‘Miyagawa’ New Bud Mutant Type: Enhances Resistance to Low-Temperature Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Observation of In Vivo Phenotypic Dynamics Under Low-Temperature Stress
2.3. Leaf Tissue Structure Observation
2.4. Observation of Cold Domestication Process and In Vitro Phenotypic Dynamics Under Low-Temperature Stress
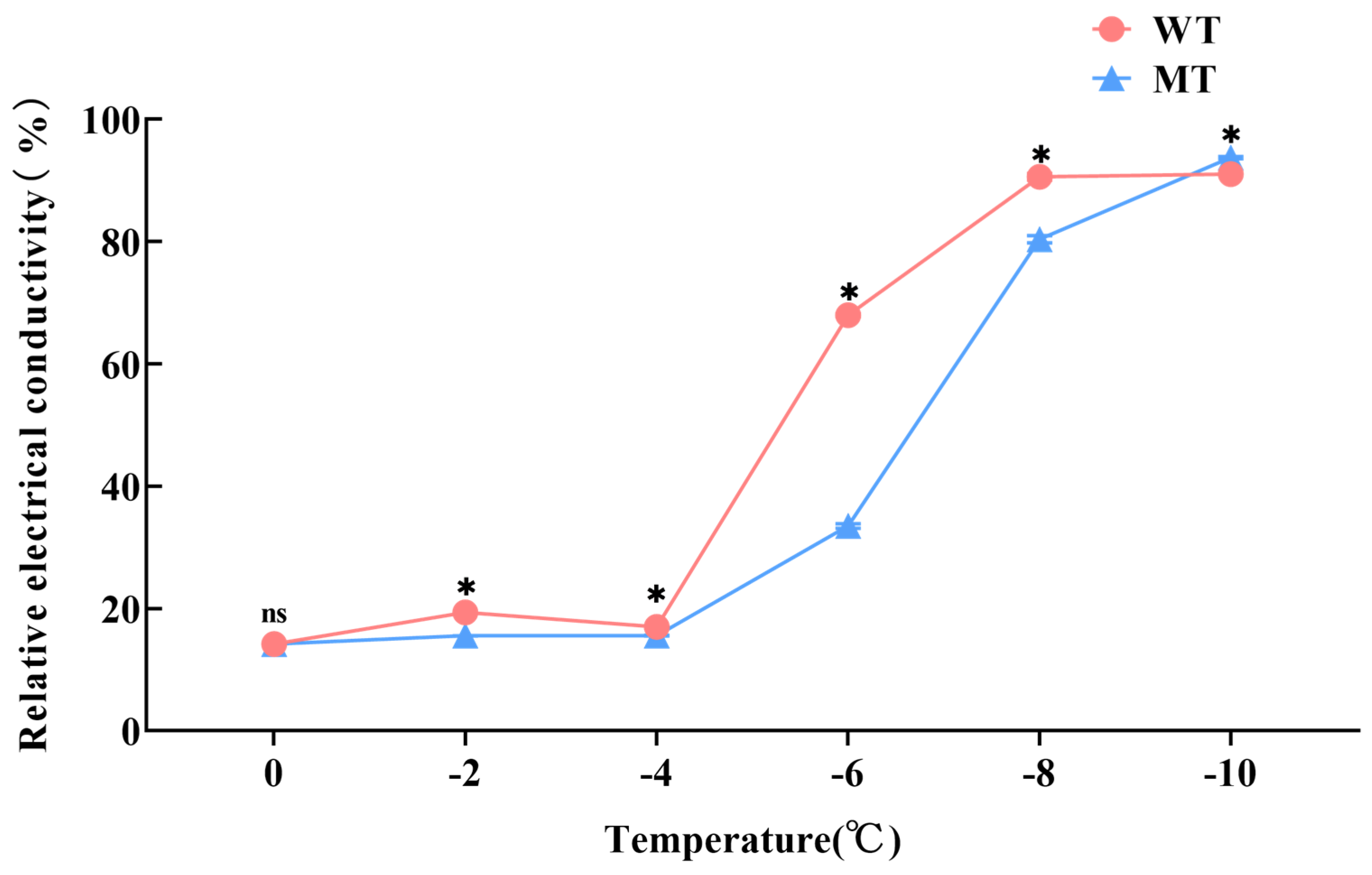
2.5. Optimal Treatment Temperature Exploration and Relative Electrical Conductivity Determination
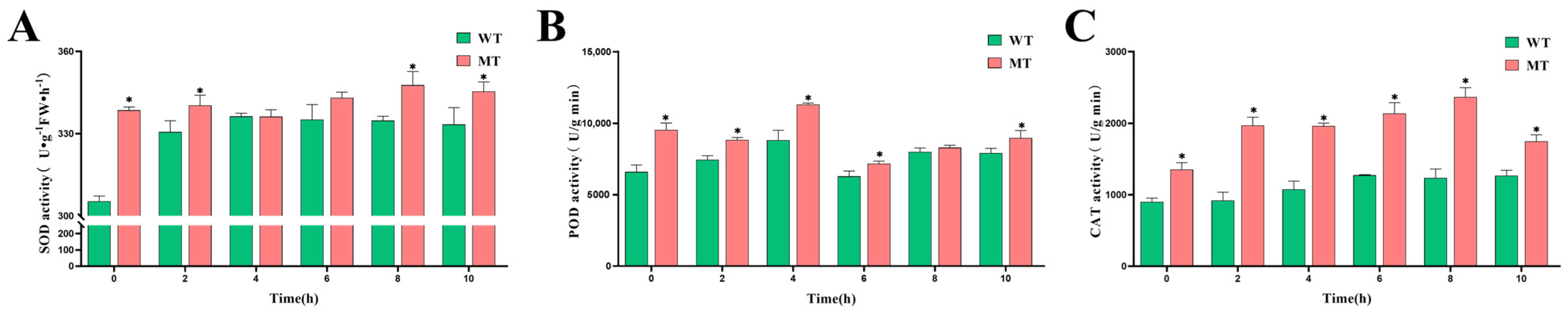
2.6. Determination of Physiological and Biochemical Indices
- SOD activity was measured by mixing 1.5 mL of 50 mmol/L phosphate buffer (pH 7.8) with 0.3 mL each of methionine (130 mmol/L), nitroblue tetrazolium (750 μmol/L), EDTA-Na2 (100 μmol/L), and riboflavin (20 μmol/L). Absorbance was recorded at 560 nm.
