Abstract
The coastal tidal flat area of Jiangsu Province, China, is vast and has great potential for carbon sequestration. Planting oat in saline–alkaline land can increase carbon sequestration from the atmosphere into soil and, thus, improve soil quality. Harvesting oats can act as a biological desalination mechanism, and long-term planting may transform saline–alkaline land into high-quality arable land. Our experiment selected two oat varieties, Caesar (V1) and Menglong (V2), and used urea, organic fertilizer, microbial inoculant, and biochar as experimental factors to investigate the effects of fertilizers and soil amendments on soil improvement and carbon sequestration when cultivating oats. The results showed that when planting V1, the carbon sequestration of the farmland ecosystem was the highest with microbial inoculant and organic fertilizer treatments, and the soil salinity decreased the most with biochar treatment. When planting V2, the carbon sequestration of the farmland ecosystem was the highest with the urea + biochar treatment, the soil salinity decreased the most with organic fertilizer + microbial inoculant treatment, and the soil organic carbon content increased the most with organic fertilizer + biochar treatment. We found that the application of organic fertilizer and biochar significantly increased soil organic carbon (SOC) content by 22.03% compared to the control treatment. Additionally, the combined treatment of urea and biochar resulted in the highest agricultural carbon sink, with a 74.62% increase in oat carbon storage compared to conventional fertilization.
1. Introduction
Soil salinization and alkalization are among the major abiotic stresses faced by crop cultivation systems, often leading to reduced crop yield and quality and posing a meaningful threat to global food security [1,2]. Soil salinization and alkalization are common problems in crop production worldwide. Currently, the global area of all saline–alkaline land exceeds 9.5 × 108 ha−1, severely restricting the sustainable development of agriculture [3]. China has 9.9 × 107 ha−1 of exploitable saline–alkaline land resources, among which the total area of unexploited saline–alkaline land reaches 7.7 × 106 ha−1, mainly distributed in the northwest, northeast, north China, and coastal regions [4]. Due to climate and more direct human activities, the area of saline–alkaline land continues to increase [5,6]. The coastal tidal flats in Jiangsu Province of China are extensive, with a high salinity and poor crop nutrition, which brings great difficulties to the development and utilization of such coastal areas. Therefore, it is urgent to make improvements [3,7].
Oat (Avena sativa L.), an annual Poaceae species belonging to the subfamily Pooideae and the genus Avena, is a long-day crop and an excellent dual-purpose species providing both food and forage [8]. Oat is rich in crude fiber, with soft and succulent stems and leaves, a high nutrient content, and good palatability. Oat plants are characterized by salt and alkali tolerance, cold resistance, a high forage yield, good quality, and strong stress resistance. Oat can effectively prevent soil erosion and reduce water evaporation and surface runoff, playing an important role in livestock production [9,10]. Studies have shown that the crude protein, digestible total nutrients, and dry matter digestibility of oat are beneficial for preventing and treating lifestyle-related diseases [11]. In recent years, the increasing demand for livestock products in China has led to huge potential for the development of the oat forage industry, which is of great significance for the sustainable development of animal husbandry and the ecological restoration of degraded grasslands [12]. Given the broad market prospects for oat products, further utilization of saline–alkaline land and the development of salt-tolerant, high-yielding, and high-quality production techniques to improve oat yield and quality constitute important opportunities [13].
Fertilizers and soil amendments have been proven to increase soil organic carbon content [14], promote soil aggregate stability [15], and alter microbial properties [16,17]. For example, organic fertilizers can enhance the formation and stability of aggregates in saline–alkaline soils [18,19], increase soil nutrient content and biological abundance, and reduce soil salinity [20,21]. Soil amendments (such as biofertilizers and decomposed straw) can change soil nutrients and physicochemical properties, shift the composition of soil microbial communities, and increase crop yield [22,23]. Lu et al. also suggested that soil amendment treatments can alter rhizosphere bacterial communities, improve oat productivity, and ameliorate soil physicochemical properties [24]. There are many microbial communities in soil that can be important contributors to carbon sequestration and stabilization [25]. Recent studies have further revealed the role and importance of the relationships between plants and soil microbes in promoting energy flow and nutrient cycling in soil ecosystems and crop yield [26,27].
The yield and economic benefits of food crops planted in coastal tidal flat areas are generally much lower than those of normal farmlands, resulting in a low overall utilization rate of saline–alkaline land. Forage planting targeting biomass yield can effectively improve the utilization rate of saline–alkaline land and climatic resources. While numerous studies have explored the effects of individual fertilizers and soil amendments on crop yield and soil health in saline–alkaline soils, there is a significant gap in understanding the combined effects of these treatments. Specifically, the interactions between different fertilizers and soil amendments and their cumulative impact on soil physicochemical properties and carbon sequestration remain underexplored. The present study is primarily designed to elucidate the effects of fertilizers and soil amendments on oat forage yield and soil physicochemical properties through an experimental investigation conducted in saline–alkaline soils of the coastal tidal flats in Yancheng, Jiangsu Province. The secondary objective of this research is to provide a theoretical foundation for the development of high-yield fertilization and soil amendment strategies aimed at ensuring sustainable oat production in these challenging environmental conditions.
2. Materials and Methods
2.1. Experimental Design and Site
This study was conducted from November 2022 to June 2024 at the DaFeng Salt Sea Forest Farm in DaFeng District, Yancheng City, Jiangsu Province (33°20′ N, 120°47′ E), with an elevation of 5 m. The region is a transitional zone between subtropical and warm temperate zones, characterized by distinct seasons, moderate temperatures, and a mean annual temperature of 14.1 °C. It has abundant rainfall, with an average annual precipitation of 1042.2 mm, a frost-free period of 213 days, and an annual sunshine duration of 2238.9 h. The soil of the current experimental plots is primarily categorized as saline–alkaline and semi-saline–alkaline. Following long-term natural processes and human-induced amelioration, most of the severely saline–alkaline soils have progressively transformed into moderately and lightly saline–alkaline soils, with varying levels of soil salinity observed across different plots. The average soil pH of the topsoil (0–20 cm) at the experimental site is 8.17, with a soil organic matter (SOM) content of 11.6 g kg−1, total nitrogen content of 5.05 g kg−1, available phosphorus content of 12.45 mg kg−1, available potassium content of 81.71 mg kg−1, and an average salt content of 3.11 g kg−1 (Table 1). The chalky sandy silt soil contains more than 50% silt, less than 50% sand, and less than 15% minimal clay content. The soil textures of the field are fine and smooth but not sticky like a clay soil, with moderate nutrient retention and a high erosion susceptibility.

Table 1.
Experimental soil properties.
Two oat varieties, Caesar (V1) and Menglong (V2), were selected for the experiment, with a seeding rate of 200 kg ha−1 and a row spacing of 30 cm for drilling. The plot size was 20 × 20 m. Before sowing, blended fertilizer (with 18% nitrogen content) was applied at a rate of 375 kg ha−1 to establish a basal fertility level. Urea (with 46% nitrogen content) was applied as top dressing at a rate of 217 kg ha−1, split into two applications during the tillering and jointing stage. The following treatments were applied: F1: urea (46% nitrogen content), S1: microbial inoculant, F2: organic fertilizer (3% nitrogen content), and S2: biochar (50% carbon content). The treatments for each variety were as follows (Table 2):

Table 2.
Experimental combination information.
2.2. Observations and Measurements
Growth Traits and Forage Yield
At the mature stage after sowing, 15 plants with consistent growth and representativeness were selected for each treatment. The fresh weight was measured, and then the samples were placed in a 105 °C oven for 30 min to terminate the living tissues, followed by drying at 85 °C until a constant weight was reached, at which time the dry weight was measured. The data were then statistically analyzed to calculate the CGR [28].
