Effects of Low-Temperature Plasma Treatment on Germination, Seedling Development, and Biochemical Parameters of Long-Term-Stored Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material Used in the Study
2.2. Plasma Treatment
2.3. Morphometric Parameters of Germinated Seeds
2.4. Biochemical Parameters of Germinated Seeds
2.4.1. Determination of Malondialdehyde
2.4.2. Extraction and Determination of Total Phenols
2.4.3. Extraction and Determination of Total Flavonoids
2.4.4. Extraction and Determination of Total Antioxidant Capacity
2.4.5. Statistical Analyses
3. Results
3.1. Evaluation of Morphometric Parameters in Germinated Seeds
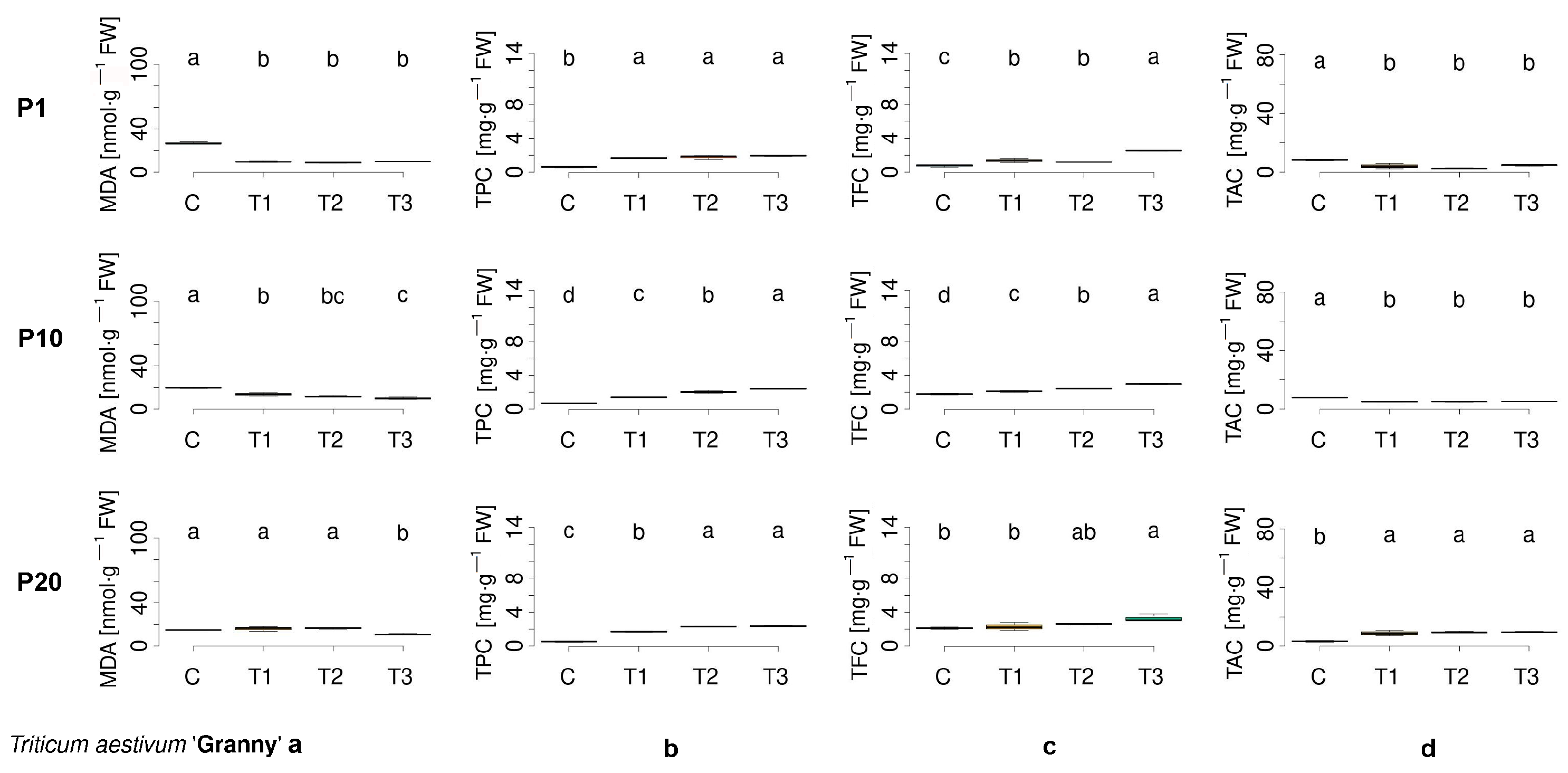
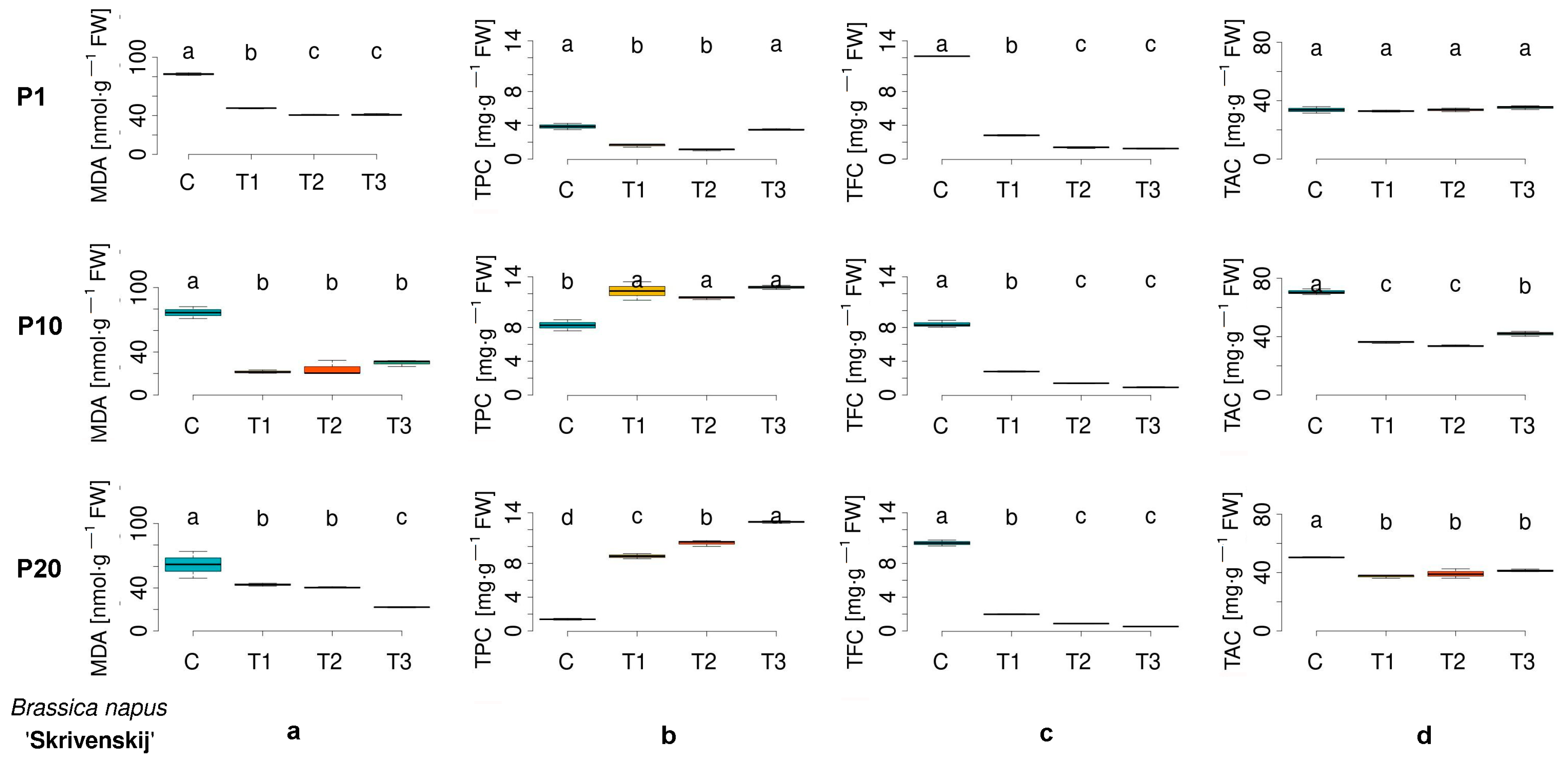
3.2. Assessment of Biochemical Parameters in Germinated Seeds
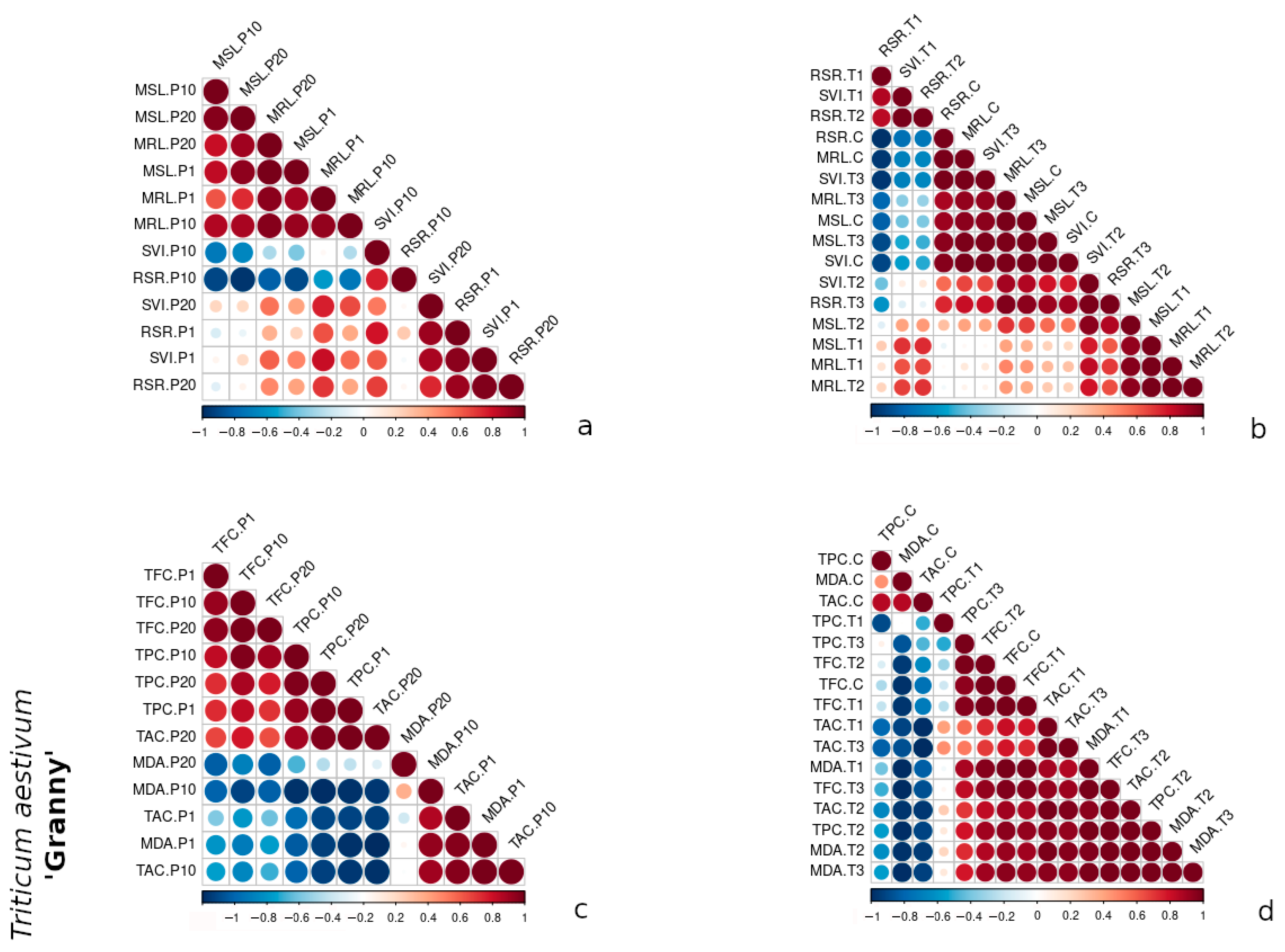
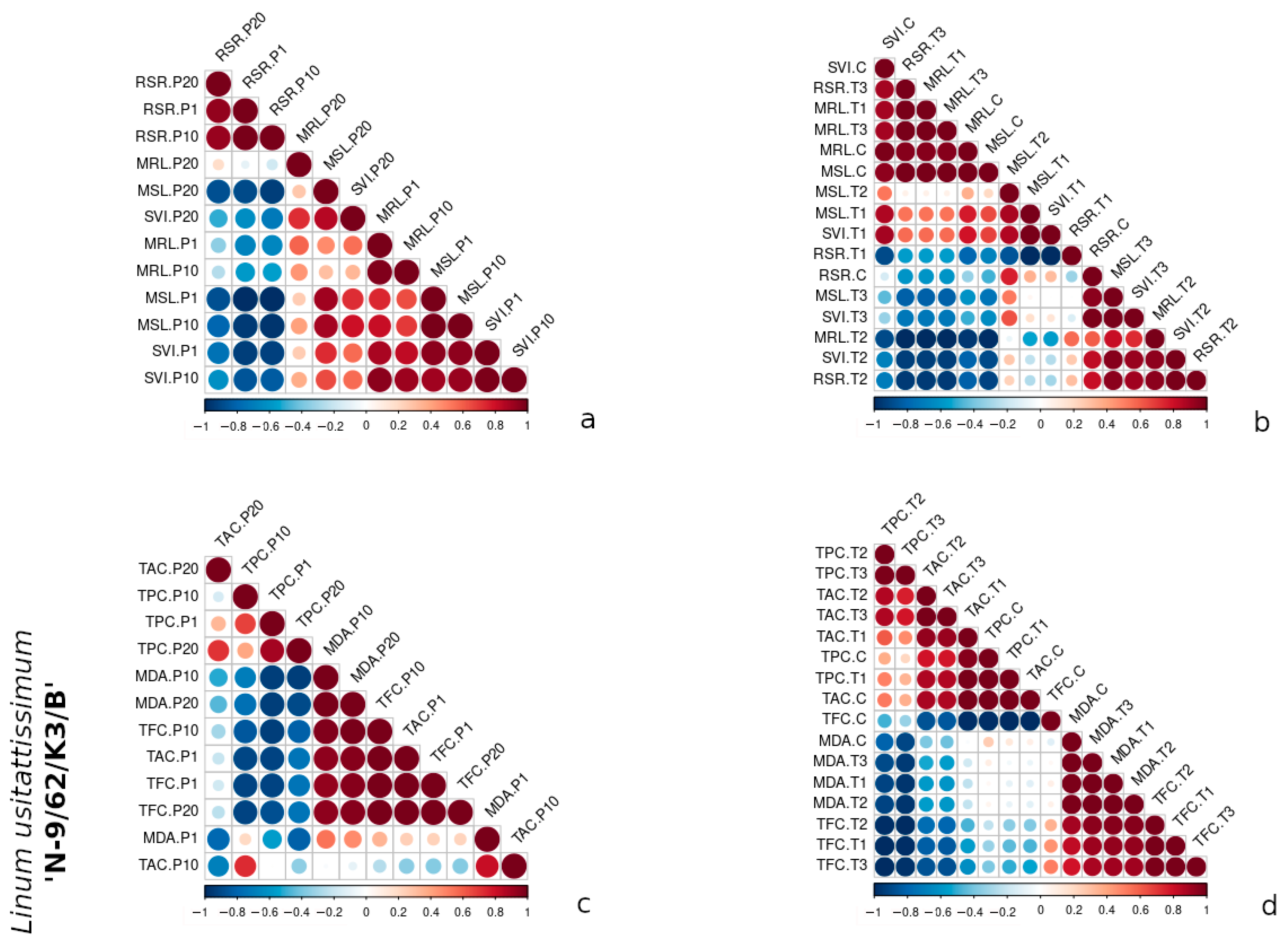
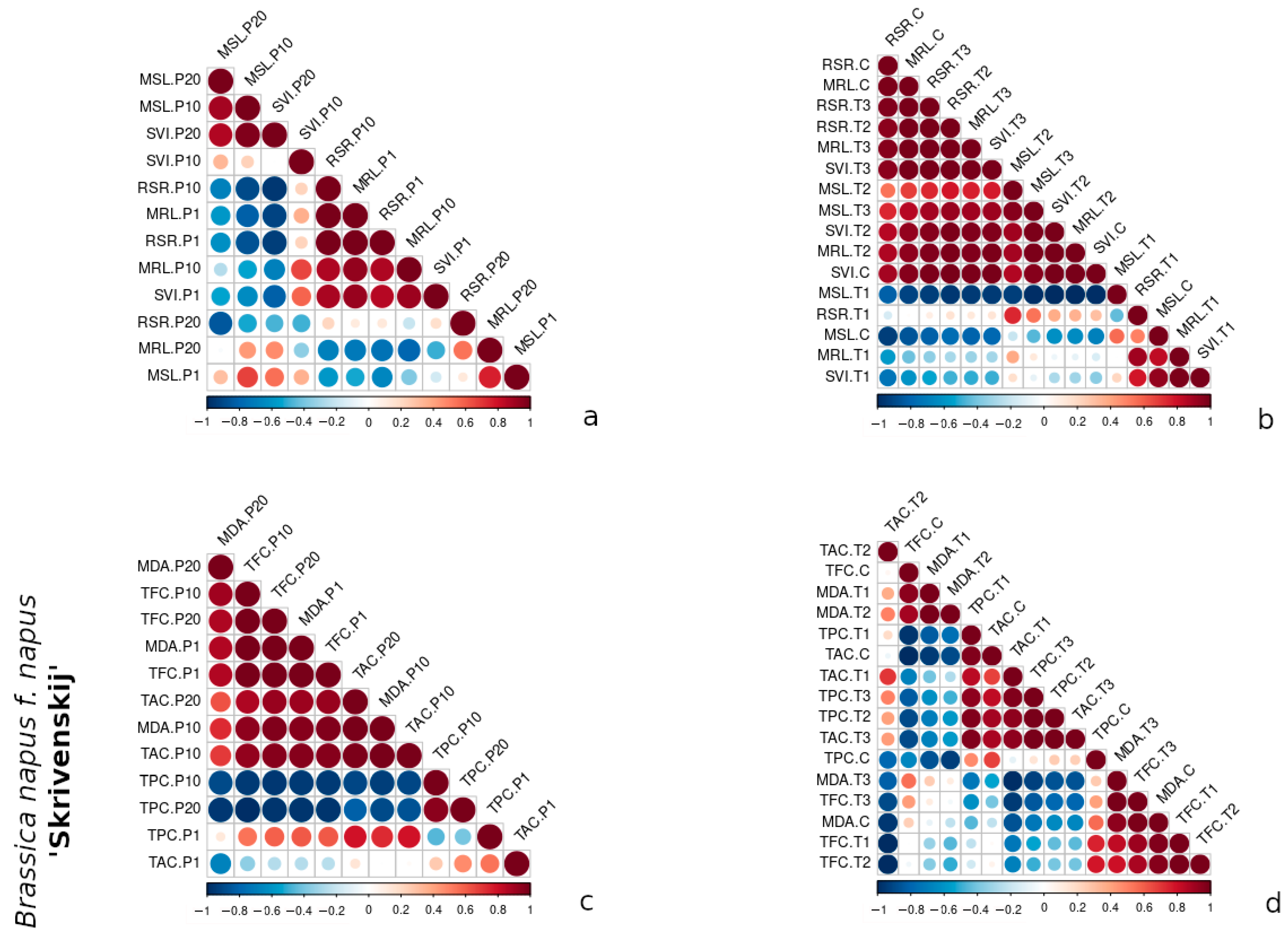
3.3. Comparative Correlation Analysis of Morphometric and Biochemical Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MRL | mean root length |
| MSL | mean shoot length |
| SVI | seedling vigor index |
| RSR | root–shoot ratio |
| LTP | low-temperature plasma (discharge) |
| SOD | superoxide dismutase |
| MDA | malondialdehyde |
| TPC | total phenol content |
| TFC | total flavonoid content |
| TAC | total antioxidant capacity |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| SG | seed germination |
| FW | fresh weight |
References
- Filatova, I.I.; Azharonok, V.V.; Goncharik, S.V.; Lushkevich, V.A.; Zhukovsky, A.G.; Gadzhieva, G.I. Effect of rf Plasma Treatment on the Germination and Phytosanitary State of Seeds. J. Appl. Spectrosc. 2014, 81, 250–256. [Google Scholar] [CrossRef]
- Guragain, R.P.; Kierzkowska-Pawlak, H.; Fronczak, M.; Kedzierska-Sar, A.; Subedi, D.P.; Tyczkowski, J. Germination improvement of fenugreek seeds with cold plasma: Exploring long-lasting effects of surface modification. Sci. Hortic. 2024, 324, 112619. [Google Scholar] [CrossRef]
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M. Plasma Agriculture from Laboratory to Farm: A Review. Processes 2020, 8, 1002. [Google Scholar] [CrossRef]
- Shashikanthalu, S.P.; Ramireddy, L.; Radhakrishnan, M. Stimulation of the germination and seedling growth of Cuminum cyminum L. seeds by cold plasma. J. Appl. Res. Med. Aromat. Plants 2020, 18, 100259. [Google Scholar] [CrossRef]