- POD activity was determined using a reaction mixture containing 200 mL of 0.2 mol/L phosphate buffer (pH 6.0), 0.076 mL of guaiacol, and 0.112 mL of 30% H2O2. Then, 30 μL of enzyme extract was added to 3 mL of the reaction solution, and absorbance was measured at 470 nm using a UV spectrophotometer.
- CAT activity was analyzed using a reaction solution composed of 200 mL of 0.15 mol/L phosphate buffer (pH 7.0) and 0.3092 mL of 30% H2O2. A volume of 0.1 mL of enzyme extract was added to 3 mL of the reaction solution, and absorbance was measured at 240 nm.
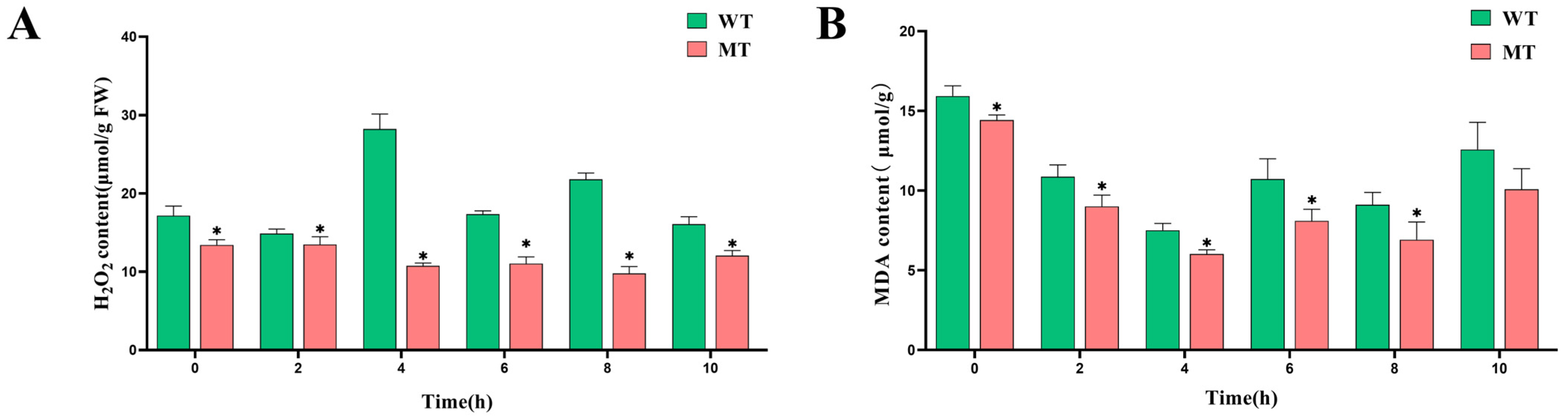
- MDA content was assessed by reacting 2 mL of enzyme extract with 2 mL of a thiobarbituric acid reaction solution, prepared by dissolving 0.5 g of thiobarbituric acid in 0.5% trichloroacetic acid. Absorbance was measured at 450 nm, 532 nm, and 600 nm.
- H2O2 content was determined using a commercially available detection kit from Beijing Solarbio Science & Technology Co., Ltd., Beijing, China. In summary, weigh approximately 0.1 g of leaf samples, add 1 mL of reagent 1, homogenize in an ice bath, centrifuge at 8000 g for 10 min (4 °C), collect all supernatant, place on ice, and proceed with the measurement steps. Measure at a wavelength of 415 nm, using a 96-well plate [39].
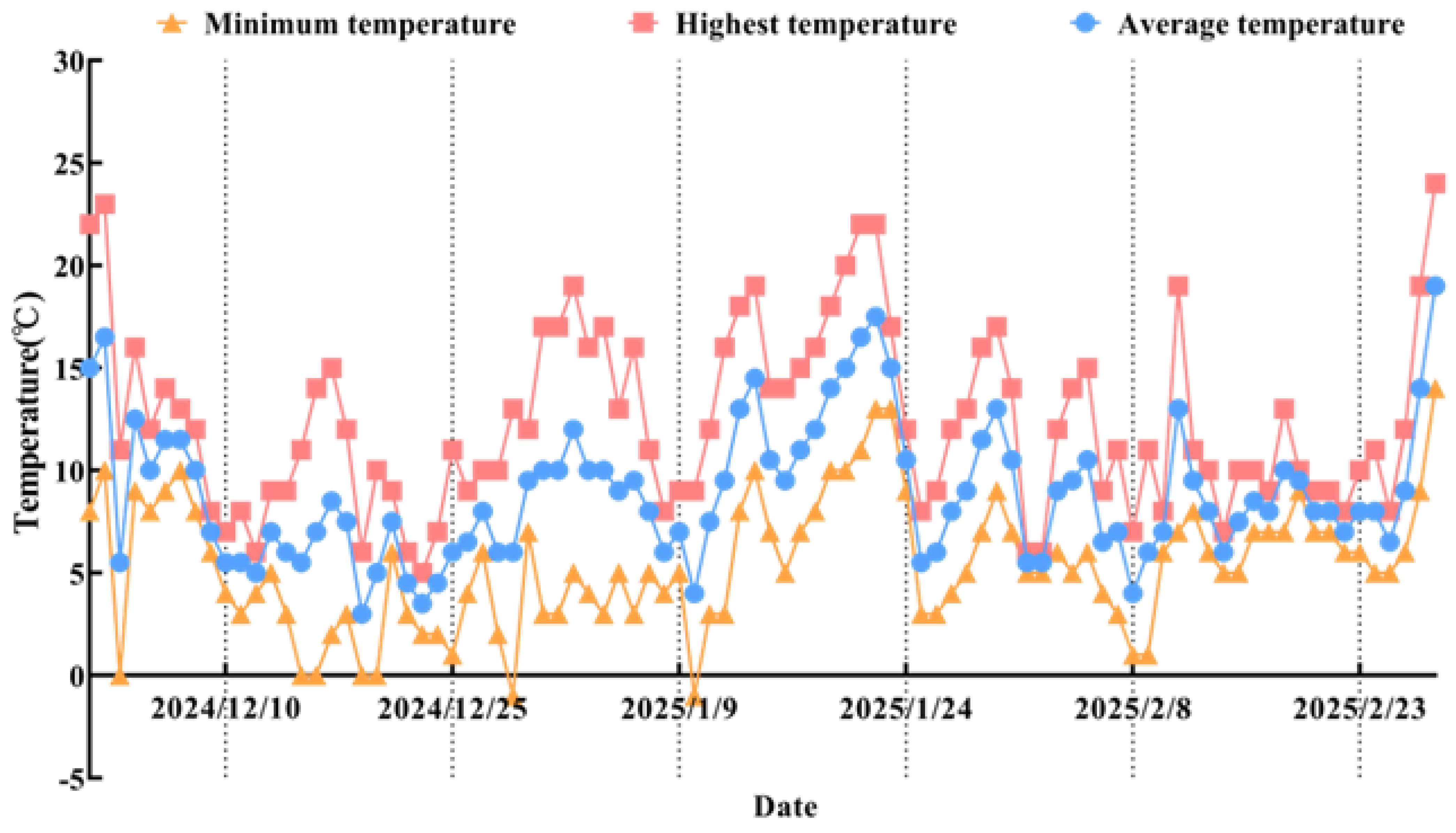
2.7. Temperature Changes During Natural Overwintering
2.8. Determination of qRT-PCR
2.9. Statistical Analyses
3. Results
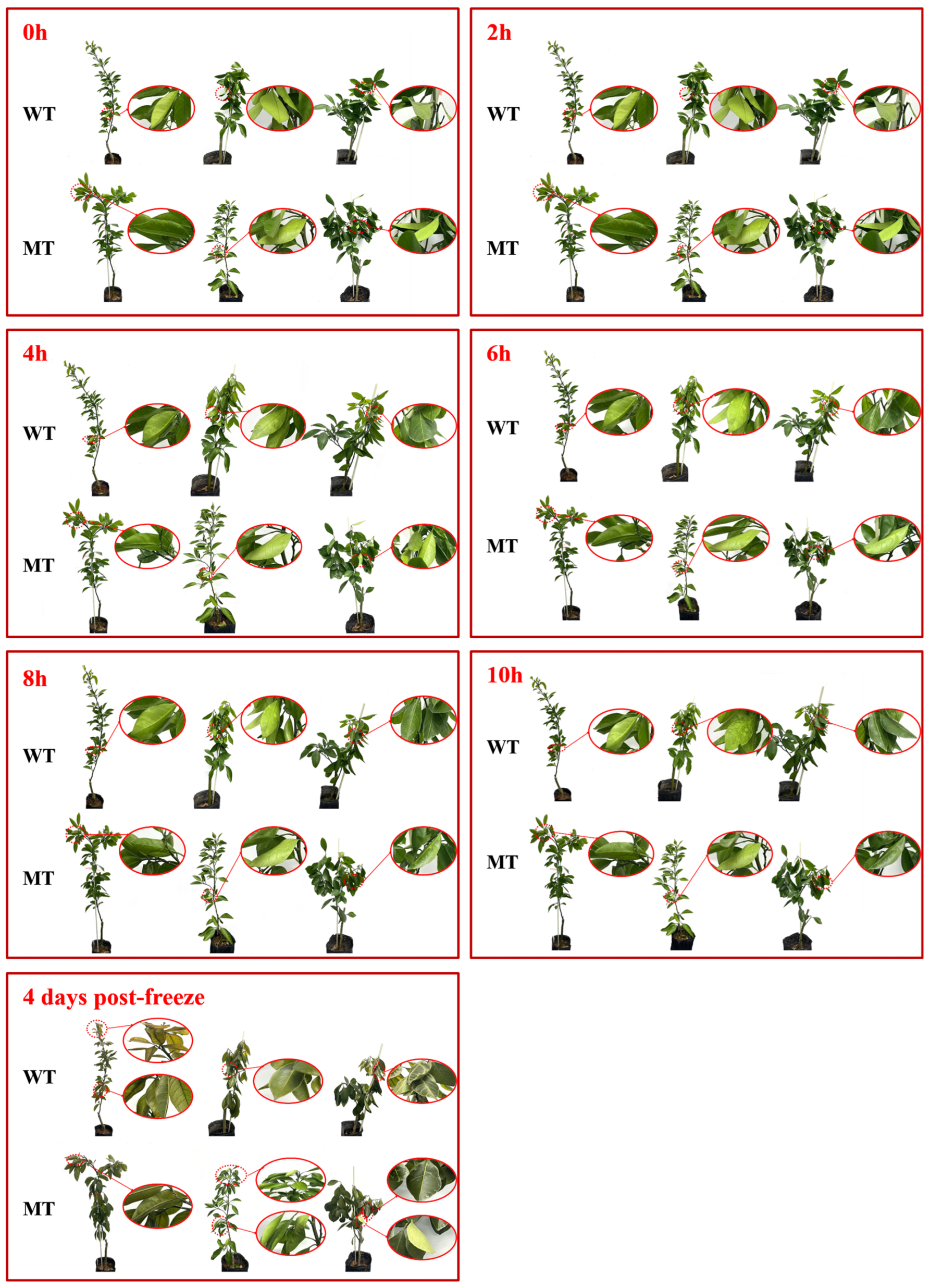
3.1. Dynamic Phenotypic Observation at Different Time Points Under Low-Temperature Treatment
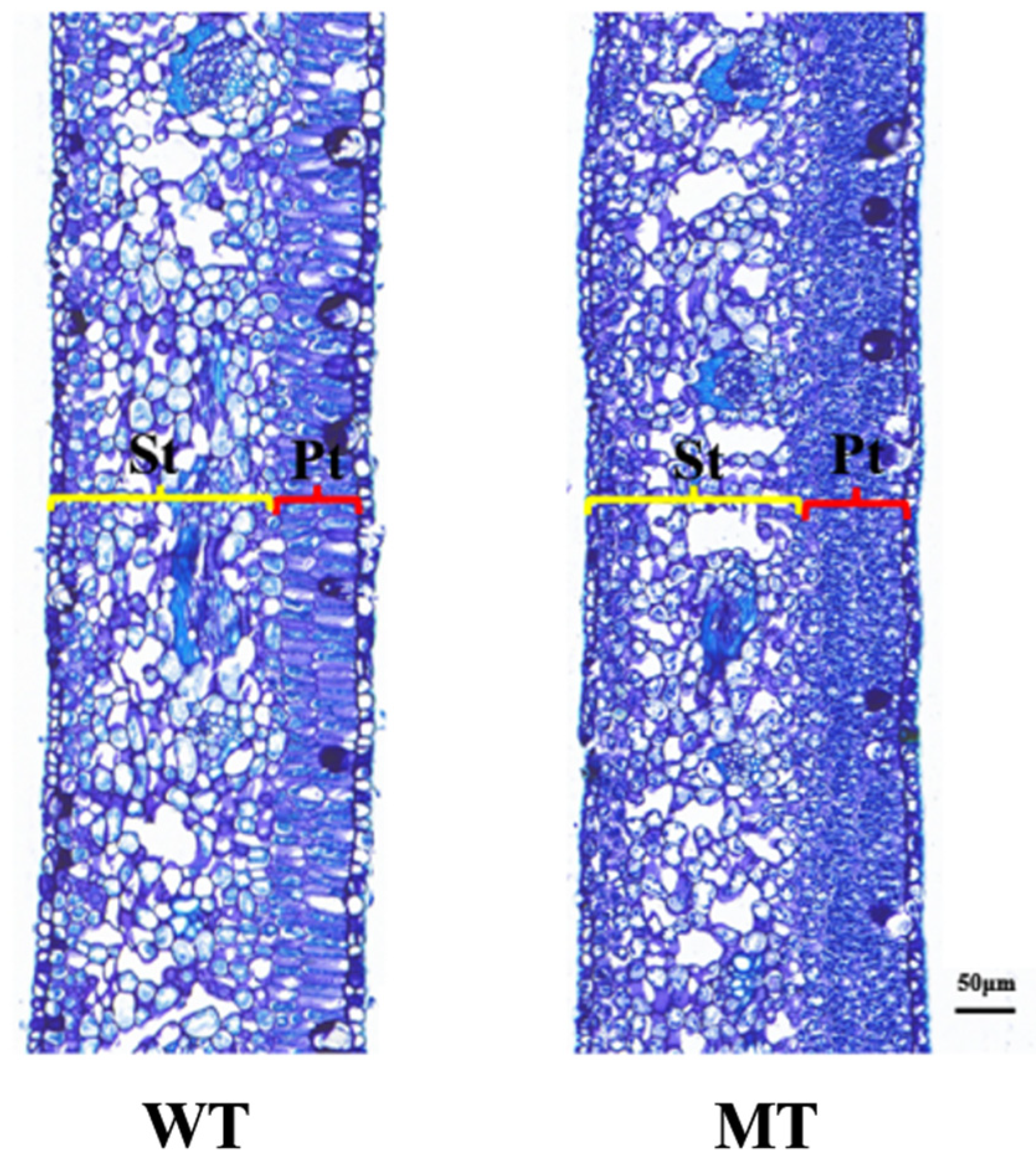
3.2. Comparison of Leaf Tissue Structure Between ‘Miyagawa’ Wild Type and Mutant Type
3.3. Changes in Relative Conductivity of ‘Miyagawa’ Mutant Under Different Low-Temperature Treatments and Determination of Optimum Temperature
3.4. Physiological and Biochemical Responses of the ‘Miyagawa’ Mutant Under Cold Stress
3.4.1. Changes in Antioxidant Enzyme Activities in the ‘Miyagawa’ Mutant Under Cold Stress
3.4.2. Changes in Cellular Damage Indicators in the ‘Miyagawa’ Mutant Under Cold Stress
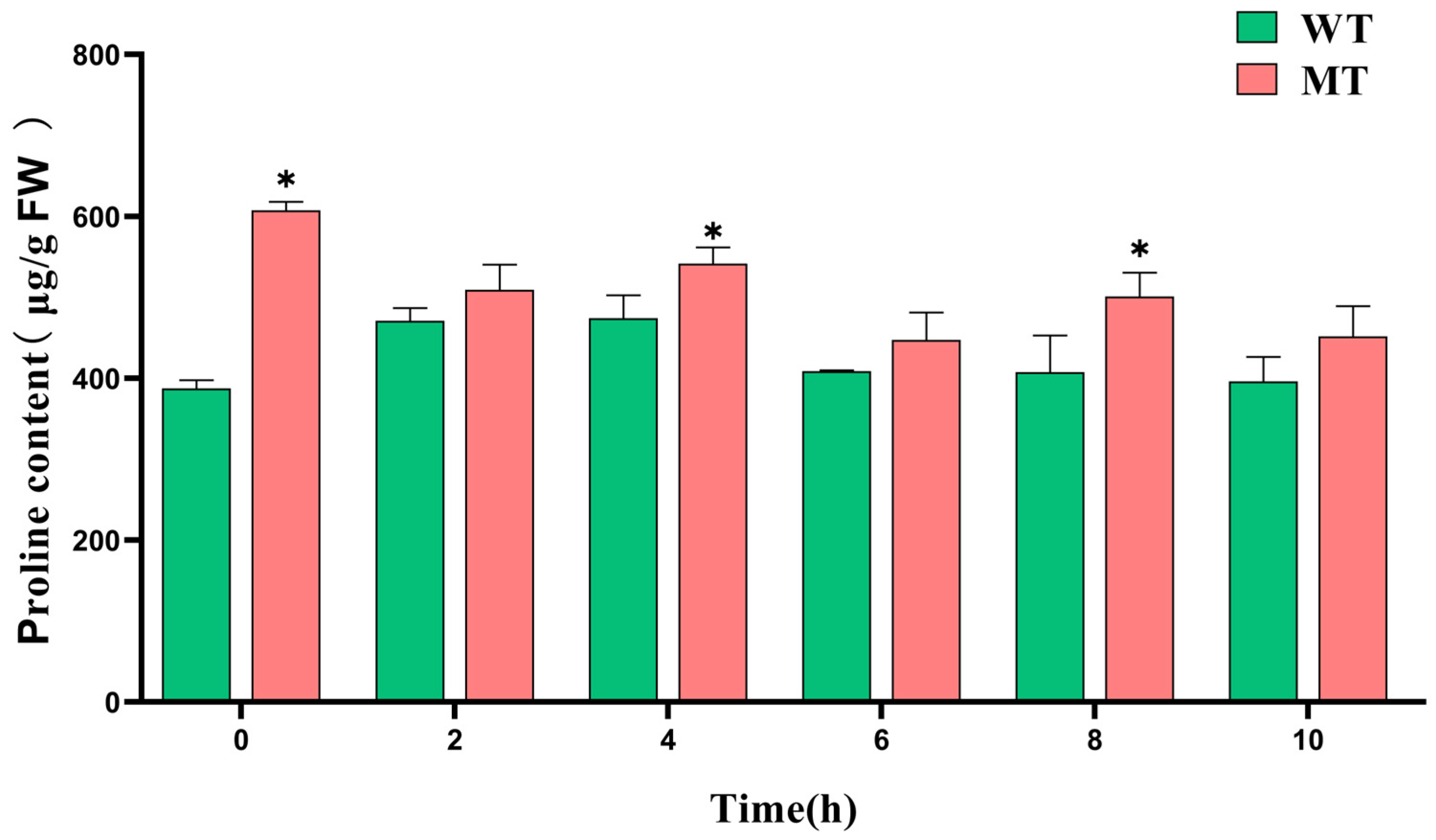
3.4.3. Changes in Proline Content in the ‘Miyagawa’ Mutant Under Cold Stress
3.5. Correlation Analysis Between Temperature Changes and Cor8 Gene Expression Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAT | Catalase (enzyme) |
| CTR | Cellular structural tightness |
| H2O2 | Hydrogen peroxide |
| MDA | Malondialdehyde |
| MT | Mutant type |
| POD | Peroxidase |
| Pro | Proline |
| ROS | Reactive oxygen species |
| SR | Cellular structural relaxation |
| SOD | Superoxide dismutase |
| WT | Wild type |
Appendix A
Appendix A.1. ‘Miyagawa’ Mutant Female Parent

Appendix A.2. Phenotypic Dynamics of Isolated Leaves of ‘Miyagawa’ Mutant at Different Time Points Under Low-Temperature Treatment

