Integrating Multi-Trait Selection Indices for Climate-Resilient Lentils: A Three-Year Evaluation of Earliness and Yield Stability Under Semi-Arid Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
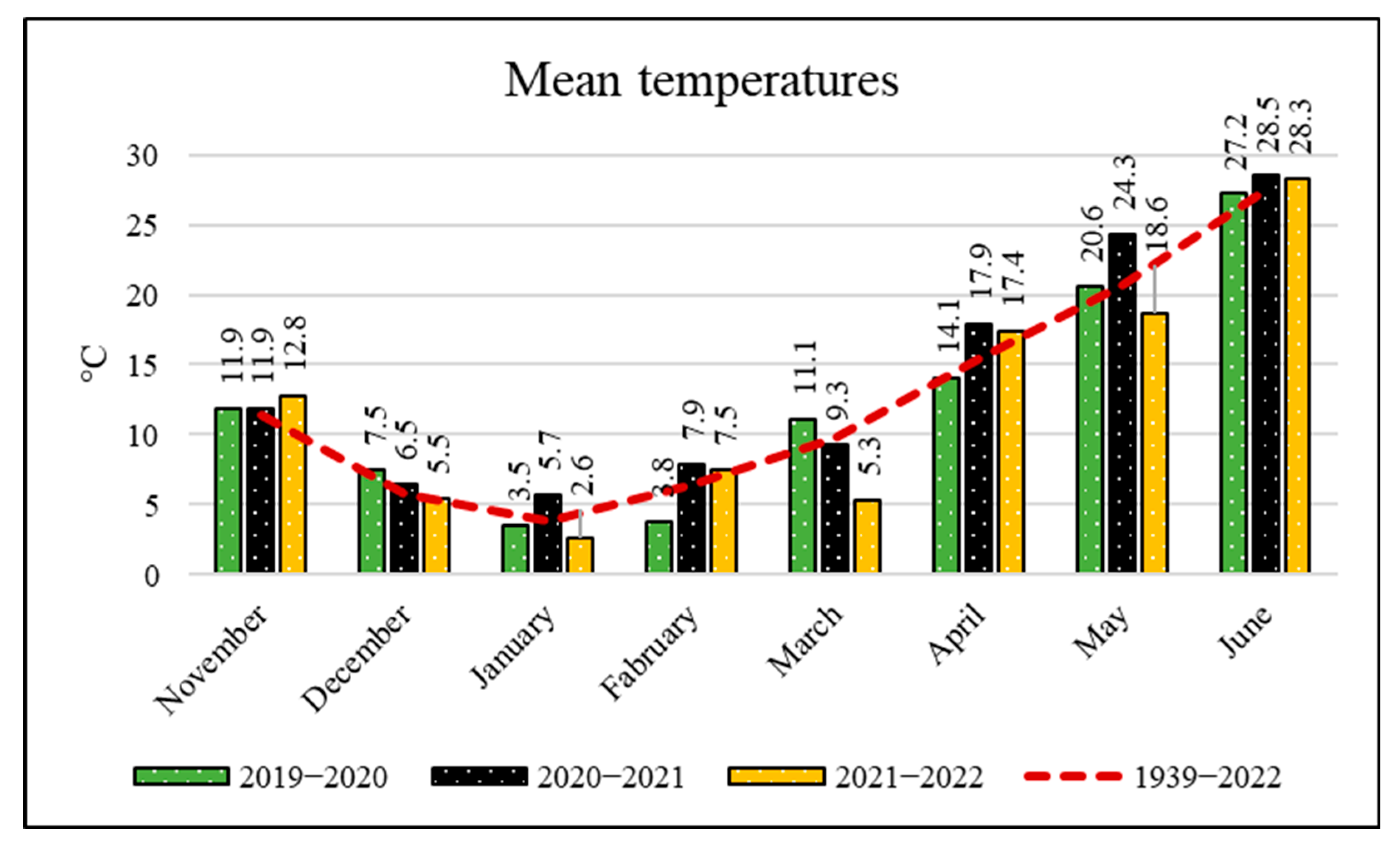
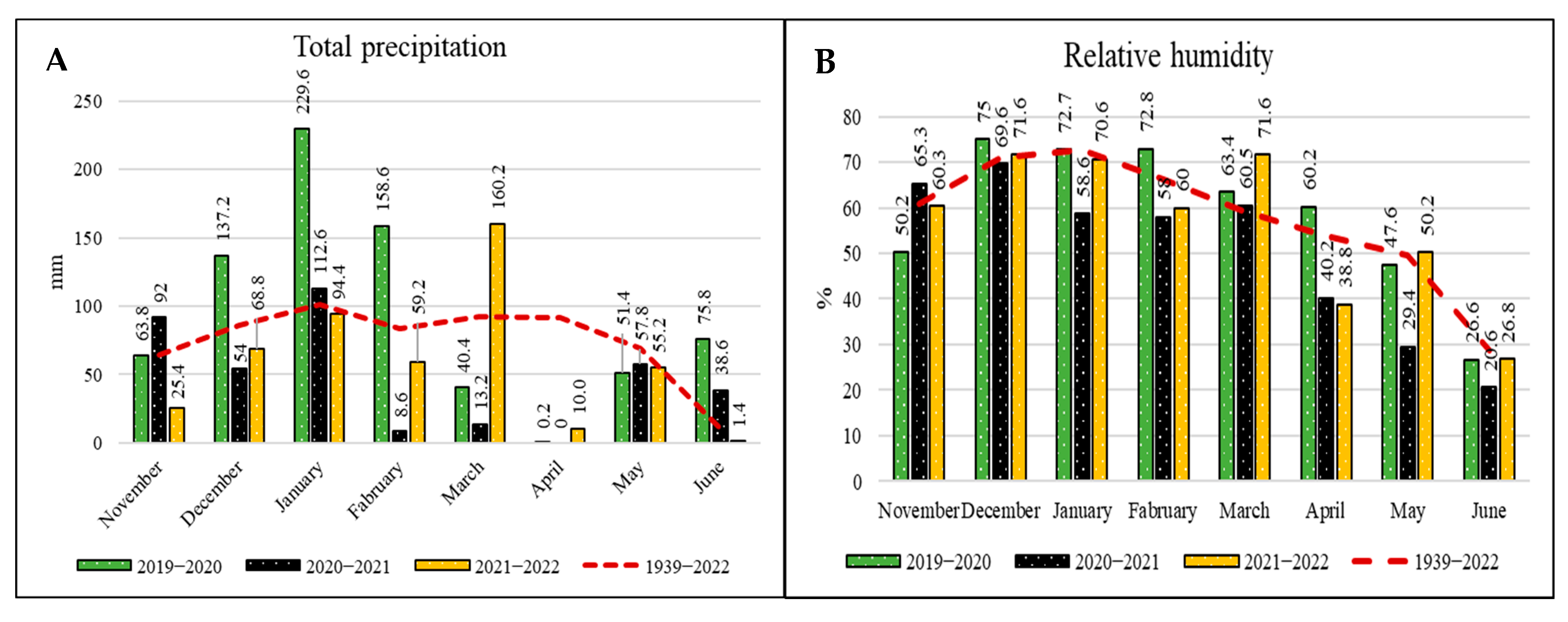
2.2. Experimental Conditions
2.3. Soil Characterization of Experimental Area
2.4. Experimental Design and Laying Out
2.5. Data Collection
2.6. Statistical Analysis
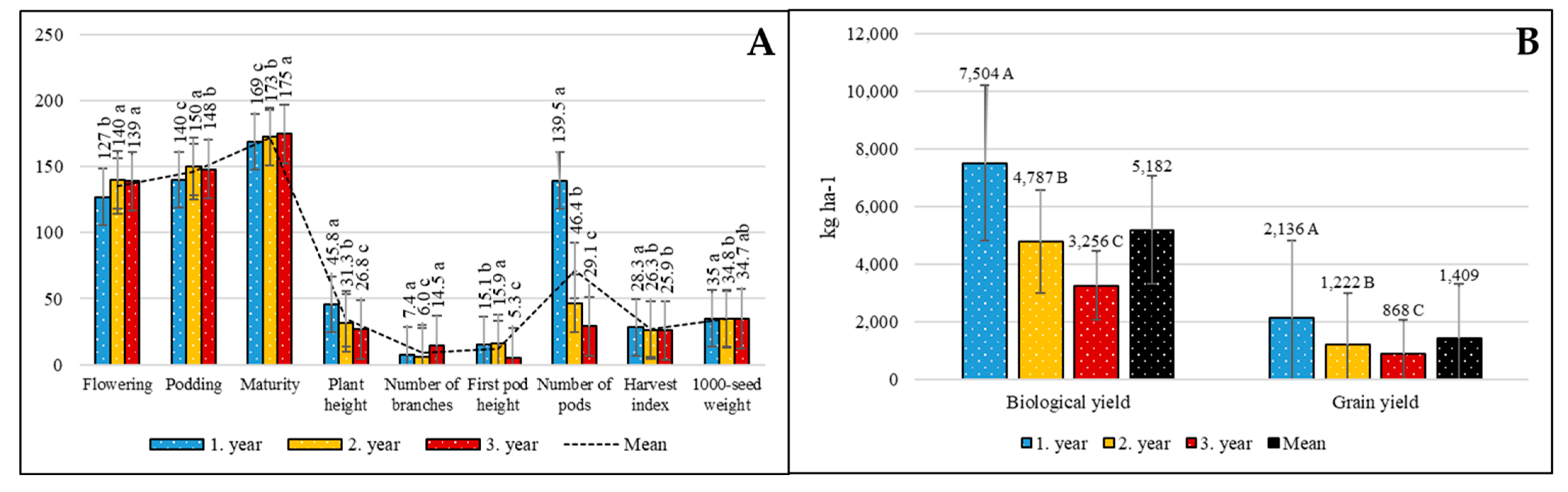
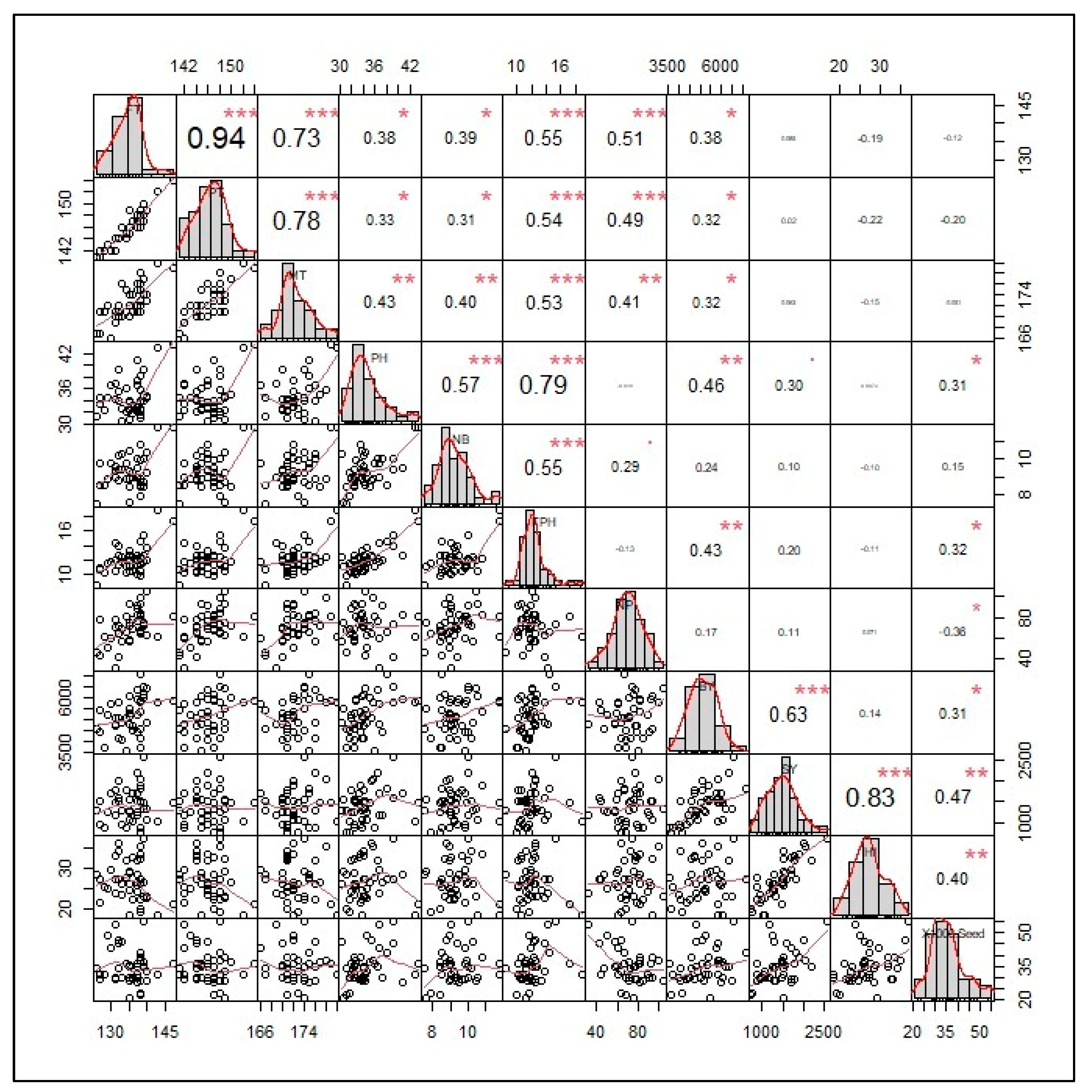
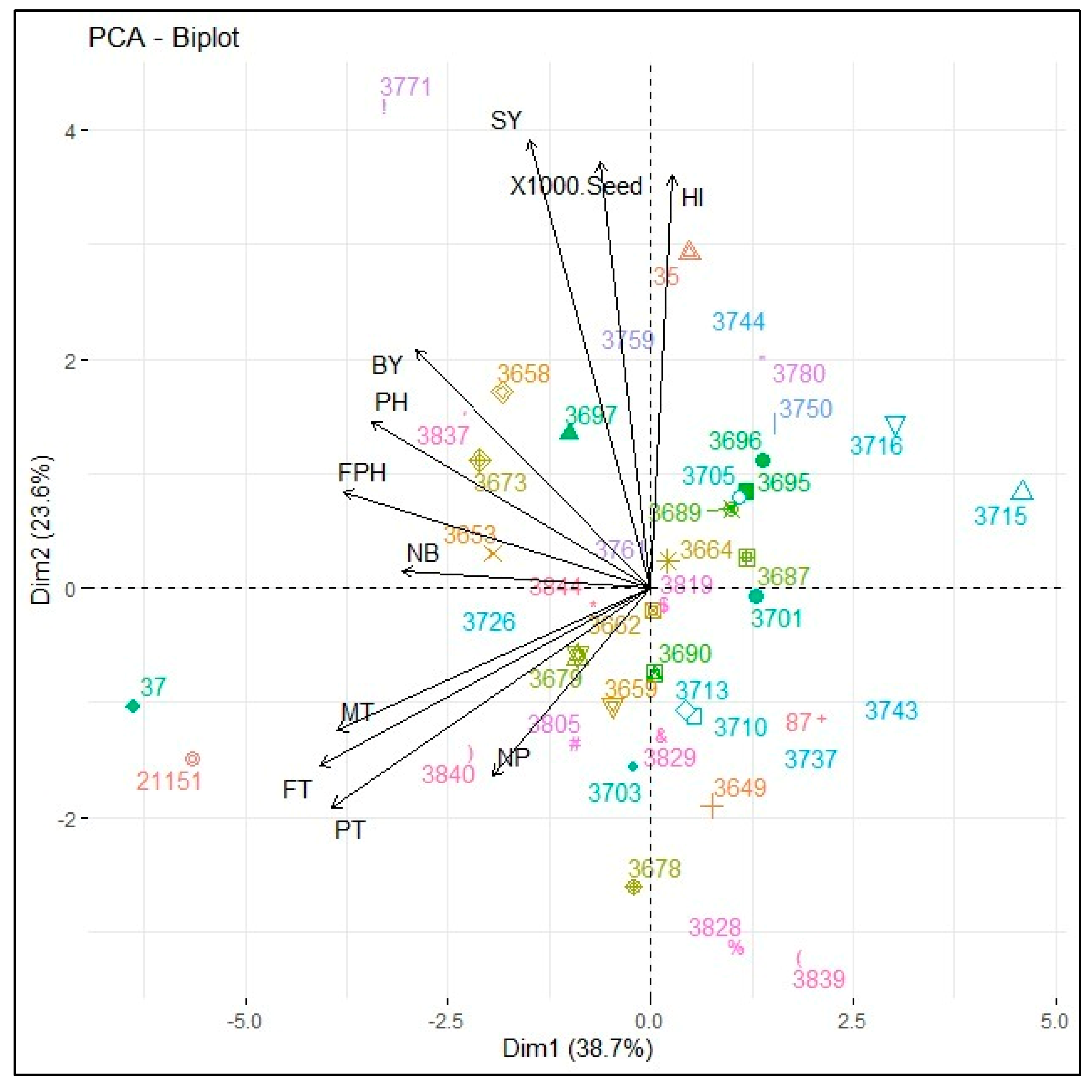
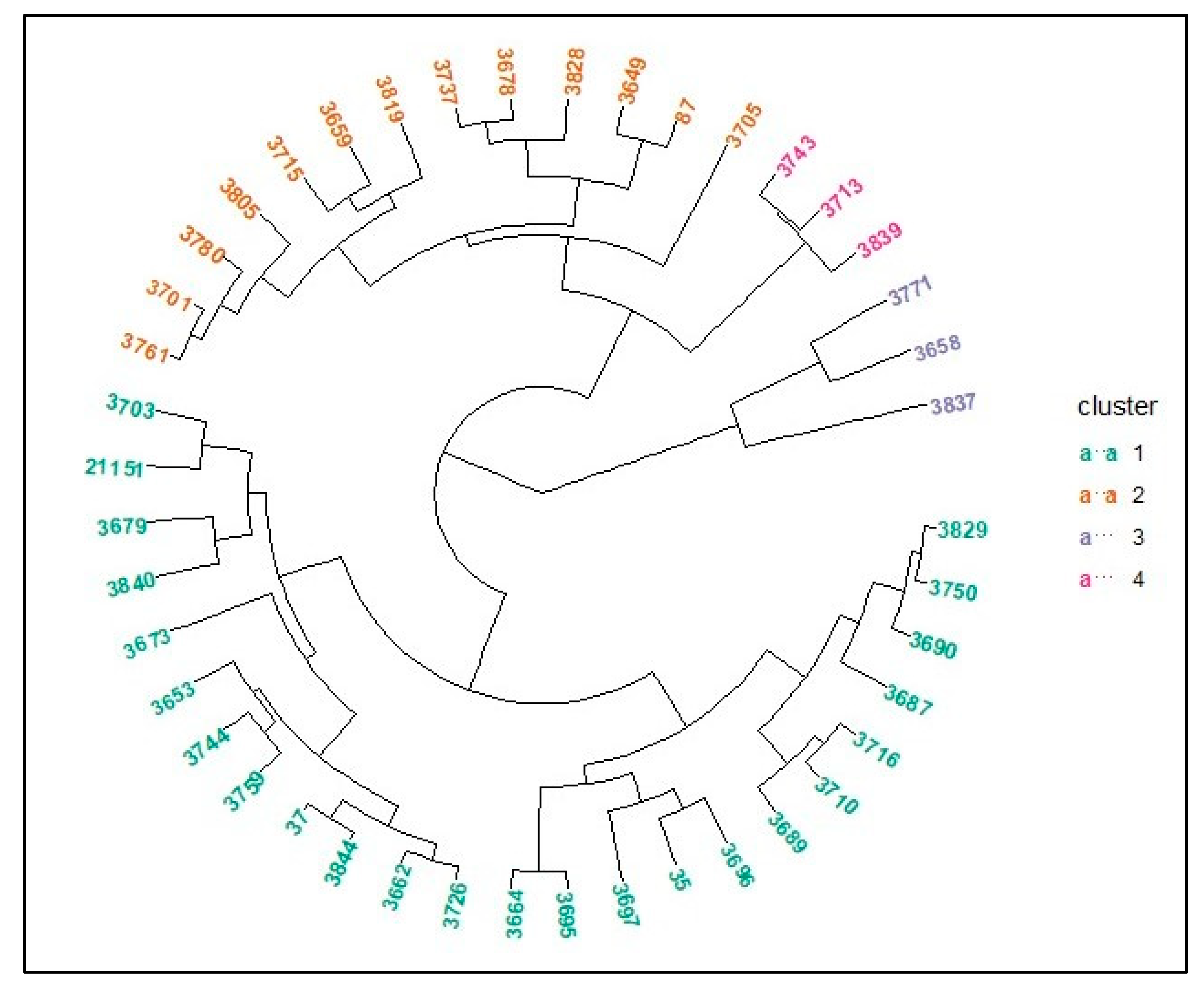
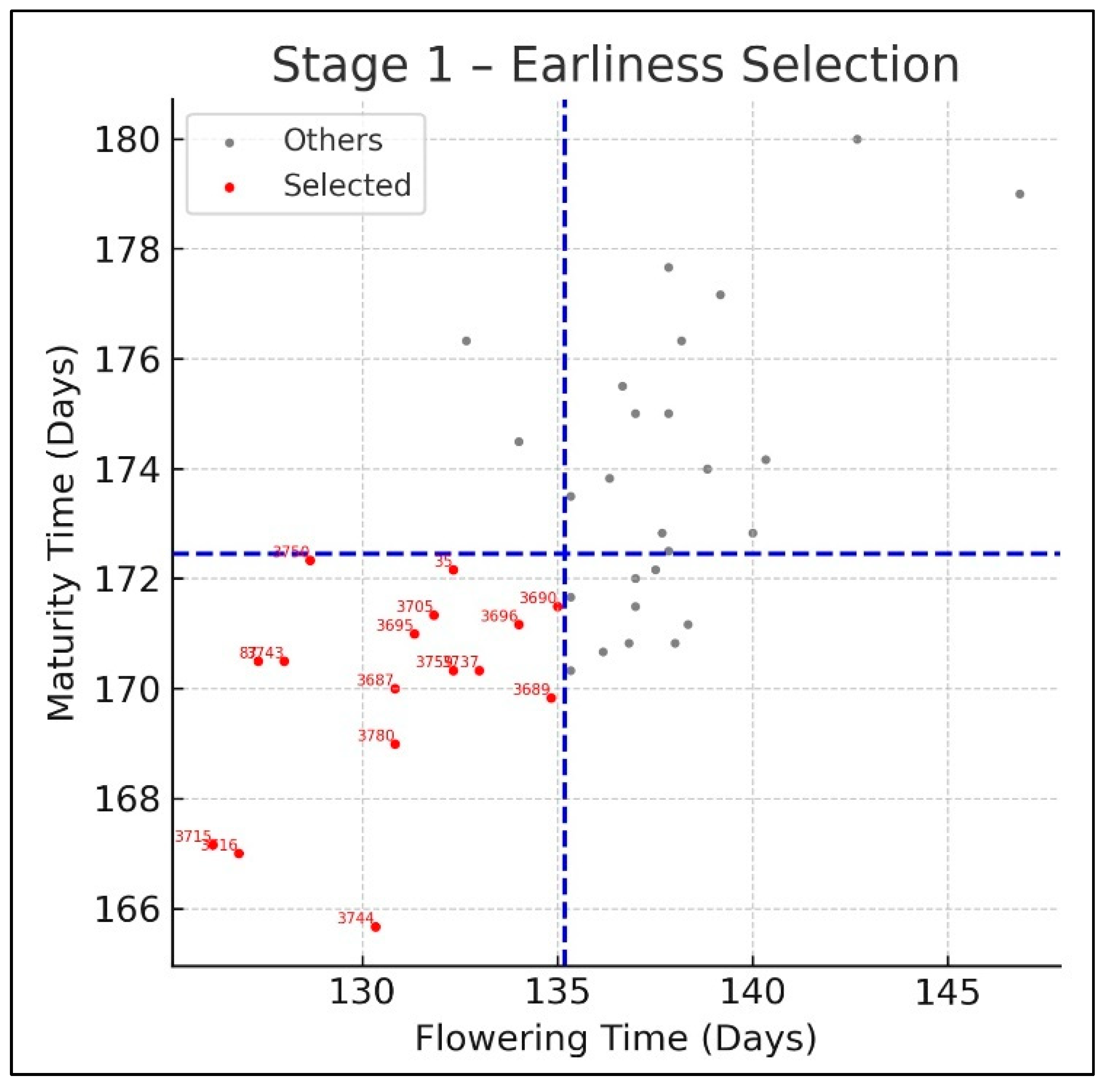
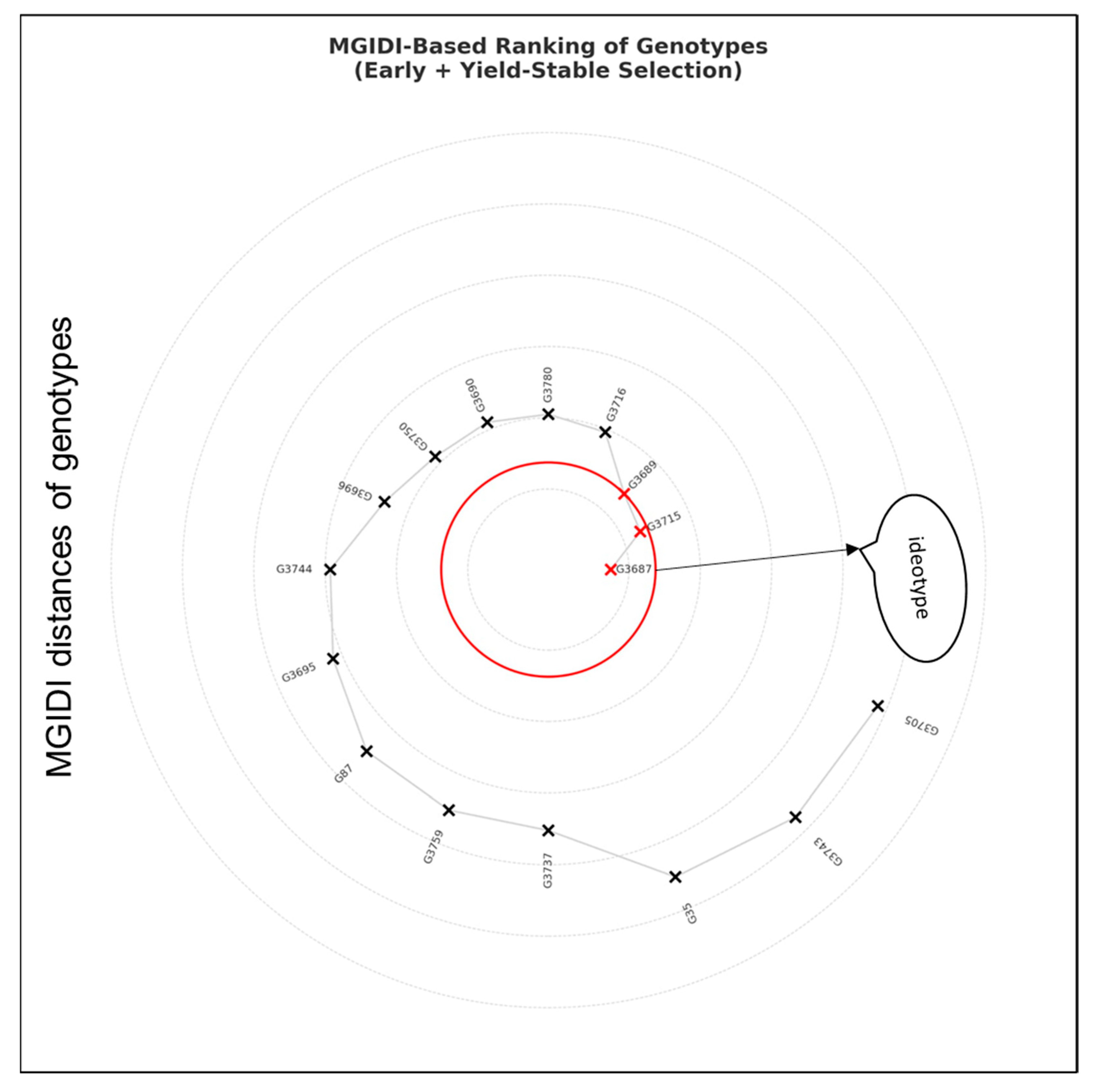
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ICARDA | International Center for Agricultural Research in the Dry Areas; |
| ICP-OES | Inductively Coupled Plasma Optical Emission spectroscopy; |
| ANOVA | Analysis of variance; |
| PCA | Principal component analysis; |
| MTSI | Modified Multi-Trait Stability Index; |
| MGIDI | Multi-Trait Genotype-Ideotype Distance Index; |
| YxG | Year x genotype interaction. |
Appendix A
| Germplasm ID | Designation | The Pedigree String of the Germplasm |
|---|---|---|
| 37 | Cv.Fırat-87 | Local check |
| 87 | ILL8006 | ILL8006 |
| 3705 | x2011s_11_12 | ILL4605XBARIMASUR-6 |
| 3703 | x2011s_110_23 | ILL8007XILL759 |
| 3689 | x2011s_129_13 | FLIP96-49LXFLIP97-33L |