- Randeniya, L.K.; de Groot, G.J.J.B. Non-Thermal Plasma Treatment of Agricultural Seeds for Stimulation of Germination, Removal of Surface Contamination and Other Benefits: A Review. Plasma Process. Polym. 2015, 12, 608–623. [Google Scholar] [CrossRef]
- Yodpitaka, S.; Mahatheeranont, S.; Boonyawan, D.; Sookwong, P.; Roytrakul, S.; Norkaew, O. Cold plasma treatment to improve germination and enhance the bioactive phytochemical content of germinated brown rice. Food Chem. 2019, 289, 328–339. [Google Scholar] [CrossRef]
- Rasooli, Z.; Barzin, G.; Mahabadi, T.D.; Entezari, M. Stimulating effects of cold plasma seed priming on germination and seedling growth of cumin plant. S. Afr. J. Bot. 2021, 142, 106–113. [Google Scholar] [CrossRef]
- Tunklová, B.; Será, B.; Srámková, P.; Durcányová, S.; Sery, M.; Kovácik, D.; Zahoranová, A.; Hnilicka, F. Growth Stimulation of Durum Wheat and Common Buckwheat by Non-Thermal Atmospheric Pressure Plasma. Plants 2023, 12, 4172. [Google Scholar] [CrossRef]
- Magureanu, M.; Sîrbu, R.; Dobrin, D.; Gîdea, M. Stimulation of the Germination and Early Growth of Tomato Seeds by Non-thermal Plasma. Plasma Chem. Plasma Process. 2018, 38, 989–1001. [Google Scholar] [CrossRef]
- Šerý, M.; Zahoranová, A.; Kerdík, A.; Será, B. Seed Germination of Black Pine (Pinus nigra Arnold) After Diffuse Coplanar Surface Barrier Discharge Plasma Treatment. IEEE Trans. Plasma Sci. 2020, 48, 939–945. [Google Scholar] [CrossRef]
- Šerá, B.; Sery, M.; Gavril, B.; Gajdova, I. Seed Germination and Early Growth Responses to Seed Pre-treatment by Non-thermal Plasma in Hemp Cultivars (Cannabis sativa L.). Plasma Chem. Plasma Process. 2017, 37, 207–221. [Google Scholar] [CrossRef]
- Mildaziene, V.; Ivankov, A.; Pauzaite, G.; Nauciene, Z.; Zukiene, R.; Degutyte-Fomins, L.; Pukalskas, A.; Venskutonis, P.R.; Filatova, I.; Lyushkevich, V. Seed treatment with cold plasma and electromagnetic field induces changes in red clover root growth dynamics, flavonoid exudation, and activates nodulation. Plasma Process. Polym. 2021, 18, e2000160. [Google Scholar] [CrossRef]