Appendix A.3. Dynamic Expression of the ‘Miyagawa’ Mutant Cor8 Gene During Natural Overwintering
References
- Vives-Peris, V.; Pérez-Clemente, R.M.; Gómez-Cadenas, A.; López-Climent, M.F. Involvement of citrus shoots in response and tolerance to abiotic stress. Hortic. Adv. 2024, 2, 3. [Google Scholar] [CrossRef]
- Khan, M.; Dahro, B.; Wang, Y.; Wang, M.; Xiao, W.; Qu, J.; Zeng, Y.; Fang, T.; Xiao, P.; Xu, X.; et al. The transcription factor ERF110 promotes cold tolerance by directly regulating sugar and sterol biosynthesis in citrus. Plant J. 2024, 119, 2385–2401. [Google Scholar] [CrossRef]
- Xiao, C.; He, L.; Qiu, W.; Wang, Z.; He, X.; Xiao, Y.; Sun, Z.; Tong, Z.; Jiang, Y. Guijing2501 (Citrus unshiu) has stronger cold tolerance due to higher photoprotective capacity as revealed by comparative transcriptomic and physiological analysis and overexpression of early light-induced protein. Int. J. Mol. Sci. 2023, 24, 15956. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, Q.; Luo, J.; Gentile, A.; Long, G.; Deng, Z.; Li, D.; Li, N. ‘Juxiangyuan’ seedless orange: A new mutant with male and female sterility. Horticulturae 2024, 10, 350. [Google Scholar] [CrossRef]
- Liu, S.; Xu, Y.; Yang, K.; Huang, Y.; Lu, Z.; Chen, S.; Gao, X.; Xiao, G.; Chen, P.; Zeng, X.; et al. Origin and de novo domestication of sweet orange. Nat. Genet. 2025, 57, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Ding, W.; Zhang, H.; Liu, X.; Zhang, H.; Zeng, J.; Xu, J. Morphological, physiological and molecular characteristics of the seedless ‘Hongjiangcheng’ sweet orange. Hortic. Plant J. 2023, 9, 437–449. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, N.; Singh, H. Pericarp and pedicel anatomy in relation to fruit cracking in lemon (Citrus limon L. Burm.). Sci. Hortic. 2019, 246, 462–468. [Google Scholar] [CrossRef]
- Schreiber, S.G.; Hamann, A.; Hacke, U.G.; Thomas, B.R. Sixteen years of winter stress: An assessment of cold hardiness, growth performance and survival of hybrid poplar clones at a boreal planting site. Plant Cell Environ. 2013, 36, 419–428. [Google Scholar] [CrossRef]
- Meng, S.Y.; Lǚ, G.Y.; Zhang, M.Z.; Zhang, Z.H.; Wei, Y.; Wang, H.T. Liu, X.M. Physiological response to cold stress and evaluation of cold resistance for five species of Euonymus linn. Acta Bot. Boreal.-Occident. Sin. 2020, 40, 11. [Google Scholar]
- Gu, K.; Hou, S.; Chen, J.; Guo, J.; Wang, F.; He, C.; Zou, C.; Xie, X. The physiological response of different tobacco varieties to chilling stress during the vigorous growing period. Sci. Rep. 2021, 11, 22136. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, K.; Gu, Y.; Zhang, L.; Li, W.; Li, Z. Effects of low-temperature stress and brassinolide application on the photosynthesis and leaf structure of tung tree seedlings. Front. Plant Sci. 2020, 10, 1767. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.L.; Deng, Q.E.; Li, J.A.; Cheng, J.Y.; Deng, X.Z.; Jiang, D.Z. Relationship between leaf anatomic structure and cold tolerancein six Camellia oleifera cultivars. Non-Wood For. Res. 2020, 38, 8. [Google Scholar]
