1. Introduction
Mulberry trees (
Morus spp.) are deciduous woody plants recognized for their high leaf yield, short gestation period, and remarkable adaptability to a wide range of environmental conditions [
1]. Owing to their resilience, mulberries have been widely cultivated and studied, particularly for their nutritional and pharmaceutical properties [
2]. Historically valued in Asia and beyond, mulberries have served as both food and medicine, with the leaves traditionally used in herbal remedies and Ajurveda [
3]. The nutritional profile of mulberry fruit varies by cultivar and maturity stage, but it typically includes essential fatty acids, amino acids, vitamins, minerals, and bioactive compounds like anthocyanins, rutin, quercetin, chlorogenic acid, and polysaccharides [
4]. Increasing scientific interest has emerged due to these compounds’ demonstrated antioxidant, neuroprotective, anti-tumor, antihyperglycemic, and other health-promoting effects in both in vitro and in vivo models [
5]. Furthermore, different parts of the mulberry tree (fruits, leaves, roots, and twigs) exhibit distinct pharmacological properties [
6]. For example, black mulberries are especially rich in anthocyanins such as cyanidin-3-O-glucoside, while leaves contain quercetin, kaempferol, and chlorogenic acid. Roots and twigs are sources of morusin, a prenylated flavonoid [
7]. These characteristics position mulberry as a promising raw material for developing functional foods with various health benefits and applications in the nutraceutical and pharmaceutical industries [
7].
To harness these properties effectively, understanding optimal growth conditions is essential. Traditionally, mulberries have been propagated in soil-based nursery systems under specific climatic and soil requirements: moderate temperatures (around 26 °C), altitudes up to 4000 m, and well-drained, loamy-clayey soils with a pH of 6.2–6.8 [
8]. However, advances in agricultural technology have introduced soil-less systems like hydroponics, aeroponics, and in vitro culture, which offer potential for higher resource efficiency and plant productivity. For instance, hydroponically grown mulberries have shown improved leaf palatability and increased 1-deoxynojirimycin (DNJ) content, though with reduced polyphenol levels compared to field-grown counterparts [
9]. Similarly, the aeroponic cultivation of V-1 mulberry resulted in superior root and shoot growth relative to conventional methods [
10,
11], while in vitro techniques have proven effective for propagating diverse mulberry genotypes [
11,
12,
13].
Building on these developments, the present study investigates for the first time the growth of Morus latifolia var. Kokuso 21 under two soil-less propagation systems: aeroponics and in vitro culture. This cultivar is known for its strong cold tolerance (−34 °C), vigorous growth, and large, nutrient-rich leaves that are ideal for silkworm rearing, making it a valuable variety for Romania’s temperate-continental climate. The study aims to compare the morphological parameters and individual phenolic profiles of Kokuso 21 propagated under aeroponic and in vitro conditions against conventional nursery-grown plants. Among the tested methods, aeroponics showed the most promising outcomes in terms of plant vigor and viability, while in vitro propagation proved effective for genotype conservation, albeit with slightly lower phenolic accumulation. To our knowledge, this is the first report evaluating Kokuso 21 using both aeroponic and in vitro propagation systems in parallel, filling a key gap in current research, which has largely focused on Morus alba and Morus nigra.
While previous studies have made important strides in improving mulberry cultivation for sericulture, few have correlated propagation strategies with biochemical profiling, particularly regarding phenolic compound accumulation. For example, Gil-Martínez et al. (2022) enhanced phenolic content in
M. alba roots via in vitro techniques [
14], but their scope was limited to root systems. Meng et al. (2025) analyzed post-harvest changes in phenolic profiles during fermentation [
15], and Karabulut and Saraçoğlu (2022) studied rooting in
M. nigra using growth regulators [
16], without exploring metabolite content or alternative propagation methods. In contrast, our study offers a novel, multidimensional perspective by evaluating morphological development and individual phenolic composition under different propagation conditions. This integrated approach provides new insights into how cultivation techniques can influence the functional qualities of lesser-studied mulberry cultivars such as