- Hui, Y.T.; Wang, D.C.; You, Y.; Shao, C.Y.; Zhong, C.S.; Wang, H.D. Effect of Low Temperature Plasma Treatment on Biological Characteristics and Yield Components of Wheat Seeds (Triticum aestivum L.). Plasma Chem. Plasma Process. 2020, 40, 1555–1570. [Google Scholar] [CrossRef]
- Perea-Brenes, A.; Garcia, J.L.; Cantos, M.; Cotrino, J.; Gonzalez-Elipe, A.R.; Gomez-Ramirez, A.; Lopez-Santos, C. Germination and First Stages of Growth in Drought, Salinity, and Cold Stress Conditions of Plasma-Treated Barley Seeds. ACS Agric. Sci. Technol. 2023, 3, 760–770. [Google Scholar] [CrossRef]
- Judickaite, A.; Venckus, J.; Koga, K.; Shiratani, M.; Mildaziene, V.; Zukiene, R. Cold Plasma-Induced Changes in Stevia rebaudiana Morphometric and Biochemical Parameter Correlations. Plants 2023, 12, 1585. [Google Scholar] [CrossRef]
- Šerá, B. Methodological contribution on seed germination and seedling initial tests in wild. Notul. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13164. [Google Scholar] [CrossRef]
- Mildaziene, V.; Ivankov, A.; Sera, B.; Baniulis, D. Biochemical and Physiological Plant Processes Affected by Seed Treatment with Non-Thermal Plasma. Plants 2022, 11, 856. [Google Scholar] [CrossRef]
- Dobrin, D.; Magureanu, M.; Mandache, N.B.; Ionita, M.D. The effect of non-thermal plasma treatment on wheat germination and early growth. Innov. Food Sci. Emerg. Technol. 2015, 29, 255–260. [Google Scholar] [CrossRef]
- Velichko, I.; Gordeev, I.; Shelemin, A.; Nikitin, D.; Brinar, J.; Pleskunov, P.; Choukourov, A.; Pazderu, K.; Pulkrábek, J. Plasma Jet and Dielectric Barrier Discharge Treatment of Wheat Seeds. Plasma Chem. Plasma Process. 2019, 39, 913–928. [Google Scholar] [CrossRef]
- Abrantes, F.L.; Machado, N.B.; Custódio, C.C. Seed moisture content can be used to accelerate dormancy release during after-ripening of Urochloa humidicola cv. Llanero spikelets. Ciencia Rural 2021, 51, e20200526. [Google Scholar] [CrossRef]
- Foyer, C.H.; Ruban, A.V.; Noctor, G. Viewing oxidative stress through the lens of oxidative signalling rather than damage. Biochem. J. 2017, 474, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Ambrico, P.F.; Simek, M.; Ambrico, M.; Moreno, M.; Prukner, V.; Minafra, A.; Allegretto, I.; Porfido, C.; Senesi, G.S.; Terzano, R. On the air atmospheric pressure plasma treatment effect on the physiology, germination and seedlings of basil seeds. J. Phys. D Appl. Phys. 2020, 53, 104001. [Google Scholar] [CrossRef]
