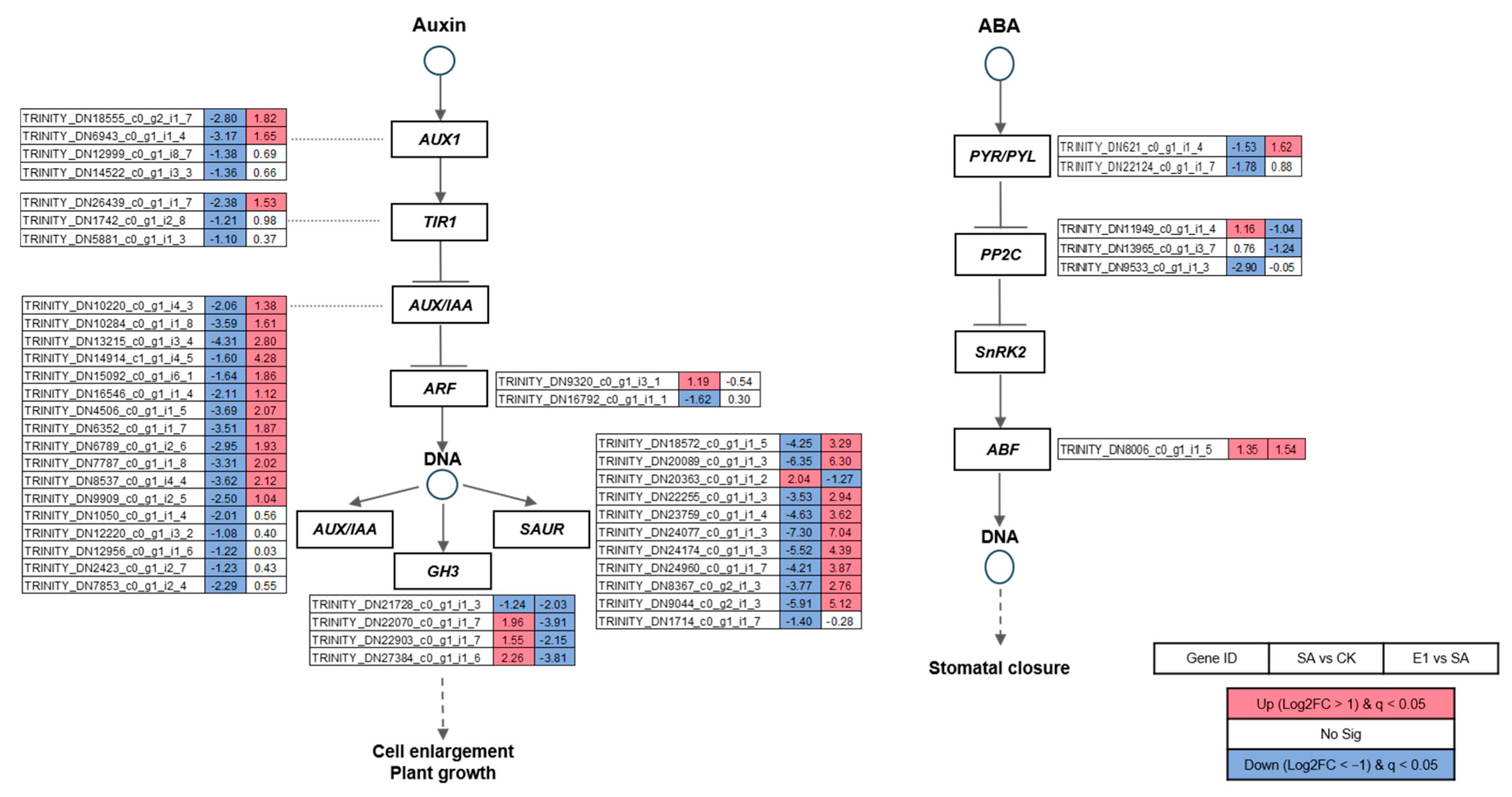
3.6. Expression Patterns of DEGs Enriched in Plant Hormone Signal Transduction
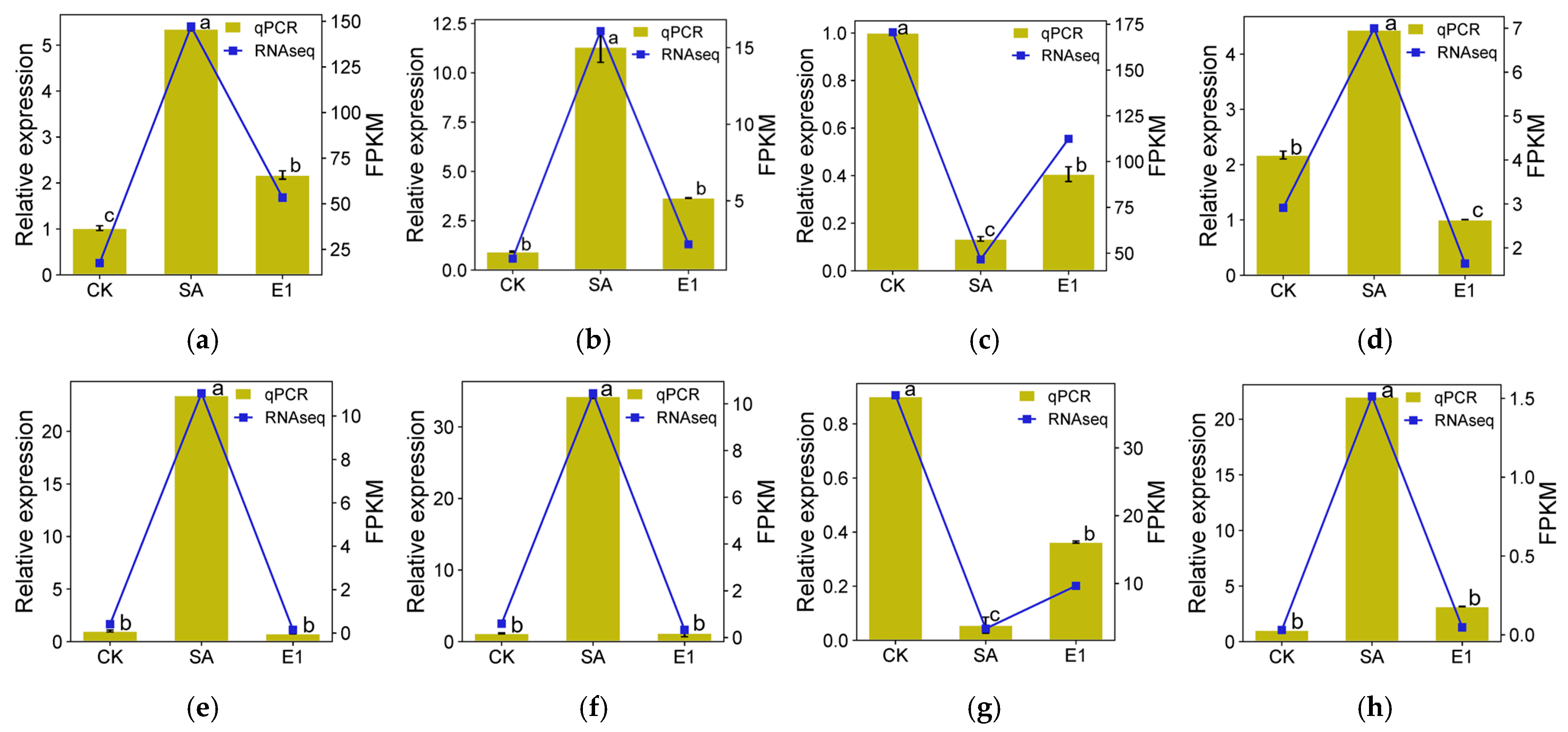
3.7. Quantitative Real-Time PCR (qRT-PCR) Analysis
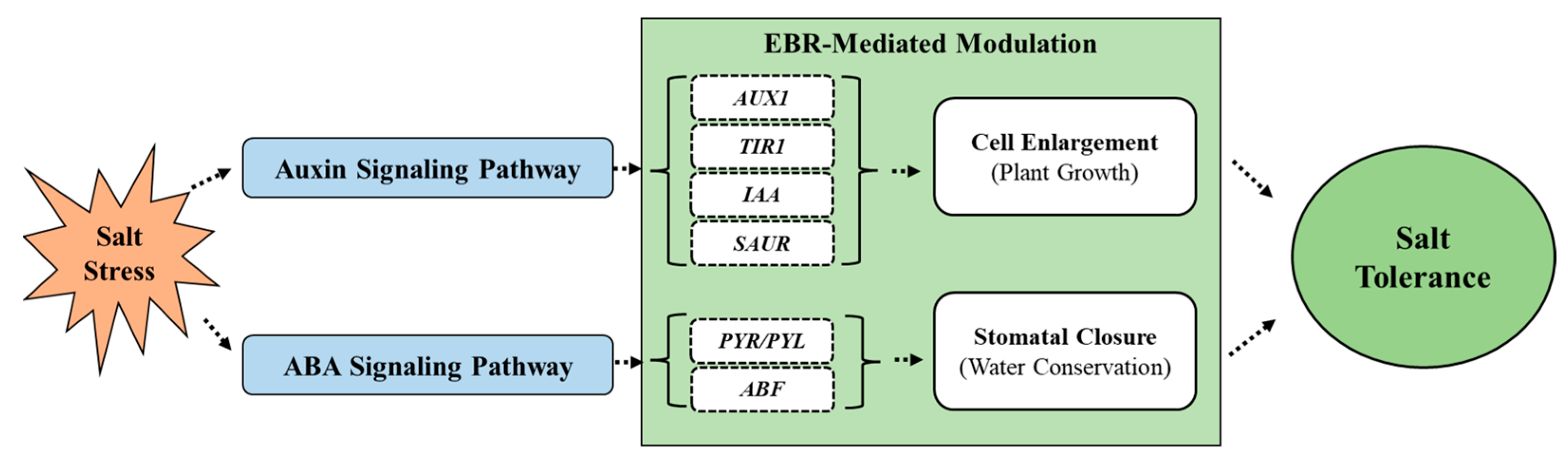
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SA vs. CK | Salt-treated seedlings versus control seedlings |
| E1 vs. CK | Salt + EBR-treated seedlings versus control seedlings |
| E1 vs. SA | Salt + EBR-treated seedlings versus salt-treated seedlings |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
References
- Haque, T.; Bhaskara, G.B.; Yin, J.; Bonnette, J.; Juenger, T.E. Natural Variation in Growth and Leaf Ion Homeostasis in Response to Salinity Stress in Panicum hallii. Front. Plant Sci. 2022, 13, 1019169. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.H.; Wang, Y.Q.; Song, J.N.; Yang, H.B. Transcriptomic Identification of Salt-Related Genes and de Novo Assembly in Common Buckwheat (F. esculentum). Plant Physiol. Biochem. 2018, 127, 299–309. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, H.; Jiang, J.; Gu, X.; Zhao, H.; Qiu, J.; Wang, Q.; Zheng, Y.; Zheng, K. Analysis of Transcriptomic Profiles and Physiological Traits of Exogenous 24-epibrassinolide Alleviating Salt Stress in Atractylodes Macrocephala Koidz. Plant Biotechnol. Rep. 2023, 18, 161–175. [Google Scholar] [CrossRef]
- Cackett, L.; Cannistraci, C.V.; Meier, S.; Ferrandi, P.; Pěnčík, A.; Gehring, C.; Novák, O.; Ingle, R.A.; Donaldson, L. Salt-Specific Gene Expression Reveals Elevated Auxin Levels in Arabidopsis Thaliana Plants Grown Under Saline Conditions. Front. Plant Sci. 2022, 13, 804716. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Li, Z.; Dai, S.; Ding, H.; Wang, Q.; Li, X.; Ding, G.; Wang, P.; Guan, Y.; Liu, W. Integrative Analyses of Transcriptomics and Metabolomics upon Seed Germination of Foxtail Millet in Response to Salinity. Sci. Rep. 2020, 10, 13660. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wei, Z.; Zhao, W.; Zhang, Z.; Chen, D.; Zhang, H.; Liu, X. Transcriptome Analysis of the Salt-Treated Actinidia deliciosa (A. Chev.) C. F. Liang and A. R. Ferguson Plantlets. Curr. Issues Mol. Biol. 2023, 45, 3772–3786. [Google Scholar] [CrossRef]