M. latifolia var. Kokuso 21.
2. Materials and Methods
The present study rigorously investigates conventional and aeroponic cultivation methods for mulberry, implemented within a controlled greenhouse facility in Cluj-Napoca, Romania (46°46′ N, 23°36′ E) and established as a case study. The area experiences a temperate continental climate, characterized by four distinct seasons. Average annual temperatures range from 8 °C to 10 °C, with summer highs reaching around 26 °C and winter lows dropping below freezing. Annual precipitation averages approximately 600–700 mm, with rainfall distributed relatively evenly throughout the year. The greenhouse and in vitro propagation laboratory settings allowed for partial control of temperature, which was set for 22 °C and light (12 h light/12 h dark cycle), reducing the influence of external climatic variability on plant development.
Open-pollinated seeds of Morus latifolia cv. Kokuso 21 was procured from mature fruits obtained from the Global Centre of Excellence for Advanced Research in Sericulture and Promotion of Silk Production, affiliated with the University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, Romania. The seeds underwent air-drying and were stored in a sealed bag at room temperature in the dark until they were used. The experimental phase started in January 2023, with the germination of seeds, while the measurements were made in July 2023. For each condition, 32 randomized plants were collected and measured to provide a reliable comparison of how the different growth systems influence plant morphology. The average number of true leaves at the time of measurements was 26 for conventional, 22 for aeroponic, and 12 for in vitro conditions.
2.1. Mulberry Grown Using Conventional Agricultural Methods
Peat substrate was used for germinating mulberry seeds in specialized alveolar tubes containing 406 cells, each measuring 17 × 17 mm. The germination rate ranged from 74.9% to 78.3% per alveolar tube, with an average value of 76.6%. Every 4 weeks, the plants received two complementary nutrient solutions. On one hand, a complex foliar fertilizer (Synergizer 8-32-4) was applied, containing micro and macro elements specifically designed to balance the nutritional ratio of plants and enhance crop yields, with the following components: total nitrogen (N) 8%; available phosphorus (P2O5) 32.0%; soluble potassium (K2O) 4.0%; Iron (Fe) 0.1%; Manganese (Mn) 0.1%; Zinc (Zn) 0.1%; and humic acids derived from leonardite 0.3%, in the form of a soluble concentrate. On the other hand, a light and quickly assimilable biostimulator (Delfan plus) was applied, which contains a well-proportioned mixture of 17 physiologically active and functional L-α essential amino acids resulting from protein hydrolysis. The biostimulator used is an ecological product, formulated with organic biomolecules of natural origin, and has the following composition: 24% free amino acids, 44% organic matter, 9% total nitrogen, 5.3% protein nitrogen, and 32.6% organic carbon.
2.2. Mulberry Grown Using Aeroponic System
For this experiment, rockwool cubes were used for germinating mulberry, and three Amazon-Twin Aeroponic Systems (
Figure 1a,b) were used to suspend plant roots in a misting chamber filled with nutrients, fostering optimal growth conditions. This method provides plants with unrestricted access to oxygen, nutrients, and water. The system’s design includes a tank measuring 1600 mm in length, 673 mm in width, and 254 mm in height, along with chamber dimensions of 2 × 750 mm in length, 750 mm in width, and 140 mm in height, holding a total volume of 100 L of nutrient solution and 32 plants each system. The pump was running continuously at 60 psi, and the nozzles atomized the water into tiny droplets, increasing the surface area for better absorption by the plant roots. The aeroponic system was placed in the greenhouse, having the same temperature, humidity, and light conditions as the plants in soil pots. This setup ensured a steady supply of nutrients to the roots while allowing excess solution to drain back into the tank, thereby preventing waterlogging and the accumulation of nutrient salts. Consequently, plants experienced unhindered uptake of nutrients and oxygen, resulting in improved absorption of water and nutrients, ultimately leading to enhanced yields compared to traditional cultivation methods. The nutrient solution was the same as prepared for conventional growing.
