Anaerobic Co-Digestion of Dairy Manure and Cucumber Residues: Methane Production Efficiency and Microbial Community Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Inoculum
2.2. Experimental Design
2.3. Measurement Indicators and Methods
2.4. Statistical Analysis
2.5. Microbial Community Analysis
3. Results
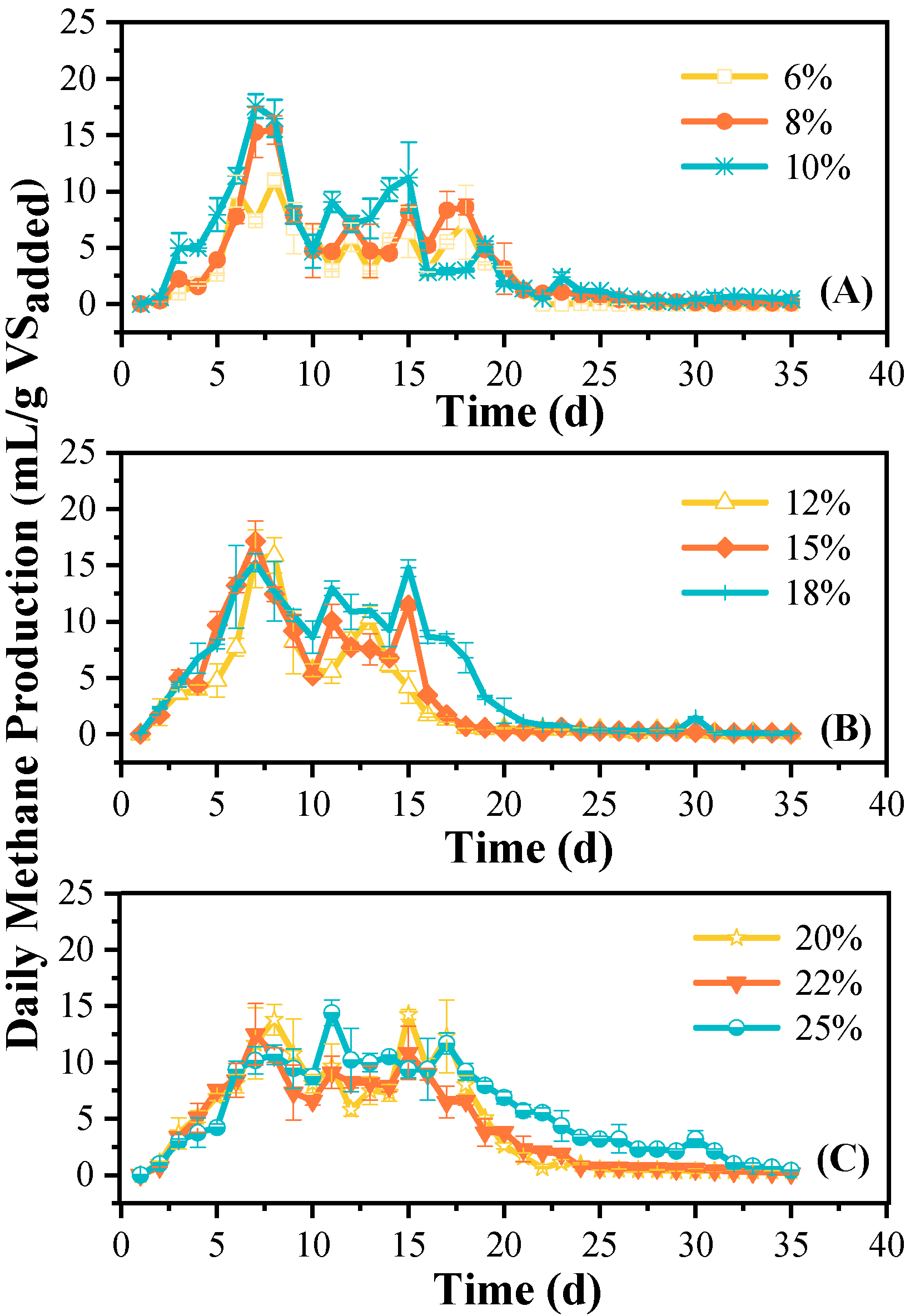
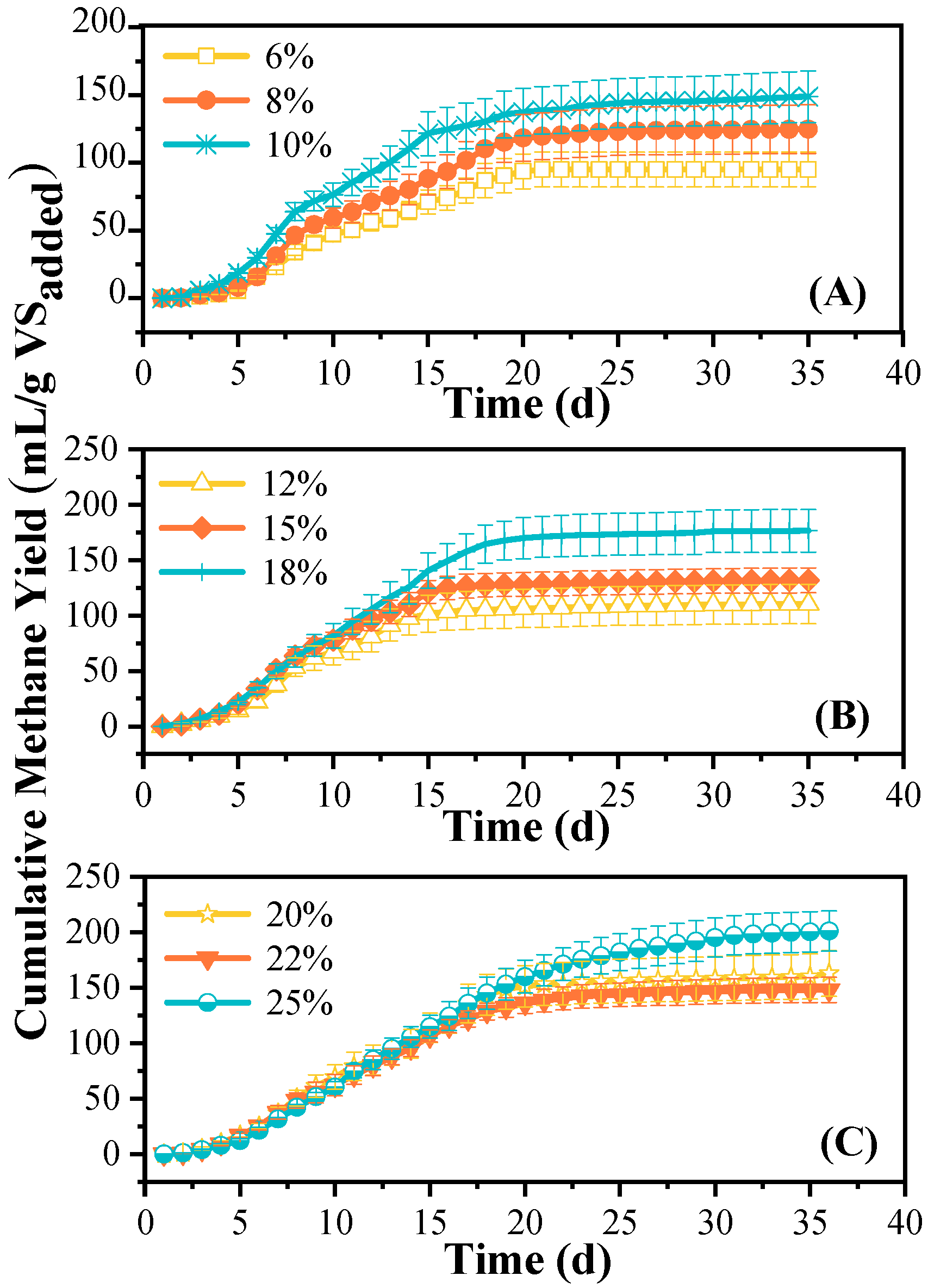
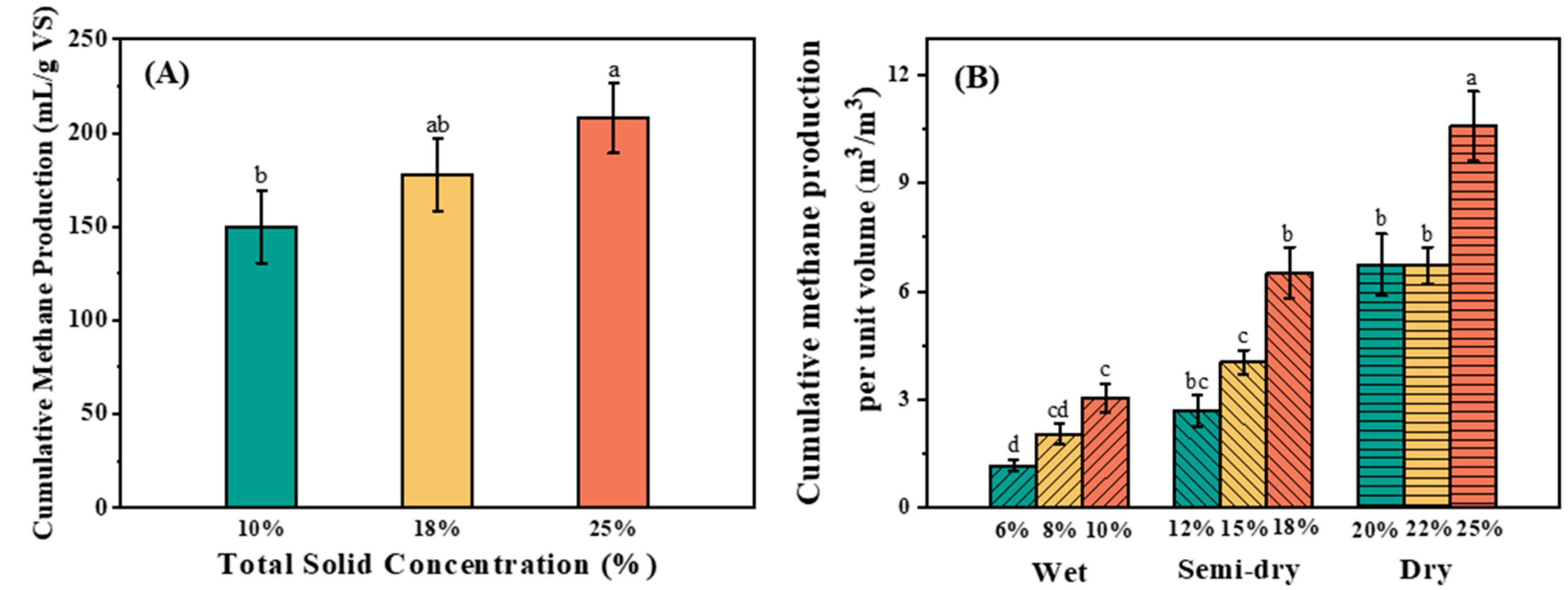
3.1. Methane Production
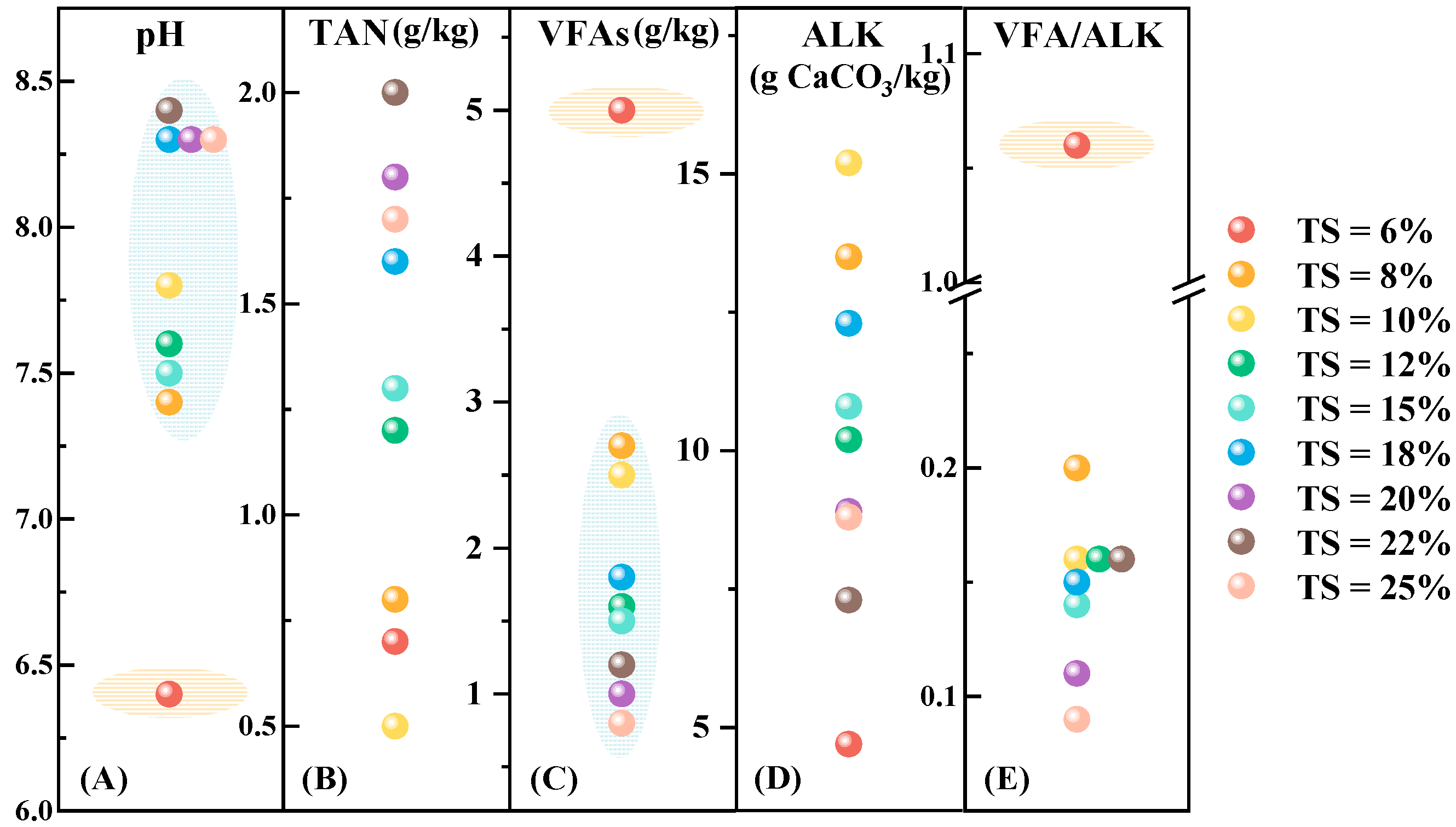
3.2. Anaerobic Digestion Physicochemical Properties
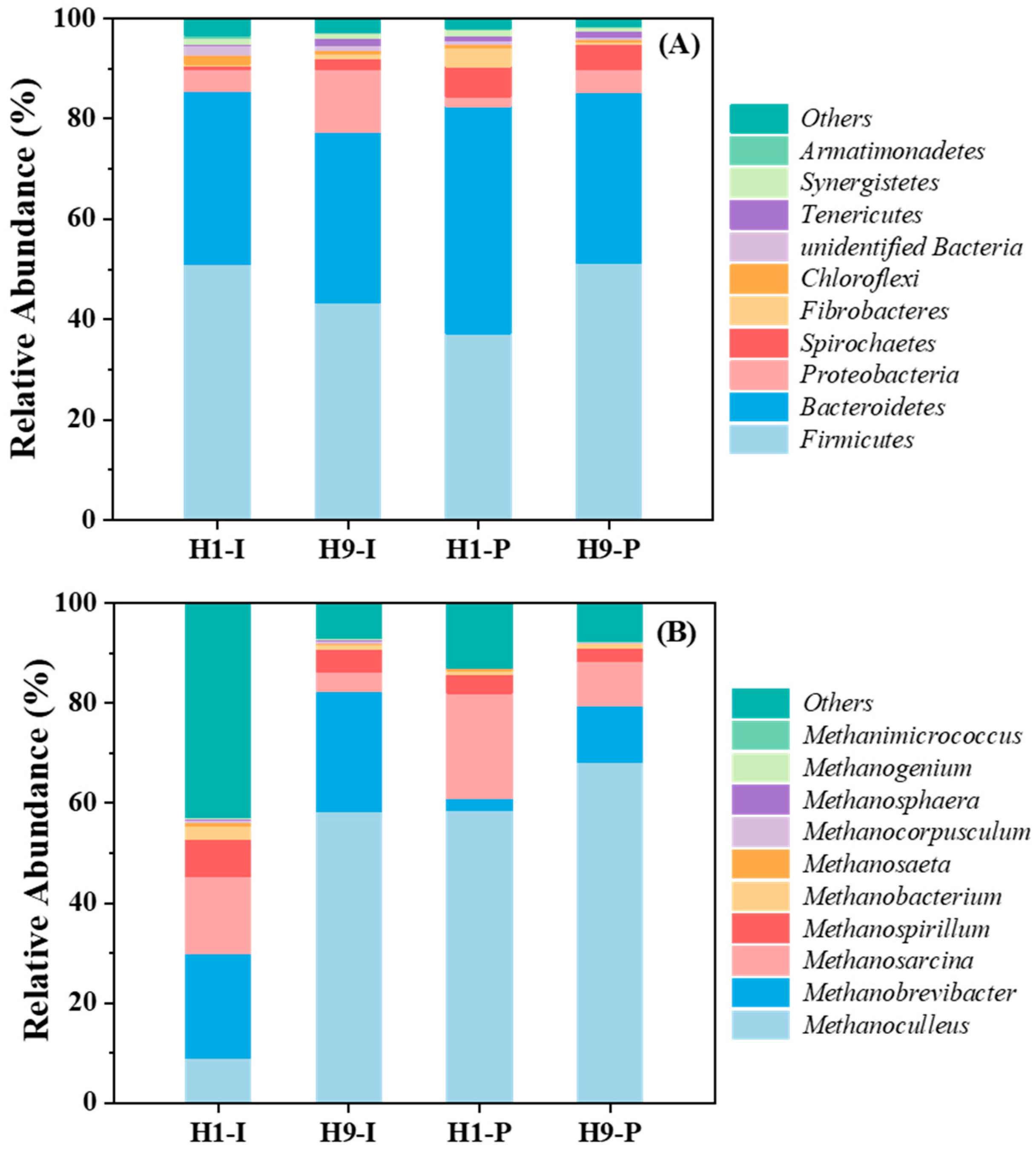
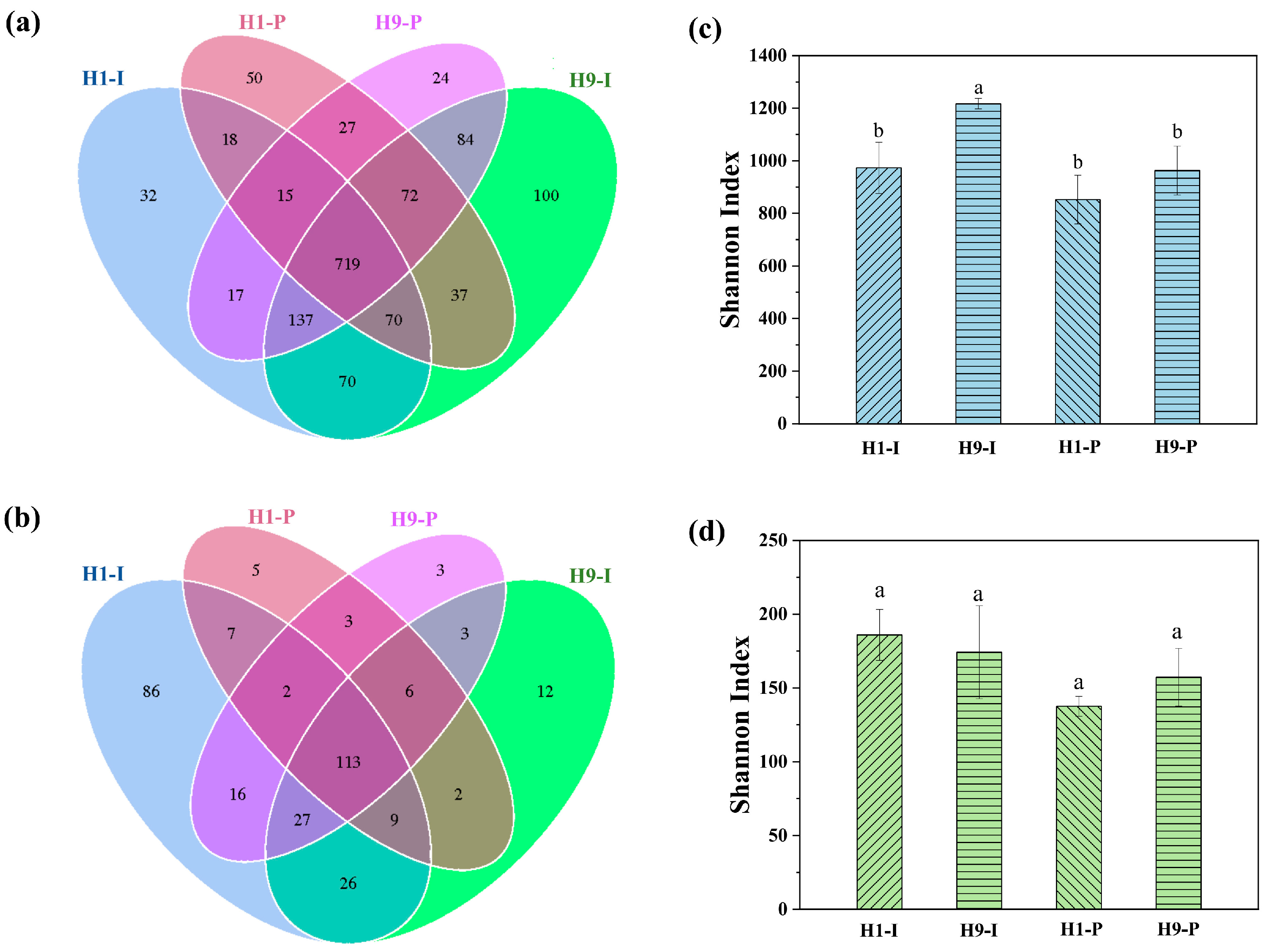
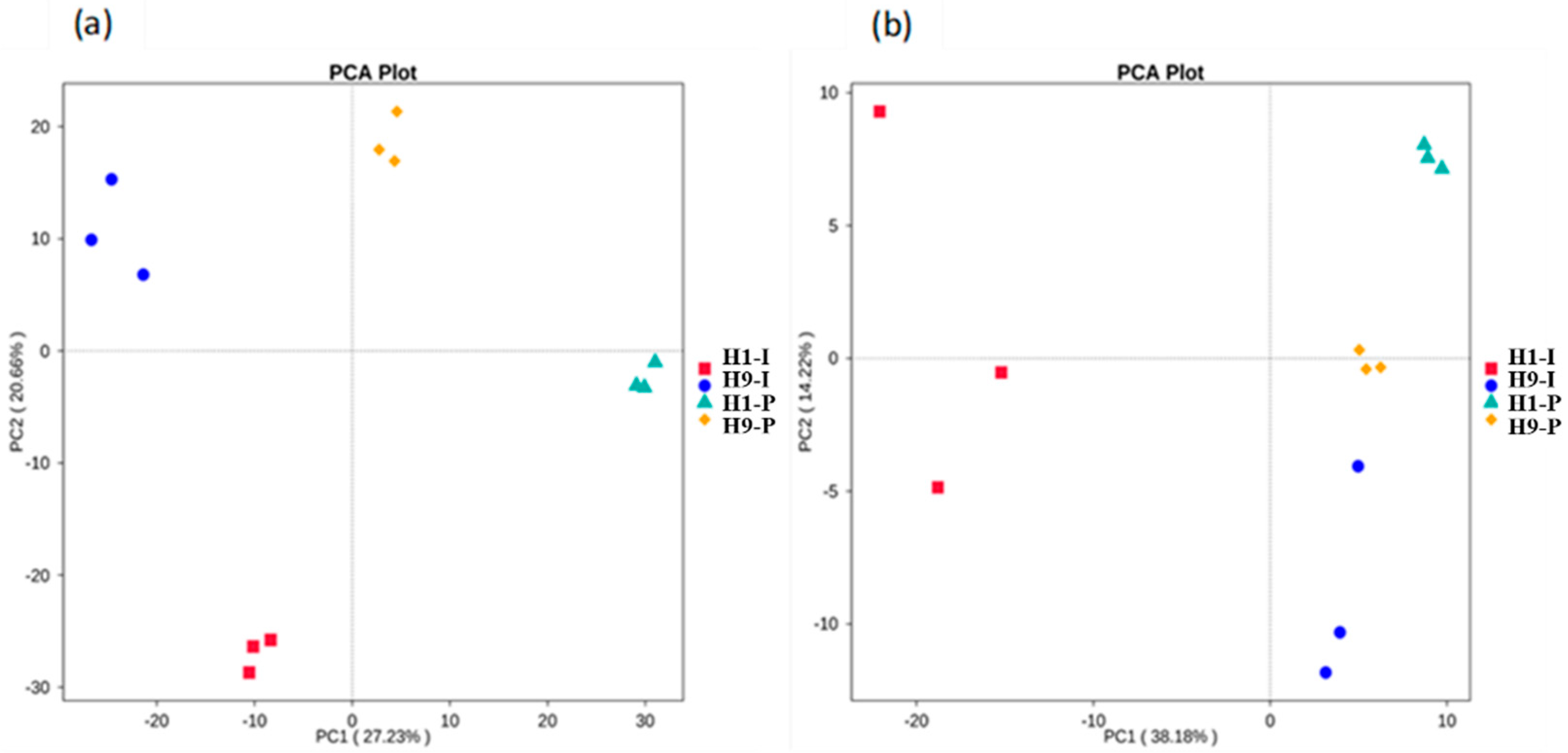
3.3. Microbial Community Structure Analysis
4. Discussion
4.1. Methane Production Efficiency
4.2. Microbial Community Characteristics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, B.; Azman, S.; Dewil, R.; Appels, L. Alkaline anaerobic digestion of livestock manure: Unveiling mechanisms, applications, and perspective. Chem. Eng. J. 2023, 477, 146852. [Google Scholar] [CrossRef]