| 3701 | x2011s_130_1 | ILL4402XILL7979 |
| 3805 | x2011s_139_4 | ILL4402XILL7950 |
| 3687 | x2011s_171_13 | Barimusor-6xL-7713 |
| 3695 | x2011s_171_2 | Barimusor-6xL-7713 |
| 3696 | x2011s_203_2 | ILL10749XILL3597 |
| 3664 | x2011s_221_5 | ILL10750XILL1959 |
| 3690 | x2011s_226_6 | ILL10800XILL4419 |
| 3649 | x2011s_59_20 | L-4147XILL4649 |
| 3839 | x2011s_60_28 | L-4147XILL4649 |
| 3697 | x2011s_72_54 | ILL7978XILL7537 |
| 3771 | x2011s133_119_15 | FLIP97-29LXFLIP97-33L |
| 3750 | x2011s17_20_3 | BARIMASUR-6XLIRL-22-46-1-1-1-0 |
| 3780 | x2011s242_230_3 | ILL10801XILL2711 |
| 3761 | x2011s91_77_6 | ILL4605XILL5597 |
| 3744 | x2013_126_54 | FLIP97-34LXFLIP97-33L |
| 3743 | x2013_171_17 | Barimusor- 6xL-7713 |
| 3737 | x2013_19_16 | BARIMASUR-6XLIRL-21-50-1-1-1-0 |
| 3715 | x2013_20_7 | BARIMASUR-6XLIRL-22-46-1-1-1-0 |
| 3716 | x2013_21_2 | BARIMASUR-6XLIRL-22-46-1-1-1-0 |
| 21151 | ILL2245 | ILL2245 |
| 35 | ILL4605 | ILL4605 |
| 3819 | x2011s_118_12 | FLIP97-29LXFLIP97-33L |
| 3713 | x2011s_119_25 | FLIP97-29LXFLIP97-33L |
| 3673 | x2011s_172_34 | ILL7723XADA’A |
| 3662 | x2011s_161_1 | ILL8008XILL8010 |
| 3653 | x2011s_172_34 | ILL7723XADA’A |
| 3840 | x2011s_183_16 | ILL7115XILL2585 |
| 3658 | x2011s_204_23 | ILL10750X33108 |
| 3679 | x2011s_204_30 | ILL10750X33108 |
| 3844 | x2011s_35_36 | ILL10731XILL4637 |
| 3710 | x2011s_55_22 | ILL4605XL-4147 |