2.3. Mulberry Grown Using In Vitro Technique
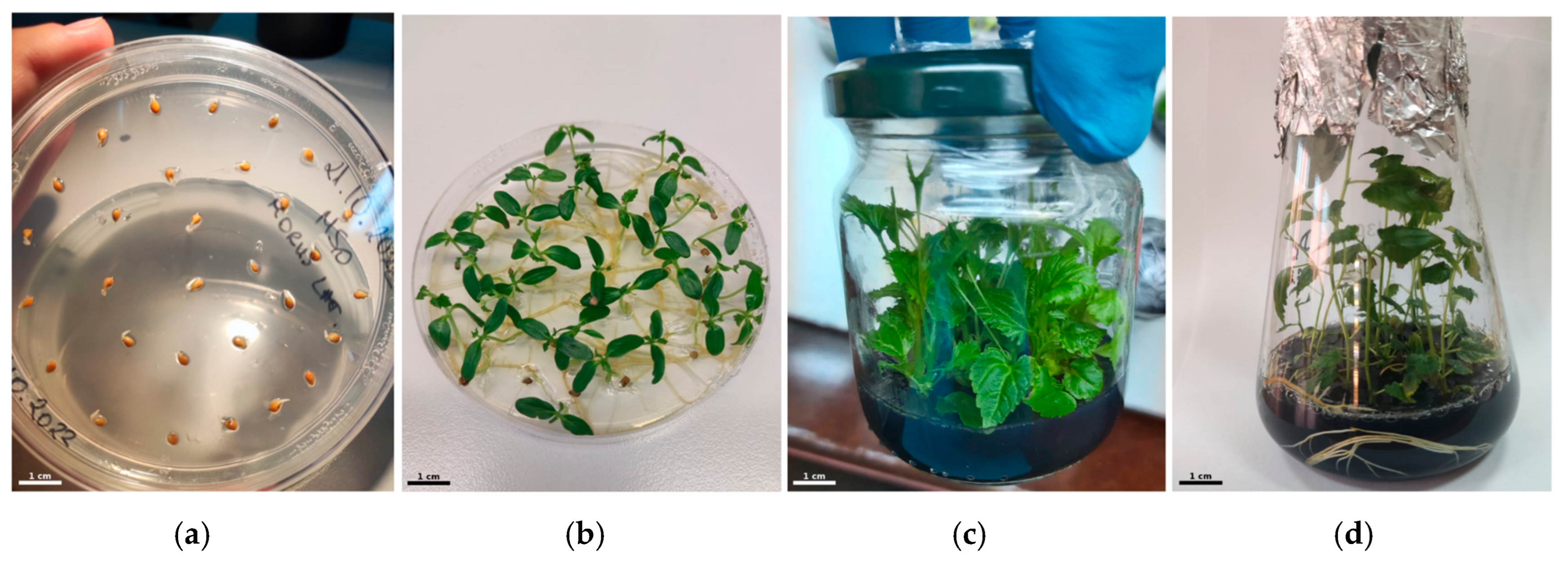
In vitro propagation of mulberry was conducted using the protocol developed by Sarkar et. al. (2022) [
11], simplified. Seeds collected in the same year were sterilized by soaking them in a 2.5% Sodium Hypochlorite solution for 26 min, followed by 3 rinses with sterile distilled water. The seeds were set for germination on Murashide–Skoog (MS) medium (Sigma-Aldrich, USA), using original recipe [
17] with an addition of sucrose (3%
w/
v) and agar (0.8%), for approximately four weeks until at least two foliage layers had developed. The leaves, together with cotyledons and hypocotyls, were subjected to a preculture on 0.5 mg/L TDZ-supplemented MS medium for expansion for another five weeks. Further, for elongation of regenerated individual shoots, an elongation medium containing MS, sucrose (3%,
w/
v), agar (0.8%) and supplemented with 6-Benzylaminopurine (BAP) 1 mg/L, gibberellic acid (GA3) 1.5 mg/L, AgNO
3 (2 mg/L), putrescine (1 mg/L), AC (0.2%), and CaCl
2 (515 mg/L) was prepared. Lastly, rooting was promoted through indole-3-butyric acid (IBA) 2 mg/L in AC-supplemented medium (
Figure 2).