CGR (kg ha−1 d−1) = (Wa − Wb)/(ta − tb)
Wa, Wb: the biomass measured at the two time points; ta, tb: the time points at which the two measurements were taken.
Soil samples were collected from the 0–20 cm soil layer under each treatment replicate before sowing and 160 days after sowing, with three replicates for each sampling. The samples were thoroughly mixed and then placed into pre-numbered plastic bags. The field-collected samples were brought back to the laboratory and spread out on kraft paper. All extraneous materials (litter, plant roots, and gravel) were removed from the samples. The soil samples were then air-dried in a cool and ventilated space. After being ground and passed through a 100-mesh sieve, the soil samples were stored in kraft paper bags for later use.
Determination of soil organic carbon content: Weighed 1 g of soil sample into a 50 mL test tube, add 5 mL of 0.8 mol L−1 K2Cr2O7 solution and 5 mL of H2SO4, shake well, and place the sample in a 100 °C constant-temperature incubator. After 90 min, take out the test tube and cool it to room temperature. Transfer the solution in the test tube to a 250 mL conical flask, rinse the test tube multiple times with distilled water to take the total volume up to 100 mL. Add 3 drops of phenanthroline indicator—the solution will turn orange-yellow. Titrate with a 0.2 mol L−1 FeSO4 standard solution until the solution changes from orange-yellow through blue-green to brick-red as the endpoint and record the volume of FeSO4 consumed (V1). Meanwhile, set up a blank test tube without soil and record the titration volume (V0) [29].
Determination of oat plant organic carbon content: Weighed 0.2 g of oat plant sample into a 50 mL test tube, the other measurement steps are the same as those for determining the soil organic carbon content [29].
Determination of soil nitrogen content: Measure the total nitrogen content using the Kjeldahl method [30]. Digest approximately 0.5 g of soil w with concentrated H2SO4 and a catalyst mixture (CuSO4 and K2SO4). Then, distill the digest with 40% NaOH, and trap the ammonia released in a boric acid solution. Determine the amount of nitrogen by titration with a standard H2SO4 solution.
Determination of soil pH value: Measure soil pH using a 1:2.5 soil-to-water ratio. Mix a 20 g soil sample with 50 mL of deionized water, shake for 30 min at room temperature (25 °C), and then filter it. Measure the pH of the filtrate using a calibrated pH meter [31].
Salt Content (Mass Fraction of Water-Soluble Salts): Precisely weigh a clean and dry glass test tube using an electronic balance (accurate to 0.1 mg). Then, accurately measure 50 mL of the above filtrate and place it into the test tube. Evaporate the filtrate to dryness in a water bath, and dry the residue in an oven set at 105 ◦C until a constant mass is achieved. Precisely weight the mass and calculate the soil salt content [32].
Determination of soil carbon storage: Estimation method of organic carbon density.
- SOCi—Soil organic carbon density of an individual soil layer (kg m−2)
- SOCI—Soil organic carbon density of a soil profile of multiple layers (kg m−2)
- Ci—Soil organic carbon content (%)
- Di—Soil bulk density (g cm−3)
- Ei—Soil thickness (cm)
To determine the SOC content, we used the Walkley–Black method. Briefly, we weighed approximately 1 g of air-dried soil into a 50 mL digestion tube, added 10 mL of 0.5 M potassium dichromate (K2Cr2O7) solution, and carefully added 20 mL of concentrated sulfuric acid (H2SO4) to avoid splashing. The contents were mixed thoroughly and then placed in a water bath at 180 °C for 30 min. After digestion, the tube was cooled in a cold water bath. Next, 100 mL of distilled water and 10 mL of 0.5 M iron(II) sulfate (FeSO4) solution were added to the cooled digest. The excess dichromate was titrated with 0.5 M ammonium ferrous sulfate (Fe(NH4)2 (SO4)2) solution until the color changed from blue-green to grayish-green [33].
To determine the organic carbon content in the oat plants, we used the combustion method. Fresh oat plant samples were collected and dried at 65 °C until a constant weight was achieved. Approximately 0.2 g of the dried plant material was weighed into a crucible and placed in a muffle furnace, where it was heated at 550 °C for 4 h to completely combust the organic matter.
Oat carbon storage = Oat biomass × Carbon content of oat plants
To determine the agricultural carbon sink, we employed a carbon balance approach, which involved calculating both the soil organic carbon storage and the carbon storage in the oat plants. For the oat plants, carbon storage was determined by multiplying the biomass of the oat plants by their carbon content. The total agricultural carbon sink was then obtained by summing the soil carbon storage and the carbon storage in the oat plants, providing a comprehensive measure of the carbon sequestration potential of the agricultural system.
Agricultural carbon sink = Oat carbon storage + Soil carbon storage
Soil organic matter was determined by the potassium dichromate external heating method [34].
2.3. Statistical Analysis
The experimental data were collated and plotted using Sigmaplot 10.0 (SPSS, Point Richmond, CA, USA), and the data were statistically analyzed using Statistix 9.0 (Analytical Software, Tallahassee, FL, USA). The mean values were compared based on the LSD test at p < 0.05. The average values are provided for each parameter for the 2-year experiments, because the tendency of each parameter was similar in each year and there was no significant difference between the two years.
3. Results
3.1. Effects of Applied Fertilizers and Soil Amendments on Oat Fresh Forage Yield, Dry Forage Yield, and Crop Growth Rate
ANOVA revealed significant differences in fresh forage yield among the V1T1 treatments (p < 0.05). Specifically, treatments V1T2, V1T3, V1T4, and V1T6 showed significant increases in fresh forage yield compared to V1T1, with V1T4 yielding the highest increase of 79.14%. The order of fresh forage yield was V1T4 > V1T2 > V1T6 > V1T3 > V1T5 > V1T1. Specifically, the fresh forage yields of V1T2, V1T3, V1T4, and V1T6 increased by 70.47, 36.20, 79.14, and 41.82%, respectively, compared with V1T1. Similarly, dry forage yield also showed significant differences among treatments (p < 0.05), with V1T2, V1T4, and V1T6 significantly outperforming V1T1. Notably, V1T2 exhibited the highest dry forage yield, which was 79.93% higher than that of V1T1. Conversely, treatments V1T5 and V1T1 did not show significant differences in either fresh or dry forage yield. The order of dry forage yield was V1T2 > V1T4 > V1T6 > V1T3 > V1T5 > V1T1. Compared with V1T1, the dry forage yields of V1T2, V1T4, and V1T6 increased by 79.93, 69.35, and 46.72%, respectively. The crop growth rate of oats under each treatment was directly related to the dry forage yield performance (Table 3).

Table 3.
Effects of different fertilizers and soil conditioners on fresh grass yield, dry grass yield, and CGR of V1.
ANOVA also revealed that the combined application of fertilizer and soil amendment significantly influenced the fresh forage yield, dry forage yield, and crop growth rate of V2 (p < 0.05). Specifically, treatments V2T2, V2T3, V2T4, V2T5, V2T6, and V2T7 showed significant increases in fresh forage yield compared to V2T1, with V2T3 yielding the highest increase of 239.01%. The order of fresh forage yield was V2T3 > V2T5 > V2T7 > V2T4 > V2T2 > V2T6 > V2T1. The fresh forage yields of these treatments increased by 239.01, 152.96, 144.97, 143.89, 121.79, and 87.24%, respectively, compared with V2T1. Similarly, dry forage yield also showed significant differences among treatments (p < 0.05), with V2T3, V2T5, and V2T7 significantly outperforming V2T1. Notably, V2T3 exhibited the highest dry forage yield, which was 239.18% higher than that of V2T1. The order of dry forage yield was V2T3 > V2T7 > V2T5 > V2T4 > V2T6 > V2T2 > V2T1. The dry forage yields increased by 239.18, 152.39, 128.33, 116.45, and 103.53%, respectively, compared with V2T1. The crop growth rate of V2 under various treatments was consistent with the dry forage yield (Table 4).