- Han, X.; Wu, Z.; Liu, F.; Wang, Y.; Wei, X.; Tian, P.; Ling, F. Transcriptomic Analysis and Salt-Tolerance Gene Mining during Rice Germination. Genes 2023, 14, 1556. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Q.; Gao, Z.; Wang, Y.; Liu, Y.; Ma, Z.; Chen, Y.; Zhang, Y.; Yan, F.; Li, J. Analysis of Phytohormone Signal Transduction in Sophora alopecuroides under Salt Stress. Int. J. Mol. Sci. 2021, 22, 7313. [Google Scholar] [CrossRef]
- Yang, Y.; Guang, Y.; Wang, F.; Chen, Y.; Yang, W.; Xiao, X.; Luo, S.; Zhou, Y. Characterization of Phytochrome-Interacting Factor Genes in Pepper and Functional Analysis of CaPIF8 in Cold and Salt Stress. Front. Plant Sci. 2021, 12, 746517. [Google Scholar] [CrossRef]
- Sicilia, A.; Testa, G.; Santoro, D.F.; Cosentino, S.L.; Piero, A.R. Lo RNASeq Analysis of Giant Cane Reveals the Leaf Transcriptome Dynamics under Long-Term Salt Stress. BMC Plant Biol. 2019, 19, 355. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, K.; Tian, Y.; Han, K.; El-Kassaby, Y.A.; Yang, H.; Si, H.; Sun, Y.; Li, Y. Time-Course Transcriptomics Analysis Reveals Key Responses of Populus to Salt Stress. Ind. Crops Prod. 2023, 194, 116278. [Google Scholar] [CrossRef]
- Alyahya, N.; Taybi, T. Comparative Transcriptomic Profiling Reveals Differentially Expressed Genes and Important Related Metabolic Pathways in Shoots and Roots of a Saudi Wheat Cultivar (Najran) under Salinity Stress. Front. Plant Sci. 2023, 14, 1225541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Z.; Song, H.; Cheng, S. From Plant Survival to Thriving: Exploring the Miracle of Brassinosteroids for Boosting Abiotic Stress Resilience in Horticultural Crops. Front. Plant Sci. 2023, 14, 1218229. [Google Scholar] [CrossRef]
- Vaishnav, A.; Shukla, A.K.; Sharma, A.; Kumar, R.; Choudhary, D.K. Endophytic Bacteria in Plant Salt Stress Tolerance: Current and Future Prospects. J. Plant Growth Regul. 2019, 38, 650–668. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Iqbal, N.; Masood, A.; Per, T.S.; Khan, N.A. Salicylic Acid Alleviates Adverse Effects of Heat Stress on Photosynthesis through Changes in Proline Production and Ethylene Formation. Plant Signal. Behav. 2013, 8, e26374. [Google Scholar] [CrossRef]