2.4. Identification and Quantification of Individual Phenolic Compounds
Individual phenolic compounds were identified and quantified using the HPLC-DAD-ESI+ method, designed and validated at the Department of Food Science, Faculty of Food Science and Technology, Cluj-Napoca [
18,
19,
20]. For sample preparation, 1 g of each sample (powdered leaves) was mixed with 10 mL of acidified ethanol, vortexed, and sonicated for 15 min, followed by centrifugation at 10,000 rpm for 10 min at 20 °C. This extraction was repeated three times, and the combined supernatants were filtered through a Chromafil Xtra PA-45/13 nylon filter (0.45 µm pore size). A 20 µL aliquot of the filtrate was injected into an Agilent 1200 HPLC system equipped with a quaternary pump, degasser, autosampler, UV–VIS photodiode array detector, and an Agilent 6110 single-quadrupole mass spectrometer. Chromatographic separation was achieved on a Kinetex XB C18 column (4.6 × 150 mm, 5 µm; Phenomenex, USA) using a gradient elution program with mobile phases consisting of water and acetonitrile, both containing 0.1% acetic acid. The gradient started at 5% B and changed as follows: 0–2 min: 5%, 2–18 min: 5–40%, 18–20 min: 40–90%, 20–24 min: 90%, 24–25 min: 90–5%, and 25–30 min: 5%. The flow rate was set at 0.5 mL/min, the column temperature at 25 °C, and spectral data were recorded in the 200–600 nm range, with chromatograms monitored at 280 and 340 nm. Mass spectrometry was performed in positive ionization mode under the following conditions: 3000 V capillary voltage, 350 °C temperature, 7 L/min nitrogen flow, 100 V fragmentor voltage, and an
m/
z scan range of 120–1200. Data acquisition and analysis were conducted using Agilent ChemStation software (Rev B.02.01—SR2). For the calibration curves, hydroxycinnamic acids were quantified as chlorogenic acid equivalents using a calibration curve with the equation y = 22.585x − 36.728 (R
2 = 0.9937), LOD = 0.41 μg/mL, LOQ = 1.64 μg/mL. For flavonols, the calibration curve with rutin was used: y = 26.935x − 33.784 (R
2 = 0.9981), LOD = 0.21 μg/mL, LOQ = 0.84 μg/mL. Lastly, anthocyanins were quantified as cyanidin equivalents: y = 55.789x − 143.21 (R