Table 4.
Effects of different fertilizers and soil conditioners on fresh grass yield, dry grass yield, and CGR of V2.
3.2. Effects of Fertilizers and Soil Amendments on Soil Organic Carbon, Organic Matter, and Nitrogen Content in Saline–Alkaline Soils
The SOC content in agricultural fields showed a downward trend before the harvest of V1. Among them, the SOC content in V1T5 increased significantly in the short term, following treatment, and then began to decline. The SOC content of the control treatment, V1T1, first decreased and then increased. At 160 days after sowing, the SOC content in V1T1 decreased the least compared with the level prior to sowing, followed by V1T3, while the SOC content in V1T2 decreased the most at 160 days after sowing (Table 5). The SOC content in agricultural fields first increased and then decreased before the harvest of V2. The short-term effect of V2T6 treatment on increasing SOC content was the best and significantly increased by 22.03% compared with V2T1, followed by V2T7. V2T2 caused the smallest increase in SOC content, with an SOC content lower than that of V2T1 at 82 days after sowing. Between 82 and 122 days after sowing, V2T4 had a better effect regarding the maintenance of SOC content, while the SOC content in V2T6 decreased most rapidly. Between 122 days and 160 days after sowing, the SOC content in soils treated with V2T3 and V2T6 increased slightly, while the SOC content of the other treatments decreased (Table 6).

Table 5.
Effects of different fertilizers and soil conditioners on soil organic carbon content in the 0–20 cm layer of V1.

Table 6.
Effects of different fertilizers and soil conditioners on soil organic carbon content in the 0–20 cm layer of V2.
As shown in Figure 1, both conventional fertilization and the application of additional fertilizers and soil amendments could increase the soil TN content and decrease the SOM content. The overall trend was a decrease in SOM with an increase in TN. At 160 days after sowing, V1T2 had the highest TN content, which was 21.84% higher than that of V1T1, and the lowest SOM content, which was 16.48% lower than that of V1T1. At the same time point, V1T3 had relatively lower TN and higher SOM contents, with no significant differences compared with V1T1.
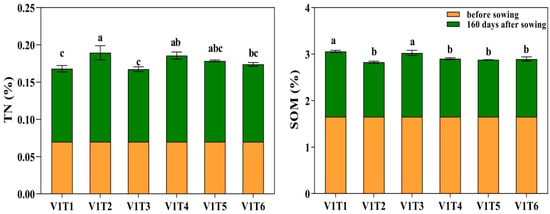
Figure 1.
Effects of different fertilizers and soil conditioners on soil nitrogen and organic matter content of V1 oats. Different letters indicate significant differences between different treatments at the same growth stage at the p < 0.05 level.
As illustrated in Figure 2, both the combined application of fertilizers and soil amendments and conventional fertilization increased the TN content while decreasing the SOM content, with an overall trend of decreasing SOM as TN increased. Among all treatments, V2T6 had the lowest TN content at 160 days after sowing, which was 31.93% lower than that of V2T1, and the lowest SOM content, which was 25.99% higher than that of V2T1. One hundred and sixty days after sowing, both the TN and SOM contents in V2T2 decreased compared with V2T1, but these reductions were not statistically significant.
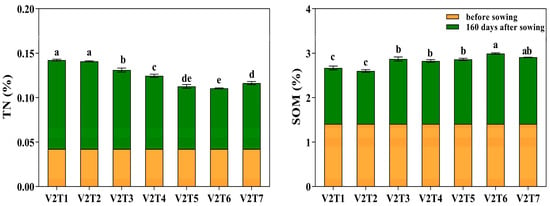
Figure 2.
Effects of different fertilizers and soil conditioners on soil nitrogen and organic matter content of V2 oats. Different letters indicate significant differences between different treatments at the same growth stage at the p < 0.05 level.
3.3. Effects of Different Fertilizers and Soil Conditioners on Soil pH and Salinity in Saline–Alkali Land
As shown in Table 7, growing oats under the evaluated treatments could reduce soil pH and salinity. At 160 days after sowing, the soil pH was most decreased by the V1T1 treatment, according to the ANOVA calculations, but soil pH did not show significant differences among the treatments (p > 0.05). The effects of fertilizers and soil amendments on soil pH reduction were in the order of V1T2 > V1T3 > V1T5 > V1T4 > V1T6. In terms of soil salinity, all treatments with fertilizers and soil amendments for oats could reduce soil salinity. The results showed significant differences in soil salinity among the treatments (p < 0.05). Among them, the soil salinity decreased the most for V1T5, while the soil salinity decreased the least as a result of treatment with V1T2, which was worse than that of V1T1.

Table 7.
Effects of different fertilizers and soil conditioners on soil pH and salinity of V1 oats.
As indicated in Table 8, planting the V2 oat variety with application of the evaluated treatments could reduce soil pH and salinity. At 160 days after sowing, according to the ANOVA calculations, the soil pH did not show significant differences among the treatments (p > 0.05). This suggests that the applied fertilizers and soil amendments did not have a significant impact on soil pH at this time point. However, the soil pH decreased the most when V2T3 and V2T7 were applied. The effects of fertilizers and soil amendments on soil pH reduction were in the order V2T3 = V2T7 > V2T5 > V2T2 = V2T6 > V2T4. In terms of soil salinity, the results showed significant differences in soil salinity among the treatments (p < 0.05). All treatments of oat with fertilizers and soil amendments reduced soil salinity. Specifically, treatment V2T7 resulted in the lowest soil salinity, while V2T3 had the highest salinity.

Table 8.
Effects of different fertilizers and soil conditioners on soil pH and salinity.
3.4. Effects of Different Fertilizers and Soil Conditioners on Carbon Sequestration in Saline–Alkaline Farmlands
As shown in Table 9, both fertilizer and soil conditioner treatments could increase the carbon storage of the oat crops. Among them, treatments V1T2, V1T4, and V1T6 caused significant increases compared with V1T1, with increases of 74.62, 74.87, and 45.68%, respectively. In terms of soil organic carbon storage (SOCi), under treatments V1T2, V1T4, V1T5, and V1T6, the SOCi was significantly lower than that of V1T1. The cropland carbon sequestration was higher under treatments V1T3 and V1T4 than other treatments. Treatments V1T6 and V1T5 caused the lowest cropland carbon sequestration.

Table 9.
Effects of different fertilizers and soil amendments on carbon sink in Caesar (V1) farmland.
As indicated in Table 10, the soil carbon storage in oats treated with the evaluated fertilizers and soil conditioners was higher than that under the V2T1 treatment. Among these, the V2T3 treatment caused the highest carbon storage in oats, with an increase of 244.54% compared to V2T1. In terms of ΔSOCi, treatments V2T3, V2T4, V2T5, V2T6, and V2T7 all resulted in increased SOCi, with V2T6 showing the greatest increase. In contrast, V2T1 and V2T2 led to a decrease in SOCi. Regarding carbon emissions, the crop land ecosystem acted as a carbon sink, with V2T3 having the highest carbon sequestration capacity, followed by V2T6 and V2T7. The other treatments also caused significant improvements compared to V2T1.

Table 10.
Effects of different fertilizers and soil amendments on carbon sink in Menglong (V2) farmland.