- Diaz-Vivancos, P.; Barba-Espín, G.; Hernández, J.A. Elucidating hormonal/ROS networks during seed germination: Insights and perspectives. Plant Cell Rep. 2013, 32, 1491–1502. [Google Scholar] [CrossRef]
- El-Maarouf-Bouteau, H.; Sajjad, Y.; Bazin, J.; Langlade, N.; Cristescu, S.M.; Balzergue, S.; Baudouin, E.; Bailly, C. Reactive oxygen species, abscisic acid and ethylene interact to regulate sunflower seed germination. Plant Cell Environ. 2015, 38, 364–374. [Google Scholar] [CrossRef]
- Kumar, S.P.J.; Prasad, S.R.; Banerjee, R.; Thammineni, C. Seed birth to death: Dual functions of reactive oxygen species in seed physiology. Ann. Bot. 2015, 116, 663–668. [Google Scholar] [CrossRef]
- Chen, K.T.; Arora, R. Dynamics of the antioxidant system during seed osmopriming, post-priming germination, and seedling establishment in Spinach (Spinacia oleracea). Plant Sci. 2011, 180, 212–220. [Google Scholar] [CrossRef]
- Jiang, J.F.; He, X.; Li, L.; Li, J.G.; Shao, H.L.; Xu, Q.L.; Ye, R.H.; Dong, Y.H. Effect of Cold Plasma Treatment on Seed Germination and Growth of Wheat. Plasma Sci. Technol. 2014, 16, 54. [Google Scholar] [CrossRef]
- Kitazaki, S.; Koga, K.; Shiratani, M.; Hayashi, N. Growth Enhancement of Radish Sprouts Induced by Low Pressure O2 Radio Frequency Discharge Plasma Irradiation. Jpn. J. Appl. Phys. 2012, 51, 01AE01. [Google Scholar] [CrossRef]
- Liu, D.; Wu, L.T.; Naeem, M.S.; Liu, H.B.; Deng, X.Q.; Xu, L.; Zhang, F.; Zhou, W.J. 5-Aminolevulinic acid enhances photosynthetic gas exchange, chlorophyll fluorescence and antioxidant system in oilseed rape under drought stress. Acta Physiol. Plant. 2013, 35, 2747–2759. [Google Scholar] [CrossRef]
- Yuan, G.F.; Jia, C.G.; Li, Z.; Sun, B.; Zhang, L.P.; Liu, N.; Wang, Q.M. Effect of brassinosteroids on drought resistance and abscisic acid concentration in tomato under water stress. Sci. Hortic. 2010, 126, 103–108. [Google Scholar] [CrossRef]
- Li, L.; Li, J.G.; Shen, M.C.; Zhang, C.L.; Dong, Y.H. Cold plasma treatment enhances oilseed rape seed germination under drought stress. Sci. Rep. 2015, 5, 13033. [Google Scholar]