2 = 0.9951), LOD = 0.36 μg/mL, LOQ = 1.44 μg/mL. Identification of phenolic compounds was based on retention times, UV-VIS spectra, and mass spectral data, supported by references from the scientific literature and the Phenol-Explorer database.
2.5. Statistical Analysis
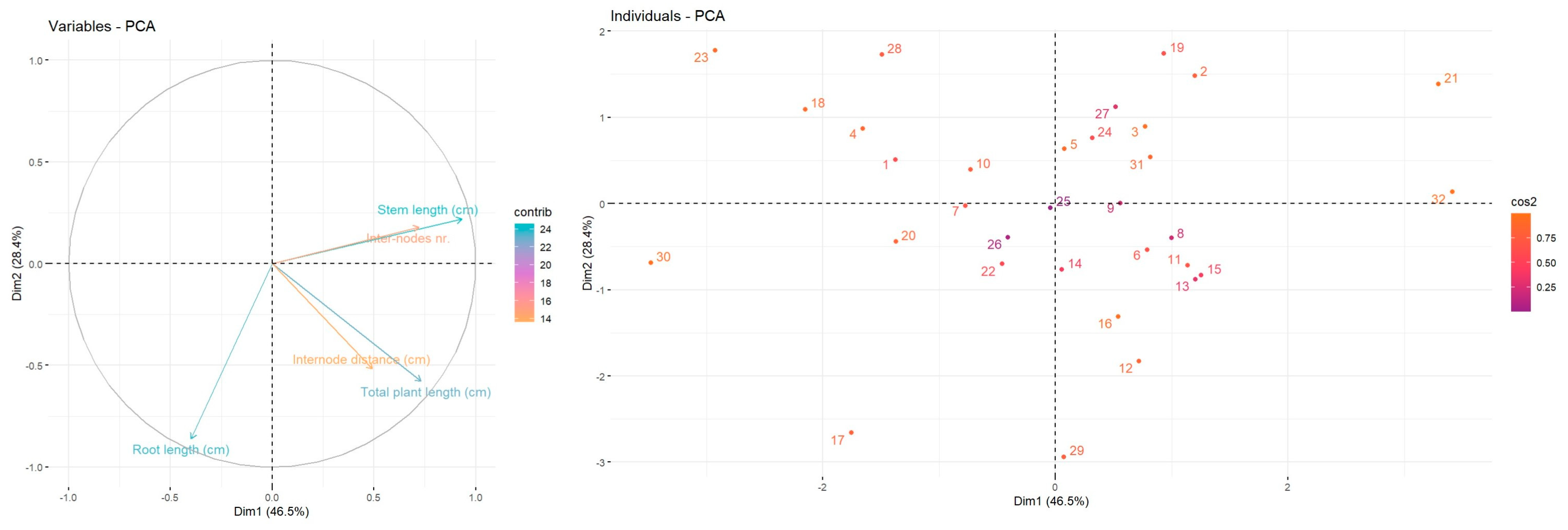
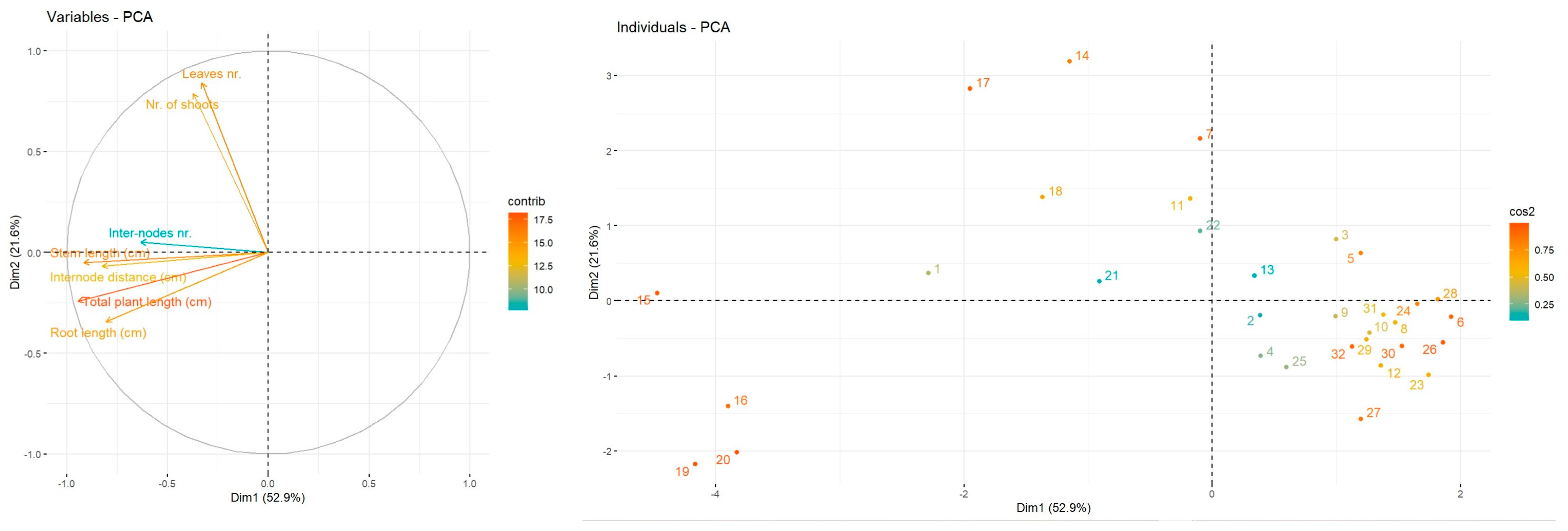
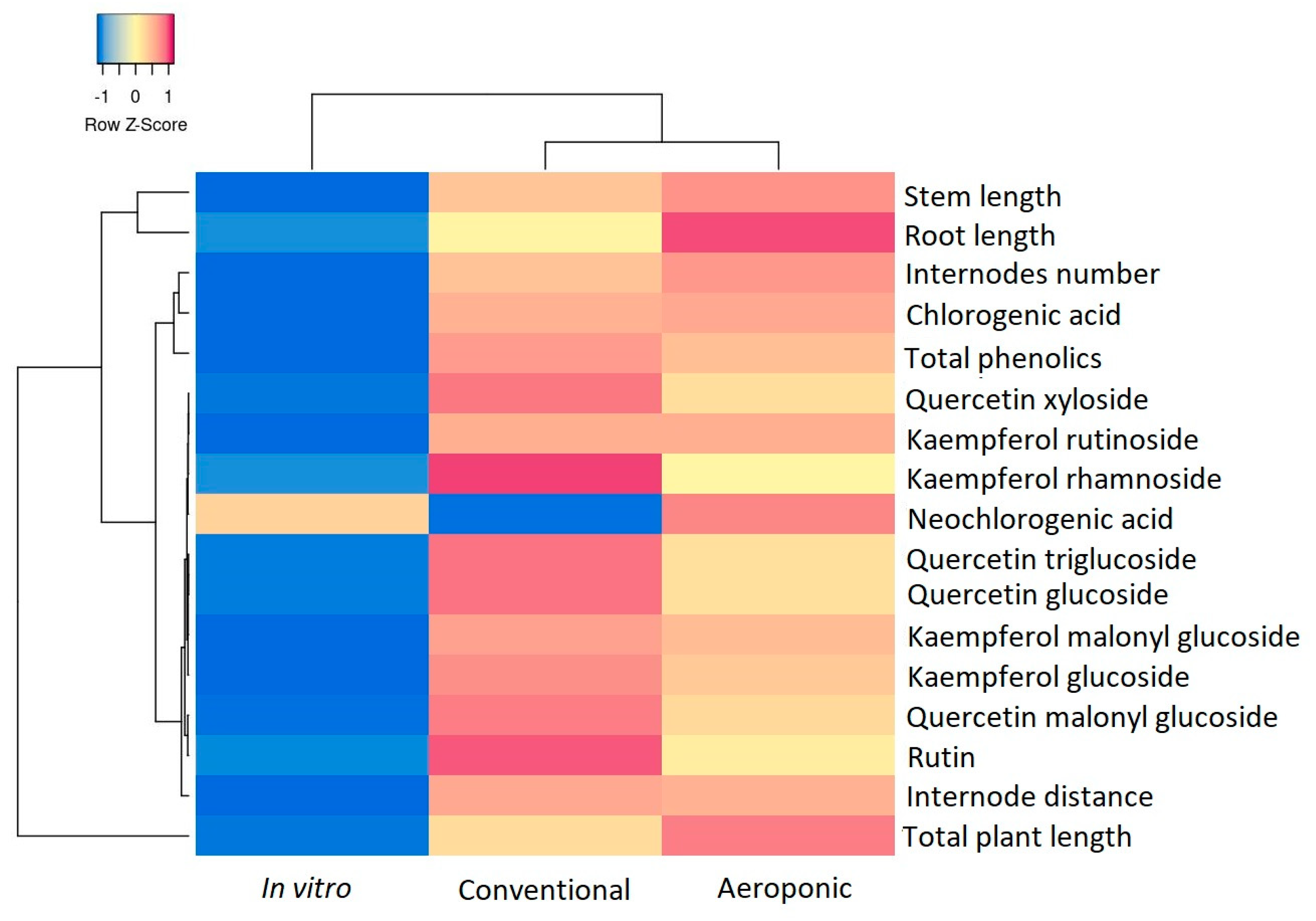
The statistical analyses were assessed using SPSS software (version 19). The data are represented as means ± standard deviation. Statistical difference between determinations was evaluated using one-way ANOVA and post hoc Tukey HSD test, p < 0.05. Principal component analysis (PCA) was employed to visualise trends associated with conventional growth and soilless culture, and was performed using the FactoMiner factoextra package. Heatmap has been employed to point out the resemblances and distinctions in the development and biochemical characterization of mulberries according to their conventional growth or soil-less technique, using the R program (version 2024.12.1).