4. Discussion
4.1. Effects of Fertilizers and Soil Conditioners on Oat Yield and Growth Rate
Saline–alkali stress is the major abiotic stress factor limiting crop production on coastal tidal flats, causing a range of detrimental effects on plant growth and yield [35,36]. The results of this study show that the fresh forage weight, dry forage weight, and CGR of crops under a range of treatments were all increased compared with the control. This indicates that fertilizers and soil amendments can mitigate the negative damage due to saline stress to some extent. The highest fresh forage yield was obtained with the V1T4 treatment, which may be related to factors such as soil fertility, irrigation conditions, or light interception by the plants given this treatment. The fresh forage, dry forage, and CGR were also relatively high for the V1T2 and V1T6 treatments. This further indicates that these treatments promoted the accumulation of crop dry matter. Previous studies have demonstrated that microbial fertilizers are utilized to enhance soil health and carbon sequestration and reduce greenhouse gas emissions. Owing to their porous nature, microbial fertilizers facilitate the formation of stable soil microaggregates, which is conducive to microbial growth and development. Moreover, they serve as a nutrient source for the soil, thereby creating a favorable soil environment for microbial diversity [37]. By applying these soil amendments reasonably, the yield and quality of wheat (Zea mays L.) [38], grape (Vitis L.) [39], and rice (Oryza. sativa L.) [40] were significantly improved. The application of organic fertilizer enhanced crop yield and soil nutrient availability in the wheat–rice rotation system, while also increasing the diversity and abundance of soil bacterial communities [41]. The long-term application of organic fertilizers can influence the distribution of soil aggregates across different particle sizes, thereby increasing the abundance of prokaryotic microorganisms associated with nitrogen cycling in the soil, including diazotrophs and denitrifying bacteria [42]. Our results show that the combined application of fertilizers and soil amendments significantly improved oat yield and growth rate. This is consistent with previous studies that have demonstrated the benefits of organic fertilizers and biochar in enhancing soil fertility and crop productivity. However, our study extends this research by examining the combined effects in saline–alkaline soils, which present unique challenges for agricultural practices.
4.2. Effects of Fertilizers and Soil Conditioners on Soil Organic Carbon, Organic Matter, and Nitrogen Content
SOC is a crucial component of soil organic matter and nutrients, playing a vital role in soil structure and fertility [43,44]. In this study, both conventional fertilization and the individual application of fertilizers or soil amendments after oat planting led to a reduction in SOC content, with the most pronounced decrease observed under the additional urea application treatment. In contrast, combinations of fertilizers and soil amendments other than urea + microbial inoculant could increase SOC content after oat production, with the organic fertilizer + biochar combinations demonstrating the most significant improvement. Zhang et al. posited that biochar exerts a significant influence on plant water conservation when applied to sandy soils, saline–alkali soils, and clay soils. The impact of biochar is not confined to its substantial effect on soil moisture content; it also significantly affects the concentration of soil salinity ions [45]. Sadegh-Zadeh et al. demonstrated that biochar significantly reduces soil electrical conductivity and the adsorption rate of sodium ions. In particular, biochar containing a high concentration of calcium and magnesium ions can exchange sodium ions on the surface of soil colloids, thereby promoting the leaching of sodium ions from saline–alkali soils and subsequently facilitating the reclamation of such soils [46]. Li et al. investigated the ameliorative effects of various types of biochar after calcium modification on saline–alkali soils. Their research findings indicated that different types of biochar were capable of reducing the content of soluble cations in the soil and providing Ca2+ to replace the exchangeable Na+ in saline–alkali soils, thereby significantly improving the salinity status of the soil [47]. Our study highlights the importance of considering the specific soil conditions, such as pH and salinity, when selecting appropriate soil amendments. This nuanced understanding is crucial for optimizing soil health and carbon sequestration in saline–alkaline environments. The study also found that the soil organic matter content decreased the most with the single nitrogen fertilizer application treatment. Except for urea and microbial inoculant application, combinations of fertilizers and soil amendments could increase the soil organic matter content. The sole application of urea can easily lead to a high N soil nutrient profile, damage soil structure, and cause the death of beneficial soil bacteria, thereby reducing soil organic matter content. The combination of urea and microbial inoculant failed to produce satisfactory results. Urea can affect soil structure and is not conducive to microbial growth and reproduction. Microbial activity can exacerbate nitrous oxide emissions, leading to nitrogen fertilizer loss [48,49,50]. This is because soil nitrogen content is not only related to the applied fertilizers and soil amendments, but also to nutrient uptake by oats. Oat yield under conventional fertilization was much lower than that under the combined application of fertilizers and soil amendments, resulting in less nitrogen fertilizer uptake by oats. Therefore, the trade-off between SOC and soil nitrogen content should be carefully considered in agricultural practices to optimize soil fertility and ecosystem services.
4.3. Effects of Fertilizers and Soil Conditioners on Soil pH and Salinity in Saline–Alkali Land
Extremely alkaline soils often exhibit high pH levels and an elevated salinity. For instance, this high pH may affect the availability of certain nutrients, influencing plant growth and soil microbial activity. Additionally, a high salinity can lead to osmotic stress, further complicating the assessment of treatment effects. Soil pH has a significant impact on plant growth and development, mainly manifested in direct effects on plant morphological characteristics, physiological metabolism, and yield and quality, as well as indirect effects on soil physicochemical properties and microbial communities [51,52]. Therefore, the normal growth and development of crops and the achievement of high yield targets are inseparable from a suitable soil pH environment. At present, organic fertilizers and soil conditioners are commonly used in crop production to regulate soil pH [53,54]. Yang et al. believed that the application of organic fertilizers can significantly reduce the soil pH of saline–alkali land, improve soil structure, and increase soil fertility [55]. In this study, the application of organic fertilizers reduced soil pH, but the effect was less than that of conventional fertilization. The possible reason for this is that the crop biomass produced under organic fertilizer treatment was larger, and the transpiration pull generated by crop transpiration was greater. The alkaline salts in the deep soil dissolved in water and entered the plow layer together with the water. Huo et al. believed that the application of microbial agents can reduce soil pH and the risk of soil salinization [56]. In this study, the capacity of microbial agents to reduce soil pH was slightly worse than that of conventional fertilization treatment, and this may be related to crop transpiration. Yu et al. demonstrated that the application of microbial inoculants can increase the diversity and abundance of soil microorganisms, accelerate the decomposition of organic matter, and enhance the uptake of nutrients by plant roots. However, an excessive or imbalanced application of microbial inoculants may lead to nutrient imbalances in the soil or fluctuations in soil pH [57]. In the experiment conducted by Li et al., the application of microbial inoculants resulted in a decrease in soil pH. However, when the combined application of microbial fertilizers exceeded a certain dosage, the pH value no longer decreased but increased with the increasing dosage of microbial fertilizers. This is consistent with the trend observed in the present study, where the soil pH initially decreased and then stabilized with an increasing dosage of microbial fertilizers [58]. Biochar is one of the most commonly used soil conditioners. However, increasing the rate of biochar application gradually raised the soil pH, likely due to the abundant base ions in biochar, which reduce the concentrations of exchangeable hydrogen and aluminum ions in the soil [59,60]. Furthermore, due to the enrichment of various hydroxides and carbonates within the internal pores of biochar, the application of biochar to soil leads to the leaching of these compounds, thereby resulting in an increase in soil pH. Gao et al. conducted a three-year continuous field experiment on winter wheat–summer maize rotations and found that the annual average pH increased by 0.02 units in the biochar-amended treatment compared to a control without biochar application, with a significant increase of 0.4 units observed in the third year [61]. Wu et al. discovered that in coastal saline–alkali soils, the pH value exhibited a slight upward trend with an increasing application rate of biochar, eventually stabilizing [62]. In this experiment, the soil pH after biochar application was significantly higher than that of conventional fertilization treatment, which is consistent with the results of previous studies. Both the combination of fertilizers and soil conditioners and conventional fertilization treatment caused a numerical decrease in the soil pH, but the differences did not reach a statistically significant level.