- Ryu, H.; Cho, Y. Plant Hormones in Salt Stress Tolerance. J. Plant Biol. 2015, 58, 147–155. [Google Scholar] [CrossRef]
- Javid, M.G.; Sorooshzadeh, A.; Moradi, F.; Sanavy, S.A.M.M.; Allahdadi, I. The Role of Phytohormones in Alleviating Salt Stress in Crop Plants. Aust. J. Crop Sci. 2011, 5, 726–734. [Google Scholar]
- Ahmad, H.; Hayat, S.; Ali, M.; Ghani, M.I.; Zhihui, C. Regulation of Growth and Physiological Traits of Cucumber (Cucumis sativus L.) through Various Levels of 28-Homobrassinolide under Salt Stress Conditions. Can. J. Plant Sci. 2018, 98, 132–140. [Google Scholar] [CrossRef]
- Sun, Z.; Zou, Y.; Xie, C.; Han, L.; Zheng, X.; Tian, Y.; Ma, C.; Liu, X.; Wang, C. Brassinolide Improves the Tolerance of Malus Hupehensis to Alkaline Stress. Front. Plant Sci. 2022, 13, 1032646. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Mir, B.A.; Yusuf, M.; Ahmad, A. 24-Epibrassinolide and/or Putrescine Trigger Physiological and Biochemical Responses for the Salt Stress Mitigation in Cucumis sativus L. Photosynthetica 2014, 52, 464–474. [Google Scholar] [CrossRef]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Biju, S.; Bhardwaj, R. 24-Epibrassinolide; an Active Brassinolide and Its Role in Salt Stress Tolerance in Plants: A Review. Plant Physiol. Biochem. 2018, 130, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liao, H. Epibrassinolide Improves the Growth Performance of Sedum Lineare upon Zn Stress through Boosting Antioxidative Capacities. PLoS ONE 2021, 16, e0257172. [Google Scholar] [CrossRef] [PubMed]
- Djemal, R.; Hanin, M.; Ebel, C. Control of Plant Responses to Salt Stress: Significance of Auxin and Brassinosteroids. In Making Plant Life Easier and Productive Under Salinity—Updates and Prospects; IntechOpen: London, UK, 2023; ISBN 978-1-83768-877-7. [Google Scholar]