- Yin, M.Q.; Huang, M.J.; Ma, B.Z.; Ma, T.C. Stimulating effects of seed treatment by magnetized plasma on tomato growth and yield. Plasma Sci. Technol. 2005, 7, 3143–3147. [Google Scholar]
- Tong, J.Y.; He, R.; Zhang, X.L.; Zhan, R.T.; Chen, W.W.; Yang, S.Z. Effects of atmospheric pressure air plasma pretreatment on the seed germination and early growth of Andrographis paniculata. Plasma Sci. Technol. 2014, 16, 260–266. [Google Scholar] [CrossRef]
- Ben Othman, K.; Cherif, M.M.; Assadi, I.; Elfalleh, W.; Khezami, L.; Ghorbal, A.; Assadi, A.A. Exploring cold plasma technology: Enhancements in carob seed germination, phytochemical composition, and antioxidant activity. Heliyon 2024, 10, e28966. [Google Scholar] [CrossRef]
- Zielinska, S.; Staniszewska, I.; Cybulska, J.; Zdunek, A.; Szymanska-Chargot, M.; Zielinska, D.; Liu, Z.L.; Pan, Z.L.; Xiao, H.W.; Zielinska, M. Modification of the cell wall polysaccharides and phytochemicals of okra pods by cold plasma treatment. Food Hydrocoll. 2022, 131, 107763. [Google Scholar] [CrossRef]
- Šerá, B.; Špatenka, P.; Šerý, M.; Vrchotová, N.; Hrušková, I. Influence of Plasma Treatment on Wheat and Oat Germination and Early Growth. IEEE Trans. Plasma Sci. 2010, 38, 2963–2968. [Google Scholar] [CrossRef]
- Roy, N.C.; Hasan, M.M.; Talukder, M.R.; Hossain, M.D.; Chowdhury, A.N. Prospective applications of low frequency glow discharge plasmas on enhanced germination, growth and yield of wheat. Plasma Chem. Plasma Process. 2018, 38, 13–28. [Google Scholar] [CrossRef]
- Matějovič, M.; Jozová, E.; Rost, M.; Čurn, V.; Hnilička, F.; Kotíková, Z.; Čepková, P.H. Evaluation of the Effect of Low-Temperature Plasma Treatment on Seed Germination of Long-Term Stored Genetic Resources. Agronomy 2024, 14, 1918. [Google Scholar] [CrossRef]
- Du, Z.Y.; Bramlage, W.J. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant-tissue extracts. J. Agric. Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Tsanova-Savova, S.; Ribarova, F.; Petkov, V. Quercetin content and ratios to total flavonols and total flavonoids in Bulgarian fruits and vegetables. Bulg. Chem. Commun. 2018, 50, 69–73. [Google Scholar]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Kassambara, A. Correlation Analyses in R STHDA. 2024. Available online: https://www.sthda.com/english/wiki/correlation-analysis-in-r (accessed on 21 March 2025).