4.4. Effects of Fertilizers and Soil Conditioners on Carbon Sequestration in Saline–Alkali Arable Land
Agricultural ecosystems can act as either carbon sources or carbon sinks [63,64]. The carbon dioxide emitted by crop respiration and soil respiration is an important source of carbon emissions. Crops absorb carbon dioxide and store the C component in the form of biomass through photosynthesis [65,66]. The enhancement of SOC sequestration primarily relies on increasing external carbon inputs and reducing the loss of indigenous soil organic carbon. By maintaining a positive imbalance between long-term inputs and losses (i.e., inputs exceeding losses), the continuous accumulation of soil organic carbon is achieved. Therefore, increasing crop biomass through rational methods can transform a carbon source into a carbon sink [67]. This study primarily investigates agricultural carbon sinks by examining crop carbon storage and changes in soil carbon storage after a single cultivation season, providing valuable reference for the development of a standardized framework for agricultural carbon sink assessment.
The experimental results showed that the carbon storage of oat crops increased when treatments with fertilizers and soil conditioners were applied, as compared with conventional fertilization, while the soil organic carbon storage decreased significantly. Considering the organic carbon contained in fertilizers and soil conditioners, the biochar treatment constituted the lowest agricultural carbon sink, lower than the conventional fertilization treatment. The primary reason for this may be attributed to the macroporous structure of biochar, which adsorbs soluble organic carbon in the soil [68]. Additionally, the biochar-amended treatments contained a certain amount of Ca2+, and soluble organic carbon in the soil is prone to forming complexes with Ca2+ [69], thereby leading to a reduction in the content of soluble organic carbon in the soil. Soil particulate organic carbon serves as an energy source for soil microorganisms and an unstable reservoir of organic carbon, the content of which is typically associated with the return flow of organic matter [70]. The predominant cause of this may be the adsorption of soluble organic carbon in the soil by the macroporous structure of biochar [68]. Furthermore, the treatments amended with biochar contained a certain concentration of Ca2+, and the soluble organic carbon in the soil readily formed complexes with Ca2+ [69], thereby resulting in a decrease in the content of soluble organic carbon in the soil. Soil particulate organic carbon serves as an energy source for soil microorganisms and constitutes an unstable reservoir of organic carbon, the content of which is generally correlated with the return flow of organic matter [70]. The organic fertilizer treatment had the highest agricultural carbon sink, followed by the microbial agent treatment. The primary mechanism underlying this phenomenon is that the input of organic fertilizers facilitates soil carbon sequestration through their own decomposition and transformation into soil organic carbon, with the release of carbon-containing substances from the decomposition of organic fertilizers being the principal cause of increased soil organic carbon retention. Additional research has indicated that the input of organic materials not only directly contributes to soil organic carbon through their own decomposition, but also indirectly promotes the accumulation of soil organic carbon by enhancing the formation of large soil aggregates [71,72]. Upon entering the soil, organic fertilizers decompose to produce a substantial amount of labile organic substances and increase microbial activity and biomass, which can serve as organic binding agents to promote the combination of soil mineral particles or colloids with organic carbon, forming large aggregates and thereby reducing the decomposition and loss of organic carbon [73,74]. Moreover, the input of organic fertilizers can indirectly enhance soil organic carbon sequestration by increasing the carbon input from crop biomass [75,76]. This is attributed to the fact that organic fertilizers, in addition to containing abundant carbonaceous substances, are also rich in various secondary, macro-, and micronutrients (such as nitrogen, phosphorus, potassium, calcium, iron, and zinc). These nutrients enable organic materials, upon entering the soil, to release additional nutrients through decomposition, thereby promoting crop biomass growth and increasing root exudates [77,78], which, in turn, enhances the input of plant residue carbon.
Therefore, to fully harness the carbon sequestration potential of agricultural ecosystems, it is essential to enhance crop biomass while also accounting for changes in soil carbon storage and the input of exogenous carbon sources. Only by finding a suitable balance among crop carbon storage, soil carbon storage, and exogenous carbon can we achieve a dual improvement in economic and ecological benefits.
Many studies have focused on the effects of the combination of fertilizers and soil conditioners on the physicochemical properties of saline–alkali soil and associated crop growth, but none have paid attention to their impact on agricultural carbon sinks. Our experimental results showed that the urea + biochar treatment resulted in the greatest agricultural carbon sinks, followed by the organic fertilizer + biochar treatment. Overall, the high carbon sink achieved by the urea + biochar treatment was mainly due to the high carbon storage of the oats. The high carbon sink capacity achieved by the organic fertilizer + biochar treatment was due to not only the high carbon storage of the oats, but also their high soil carbon storage. The reason for this may be that the fertilizers provided sufficient nutrients, which increased the crop carbon storage by enhancing biomass. Biochar improved the soil environment, promoted soil microbial activity and crop root growth, and increased soil organic carbon storage. All combinations of biochar with fertilizers enhanced both crop and soil carbon storage. Conducting more experiments with different ratios of biochar and fertilizers may lead to the identification of an optimal combination. Our results suggest that fertilizers and soil amendments may mitigate saline stress, as indicated by improvements in soil physicochemical properties. However, further research is needed to establish causal relationships and understand the underlying biological and chemical mechanisms. We have added a discussion on the potential mechanisms involved in the effects of fertilizers and soil amendments. The observed improvements in soil health and crop yield may be attributed to enhanced microbial activity and improved nutrient uptake pathways. For example, organic fertilizers can increase the abundance of beneficial soil microorganisms, which, in turn, promote nutrient cycling and plant growth. Biochar can improve soil structure and water retention, further supporting plant health. We have also included references to studies that have explored these mechanisms in more detail.
5. Conclusions
For Caesar (V1), as the growth stage advanced, the SOC storage showed a declining trend for all treatments except at the tillering stage in the V1T5 treatment, with the largest decrease observed for the V1T2 treatment. The plant carbon storage associated with V1T2 and V1T4 was significantly higher than that for V1T1. The carbon sink capacity of V1T3 and V1T4 increased the most compared to V1T1. With an increase in the input of exogenous organic carbon into the soil and plant carbon storage, soil basal respiration and SOC formation gradually increased, leading to significant differences in the carbon sink capacity of the oat agroecosystem under specific treatments. The largest surplus was observed with the V1T4 treatment, while the smallest surplus was with V1T5, with no treatments showing a deficit.
For Menglong (V2), all six treatments significantly increased plant carbon storage, changes in SOC storage, and carbon sink capacity. Specifically, V2T2 exacerbated the decline in SOC storage, while V2T6 showed the largest increase in SOC storage compared to V2T1. The V2T3 treatment resulted in the largest increase in plant carbon storage compared to the control V2T1. The largest surplus in the carbon sink capacity of the agroecosystem was observed for V2T3, while the smallest surplus resulted from treatment with V1T5, with no treatments resulting in a deficit. Overall, the surplus of the SOC pool increased with an increasing application of exogenous organic matter.
The conclusion of this study is that the combined application of fertilizers and soil amendments can significantly enhance oat yield and soil quality in saline–alkaline lands. Specifically, treatments involving organic fertilizer and biochar demonstrated the most effective improvements in soil organic carbon sequestration and overall carbon sink capacity. These findings directly address the main research goal of identifying effective fertilization and soil amendment strategies for sustainable oat production in saline–alkaline soils.
Author Contributions
J.L. wrote the manuscript. Y.Z. assisted in the literature search and design of the work, H.W. helped in drawing figures and data collection. G.D. collected and analyzed the data, D.L.S. assisted in data interpretation. G.Z. conceptualized the idea and edited the revised manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially funded by the Jiangsu Provincial Fund for Realizing Carbon Emission Peaking and Neutralization (BE2022305), from the Department of Science and Technology of Jiangsu Province, the “Belt and Road” innovation talent exchange for foreign experts program of the Ministry of Science and Technology (DL2023014011L), from Ministry of Science and Technology of the People’s Republic of China, and the Postgraduate Research and Practice Innovation Program of Jiangsu Province (KYCX23_3570), from Jiangsu Provincial Department of Education.