- Liu, E.; Liu, S. Process optimization and study of biogas fermentation with a mixture of duck manure and straw. Renew. Sust. Energ. Rev. 2017, 72, 439–444. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Wu, J.; Li, H.Z.; Poncin, S.; Wang, K.J.; Zuo, J.E. Study of an enhanced dry anaerobic digestion of swine manure: Performance and microbial community property. Bioresour. Technol. 2019, 282, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Sillero, L.; Solera, R.; Perez, M. Anaerobic co-digestion of sewage sludge, wine vinasse and poultry manure for bio-hydrogen production. Int. J. Hydrogen Energy. 2022, 47, 3667–3678. [Google Scholar] [CrossRef]
- Liu, C.; Li, H.; Zhang, Y.Y.; Chen, Q.W. Characterization of methanogenic activity during high-solids anaerobic digestion of sewage sludge. Biochem. Eng. J. 2016, 109, 96–100. [Google Scholar] [CrossRef]
- Yao, L.; Wang, Y.; Li, R.; Fu, L.; Liu, Z.; Gao, X. Effects of Total Solid Content on Anaerobic Fermentation Performance and Biogas Productivity of Tail Vegetables. Fermentation 2024, 10, 437. [Google Scholar] [CrossRef]
- Guo, E.; Chen, G.; Yu, D.; Qiu, Y.; Li, S.; Yu, Y. Optimization of dry anaerobic co-fermentation of sludge and corn straw with magnetite (Fe3O4). J. Environ. Chem. Eng. 2022, 10, 108618. [Google Scholar] [CrossRef]
- Pakarinen, O.; Lehtomaki, A.; Rissanen, S.; Rintala, J. Storing energy crops for methane production: Effects of solids content and biological additive. Bioresour. Technol. 2008, 99, 7074–7082. [Google Scholar] [CrossRef]
- Wang, Z.; Lv, Z.; Du, J.; Mo, C.; Yang, X.; Tian, S. Combined process for ethanol fermentation at high-solids loading and biogas digestion from unwashed steam-exploded corn stover. Bioresour. Technol. 2014, 166, 282–287. [Google Scholar] [CrossRef]