- Waskow, A.; Avino, F.; Howling, A.; Furno, I. Entering the plasma agriculture field: An attempt to standardize protocols for plasma treatment of seeds. Plasma Process. Polym. 2022, 19, e2100152. [Google Scholar] [CrossRef]
- Bradshaw, J.E. Plant breeding: Past, present and future. Euphytica 2017, 213, 60. [Google Scholar] [CrossRef]
- Aribi, M.M. Plant Gene Banks: Conservation of Genetic Resources. In Sustainable Utilization and Conservation of Plant Genetic Diversity; Sustainable Development and Biodiversity; Al-Khayri, J.M., Jain, S.M., Penna, S., Eds.; Springer: Singapore, 2024; Volume 35, pp. 753–775. [Google Scholar]
- Tamosiune, I.; Gelvonauskiene, D.; Haimi, P.; Mildaziene, V.; Koga, K.; Shiratani, M.; Baniulis, D. Cold plasma treatment of sunflower seeds modulates plant-associated microbiome and stimulates root and lateral organ growth. Front. Plant Sci. 2020, 11, 568924. [Google Scholar] [CrossRef]
- Matra, K. Atmospheric non-thermal argon-oxygen plasma for sunflower seedling growth improvement. Jpn. J. Appl. Phys. 2018, 57, 01AG03. [Google Scholar] [CrossRef]
- Bozhanova, V.; Marinova, P.; Videva, M.; Nedjalkova, S.; Benova, E. Effect of cold plasma on the germination and seedling growth of durum wheat genotypes. Processes 2024, 12, 544. [Google Scholar] [CrossRef]
- Singh, R.; Kishor, R.; Singh, V.; Singh, V.; Prasad, P.; Aulakh, N.S.; Tiwari, U.K.; Kumar, B. Radio-frequency (RF) room temperature plasma treatment of sweet basil seeds (Ocimum basilicum L.) for germination potential enhancement by immaculation. J. Appl. Res. Med. Aromat. Plants 2022, 26, 100350. [Google Scholar] [CrossRef]
- Strejčkova, M.; Bohatá, A.; Olšan, P.; Havelka, Z.; Křiž, P.; Beran, P.; Bartoš, P.; Čurn, V.; Špatenka, P. Enhancement of the yield of crops by plasma and using of entomopathogenic and mycoparasitic fungi: From laboratory to large-field experiments. J. Biomater. Tissue Eng. 2018, 8, 829–836. [Google Scholar] [CrossRef]