Data Availability Statement
Data used in this article are present in the tables and figures.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, X.G.; Sun, R.B.; Tian, Y.P.; Guo, K.; Sun, H.Y.; Liu, X.J.; Chu, H.Y.; Liu, B.B. Long-term phytoremediation of coastal saline soil reveals plant species-specific patterns of microbial community recruitment. mSystems 2020, 5, e00719–e00741. [Google Scholar] [CrossRef] [PubMed]
- He, K.; He, G.; Wang, C.P.; Zhang, H.P.; Xu, Y.; Wang, S.M.; Kong, Y.Z.; Zhou, G.K.; Hu, R.B. Biochar amendment ameliorates soil properties and promotes miscanthus growth in a coastal saline-alkali soil. Appl. Soil Ecol. 2020, 155, e103674. [Google Scholar] [CrossRef]
- An, X.C.; Sun, M.L.; Ren, K.Y.; Xu, M.; Wang, Z.F.; Li, Y.; Liu, H.L.; Lian, B. Effect and mechanism of the improvement of coastal silt soil by application of organic fertilizer and gravel combined with Sesbania cannabina cultivation. Front. Plant Sci. 2023, 13, 1092089. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Xia, J.B.; Yang, H.J.; Liu, J.T.; Shao, P.S. Biochar and effective microorganisms promote Sesbania cannabina growth and soil quality in the coastal saline-alkali soil of the yellow river delta, China. Sci. Total Environ. 2021, 756, e143801. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Cheng, S.F.; Liu, X.Y.; Du, H.; Dai, M.Q.; Zhou, D.X.; Yang, W.J.; Zhao, Y. The R2R3-type MYB gene OsMYB91 has a function in coordinating plant growth and salt stress tolerance in rice. Plant Sci. 2015, 236, 146–156. [Google Scholar] [CrossRef]
- An, X.C.; Wang, Z.F.; Teng, X.M.; Zhou, R.R.; Wang, X.X.; Xu, M.; Lian, B. Rhizosphere bacterial diversity and environmental function prediction of wild salt-tolerant plants in coastal silt soil. Ecol. Indic. 2022, 137, 108503. [Google Scholar] [CrossRef]
- Gong, W.L.; Ju, Z.L.; Chai, J.K.; Zhou, X.R.; Lin, D.D.; Su, W.J.; Zhao, G.Q. Physiological and transcription analyses reveal the regulatory mechanism in Oat (Avena sativa) seedlings with different drought resistance under PEG-induced drought stress. Agronomy 2022, 12, 1005. [Google Scholar] [CrossRef]
- Bai, J.H.; Liu, J.H.; Zhang, N.; Yang, J.H.; Sa, R.L.; Wu, L. Effect of alkali stress on soluble sugar, antioxidant enzymes and yield of oat. J. Integr. Agr. 2013, 12, 1441–1449. [Google Scholar] [CrossRef]
- Sign, R.; De, S.; Belkheir, A. Avena sativa (oat), A potential neutraceutical and therapeutic agent: An overview. Crit. Rev. Food Sci. Nutr. 2013, 53, 126–144. [Google Scholar] [CrossRef]
- Rasane, P.; Jha, A.; Sabikhi, L.; Kumar, A.; Unnikrishnan, V.S. Nutritional advantages of oats and opportunities for its processing as value added foods—a review. J. Food Sci. Tech. Mys. 2015, 52, 662–675. [Google Scholar] [CrossRef] [PubMed]
- Holopainen-Mantila, U.; Vanhatalo, S.; Lehtinen, P.; Sozer, N. Oats as a source of nutritious alternative protein. J. Cereal Sci. 2024, 116, 103862. [Google Scholar] [CrossRef]
- Song, X.D.; Zhou, G.S.; Ma, B.L.; Wu, W.; Ahmad, I.; Zhu, G.L.; Yan, W.K.; Jiao, X.R. Nitrogen Application Improved Photosynthetic Productivity, Chlorophyll Fluorescence, Yield and Yield Components of Two Oat Genotypes under Saline Conditions. Agronomy 2019, 9, 115. [Google Scholar] [CrossRef]
- Xu, P.D.; Zhu, J.; Fu, Q.L.; Chen, J.Z.; Hu, H.Q.; Huang, Q.Y. Structure and biodegradability of dissolved organic matter from Ultisol treated with long-term fertilizations. J. Soil. Sediment. 2018, 18, 1865–1872. [Google Scholar] [CrossRef]
- Tripathi, R.; Nayak, A.K.; Bhattacharyya, P.; Shukl, A.K.; Shahid, M.; Raja, R.; Panda, B.B.; Mohanty, S.; Kumara, A.; Thilagama, V.K. Soil aggregation and distribution of carbon and nitrogen in different fractions after 41 years longterm fertilizer experiment in tropical rice-rice system. Geoderma 2014, 213, 280–286. [Google Scholar] [CrossRef]
- Tian, S.Y.; Zhu, B.J.; Yin, R.; Wang, M.W.; Jiang, Y.J.; Zhang, C.Z.; Li, D.M.; Chen, X.; Kardol, P.; Liu, M.Q. Organic fertilization promotes crop productivity through changes in soil aggregation. Soil Biol. Biochem. 2022, 165, 108533. [Google Scholar] [CrossRef]
- Zhang, H.F.; Pang, H.C.; Song, J.S.; Chang, F.D.; Wang, J.; Wang, X.; Zhang, Y.T.; Peixoto, L.; Li, Y.Y. Subsurface organic ameliorant plus polyethylene mulching strengthened soil organic carbon by altering saline soil aggregate structure and regulating the fungal community. Land Degrad. Dev. 2022, 33, 2543–2553. [Google Scholar] [CrossRef]
- Zhang, J.C.; Zhang, L.; Wang, P.; Huang, Q.W.; Yu, G.H.; Li, D.C.; Shen, Q.R.; Ran, W. The role of non-crystalline iron in the increase of SOC after long-term organic manure application to the red soil of southern China. Eur. J. Soil Sci. 2013, 64, 797–804. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Wu, Y.P.; Li, Y.F.; Zheng, C.Y.; Zhang, Y.F.; Sun, Z.J. Organic amendment application influence soil organism abundance in saline alkali soil. Eur. J. Soil Biol. 2013, 54, 32–40. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Liu, H.; Liu, X.X.; Chen, Y.; Lu, Y.; Shen, M.C.; Dang, K.K.; Zhao, Y.; Dong, Y.H.; Li, Q.Y.; et al. Organic fertilizer enhances rice growth in severe saline-alkali soil by increasing soil bacterial diversity. Soil Use. Manag. 2022, 38, 964–977. [Google Scholar] [CrossRef]
- Sun, D.; Hale, L.; Crowley, D. Nutrient supplementation of pinewood biochar for use as a bacterial inoculum carrier. Biol. Fert. Soils 2016, 52, 515–522. [Google Scholar] [CrossRef]
- Celestina, C.; Hunt, J.R.; Sale, P.W.G.; Franks, A.E. Attribution of crop yield responses to application of organic amendments: A critical review. Soil Tillage Res. 2019, 186, 135–145. [Google Scholar] [CrossRef]
- Lu, P.N.; Bainard, L.D.; Ma, B.; Liu, J.H. Bio-fertilizer and rotten straw amendments alter the rhizosphere bacterial community and increase oat productivity in a saline–alkaline environment. Sci. Rep. 2020, 10, 19896. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.; Agrawal, M.; Singh Bohra, J.; Adhya, T.K.; Bhattacharyya, P. Recalcitrant and labile carbon pools in a sub-humid tropical soil under different tillage combinations: A case study of rice–wheat system. Soil Tillage Res. 2014, 143, 116–122. [Google Scholar] [CrossRef]