- Wang, Q.; Yu, F.; Xie, Q. Balancing Growth and Adaptation to Stress: Crosstalk between Brassinosteroid and Abscisic Acid Signaling. Plant Cell Environ. 2020, 43, 2325–2335. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhou, J.; Li, Q.; Quan, D.; Wang, Q.; Gao, Y. Auxin–Brassinosteroid Crosstalk: Regulating Rice Plant Architecture and Grain Shape. Crop J. 2024, 12, 953–963. [Google Scholar] [CrossRef]
- Bai, Y.; Yu, H.; Chen, L.; Meng, Y.; Ma, Y.; Wang, D.; Qian, Y.; Zhang, D.; Feng, X.; Zhou, Y. Time-Course Transcriptome Analysis of Aquilegia vulgaris Root Reveals the Cell Wall’s Roles in Salinity Tolerance. Int. J. Mol. Sci. 2023, 24, 16450. [Google Scholar] [CrossRef]
- Mo, S.; Biao, A.; Wang, Z.; Lin, S.; Yang, T.; Pan, L.; Wang, Y.; Zeng, S. Spatio Transcriptome Uncover Novel Insight into the Lycium Ruthenicum Seedling Tolerant to Salt Stress. Ind. Crops Prod. 2022, 177, 114502. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Du, S.; Jiang, J.; Wang, G. Transcriptome and Metabonomic Analysis of Tamarix ramosissima Potassium (K+) Channels and Transporters in Response to NaCl Stress. Genes 2022, 13, 1313. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, M.; Ren, T.; Li, K.; Li, Y.; Marowa, P.; Zhang, C. Comparative Transcriptome Analysis Reveals the Molecular Mechanism of Salt Tolerance in Apocynum Venetum. Plant Physiol. Biochem. 2021, 167, 816–830. [Google Scholar] [CrossRef]
- Zhang, X.; Han, C.; Gao, H.; Cao, Y. Comparative Transcriptome Analysis of the Garden Asparagus (Asparagus officinalis L.) Reveals the Molecular Mechanism for Growth with Arbuscular Mycorrhizal Fungi under Salinity Stress. Plant Physiol. Biochem. 2019, 141, 20–29. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Guo, D.; Zhang, H.; Che, Y.; Li, Y.; Tian, B.; Wang, Z.; Sun, G.; Zhang, H. Physiological and Comparative Transcriptome Analysis of Leaf Response and Physiological Adaption to Saline Alkali Stress across PH Values in Alfalfa (Medicago sativa). Plant Physiol. Biochem. 2021, 167, 140–152. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.; Qin, B.; Sun, H.; Yuan, X.; Wang, Q.; Xu, J.; Yin, Z.; Du, Y.; Du, J.; et al. Analysis of the Transcriptome and Metabolome Reveals Phenylpropanoid Mechanism in Common Bean (Phaseolus vulgaris) Responding to Salt Stress at Sprout Stage. Food Energy Secur. 2023, 12, e481. [Google Scholar] [CrossRef]