- Abu Yazid, N.; Barrena, R.; Komilis, D.; Sanchez, A. Solid-State Fermentation as a Novel Paradigm for Organic Waste Valorization: A Review. Sustainability 2017, 9, 224. [Google Scholar] [CrossRef]
- Gervasi, T.; Mandalari, G. Valorization of Agro-Industrial Orange Peel By-Products through Fermentation Strategies. Fermentation 2024, 10, 224. [Google Scholar] [CrossRef]
- Wu, D.; Liu, H.; Xing, T.; Xiao, F.; Liu, Y.; Zhen, F.; Sun, Y. An integrated evaluation strategy for anaerobic digestion monitoring based on acid-base balance and thermodynamics of volatile fatty acid degradation. Chem. Eng. J. 2024, 486, 150340. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, Y.; Li, L.; Zhou, H.; Wang, K.; Ding, J.; Jiang, J.; Wei, L.; Zhao, Q. The stress of bioactive compounds on microbes in anaerobic digestion of food waste and mitigation strategies: A critical review. Chem. Eng. J. 2024, 493, 152764. [Google Scholar] [CrossRef]
- Yang, F.; Li, W.; Sun, M.; Li, Q.; Wang, M.; Sun, Y. Improved Buffering Capacity and Methane Production by Anaerobic Co-Digestion of Corn Stalk and Straw Depolymerization Wastewater. Energies 2018, 11, 1751. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Macé, S.; Llabrés, P. Anaerobic digestion of organic solid wastes: An overview of research achievements and perspectives. Bioresour. Technol. 2000, 74, 3–16. [Google Scholar] [CrossRef]
- Cai, F.; Gu, Y.; Yan, H.; Chen, C.; Liu, G. Impact of different pretreatments on the anaerobic digestion performance of cucumber vine. Environ. Sci. Pollut. R 2022, 29, 88507–88518. [Google Scholar] [CrossRef]
- Nleya, Y.; Young, B.; Nooraee, E.; Baroutian, S. Anaerobic digestion of dairy cow and goat manure: Comparative assessment of biodegradability and greenhouse gas mitigation. Fuel 2025, 381, 133458. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Liang, Y.; Yang, S.; Liu, X. Evaluation of Semi-dry Mesophilic Anaerobic Co-digestion of Corn Stover and Vegetable Waste by a Single-Phase Process. Waste. Biomass. Valori. 2019, 10, 1159–1166. [Google Scholar] [CrossRef]
- Tian, P.; Gong, B.; Bi, K.; Liu, Y.; Ma, J.; Wang, X.; Ouyang, Z.; Cui, X. Anaerobic Co-Digestion of Pig Manure and Rice Straw: Optimization of Process Parameters for Enhancing Biogas Production and System Stability. Int J Env. Res Public Health. 2023, 20, 804. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Yao, L.; Fu, L.; Liu, Z. The Biogas Production Potential and Community Structure Characteristics of the Co-Digestion of Dairy Manure and Tomato Residues. Agronomy 2024, 14, 881. [Google Scholar] [CrossRef]
- ISO-5664; Water Quality-Determination of Ammonium-Distillation and Titration Method. International Organization for Standardization: Geneva, Switzerland, 1984.