| 3678 | x2011s_55_9 | ILL4605XL-4147 |
| 3659 | x2011s_60_48 | L-4147XILL4649 |
| 3829 | x2011s_63_9 | ILL3796XILL4605 |
| 3828 | x2011s_75_17 | ILL7978XILL5888 |
| 3837 | x2011s_97_17 | ILL5883XILL10750 |
| 3759 | x2011s91_76_4 | ILL4605XILL5597 |
| 3726 | x2013_126_8 | FLIP97-34LXFLIP97-33L |
| Sum of Square/F Prob. | |||
|---|---|---|---|
| Traits | Year | Genotype | Y × G |
| Flowering time | 8877.5 ** | 224.3 ** | 29.5 ** |
| Podding time | 5604.0 ** | 92.9 ** | 10.2 ** |
| Maturity time | 1774.5 ** | 109.8 ** | 28.4 ** |
| Plant height | 16,885 ** | 110.6 ** | 29.1 ** |
| Number of branches per plant | 3573.4 ** | 10.6 ** | 11.8 ** |
| First pod height | 6052.6 ** | 43.1 ** | 20.9 ** |
| Number of pods per plant | 606,085 ** | 2926 ** | 2427 ** |
| Biological yield | 796,196,793 ** | 8,544,848 ** | 6,849,222 ** |
| Seed yield | 73,658,759 ** | 1,936,561 ** | 1,060,640 ** |
| Harvest index | 275.5 ** | 287.5 ** | 169.8 ** |
| 1000-seed weight | 3.68 ** | 685.6 ** | 53.7 ** |
| Genotype | Flowering | Maturity | Biological Yield | Seed Yield | Harvest Index | MGIDI |
|---|---|---|---|---|---|---|
| 3687 | 130.83 | 170.00 | 5058.83 | 1462.86 | 27.33 | 0.39 |
| 3715 | 126.17 | 167.17 | 4591.17 | 1298.93 | 28.31 | 0.62 |
| 3689 | 134.83 | 169.83 | 5315.67 | 1545.73 | 28.69 | 0.67 |
| 3716 | 126.83 | 167.00 | 5414.33 | 1519.64 | 27.45 | 0.92 |