- Tan, Y.Y.; Jin, X.L.; Yu, Q.X.; Sun, L.X. Screening of leaf cold-resistant structural indexes and cold-resistance evaluation of five Michelia species. Guihaia 2021, 41, 1296–1305. [Google Scholar]
- Jiang, J.; Zhang, N.; Srivastava, A.K.; He, G.; Tai, Z.; Wang, Z.; Yang, S.; Xie, X.; Li, X. Superoxide dismutase positively regulates Cu/Zn toxicity tolerance in Sorghum bicolor by interacting with Cu chaperone for superoxide dismutase. J. Hazard. Mater. 2024, 480, 135828. [Google Scholar] [CrossRef]
- Rao, M.J.; Ahmed, U.; Ahmed, M.H.; Duan, M.; Wang, J.; Wang, Y.; Wang, L. Comparison and quantification of metabolites and their antioxidant activities in young and mature leaves of sugarcane. ACS Food Sci. Technol. 2021, 1, 362–373. [Google Scholar] [CrossRef]
- Rao, M.J.; Tahir ul Qamar, M.; Wang, D.; Ali, Q.; Ma, L.; Han, S.; Duan, M.; Hu, L.; Wang, L. A high-throughput lipidomics and transcriptomic approach reveals novel compounds from sugarcane linked with promising therapeutic potential against COVID-19. Front. Nutr. 2022, 9, 988249. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.J.; Wu, S.; Duan, M.; Wang, L. Antioxidant metabolites in primitive, wild, and cultivated citrus and their role in stress tolerance. Molecules 2021, 26, 5801. [Google Scholar] [CrossRef]
- Gubanova, T.B.; Paliy, A.E. Physiological and biochemical aspects of frost resistance in Olea europaea L. Russ. J. Plant Physiol. 2020, 67, 671–679. [Google Scholar]
- Herrera, C.M.; Voss, B.J.; Trent, M.S. Homeoviscous adaptation of the acinetobacter baumannii outer membrane: Alteration of lipooligosaccharide structure during cold stress. mBio 2021, 12, e0129521. [Google Scholar] [CrossRef]
- Manchanda, P.; Chaudhary, P.; Deswal, R. Photosynthesis regulation, cell membrane stabilization and methylglyoxal detoxification seems major altered pathways under cold stress as revealed by integrated multi-omics meta-analysis. Physiol. Mol. Biol. Plants 2023, 29, 1395–1407. [Google Scholar] [CrossRef]
- Mishra, G.; Mohapatra, S.K.; Rout, G.R. Plant membrane transporters function under abiotic stresses: A review. Planta 2024, 260, 125. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Bodjrenou, D.M.; Zhang, S.; Wang, B.; Pan, H.; Yeh, K.-W.; Lai, Z.; Cheng, C. The endophytic fungus piriformospora indica reprograms banana to cold resistance. Int. J. Mol. Sci. 2021, 22, 4973. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Basal, O.; Zargar, T.B.; Veres, S. Elevated tolerance of both short-term and continuous drought stress during reproductive stages by exogenous application of hydrogen peroxide on soybean. Sci. Rep. 2024, 14, 2200. [Google Scholar] [CrossRef]