- Gan, T.; Lin, Z.; Bao, L.; Hui, T.; Cui, X.; Huang, Y.; Wang, H.; Su, C.; Jiao, F.; Zhang, M.; et al. Comparative Proteomic Analysis of Tolerant and Sensitive Varieties Reveals That Phenylpropanoid Biosynthesis Contributes to Salt Tolerance in Mulberry. Int. J. Mol. Sci. 2021, 22, 9402. [Google Scholar] [CrossRef]
- Cao, B.-L.; Li, N.; Xu, K. Crosstalk of Phenylpropanoid Biosynthesis with Hormone Signaling in Chinese Cabbage Is Key to Counteracting Salt Stress. Environ. Exp. Bot. 2020, 179, 104209. [Google Scholar] [CrossRef]
- Li, W.; Sun, J.; Zhang, X.; Ahmad, N.; Hou, L.; Zhao, C.; Pan, J.; Tian, R.; Wang, X.; Zhao, S. The Mechanisms Underlying Salt Resistance Mediated by Exogenous Application of 24-Epibrassinolide in Peanut. Int. J. Mol. Sci. 2022, 23, 6376. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Ren, X.Q.; Liu, J.X.; Yang, F.; Wang, Y.P.; Xiong, A.S. Transcript Profiling Reveals an Important Role of Cell Wall Remodeling and Hormone Signaling under Salt Stress in Garlic. Plant Physiol. Biochem. 2019, 135, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Yang, X.; Yu, W.; Xiang, P.; Zhang, S.; Wang, G. The Diversity of Melia azedarach L. from China Based on Transcriptome-Developed SSR Marker. Forests 2022, 13, 1011. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Costa, M.; Araújo, M.; Santos, C.; Silva, A.M.S. Phytochemical and Antioxidant Profile of the Medicinal Plant Melia azedarach Subjected to Water Deficit Conditions. Int. J. Mol. Sci. 2022, 23, 13611. [Google Scholar] [CrossRef]
- Han, C.; Chen, J.; Liu, Z.; Chen, H.; Yu, F.; Yu, W. Morphological and Physiological Responses of Melia azedarach Seedlings of Different Provenances to Drought Stress. Agronomy 2022, 12, 1461. [Google Scholar] [CrossRef]
- Dias, M.C.; Azevedo, C.; Costa, M.; Pinto, G.; Santos, C. Melia azedarach Plants Show Tolerance Properties to Water Shortage Treatment: An Ecophysiological Study. Plant Physiol. Biochem. 2014, 75, 123–127. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Han, G.; Zhang, Y.; Wang, B.; Chen, M. Salt Tolerance Mechanisms in Trees: Research Progress. Trees 2021, 35, 717–730. [Google Scholar] [CrossRef]