| 3780 | 130.83 | 169.00 | 4790.00 | 1175.99 | 23.72 | 0.96 |
| 3690 | 135.00 | 171.50 | 5147.22 | 1280.61 | 24.58 | 0.99 |
| 3750 | 128.67 | 172.33 | 5217.67 | 1243.48 | 24.96 | 0.99 |
| 3696 | 134.00 | 171.17 | 5067.00 | 1772.01 | 31.88 | 1.10 |
| 3744 | 130.33 | 165.67 | 5729.33 | 1689.92 | 29.45 | 1.35 |
| 3695 | 131.33 | 171.00 | 4757.17 | 1774.25 | 33.30 | 1.45 |
| 87 | 127.33 | 170.50 | 4396.17 | 943.32 | 21.29 | 1.59 |
| 3759 | 132.33 | 170.33 | 5825.00 | 1598.39 | 27.12 | 1.61 |
| 3737 | 133.00 | 170.33 | 4044.89 | 941.67 | 23.51 | 1.62 |
| 35 | 132.33 | 172.17 | 5112.50 | 2004.34 | 36.22 | 2.06 |
| 3743 | 128.00 | 170.50 | 3700.72 | 870.87 | 25.20 | 2.17 |
| 3705 | 131.83 | 171.33 | 4116.72 | 1522.12 | 34.24 | 2.21 |
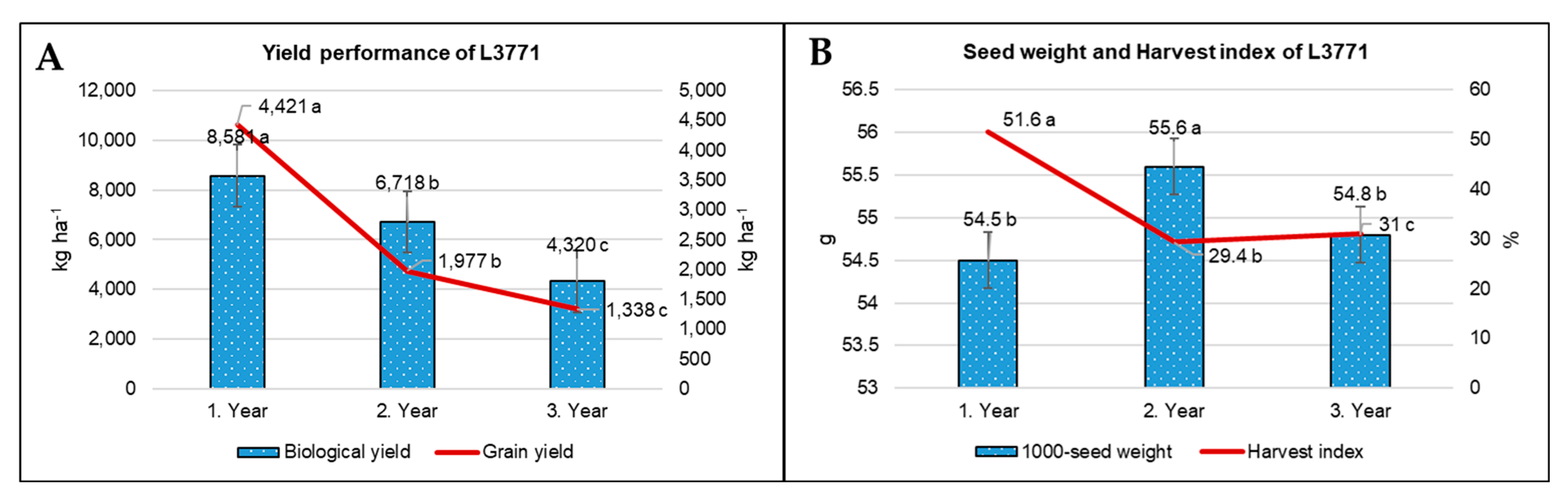
References
- Singh, M.; Bisht, I.S.; Kumar, S.; Dutta, M.; Bansal, K.C.; Singh, A.K.; Sarker, A. Global wild annual Lens collection: A potential resource for lentil genetic base broadening and yield enhancement. PLoS ONE 2014, 9, e107781. [Google Scholar] [CrossRef] [PubMed]
- Fratini, R.M.; Pérez de la Vega, M. Lentil. In Broadening the Genetic Base of Grain Legumes; Singh, M., Upadhyaya, H.D., Bisht, I.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 123–149. [Google Scholar] [CrossRef]
- Dissanayake, R.; Braich, S.; Cogan, N.O.I.; Smith, K.; Spangenberg, G.C.; Kaur, S. Characterization of genetic and allelic diversity amongst cultivated and wild lentil accessions for germplasm enhancement. Front. Genet 2020, 11, 546. [Google Scholar] [CrossRef] [PubMed]