- Zhang, S.; Shan, Y.; Li, Y.; He, J.; Jiang, Y. Hydrogen peroxide receptors regulate chilling injury of banana fruit during low-temperature storage. Postharvest Biol. Technol. 2024, 214, 112985. [Google Scholar] [CrossRef]
- Liu, J.; Wu, H.; Wang, B.; Zhang, Y.; Wang, J.; Cheng, C.; Huang, Y. Exogenous melatonin enhances cold resistance by improving antioxidant defense and cold-responsive genes’ expression in banana. Horticulturae 2022, 8, 260. [Google Scholar] [CrossRef]
- Li, M.; Yue, T.; Han, J.; Wang, J.; Xiao, H.; Shang, F. Exogenous glucose irrigation alleviates cold stress by regulating soluble sugars, ABA and photosynthesis in melon seedlings. Plant Physiol. Biochem. 2024, 217, 109214. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.H.; Locy, R.D.; Rychter, A. Plant Tolerance to Abiotic Stresses in Agriculture: Role of Genetic Engineering; Springer: Dordrecht, The Netherlands, 2000. [Google Scholar] [CrossRef]
- Zalunskaitė, I.; Rugienius, R.; Gelvonauskienė, D.; Bendokas, V.; Vinskienė, J.; Stanys, V. Cloning properties of cold induced COR47 gene and investigation of its homologs. Sodinink. Ir Darzinink. 2006, 25, 25–31. [Google Scholar]
- Ma, X.; Chen, C.; Yang, M.; Dong, X.; Lv, W.; Meng, Q. Cold-regulated protein (SlCOR413IM1) confers chilling stress tolerance in tomato plants. Plant Physiol. Biochem. 2018, 124, 29–39. [Google Scholar] [CrossRef]
- Tang, K.; Zhao, L.; Ren, Y.; Yang, S.; Zhu, J.K.; Zhao, C. The transcription factor ICE1 functions in cold stress response by binding to the promoters of CBF and COR genes. J. Integr. Plant Biol. 2020, 62, 258–263. [Google Scholar] [CrossRef]
- Zhang, X.C.; Wang, L.H.; Ni, H.Z. 2016 spring citrus freeze damage survey in Huangyan district. Zhejiang Citrus 2016, 33, 17–18. [Google Scholar]
- Shuai, H.L.; Yang, T.X.; Wei, A.Z.; Wang, J.; Li, X.; Zhang, Y. A modified technique of paraffin section for apricot flower buds. J. Fruit Sci. 2011, 28, 536–539+552. [Google Scholar]
- Hou, R.P.; Jiang, J.L.; Yang, N.; Li, L.; Deng, J.R.; Ding, D.K.; Sun, W.; Dong, Y.X. Physiological response and cold resistance evaluation of eight species ofcitrus under low temperature stress. Mol. Plant Breed. 2021, 19, 2013–2022. [Google Scholar]
- Yang, N.; Jiang, J.L.; Li, L.; Hou, R.P.; Ding, D.K.; Deng, J.R. Physiological response and evaluation of cold resistance of three citrus varieties citrus under low temperature stress. China Fruits 2020, 5, 32–39+143. [Google Scholar]
- Xu, G.; Li, L.; Zhou, J.; Lyu, D.; Zhao, D.; Qin, S. Comparison of transcriptome and metabolome analysis revealed differences in cold resistant metabolic pathways in different apple cultivars under low temperature stress. Hortic. Plant J. 2023, 9, 183–198. [Google Scholar] [CrossRef]