4. Discussion
The results of this study demonstrate a clear advantage of aeroponic systems over conventional greenhouse and in vitro cultivation methods for the propagation of mulberry. Among the observed parameters—root development, biomass accumulation, and overall plant vigor—aeroponic cultivation consistently yielded superior outcomes. These findings align with recent studies, such as those by Nishchitha et al. (2023), which also reported enhanced rooting and growth characteristics in mulberry saplings propagated through aeroponics [
10]. The physiological benefits of this method, particularly the increased oxygenation of the root zone and the efficient, targeted delivery of nutrients, likely underpin these results. Unlike soil-based, aeroponics ensures that plant roots are suspended in air and misted intermittently with nutrient solutions, promoting faster root initiation, greater nutrient absorption, and more robust overall development.
In vitro and aeroponic cultivation methods have shown significant potential in enhancing plant development. Numerous studies have demonstrated that in vitro propagation can lead to increased secondary metabolite production in various crops. For instance, in vitro propagated
Rheum rhabarbarum (rhubarb) showed high proliferation rates and substantial levels of resveratrol (229.4–371.7 μg/g dry weight), along with excellent rooting efficiency and genetic stability [
24]. Similar results were reported for
Cichorium intybus (chicory), where cultures enriched with indole-3-acetic acid (IAA) and benzylaminopurine (BAP) promoted the production of phenolic compounds, particularly under abiotic stress conditions [
25,
26,
27,
28]. In contrast to these trends, our study on mulberry revealed that in vitro propagation resulted in lower phenolic compound accumulation compared to aeroponic cultivation. This finding underscores the species-specific nature of metabolic responses to cultivation conditions. Unlike rhubarb, chicory, or
Lycium species, mulberry may not receive the necessary physiological cues or environmental stimuli in vitro to effectively trigger phenolic biosynthesis. On the other hand, the results show that in vitro-grown Kokuso 21 plants exhibited a significantly higher number of shoots (2.59 ± 1.04), reflecting the strong influence of exogenous plant growth regulators typically used in tissue culture. However, this enhanced shoot multiplication came at the cost of markedly lower phenolic content, with 5-Caffeoylquinic acid levels reaching only 1.86 mg/g, substantially lower than in plants grown under conventional (12.66 mg/g) and aeroponic (13.04 mg/g) conditions. This contrast suggests a physiological trade-off, where hormonal conditions favoring shoot initiation may suppress secondary metabolite biosynthesis, likely due to altered metabolic priorities or enzyme expression. Complementary, the aeroponic system, which offers a well-aerated, stress-responsive environment, may better simulate natural conditions conducive to phenolic accumulation in mulberry. These results highlight the importance of tailoring propagation and cultivation systems to the specific metabolic and developmental characteristics of each crop species when the goal is to optimize the production of health-promoting secondary metabolites.
From a broader perspective, these findings contribute to the advancement of sustainable and high-efficiency agricultural practices. Aeroponic systems, due to their design, require significantly less water and eliminate the need for traditional growing substrates, making them highly suitable for regions with limited water availability or declining soil health. The controlled environment intrinsic to aeroponic setups allows for standardization in plant production, reduces susceptibility to soil-borne pathogens, and enables year-round cultivation. This positions aeroponics as a promising tool for the commercial propagation of mulberry, especially in urban and peri-urban settings, where vertical farming and space efficiency are becoming increasingly important.