- Tullu, A.; Diederichsen, A.; Suvorova, G.; Vandenberg, A. Genetic and genomic resources of lentil: Status, use and prospects. Plant Genet. Res. 2011, 9, 19–29. [Google Scholar] [CrossRef]
- Neupane, S.; Dhakal, R.; Wright, D.M.; Shrestha, D.K.; Shrestha, J. Strategic identification of new genetic diversity to expand lentil (Lens culinaris Medik.) production (using Nepal as an example). Agronomy 2021, 11, 1933. [Google Scholar] [CrossRef]
- Dikshit, H.K.; Singh, A.; Singh, D.; Aski, M.S.; Prakash, P. Genetic diversity in Lens species revealed by EST and genomic simple sequence repeat analysis. PLoS ONE 2015, 10, e0138101. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, M.; Materne, M.; Cogan, N.O.I.; Rodda, M.S.; Daetwyler, H.D.; Slater, A.T. Assessment of genetic variation within a global collection of lentil (Lens culinaris Medik.) cultivars and landraces using SNP markers. BMC Genet. 2014, 15, 150. [Google Scholar] [CrossRef]
- Kumar, J.; Gupta, S.; Dubey, S.; Gupta, P.; Gupta, D.S. Genetic diversity changes in Indian lentils over the times. J. Plant Biochem. Biotechnol. 2018, 27, 456–464. [Google Scholar] [CrossRef]
- Guerra-Garcia, A.; Haile, T.; Ogutcen, E.; von Wettberg, E.; Wright, D. An evolutionary look into the history of lentil reveals unexpected diversity. Evol. Appl. 2022, 15, 822–835. [Google Scholar] [CrossRef]