- Li, L.; Yang, B.; Zhao, X.; Wang, P.; Lyu, D.; Qin, S. Auxin participates in the regulation of the antioxidant system in malus baccata borkh. roots under sub-low temperature by exogenous sucrose application. Horticulturae 2023, 9, 297. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Ma, C.; Zhang, Y.; Polle, A.; Rennenberg, H.; Cheng, X.; Luo, Z.B. Overexpression of bacterial g-glutamylcysteine synthetase mediates changes in cadmium influx, allocation and detoxification in poplar. New Phytol. 2015, 205, 240–245. [Google Scholar] [CrossRef]
- Zhou, J.; Wan, H.; He, J.; Lyu, D.; Li, H. Integration of cadmium accumulation, subcellular distribution, and physiological responses to understand cadmium tolerance in apple rootstocks. Front. Plant Sci. 2017, 8, 966. [Google Scholar]
- Xu, G.; He, M.; Zhao, D.; Lyu, D.; Qin, S. Physiological and structural changes in apple tree branches of different varieties during dormancy. Horticulturae 2023, 9, 947. [Google Scholar] [CrossRef]
- Zhang, C.M. Structure and Expression Analysis of Gene Cor8 in Different Citrus Genotypes with Different Cold Tolerance Subjected to Cold Acclimation. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2021. [Google Scholar]
- Saijo, Y.; Loo, E.P.-i. Plant immunity in signal integration between biotic and abiotic stress responses. New Phytol. 2020, 225, 87–104. [Google Scholar] [CrossRef]
- Sharma, A.; Kapoor, D.; Gautam, S.; Landi, M.; Kandhol, N.; Araniti, F.; Ramakrishnan, M.; Satish, L.; Singh, V.P.; Sharma, P.; et al. Heavy metal induced regulation of plant biology: Recent insights. Physiol. Plant. 2022, 174, e13688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.L.; Li, N.; Yan, Y.Y.; Gou, J.; Wang, C.; Huang, W.; Gong, L.; Long, G.Y. Preliminary identification and evaluation of putative cold- resistant mutant in Newhall Navel orange. J. Fruit Sci. 2022, 39, 1183–1190. [Google Scholar]
- Liu, X.H.; Wang, H.H. Relationship between cellular structure and physiological characteristics of banana leaf tissues and cold hardiness. J. Fujian Agric. Coll. 1990, 19, 5. [Google Scholar]
- Kang, Z.; Cai, H.; Guo, G.; Zeng, H.; Wang, W.; Tu, X. Physiological response of macadamia (Macadamia integrifolia) seedlings to drought stress. Horticulturae 2025, 11, 347. [Google Scholar] [CrossRef]
- Khanna, K.; Bhardwaj, R.; Alam, P.; Reiter, R.J.; Ahmad, P. Phytomelatonin: A master regulator for plant oxidative stress management. Plant Physiol. Biochem. 2023, 196, 260–269. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, Y.-H.; Ai, C.-X.; Zhang, H.; Huang, Y.-C.; Zou, W.-G. Different cold tolerances among three strains of large yellow croaker: Related to antioxidant defense and energy metabolism. Fish Physiol. Biochem. 2023, 49, 471–486. [Google Scholar] [CrossRef]
- Marta, B.; Szafrańska, K.; Posmyk, M.M. Exogenous melatonin improves antioxidant defense in cucumber seeds (Cucumis sativus L.) germinated under chilling stress. Front. Plant Sci. 2016, 7, 575. [Google Scholar] [CrossRef]