- Ongrak, P.; Poolyarat, N.; Suksaengpanomrung, S.; Saidarasamoot, K.; Jirakiattikul, Y.; Rithichai, P. Germination, physicochemical properties, and antioxidant enzyme activities in kangkong (Ipomoea aquatica Forssk.) seeds as affected by dielectric barrier discharge plasma. Horticulturae 2023, 9, 11269. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Ali, N.; Nungula, E.Z.; Gitari, H.I.; Alhammad, B.A.; Battaglia, M.L. Enhancing germination and seedling growth of barley using plasma-activated water (PAW) with neutralized pH. Cogent Food Agric. 2024, 10, 2390162. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Zhang, H.R.; Qu, G.; Wang, T.C.; Sun, Q.H.; Liang, D.L. Alleviation of adverse effects of drought stress on wheat seed germination using atmospheric dielectric barrier discharge plasma treatment. Sci. Rep. 2017, 7, 16680. [Google Scholar] [CrossRef] [PubMed]
- Rithichai, P.; Jirakiattikul, Y.; Singhawiboon, M.; Poolyarat, N. Enhancement of seed quality and bioactive compound accumulation in sunflower sprouts by dielectric barrier discharge plasma treatment. ScienceAsia 2021, 47, 441–448. [Google Scholar] [CrossRef]
- Mildaziene, V.; Pauzaite, G.; Nauciene, Z.; Malakauskiene, A.; Zukiene, R.; Januskaitiene, I.; Jakstas, V.; Ivanauskas, L.; Filatova, I.; Lyushkevich, V. Pre-sowing seed treatment with cold plasma and electromagnetic field increases secondary metabolite content in purple coneflower (Echinacea purpurea) leaves. Plasma Process. Polym. 2018, 15, 1700059. [Google Scholar] [CrossRef]
- Guragain, R.P.; Baniya, H.B.; Pradhan, S.P.; Pandey, B.P.; Shrestha, B.; Fronczak, M.; Kierzkowska-Pawlak, H.; Subedi, D.P. Growth Enhancement of Radish Seed Induced by Low-Temperature Argon Plasma. Plasma Chem. Plasma Process. 2023, 43, 111–137. [Google Scholar] [CrossRef]