- Wang, Y.Y.; Li, G.X.; Chi, M.H.; Sun, Y.B.; Zhang, J.X.; Jiang, S.X.; Cui, Z.J. Effect of co-digestion of cucumber residues to corn stover and pig manure ratio on methane production in solid state anaerobic digestion. Bioresour. Technol. 2018, 250, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.M.M.; Fonseca, Y.A.; Faria, M.V.; Aquino, S.F.de.; Herrera Adarme, O.F.; Baêta, B.E.L. Methane production by anaerobic co-digestion of dairy manure and cassava wastes for energy recovery. J. Chem. Technol. Biotechnol. 2022, 98, 797–806. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y. Anaerobic digestion of giant reed for methane production. Bioresour. Technol. 2014, 171, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Li, Y.B. Evaluation of methane production and macronutrient degradation in the anaerobic co-digestion of algae bio-mass residue and lipid waste. Bioresour. Technol. 2012, 111, 42–48. [Google Scholar] [CrossRef]
- Al-Sulaimi, I.N.; Nayak, J.K.; Alhimali, H.; Sana, A.; Al-Mamun, A. Production by Sludge-Feeding Thermophilic Anaerobic Digester and Predicting Process Parameters. Fermentation 2022, 8, 184. [Google Scholar] [CrossRef]
- Lahav, O.; Morgan, B. Titration methodologies for monitoring of anaerobic digestion in developing countries: A review. J. Chem. Technol. Biotechnol. 2004, 79, 1331–1341. [Google Scholar] [CrossRef]
- He, S.; Deng, Q.; Xian, P.; Li, Z.; Tan, S.; Liu, Q. Volatile fatty acid (VFA) and methane generation from sewage sludge and banana straw: Influence of pH and two-phase anaerobic fermentation. Desalin. Water. Treat. 2019, 152, 208–213. [Google Scholar] [CrossRef]
- Meng, L.Y.; Xie, L.; Suenaga, T.; Riya, S.; Terada, A.; Hosomi, M. Eco-compatible biochar mitigates volatile fatty acids stress in high load thermophilic solid-state anaerobic reactors treating agricultural waste. Bioresour. Technol. 2020, 309, 123366. [Google Scholar] [CrossRef]
- Poggi-Varaldo, H.M.; Rodriguez-Vazquez, R.; Fernandez-Villagomez, G.; Esparza-Garcia, F. Inhibition of mesophilic solid-substrate anaerobic digestion by ammonia nitrogen. Appl. Microbiol. Biotechnol. 1997, 47, 284–291. [Google Scholar] [CrossRef]
- Ren, J.W.; Yuan, X.F.; Li, J.; Ma, X.G.; Zhao, Y.; Zhu, W.B.; Wang, X.F.; Cui, Z.J. Performance and microbial community dynamics in a two-phase anaerobic co-digestion system using cassava dregs and pig manure. Bioresour. Technol. 2014, 155, 342–351. [Google Scholar] [CrossRef]
- Woraruthai, T.; Kunno, J.; Pongsopon, M.; Yansakon, K.; Phoopraintra, P.; Chantiwas, R.; Leartsakulpanich, U.; Chaiyen, P.; Wongnate, T. Identification and cultivation of hydrogenotrophic methanogens from palm oil mill effluent for high methane production. Int. J. Energy Res. 2020, 44, 10058–10070. [Google Scholar] [CrossRef]
- Halim, M.F.A.; Day, L.A.; Costa, K.C. Formate-Dependent Heterodisulfide Reduction in a Methanomicrobiales Archaeon. Appl. Environ. Microbiol. 2021, 87, e02698–20. [Google Scholar]
- Liu, W.; Chen, L. Community Detection in Disease-Gene Network Based on Principal Component Analysis. Tsinghua. Sci. Technol. 2013, 18, 454–461. [Google Scholar] [CrossRef]
- Ma, G.; Ndegwa, P.; Harrison, J.H.; Chen, Y. Methane yields during anaerobic co-digestion of animal manure with other feedstocks: A meta-analysis. Sci. Total. Environ. 2020, 728, 138224. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yu, M.; Yang, M.; Gao, M.; Wu, C.; Wang, Q. Synergistic effect from anaerobic co-digestion of food waste and Sophora flavescens residues at different co-substrate ratios. Environ. Sci. Pollut. Res. 2019, 26, 37114–37124. [Google Scholar] [CrossRef]
- Lim, E.Y.; Lee, J.T.E.; Zhang, L.; Tian, H.L.; Ong, K.C.; Tio, Z.K.; Zhang, J.X.; Tong, Y.W. Abrogating the inhibitory effects of volatile fatty acids and ammonia in overloaded food waste anaerobic digesters via the supplementation of nano-zero valent iron modified biochar. Sci. Total. Environ. 2022, 817, 152968. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Yi, J.; Dong, B.; Jin, J.; Dai, X. Effect of increasing total solids contents on anaerobic digestion of food waste under mesophilic conditions: Performance and microbial characteristics analysis. PLoS ONE 2014, 9, e102548. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Y.; Wang, S.; Zhang, Y.; Hu, Y.; Hu, Z.; Wu, G.; Zhan, X. Impact of total solids content on anaerobic co-digestion of pig manure and food waste: Insights into shifting of the methanogenic pathway. Waste. Manage. 2020, 114, 96–106. [Google Scholar] [CrossRef]