- Htet, Z.M.; Li, X.; Yu, F. Enhanced Seedling Growth and Physiological Performances of Melia azedarach L. by Foliar Application of 24-Epibrassinolide under Salt Stress. Forests 2024, 15, 427. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Kuang, L.; Li, X.; Wu, L.; Wu, D.; Zhang, G. Metabolomic and Transcriptomic Analyses Reveal the Reasons Why Hordeum Marinum Has Higher Salt Tolerance than Hordeum Vulgare. Environ. Exp. Bot. 2018, 156, 48–61. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-Length Transcriptome Assembly from RNA-Seq Data without a Reference Genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A New Generation of Protein Database Search Programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential Expression Analysis for Sequence Count Data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Qian, C.; Zhang, C.; Wang, H.; Li, W.; Zhao, H.; Ju, Y. Physiological and Molecular Responses of Apocynum venetum L. (Apocynaceae) on Salt Stress. Horticulturae 2023, 9, 1010. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Du, S.; Wang, G.; Zhang, J.; Jiang, J. Effects of Exogenous (K+) Potassium Application on Plant Hormones in the Roots of Tamarix ramosissima under NaCl Stress. Genes 2022, 13, 1803. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Sun, S.; Li, Y.; Jia, L.; Ye, S.; Yu, Y.; Dossa, K.; Luan, Y. Transcriptome Analysis Reveals the Key Pathways and Candidate Genes Involved in Salt Stress Responses in Cymbidium Ensifolium Leaves. BMC Plant Biol. 2023, 23, 64. [Google Scholar] [CrossRef]
- Liu, J.-G.; Han, X.; Yang, T.; Cui, W.-H.; Wu, A.-M.; Fu, C.-X.; Wang, B.-C.; Liu, L.-J. Genome-Wide Transcriptional Adaptation to Salt Stress in Populus. BMC Plant Biol. 2019, 19, 367. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, I.A.A.; Shalby, N.; El-Badri, A.M.; Batool, M.; Wang, C.; Wang, Z.; Salah, A.; Rady, M.M.; Jie, K.; Wang, B.; et al. RNA-Seq Analysis Revealed Key Genes Associated with Salt Tolerance in Rapeseed Germination through Carbohydrate Metabolism, Hormone, and MAPK Signaling Pathways. Ind. Crops Prod. 2022, 176, 114262. [Google Scholar] [CrossRef]