- Wu, Z.-X.; Xu, N.-W.; Yang, M.; Li, X.-L.; Han, J.-L.; Lin, X.-H.; Yang, Q.; Lv, G.-H.; Wang, J. Responses of photosynthesis, antioxidant enzymes, and related gene expression to nicosulfuron stress in sweet maize (Zea mays L.). Environ. Sci. Pollut. Res. 2022, 29, 37248–37265. [Google Scholar] [CrossRef]
- Sodhi, G.K.; Saxena, S. Plant growth-promoting endophyte Nigrospora oryzae mitigates abiotic stress in rice (Oryza sativa L.). FEMS Microbiol. Ecol. 2023, 99, fiad094. [Google Scholar] [CrossRef]
- Jing, J.; Liu, M.; Yin, B.; Liang, B.; Li, Z.; Zhang, X.; Xu, J.; Zhou, S. Effects of 10 dwarfing interstocks on cold resistance of ‘tianhong 2’ apple. Horticulturae 2023, 9, 827. [Google Scholar] [CrossRef]
- Agurla, S.; Gahir, S.; Munemasa, S.; Murata, Y.; Raghavendra, A.S. Mechanism of stomatal closure in plants exposed to drought and cold stress. Adv. Exp. Med. Biol. 2018, 1081, 215–232. [Google Scholar]
- Wang, R.; Liu, J.P.; Zhao, H.Q. Evaluation of cold resistance of Piper spp. in Hainan. J. Trop. Biol. 2013, 4, 119–123. [Google Scholar]
- Devireddy, A.R.; Tschaplinski, T.J.; Tuskan, G.A.; Muchero, W.; Chen, J.G. Role of reactive oxygen species and hormones in plant responses to temperature changes. Int. J. Mol. Sci. 2021, 22, 8843. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Wang, J.-W.; Ma, L.-Y.; Gómez-del-Campo, M.; Zhang, D.-S.; Deng, Y.; Jia, Z.-K. Youth tree behavior of olive (Olea europaea L.) cultivars in Wudu, China: Cold and drought resistance, growth, fruit production, and oil quality. Sci. Hortic. 2018, 236, 106–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Yang, J.; Yang, X.; Chen, S.; Xie, Z.; Zhang, M.; Huang, Y.; Zhang, J.; Huang, X. Genome-wide analysis and expression of cyclic nucleotide–gated ion channel (cngc) family genes under cold stress in mango (Mangifera indica). Plants 2023, 12, 592. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Shi, J.C.; Li, Y.C. Cold resistance of Zelkova sinica under low temperature stress. J. West China For. Sci. 2019, 48, 6. [Google Scholar]
- Shi, Q.H.; Gao, J.S.; Wang, J.; Fu, Y.Q. Determination and evaluation of cold resistance of 5 poplar clones. Acta Bot. Boreal.-Occident. Sin. 2003, 23, 5. [Google Scholar]
- Yu, M.; Luobu, Z.; Zhuoga, D.; Wei, X.; Tang, Y. Advances in plant response to low-temperature stress. Plant Growth Regul. 2025, 105, 167–185. [Google Scholar] [CrossRef]
- Renzetti, M.; Funck, D.; Trovato, M. Proline and ROS: A unified mechanism in plant development and stress response? Plants 2025, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Du, W.H.; Zhang, C.Z. Evaluation of physiological and biochemical indicators of cold resistance in forage-type small rye varieties (lines). Acta Agric. Boreali-Occident. Sin. 2024, 33, 2231–2243. [Google Scholar]