- Jílkova, V.; Jandova, K.; Kukla, J. Responses of microbial activity to carbon, nitrogen, and phosphorus additions in forest mineral soils differing in organic carbon content. Biol. Fert. Soils 2021, 57, 513–521. [Google Scholar] [CrossRef]
- Li, Y.M.; Duan, Y.; Wang, G.L.; Wang, A.Q.; Shao, G.Z.; Meng, X.H.; Hu, H.Y.; Zhang, D.M. Straw alters the soil organic carbon composition and microbial community under different tillage practices in a meadow soil in Northeast China. Soil Tillage Res. 2021, 208, 104879. [Google Scholar] [CrossRef]
- Zhu, G.L.; Liu, J.; Wu, H.; Zhu, Y.M.; Nimir, N.E.A.; Zhou, G.S. The Optimum Mixed Cropping Ratio of Oat and Alfalfa Enhanced Plant Growth, Forage Yield, and Forage Quality in Saline Soil. Plants 2024, 13, 3103. [Google Scholar] [CrossRef]
- Walkley, A. Studies on the Organic Matter of Soils; University of London: London, UK, 1933. [Google Scholar]
- Lynch, J.M.; Barbano, D.M. Kjeldahl nitrogen analysis as a reference method for protein determination in dairy products. J. Aoac Int. 1999, 82, 1389–1398. [Google Scholar] [CrossRef]
- Bolt, G.H. Soil pH, an early diagnostic tool: Its determination and interpretation. Adv. Geoecology 1997, 177–210. [Google Scholar] [CrossRef]
- Hernández, T.D.B.; Slater, B.K.; Shaffer, J.M.; Basta, N. Comparison of methods for determining organic carbon content of urban soils in Central Ohio. Geoderma. Reg. 2023, 34, e00680. [Google Scholar] [CrossRef]
- Green, J.K.; Seneviratne, S.I.; Berg, A.M.; Findell, K.L.; Hagemann, S.; Lawrence, D.M.; Gentine, P. Large influence of soil moisture on long-term terrestrial carbon uptake. Nature 2019, 565, 476–479. [Google Scholar] [CrossRef]
- Zhang, M.K.; Walelign, D.B.; Tang, H.J. Effects of biochar’s application on active organic carbon fractions in soil. J. Soil Water Conserv. 2012, 26, 127–137, (ln Chinese). [Google Scholar]
- Liu, J.; Lu, F.G.; Zhu, Y.M.; Wu, H.; Ahmad, I.; Dong, G.C.; Zhou, G.S.; Wu, Y.Q. The Effects of Planting Density and Nitrogen Application on the Growth Quality of Alfalfa Forage in Saline Soils. Agriculture 2024, 14, 302. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Wu, H.; Zhu, Y.M.; Ahmad, I.; Dong, G.C.; Zhou, G.S.; Wu, Y.Q. Association between Reactive Oxygen Species, Transcription Factors, and Candidate Genes in Drought-Resistant Sorghum. Int. J. Mol. Sci. 2024, 25, 6464. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.L.; Gong, T.; Wang, J.W.; Li, G.J.; Liu, Y.Y.; Zhen, J.; Ning, M.; Yue, D.D.; Du, Z.M.; Chen, G.C. Effects of Compound Microbial Fertilizer on Soil Characteristics and Yield of Wheat (Triticum aestivum L.). J. Soil Sci. Plant Nut. 2020, 20, 2740–2748. [Google Scholar] [CrossRef]
- Maftu’ah, E.; Saleh, M.; Sulaseman, Y.; Napisah, K.; Agustina, R.; Mukhlis, M.; Anwar, K.; Ningsih, R.D.; Masganti, M.; Masganti, M.; et al. Si-Humate as soil ameliorant to improve the properties of acid sulfate soil, growth, and rice yield. Chil. J. Agr. Res. 2024, 84, 267–280. [Google Scholar]
- Myburgh, P.A.; Howell, C.L. Effects of Soil Ameliorants Produced from Recycled Glass on the Establishment of Table Grapes. S. Afr. J. Enol. Vitic. 2023, 44, 101–111. [Google Scholar] [CrossRef]
- Danapriatna, N.; Ismarani, I.; Dede, M. Application of biochar and biological fertilizer to improve soil quality and Oryza sativa L. productivity. Cogent Food Agr. 2023, 9, 2207416. [Google Scholar] [CrossRef]
- Li, X.Y.; Li, B.; Chen, L.; Liang, J.Y.; Huang, R.; Tang, X.Y.; Zhang, X.; Wang, C.Q. Partial substitution of chemical fertilizer with organic fertilizer over seven years increases yields and restores soil bacterial community diversity in wheat-rice rotation. Eur. J. Agron. 2022, 133, 126445. [Google Scholar] [CrossRef]
- Luo, G.W.; Friman, V.P.; Chen, H.; Liu, M.Q.; Wang, M.; Guo, S.W.; Ling, N.; Shen, Q.R. Long-term fertilization regimes drive the abundance and composition of N-cycling-related prokaryotic groups via soil particle-size differentiation. Soil Biol. Biochem. 2018, 116, 213–223. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, K.; Han, L.F.; Chen, Y.L.; Liu, J.; Xing, B.S. Biochar stability and impact on soil organic carbon mineralization depend on biochar processing, aging and soil clay content. Soil Biol. Biochem. 2022, 169, 108657. [Google Scholar] [CrossRef]
- Ma, Y.Q.; Woolf, D.; Fan, M.S.; Qian, L.; Li, R.; Lehmann, J. Global crop production increase by soil organic carbon. Nat. Geosci. 2023, 16, 1159–1165. [Google Scholar] [CrossRef]
- Zhang, J.; Amonette, J.E.; Flury, M. Effect of biochar and biochar particle size on plant-available water of sand, silt loam, and clay soil. Soil Till. Res. 2021, 212, 104992. [Google Scholar] [CrossRef]
- Sadegh-Zadeh, F.; Parichehreh, M.; Jalili, B.; Bahmanyar, M.A. Rehabilitation of calcareous saline-sodic soil by means of biochars and acidified biochars. Land Degrad. Dev. 2018, 29, 3262–3271. [Google Scholar] [CrossRef]
- Li, H.; Wang, B.J.; Siri, M.; Liu, C.; Feng, C.L.; Shao, X.Q.; Liu, K.S. Calcium-modified biochar rather than original biochar decreases salinization indexes of saline-alkaline soil. Environ. Sci. Pollut. R. 2023, 30, 74966–74976. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Kabir, E.; Kim, K.H.; Kwon, E.E. Biochar as a tool for the improvement of soil and environment. Front. Environ. Sci. 2023, 11, 1324533. [Google Scholar] [CrossRef]
- Gong, H.Y.; Li, Y.F.; Li, S.J. Effects of the interaction between biochar and nutrients on soil organic carbon sequestration in soda saline-alkali grassland: A review. Glob. Ecol. Conserv. 2021, 26, e01449. [Google Scholar] [CrossRef]
- Naz, M.; Dai, Z.C.; Hussain, S.; Tariq, M.; Danish, S.; Khan, I.U.; Qi, S.S.; Du, D.L. The soil pH and heavy metals revealed their impact on soil microbial community. J. Environ. Manag. 2022, 321, 115770. [Google Scholar] [CrossRef]
- Malik, A.A.; Puissant, J.; Buckeridge, K.M.; Goodall, T.; Jehmlich, N.; Chowdhury, S.; Gweon, H.S.; Peyton, J.M.; Mason, K.E.; Van Agtmaal, M.; et al. Land use driven change in soil pH affects microbial carbon cycling processes. Nat. Commun. 2018, 9, 3591. [Google Scholar] [CrossRef]