Moreover, while aeroponics offers distinct advantages for rapid and scalable production, in vitro propagation techniques play an equally critical yet complementary role in the context of mulberry cultivation and conservation. Although more labor-intensive and technically demanding, in vitro propagation is invaluable for the preservation and multiplication of genetically important or rare mulberry germplasm. Other studies have also shown successful micropropagation of
Morus alba cultivars using shoot-tip culture, achieving high multiplication rates and satisfactory rooting outcomes under sterile conditions [
11,
12,
29]. In vitro methods play a crucial role in producing disease-free planting material and offer a dependable strategy for the long-term conservation of genetic resources, thereby preserving the genetic diversity of mulberry. This is particularly vital for breeding programs, biotechnological research, and the development of resilient crop populations capable of withstanding climate change and emerging pests. Given the wide array of bioactivities exhibited by mulberry, the data presented in this study represent a significant advancement not only for agriculture but also for biotechnology. Mulberry is especially noted for its antioxidative, anticancer, antidiabetic, and hepatoprotective properties [
11]. These diverse health benefits underscore the growing demand for expanded mulberry cultivation, exploration of alternative growing techniques, and maximizing the production of valuable health-promoting compounds such as phenols, anthocyanins, polysaccharides, and flavonoids.
Together, the results of this study suggest that aeroponic systems may be best suited for high-efficiency commercial production of mulberry, whereas in vitro techniques should be prioritized for germplasm preservation, elite cultivar maintenance, and the propagation of genetically sensitive lines. To integrate aeroponics and in vitro systems into a commercial setup, a synergistic model can be established combining vertical farming, pharmacological compound production, and genetic conservation. Aeroponics is ideal for vertical farming, enabling space-efficient, year-round cultivation of Kokuso 21 mulberry in stacked layers under controlled conditions, producing nutrient-rich biomass for sericulture and industrial use. This system can be optimized to enhance specific phytochemicals, supporting pharmacological compounds, by using elicitors or nutrient adjustments to boost metabolite production. In parallel, in vitro culture serves as a foundation for maintaining a healthy gene pool, allowing for disease-free micropropagation, genotype conservation, and the rapid scaling of elite clones for commercial use. The integration of both approaches could therefore support a more resilient and dynamic mulberry cultivation system that addresses both short-term productivity and long-term sustainability.
Looking ahead, future research should explore the optimization of environmental parameters within aeroponic systems, such as misting intervals, nutrient formulations, and light regimes, to further enhance plant performance. The incorporation of precision agriculture technologies, including sensor-based monitoring and automated nutrient delivery, could improve system efficiency and scalability. Long-term studies evaluating the economic viability and sustainability of aeroponic systems for mulberry, especially under commercial production conditions, will be essential. Moreover, genotype-specific responses to aeroponic cultivation warrant investigation, as different mulberry cultivars may vary in their adaptability to soilless systems. Another promising avenue is the assessment of secondary metabolite production in aeroponically and in vitro grown mulberry plants, which could have implications for pharmaceutical and nutraceutical applications.
5. Conclusions
Aeroponically grown Kokuso 21 plants demonstrated the most vigorous development, achieving an average plant height of 108.36 ± 12.56 cm, 41% taller than plants grown conventionally (76.58 ± 5.72 cm). Additionally, aeroponic plants developed root systems nearly twice as long (44.71 ± 9.25 cm) as those in soil (23.40 ± 5.59 cm), indicating enhanced underground biomass likely due to superior oxygen and nutrient availability. Although in vitro propagation resulted in significantly shorter plants (7.14 ± 4.54 cm), it produced the highest shoot proliferation rate (2.59 ± 1.04), emphasizing its utility for rapid multiplication and germplasm maintenance. However, in vitro conditions led to a drastic reduction in phenolic accumulation, with chlorogenic acid levels nearly 85% lower than in the other two systems. These findings represent the first parallel evaluation of Kokuso 21 under aeroponic and in vitro systems, revealing critical physiological trade-offs and establishing aeroponics as the most effective approach for robust plant development and bioactive compound retention in temperate climates.
In conclusion, the superior performance of mulberry under aeroponic conditions underscores the potential of this technology to transform conventional propagation practices. At the same time, in vitro techniques offer critical support for the preservation and careful manipulation of genetic resources. Together, these approaches can be strategically combined to support a sustainable, high-performing, and resilient mulberry cultivation framework suitable for both commercial and conservation purposes.