- Abbassi-Guendouz, A.; Brockmann, D.; Trably, E.; Dumas, C.; Delgenès, J.P.; Steyer, J.P.; Escudié, R. Total solids content drives high solid anaerobic digestion via mass transfer limitation. Bioresour. Technol. 2012, 111, 55–61. [Google Scholar] [CrossRef]
- Vavilin, V.; Fernandez, B.; Palatsi, J.; Flotats, X. Hydrolysis kinetics in anaerobic degradation of particulate organic material: An overview. Waste Manag. 2008, 28, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Kallistova, A.Y.; Goel, G.; Nozhevnikova, A.N. Microbial diversity of methanogenic communities in the systems for anaerobic treatment of organic waste. Microbiology 2014, 83, 462–483. [Google Scholar] [CrossRef]
- Magdalena, J.A.; González-Fernández, C. Microalgae Biomass as a Potential Feedstock for the Carboxylate Platform. Molecules. 2019, 24, 4404. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.N.; Nelson, B.K. Invited review: Anaerobic fermentation of dairy food wastewater. J. Dairy Sci. 2012, 95, 6188–6203. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Zhang, P.; Zhou, Y. Quorum quenching altered microbial diversity and activity of anaerobic membrane bioreactor (AnMBR) and enhanced methane generation. Bioresour. Technol. 2020, 315, 123862. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.; Hunger, S.; Brown, G.; Tsai, S.; Cerri, C.; Conrad, R.; Drake, H.L. Methanogenic food web in the gut contents of methane-emitting earthworm Eudrilus eugeniae from Brazil. ISME J. 2015, 9, 1778–1792. [Google Scholar] [CrossRef]
- Ziganshin, A.M.; Liebetrau, J.; Pröter, J.; Kleinsteuber, S. Microbial community structure and dynamics during anaerobic digestion of various agricultural waste materials. Appl. Microbiol. Biotechnol. 2013, 97, 5161–5174. [Google Scholar] [CrossRef]
- Song, Y.; Qiao, W.; Zhang, J.; Dong, R. Process Performance and Functional Microbial Community in the Anaerobic Digestion of Chicken Manure: A Review. Energies 2023, 16, 4675. [Google Scholar] [CrossRef]
- Huang, Z.; Yu, X.; Miao, H.; Ren, H.; Zhao, M.; Ruan, W. Enzymatic dynamics of microbial acid tolerance response (ATR) during the enhanced biohydrogen production process via anaerobic digestion. Int. J. Hydrog. Energy. 2012, 37, 10655–10662. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Sun, Y. Acclimation of Acid-Tolerant Methanogenic Culture for Bioaugmentation: Strategy Comparison and Microbiome Succession. ACS Omega. 2020, 5, 6062–6068. [Google Scholar] [CrossRef]
| Group | Treatment | TS (%) |
|---|---|---|
| Low TS | H1 | 6 |
| H2 | 8 | |
| H3 | 10 | |
| Medium TS | H4 | 12 |
| H5 | 15 | |
| H6 | 18 | |
| High TS | H7 | 20 |
| H8 | 22 | |
| H9 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, Y.; Qi, Y.; Fu, L.; Li, G.; Liu, Z.; Bo, L.; Jing, Y. Anaerobic Co-Digestion of Dairy Manure and Cucumber Residues: Methane Production Efficiency and Microbial Community Characteristics. Agronomy 2025, 15, 1610. https://doi.org/10.3390/agronomy15071610
Wang Y, Li Y, Qi Y, Fu L, Li G, Liu Z, Bo L, Jing Y. Anaerobic Co-Digestion of Dairy Manure and Cucumber Residues: Methane Production Efficiency and Microbial Community Characteristics. Agronomy. 2025; 15(7):1610. https://doi.org/10.3390/agronomy15071610
Chicago/Turabian StyleWang, Yanqin, Yan Li, Yumeng Qi, Longyun Fu, Guangjie Li, Zhaodong Liu, Luji Bo, and Yongping Jing. 2025. "Anaerobic Co-Digestion of Dairy Manure and Cucumber Residues: Methane Production Efficiency and Microbial Community Characteristics" Agronomy 15, no. 7: 1610. https://doi.org/10.3390/agronomy15071610
APA StyleWang, Y., Li, Y., Qi, Y., Fu, L., Li, G., Liu, Z., Bo, L., & Jing, Y. (2025). Anaerobic Co-Digestion of Dairy Manure and Cucumber Residues: Methane Production Efficiency and Microbial Community Characteristics. Agronomy, 15(7), 1610. https://doi.org/10.3390/agronomy15071610