- Cooper, J.M.; Warman, P.R. Effects of three fertility amendments on soil dehydrogenase activity, organic C and pH. Can. J. Soil Sci. 1997, 77, 281–283. [Google Scholar] [CrossRef]
- Zhang, K.; Wei, H.Y.; Chai, Q.; Li, L.L.; Wang, Y. Biological soil conditioner with reduced rates of chemical fertilization improves soil functionality and enhances rice production in vegetable-rice rotation. Appl. Soil Ecol. 2024, 195, 105242. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Z.Y.; Dai, J.; Liu, F.; Zhu, J. Effects of Long-Term Organic Fertilizer Application on Tea Plantation Soil of Its Physical and Chemical Properties and Microbial Communities. Pol. J. Environ. Stud. 2025, 34, 905–916. [Google Scholar] [CrossRef]
- Huo, Q.Y.; Gong, M.; Jiang, Y.W.; Yang, X.; Kong, M.; He, J.X.; Zhang, Q.; Song, J.Q.; Li, X.Z.; Han, W.; et al. Microencapsulated Microbial Seed Coating Could Improve Soil Environment and Maize Grain Yield in Saline Soil. Plants 2024, 13, 3139. [Google Scholar] [CrossRef]
- Yu, Q.; Hu, X.; Ma, J.; Ye, J.; Sun, W.; Wang, Q.; Lin, H. Effects of long-term organic material applications on soil carbon and nitrogen fractions in paddy field. Soil Tillage Res. 2020, 196, 104483. [Google Scholar] [CrossRef]
- Li, X.; Kang, X.F.; Zou, J.Z.; Yin, J.H.; Wang, Y.C.; Li, A.; Ma, X.D. Allochthonous arbuscular mycorrhizal fungi promote Salix viminalis L. -mediated phytoremediation of polycyclic aromatic hydrocarbons characterized by increasing the release of organic acids and enzymes in soils. Ecotox. Environ. Safe. 2023, 249, 114461. [Google Scholar] [CrossRef]
- Lin, Y.C.; Yu, C.L.; Zhang, Y.B.; Lu, L.; Xu, D.; Peng, X.L. Biochar modification methods and mechanisms for salt-affected soil and saline-alkali soil improvement: A review. Soil Use Manag. 2024, 40, e12992. [Google Scholar] [CrossRef]
- Ababsa, N.; Boudjabi, S.; Chenchouni, H. Biochar Amendments Changed Soil Properties and Improved Cereal Crop Growth Under Salt Stress. J. Soil Sci. Plant Nut. 2023, 23, 4912–4925. [Google Scholar] [CrossRef]
- Gao, S.J.; Liu, X.R.; Li, Y.C.; Liu, X.W. Effects of biochar and straw return on greenhouse gas emissions and global warming potential in the farmland. Sci. Agric. Sin. 2024, 57, 935–949. (In Chinese) [Google Scholar] [CrossRef]
- Wu, D.; Sun, P.; Lu, P.Z.; Chen, Y.Y.; Guo, J.M.; Liu, M.; Wang, L.; Zhang, C.J. Effect and approach of Enteromorpha prolifera biochar to improve coastal saline soil. Environ. Sci. 2020, 41, 1941–1949. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Y.F.; Liang, S.; Liang, Y.X.; Liu, X.X. A Comprehensive Accounting of Carbon Emissions and Carbon Sinks of China’s Agricultural Sector. Land 2024, 13, 1452. [Google Scholar] [CrossRef]
- She, W.; Wu, Y.; Huang, H.; Chen, Z.D.; Cui, G.X.; Zheng, H.B.; Guan, C.Y.; Chen, F. Integrative analysis of carbon structure and carbon sink function for major crop production in China’s typical agriculture regions. J. Clean. Prod. 2017, 162, 702–708. [Google Scholar] [CrossRef]
- Burnett, A.C.; Rogers, A.; Rees, M.; Osborne, C.P. Carbon source-sink limitations differ between two species with contrasting growth strategies. Plant Cell Environ. 2016, 39, 2460–2472. [Google Scholar] [CrossRef]
- Ciais, P.; Bousquet, P.; Freibauer, A.; Naegler, T. Horizontal displacement of carbon associated with agriculture and its impacts on atmospheric CO2. Global Biogeochem. Cy. 2014, 21, GB2014. [Google Scholar] [CrossRef]
- Wang, L.J.; Sheng, M.Y. Phytolith occluded organic carbon in Fagopyrum (Polygonaceae) plants: Insights on the carbon sink potential of cultivated buckwheat planting. Front. Plant Sci. 2022, 3, 1014980. [Google Scholar] [CrossRef]
- Cao, X.D.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D.; Lan, Y.; Meng, J.; Jiang, L.; Sun, Q.; Cao, D.Y.; Sun, Y.Y.; Chen, W.F. Labile organic carbon fractions and carbon pool management index in a 3-year field study with biochar amendment. J. Soil Sediment 2018, 18, 1569–1578. [Google Scholar] [CrossRef]
- Curtin, D.; Beare, M.H.; Qiu, W.W.; Sharp, J. Does particulate organic matter fraction meet the criteria for a model soil organic matter pool. Pedosphere 2019, 29, 195–203. [Google Scholar] [CrossRef]
- Huang, X.L.; Jia, Z.X.; Jiao, X.Y.; Wang, J.L.; Huang, X.F. Long-term manure applications to increase carbon sequestration and macroaggregate-stabilized carbon. Soil Biol. Biochem. 2022, 174, 108827. [Google Scholar] [CrossRef]
- Yu, H.Y.; Ding, W.X.; Luo, J.F.; Geng, R.L.; Ghani, A.; Cai, Z.C. Effects of long-term compost and fertilizer application on stability of aggregate-associated organic carbon in an intensively cultivated sandy loam soil. Biol. Fertil. Soils 2012, 48, 325–336. [Google Scholar] [CrossRef]
- Li, G.; Chen, W.J.; Xu, S.Q.; Xiong, S.G.; Zhao, J.Y.; Liu, D.L.; Ding, G.C.; Li, J.; Wei, Y.Q. Role of fungal communities and their interaction with bacterial communities on carbon and nitrogen component transformation in composting with different phosphate additives. Environ. Sci. Pollut. R. 2023, 30, 44112–44120. [Google Scholar] [CrossRef] [PubMed]
- Six, J.; Elliott, E.T.; Paustian, K. Soil macroaggregate turnover and microaggregate formation: A mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Li, B.Z.; Liang, F.; Wang, Y.J.; Cao, W.C.; Song, H.; Chen, J.S.; Guo, J.H. Magnitude and efficiency of straw return in building up soil organic carbon: A global synthesis integrating the impacts of agricultural managements and environmental conditions. Sci. Total Environ. 2023, 875, 162670. [Google Scholar] [CrossRef]
- Liu, C.; Lu, M.; Cui, J.; Li, B.; Fang, C.M. Effects of straw carbon input on carbon dynamics in agricultural soils: A meta-analysis. Global Change Biol. 2014, 20, 1366–1381. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.F.; Jiang, J.; Wang, Y.G.; Colinet, G.; Feng, W.T. Small straw addition enhances straw decomposition and carbon stabilized in soil aggregates over time. Soil Tillage Res. 2024, 238, 106022. [Google Scholar] [CrossRef]
- Liu, J.; Fang, L.C.; Qiu, T.Y.; Chen, J.; Wang, H.; Liu, M.X.; Yi, J.; Zhang, H.L.; Wang, C.; Sardans, J.; et al. Crop residue return achieves environmental mitigation and enhances grain yield: A global meta-analysis. Agron. Sustain. Dev. 2023, 43, 78. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).