- Zhao, G.L.; Tang, J.Q.; Li, R.T.; Bu, Q.Y.; Lǔ, Z.Q. Molecular mechanism of ICE-CBF-COR-mediated cold tolerance regulationin plants under cold stress: A review. Chin. Agric. Sci. Bull. 2025, 41, 132–139. [Google Scholar]
- Aslam, M.; Fakher, B.; Ashraf, M.A.; Cheng, Y.; Wang, B.; Qin, Y. Plant low-temperature stress: Signaling and response. Agronomy 2022, 12, 702. [Google Scholar] [CrossRef]

| Components | Volume (μL) |
|---|---|
| 2 × Universal SYBR qPCR Master Mix | 5 |
| cDNA | 1 |
| Upstream primer | 0.2 |
| Downstream primer | 0.2 |
| Sterilized water | 3.6 |
| Primer Name | Primer Sequence |
|---|---|
| Actin-F | CACACTGGAGTGATGGTTGG |
| Actin-R | ATTGGCCTTGGGGTTAAGAG |
| Cor8-q-F | TGGTTGCTCCCAGTTT |
| Cor8-q-R | GCCCAGGCAGGATAGA |
| Genotypes | Fence Tissue Thickness (μm) | Spongy Tissue Thickness (μm) | Blade Thickness (μm) | Cellular Structural Tightness | Cell Structure Relaxation | Ratio of Fenestrated to Spongy Tissue |
|---|---|---|---|---|---|---|
| WT | 61.08 ± 2.96 | 176.44 ± 6.81 | 264.2 ± 7.01 | 0.23 ± 0.01 | 0.67 ± 0.02 | 0.35 ± 0.01 |
| MT | 94.1 ± 2.68 *** | 195.12 ± 8.12 ** | 321.05 ± 6.74 *** | 0.3 ± 0.01 *** | 0.61 ± 0.01 *** | 0.48 ± 0.03 *** |
| Type | Weather_Window_Days | Mean_Temp | Max_Temp | Min_Temp |
|---|---|---|---|---|
| WT | 10 | −0.89 * | −0.66 | −0.96 ** |
| 9 | −0.86 * | −0.67 | −0.94 ** | |
| 8 | −0.81 * | −0.64 | −0.91 * | |
| 7 | −0.86 * | −0.71 | −0.94 ** | |
| 6 | −0.83 * | −0.67 | −0.91 * | |
| 5 | −0.81 * | −0.69 | −0.88 * | |
| 4 | −0.8 * | −0.67 | −0.88 * | |
| 3 | −0.75 | −0.64 | −0.81 * | |
| 2 | −0.64 | −0.4 | −0.76 | |
| 1 | −0.41 | 0.11 | −0.67 | |
| MT | 10 | −0.74 | −0.64 | −0.71 |
| 9 | −0.77 | −0.68 | −0.75 | |
| 8 | −0.76 | −0.68 | −0.76 | |
| 7 | −0.74 | −0.65 | −0.77 | |
| 6 | −0.68 | −0.55 | −0.76 | |
| 5 | −0.66 | −0.51 | −0.77 | |
| 4 | −0.66 | −0.52 | −0.77 | |
| 3 | −0.67 | −0.51 | −0.8 * | |
| 2 | −0.61 | −0.28 | −0.8 * | |
| 1 | −0.68 | −0.18 | −0.88 * | |
| 1 | −0.68 | −0.18 | −0.88 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhang, Y.; Zhang, B.; Luo, W.; Liu, X.; Dai, S.; Li, D.; Li, N. ‘Miyagawa’ New Bud Mutant Type: Enhances Resistance to Low-Temperature Stress. Agronomy 2025, 15, 1570. https://doi.org/10.3390/agronomy15071570
Wang S, Zhang Y, Zhang B, Luo W, Liu X, Dai S, Li D, Li N. ‘Miyagawa’ New Bud Mutant Type: Enhances Resistance to Low-Temperature Stress. Agronomy. 2025; 15(7):1570. https://doi.org/10.3390/agronomy15071570
Chicago/Turabian StyleWang, Shuangyou, Yingzi Zhang, Ben Zhang, Weiqi Luo, Xiang Liu, Suming Dai, Dazhi Li, and Na Li. 2025. "‘Miyagawa’ New Bud Mutant Type: Enhances Resistance to Low-Temperature Stress" Agronomy 15, no. 7: 1570. https://doi.org/10.3390/agronomy15071570
APA StyleWang, S., Zhang, Y., Zhang, B., Luo, W., Liu, X., Dai, S., Li, D., & Li, N. (2025). ‘Miyagawa’ New Bud Mutant Type: Enhances Resistance to Low-Temperature Stress. Agronomy, 15(7), 1570. https://doi.org/10.3390/agronomy15071570