- Singh, P.; Choudhary, K.K.; Chaudhary, N.; Gupta, S.; Sahu, M.; Tejaswini, B.; Sarkar, S. Salt Stress Resilience in Plants Mediated through Osmolyte Accumulation and Its Crosstalk Mechanism with Phytohormones. Front. Plant Sci. 2022, 13, 1006617. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, N.; Li, W.; Gao, X.; Cha, M.; Qin, L.; Liu, L. Advances in Transcriptomics in the Response to Stress in Plants. Glob. Med. Genet. 2020, 7, 30–34. [Google Scholar] [CrossRef]
- Li, N.; Shao, T.; Xu, L.; Long, X.; Rengel, Z.; Zhang, Y. Transcriptome Analysis Reveals the Molecular Mechanisms Underlying the Enhancement of Salt-Tolerance in Melia azedarach under Salinity Stress. Sci. Rep. 2024, 14, 10981. [Google Scholar] [CrossRef]
- Ribba, T.; Garrido-Vargas, F.; O’Brien, J.A. Auxin-Mediated Responses under Salt Stress: From Developmental Regulation to Biotechnological Applications. J. Exp. Bot. 2020, 71, 3843–3853. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.; Yang, T.; Wang, J.; Dai, Q.; Zhang, F.; Xi, R.; Yu, Q.; Li, N. The Transcriptional Regulatory Network of Hormones and Genes under Salt Stress in Tomato Plants (Solanum lycopersicum L.). Front. Plant Sci. 2023, 14, 1115593. [Google Scholar] [CrossRef]
- Chen, Y.; Xiang, Y.; Hu, Z.; Gao, Y.; Zhang, Y.; Chen, M.; Khaldun, A.B.M.; Yan, X.; Fan, J. Transcriptomic Profiling Revealed the Role of 24-Epibrassinolide in Alleviating Salt Stress Damage in Tall Fescue (Festuca arundinacea). Front. Plant Sci. 2022, 13, 976341. [Google Scholar] [CrossRef]
- Park, J.E.; Park, J.Y.; Kim, Y.S.; Staswick, P.E.; Jeon, J.; Yun, J.; Kim, S.Y.; Kim, J.; Lee, Y.H.; Park, C.M. GH3-Mediated Auxin Homeostasis Links Growth Regulation with Stress Adaptation Response in Arabidopsis. J. Biol. Chem. 2007, 282, 10036–10046. [Google Scholar] [CrossRef]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.H.; Lee, S.C. Function of ABA in Stomatal Defense against Biotic and Drought Stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Y.; Zhao, X.; Wang, J.; Gu, M.; Yuan, Z. Transcriptomic Profiling of Pomegranate Provides Insights into Salt Tolerance. Agronomy 2019, 10, 44. [Google Scholar] [CrossRef]