- Wright, D.M.; Neupane, S.; Heidecker, T.; von Wettberg, E.J.B. Understanding photothermal interactions will help expand production range and increase genetic diversity of lentil (Lens culinaris Medik.). Plants People Planet 2021, 3, 75–89. [Google Scholar] [CrossRef]
- Khazaei, H.; Caron, C.T.; Fedoruk, M.; Diapari, M.; Vandenberg, A.; Bett, K.E. Genetic diversity of cultivated lentil (Lens culinaris Medik.) and its relation to the world’s agro-ecological zones. Front. Plant Sci. 2016, 7, 1093. [Google Scholar] [CrossRef]
- FAO 2025. Lentil Production on the World. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 13 June 2025).
- Anonymous. Crop Report-Lentil; Ministry of Agriculture and Forestry of Repablic of Türkiye: Ankara, Türkiye, 2022.
- GAPUTAEM. Registration Information of Fırat-87 Lentil Cultivar. Available online: https://arastirma.tarimorman.gov.tr/gaputaem/Belgeler/%C3%A7e%C5%9Fit%20belgeleri/t%C3%BCrk%C3%A7e/mercimek/f%C4%B1rat%2087%20tr.pdf (accessed on 25 May 2024).
- Bouyoucos, G.J. Hydrometer method improved for making particle size analyses of soils. J. Agron. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Burt, R. Soil Survey Laboratory Methods Manual; USDA: Washington, DC, USA, 1992.