- Bussmann, F.; Krüger, A.; Scholz, C.; Brust, H.; Stöhr, C. Long-term effects of cold atmospheric plasma-treated water on the antioxidative system of Hordeum vulgare. J. Plant Growth Regul. 2023, 42, 3274–3290. [Google Scholar] [CrossRef]
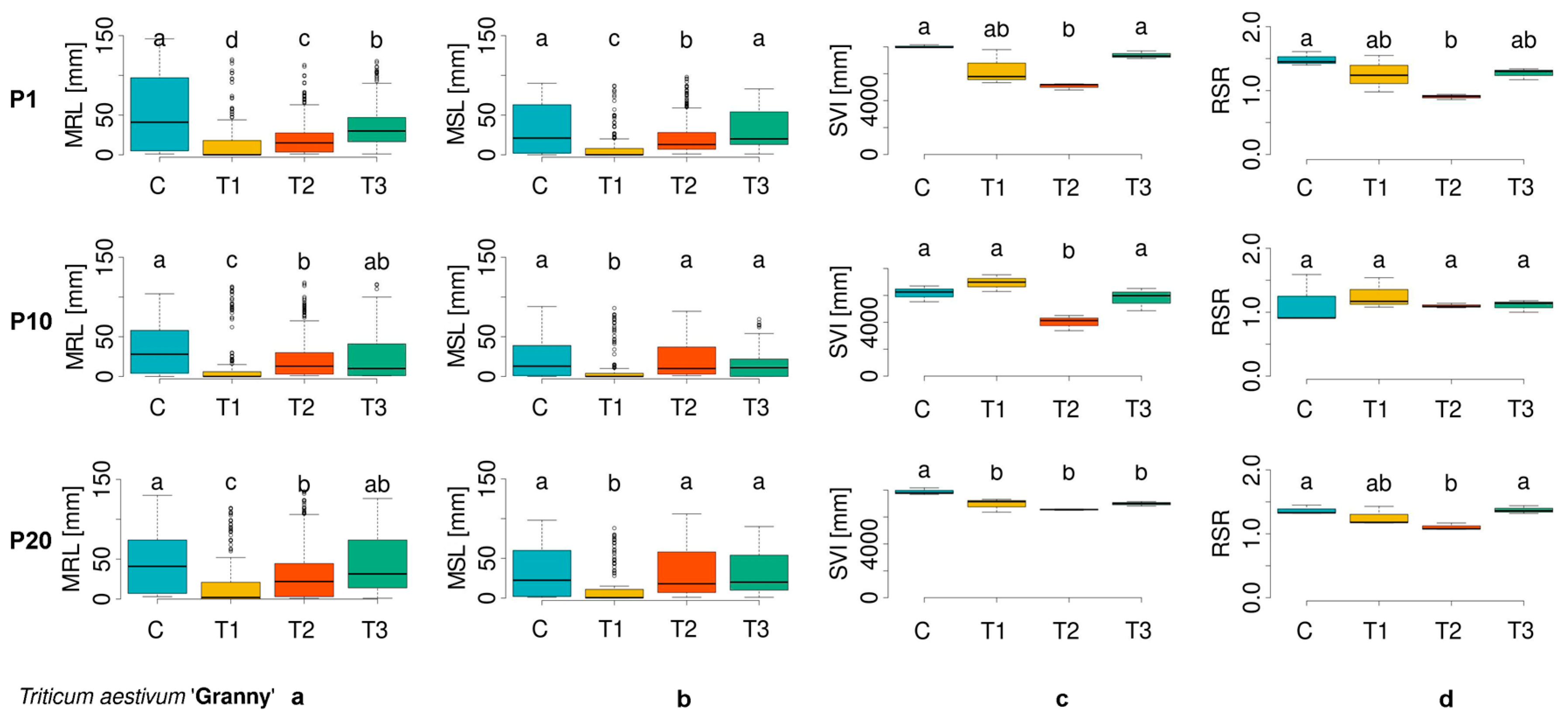
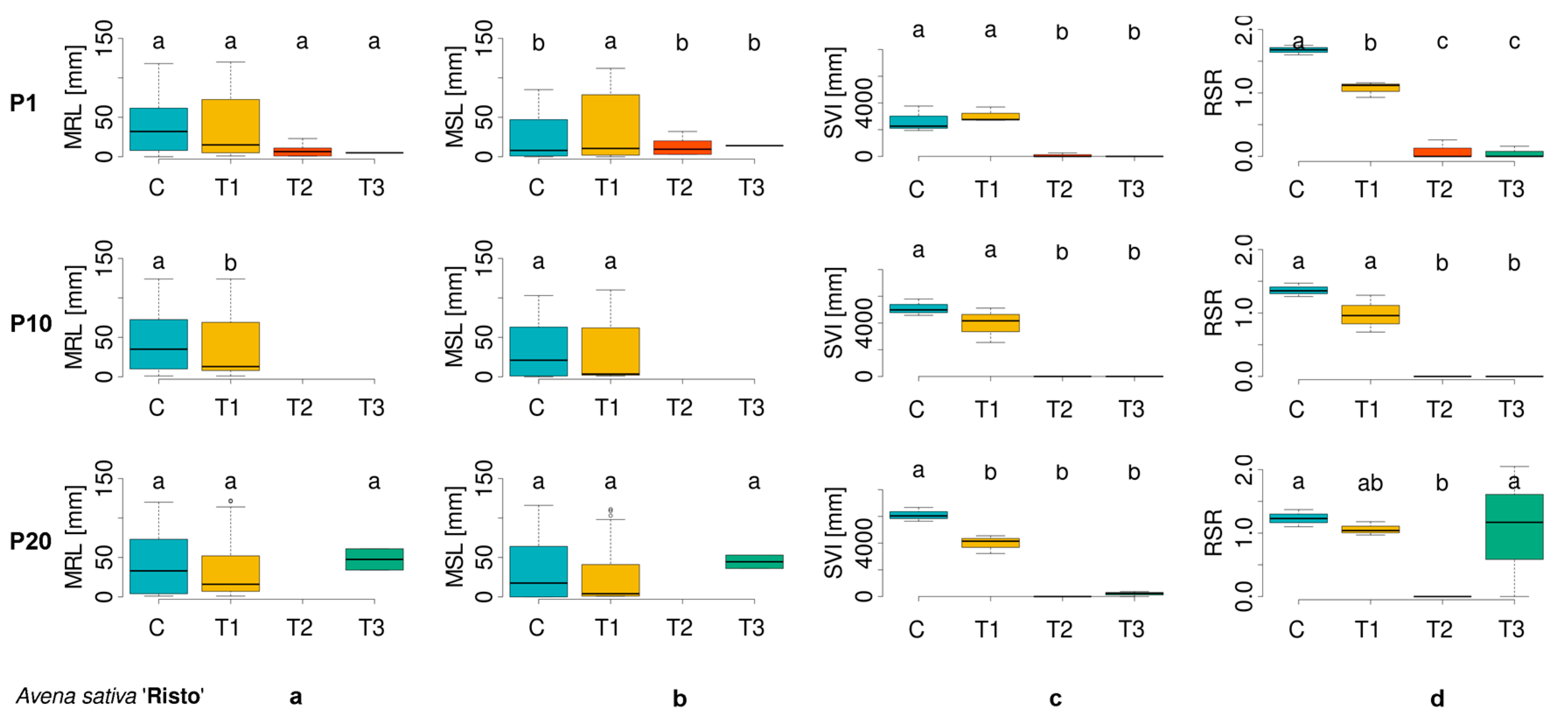
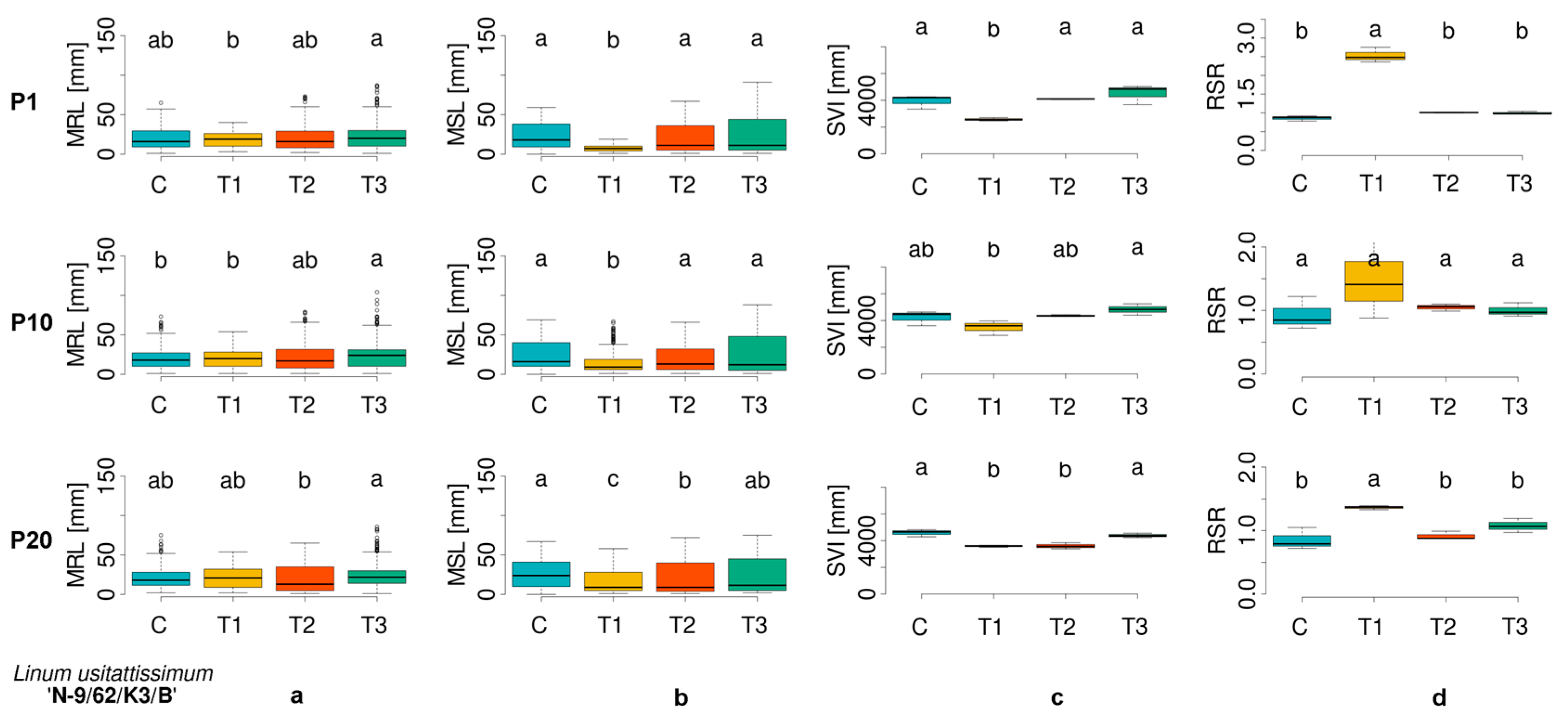
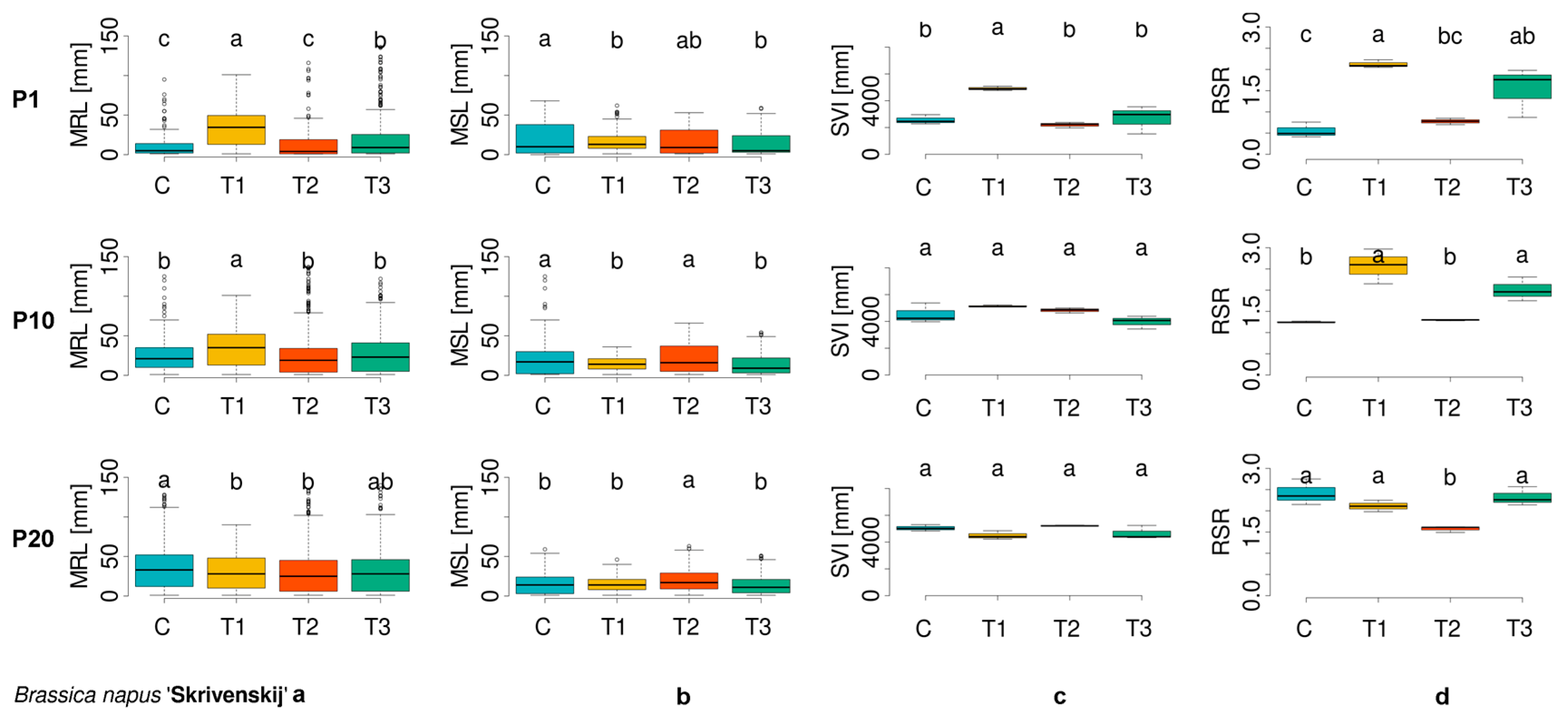



| Cultivar Number | ECN | Plant Species | Cultivar |
|---|---|---|---|
| TA_1 | 01C0100139 | Triticum aestivum | Granny |
| AS_2 | 03C0700967 | Avena sativa | Risto |
| LU_3 | 05X1100390 | Linum usitatissimum | N-9/62/K3/B |
| BN_4 | 15O0100097 | Brassica napus f. napus | Skrivenskij |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matějovič, M.; Čurn, V.; Kubeš, J.; Jozová, E.; Kotíková, Z.; Hlásná Čepková, P. Effects of Low-Temperature Plasma Treatment on Germination, Seedling Development, and Biochemical Parameters of Long-Term-Stored Seeds. Agronomy 2025, 15, 1637. https://doi.org/10.3390/agronomy15071637
Matějovič M, Čurn V, Kubeš J, Jozová E, Kotíková Z, Hlásná Čepková P. Effects of Low-Temperature Plasma Treatment on Germination, Seedling Development, and Biochemical Parameters of Long-Term-Stored Seeds. Agronomy. 2025; 15(7):1637. https://doi.org/10.3390/agronomy15071637
Chicago/Turabian StyleMatějovič, Martin, Vladislav Čurn, Jan Kubeš, Eva Jozová, Zora Kotíková, and Petra Hlásná Čepková. 2025. "Effects of Low-Temperature Plasma Treatment on Germination, Seedling Development, and Biochemical Parameters of Long-Term-Stored Seeds" Agronomy 15, no. 7: 1637. https://doi.org/10.3390/agronomy15071637
APA StyleMatějovič, M., Čurn, V., Kubeš, J., Jozová, E., Kotíková, Z., & Hlásná Čepková, P. (2025). Effects of Low-Temperature Plasma Treatment on Germination, Seedling Development, and Biochemical Parameters of Long-Term-Stored Seeds. Agronomy, 15(7), 1637. https://doi.org/10.3390/agronomy15071637