- Gu, R.; Wan, Z.Q.; Tang, F.; Liu, X.T.; Yang, Y.T.; Shi, F.L. Physiological and Transcriptomic Analysis of Salt Tolerant Glaux Maritima Grown under High Saline Condition. Front. Plant Sci. 2023, 14, 1173191. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Zhou, H. Plant Salt Response: Perception, Signaling, and Tolerance. Front. Plant Sci. 2023, 13, 1053699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, D.; Zhou, R.; Wang, X.; Dossa, K.; Wang, L.; Zhang, Y.; Yu, J.; Gong, H.; Zhang, X.; et al. Transcriptome and Metabolome Analyses of Two Contrasting Sesame Genotypes Reveal the Crucial Biological Pathways Involved in Rapid Adaptive Response to Salt Stress. BMC Plant Biol. 2019, 19, 66. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Htet, Z.M.; Chen, H.; Liu, J.; Yu, F. Transcriptomic Insights into Salt Stress Tolerance Mechanisms in Melia azedarach: 24-Epibrassinolide-Mediated Modulation of Auxin and ABA Signaling Pathways. Agronomy 2025, 15, 1653. https://doi.org/10.3390/agronomy15071653
Li X, Htet ZM, Chen H, Liu J, Yu F. Transcriptomic Insights into Salt Stress Tolerance Mechanisms in Melia azedarach: 24-Epibrassinolide-Mediated Modulation of Auxin and ABA Signaling Pathways. Agronomy. 2025; 15(7):1653. https://doi.org/10.3390/agronomy15071653
Chicago/Turabian StyleLi, Xiaoxian, Zin Myo Htet, Hong Chen, Jianbing Liu, and Fangyuan Yu. 2025. "Transcriptomic Insights into Salt Stress Tolerance Mechanisms in Melia azedarach: 24-Epibrassinolide-Mediated Modulation of Auxin and ABA Signaling Pathways" Agronomy 15, no. 7: 1653. https://doi.org/10.3390/agronomy15071653
APA StyleLi, X., Htet, Z. M., Chen, H., Liu, J., & Yu, F. (2025). Transcriptomic Insights into Salt Stress Tolerance Mechanisms in Melia azedarach: 24-Epibrassinolide-Mediated Modulation of Auxin and ABA Signaling Pathways. Agronomy, 15(7), 1653. https://doi.org/10.3390/agronomy15071653