- Leal, O.; Trujillo, G.; Moreno, D.A. Assessment of calcium carbonate content in calcareous soils using the Bernard calcimeter method. Geoderma Reg. 2021, 25, e00432. [Google Scholar] [CrossRef]
- De Vos, B.; Lettens, S.; Muys, B.; Deckers, J.A. Walkley-Black analysis of forest soil organic carbon: Recovery, limitations and uncertainty. Soil Use Manag. 2007, 23, 221–229. [Google Scholar] [CrossRef]
- Kacar, B. Soil Analyses; Nobel Publishing: Ankara/Türkiye, 2009. [Google Scholar]
- Kraska, P.; Andruszczak, S.; Kwiecińska-Poppe, E.; Staniak, M.; Różyło, K.; Rusecki, H. Supporting crop and different row spacing as factors influencing weed infestation in lentil crop and seed yield under organic farming conditions. Agronomy 2020, 10, 9. [Google Scholar] [CrossRef]
- Togay, N.; Anlarsal, A.E. The effects of different planting densities and sowing methods on yield and yield components of lentil (Lens culinaris Medic.) in Van conditions. Tarım Bilim. Derg. 2008, 18, 35–47. [Google Scholar]
- Dona, W.H.G.; Schoenau, J.J.; King, T. Effect of starter fertilizer in seed-row on emergence, biomass and nutrient uptake by six pulse crops grown under controlled environment conditions. J. Plant Nutr. 2020, 43, 879–895. [Google Scholar] [CrossRef]
- Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research; R Package Version 1.1-8. 2010. Available online: https://cran.r-project.org/web/packages/agricolae/agricolae.pdf (accessed on 25 May 2025).
- Peterson, B.G.; Carl, P.; Boudt, K.; Bennett, R.; Ulrich, J.; Zivot, E.; Lestel, M.; Balkissoon, K.; Wuertz, D. Performance Analytics: Econometric Tools for Performance and Risk Analysis; R Package Version 2.0.4. 2014. Available online: https://cran.r-project.org/web/packages/PerformanceAnalytics/PerformanceAnalytics.pdf (accessed on 11 March 2025).
- Benakanahalli, N.K.; Sridhara, S.; Ramesh, N.; Olivoto, T.; Sreekantappa, G.; Tamam, N.; Abdelbacki, A.M.M.; Elansary, H.O.; Abdelmohsen, S.A.M. A framework for identification of stable genotypes based on MTSI and MGDII indexes: An example in guar (Cymopsis tetragonoloba L.). Agronomy 2021, 11, 1221. [Google Scholar] [CrossRef]
- Olivoto, T.; Lúcio, A.D. metan: An R package for multi-environment trial analysis. Methods Ecol. Evol. 2020, 11, 783–789. [Google Scholar] [CrossRef]
- Karademir, E.; Karademir, Ç.; Ekinci, R.; Karahan, H. Determining the appropriate cotton varieties for second crop cultivation in the Southeastern Anatolia Region. Çukurova J. Agric. Food Sci. 2017, 21, 119–126. (In Turkish) [Google Scholar]
- Wang, Y.; Tao, H.; Tian, B.; Sheng, D.; Xu, C.; Zhou, H.; Huang, S.; Wang, P. Flowering dynamics, pollen, and pistil contribution to grain yield in response to high temperature during maize flowering. Environ. Exp. Bot. 2019, 158, 80–88. [Google Scholar] [CrossRef]
- Kuzbakova, M.; Khassanova, G.; Oshergina, I.; Ten, E.; Jatayev, S.; Yerzhebayeva, R.; Bulatova, K.; Khalbayeva, S.; Schramm, C.; Anderson, P.; et al. Height to first pod: A review of genetic and breeding approaches to improve combine harvesting in legume crops. Front. Plant Sci. 2022, 13, 948099. [Google Scholar] [CrossRef] [PubMed]
- Silva-Perez, V.; Shunmugam, A.S.K.; Rao, S.; Cossani, C.M.; Tefera, A.T.; Fitzgerald, G.J.; Armstrong, R.; Rosewarne, G.M. Breeding has selected for architectural and photosynthetic traits in lentils. Front. Plant Sci. 2022, 13, 925987. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Ryan, M.H.; Lambers, H.; Siddique, K.H.M. Phosphorus acquisition and utilisation in crop legumes under global change. Curr. Opin. Plant Biol. 2018, 45, 648379. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Showalter, A.M.; Zhou, D. Potassium nutrition and drought stress in plants: Current status and perspectives. Front. Plant Sci. 2020, 11, 543. [Google Scholar] [CrossRef]
- Tariq, A.; Liu, Y.; Han, C.; Xie, Z.; Chen, M.; Abbas, F.; Xie, Y. Combined application of phosphorus and potassium improves drought tolerance in chickpea (Cicer arietinum L.) by enhancing photosynthesis and antioxidant enzyme activities. Plants 2021, 10, 796. [Google Scholar] [CrossRef]
- Berzsenyi, Z.; Dang, Q.L. Effect of various crop production factors on the yield and yield stability of maize in a long-term experiment. Cereal Res. Commun. 2008, 36, 167–176. [Google Scholar] [CrossRef]
- Ceritoglu, M.; Erman, M. Effect of vermicompost application at different sowing dates on some phenological, agronomic and yield traits in lentil. J. Int. Environ. Appl. Sci. 2020, 15, 158–166. [Google Scholar]
- Ceritoglu, M.; Erman, M.; Çığ, F. Seed priming boosts plant growth, yield attributes, seed chemical and antioxidant composition in lentil under low-phosphorus field conditions. Int. J. Plant Prod. 2024, 18, 513–530. [Google Scholar] [CrossRef]
- Alexandru, A.M.; Mihai, G.; Stoica, E.; Curtu, A.L. Multi-trait selection and stability in norway spruce (Picea abies) provenance trials in Romania. Forests 2023, 14, 456. [Google Scholar] [CrossRef]
- Behera, P.; Singode, A.; Bhat, B.V.; Ronda, V.; Borah, N.; Verma, H.; Gogoi, L.R.; Borah, J.; Majhi, P.K.; Saharia, N.; et al. Genetic gains in forage sorghum for adaptive traits for non-conventional areas through multi-trait-based stability selection methods. Front. Plant Sci. 2024, 15, 1248663. [Google Scholar] [CrossRef]
- Olivoto, T.; Nardino, M. MGIDI: Toward an effective multivariate selection in biological experiments. Bioinformatics 2021, 37, 1383–1389. [Google Scholar] [CrossRef] [PubMed]

| Year | Sand | Silt | Clay | T | pH | EC | Lime | OM | P | K | Ca | Mg | Zn | Mn | Fe | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | dS m- | % | % | kg ha- | kg ha- | mg kg- | mg kg- | mg kg- | mg kg- | mg kg- | mg kg- | |||||
| 2019–2020 | 46 | 22 | 32 | SCL | 7.8 | 0.19 | 22.7 | 1.47 | 11.3 | 156.1 | 2126 | 56.9 | 0.57 | 20.71 | 2.95 | 0.72 |
| 2020–2021 | 54 | 23 | 23 | SIL | 7.3 | 0.22 | 1.88 | 1.14 | 3.49 | 81.1 | 2347 | 57.4 | 0.61 | 21.30 | 2.56 | 0.69 |
| 2021–2022 | 36 | 33 | 31 | L | 8.0 | 0.16 | 8.44 | 1.55 | 3.37 | 55.1 | 2299 | 57.0 | 0.60 | 20.93 | 2.68 | 0.74 |
| Trait | Best Genotypes | Worst Genotypes |
|---|---|---|
| Flowering time (day) | G3715 (126.2) followed by G3716 (126.8) and G87 (127.3) | G37 (146.8) followed by G21151 (142.7) and 3840 (140.3) |
| Podding time (day) | G3715 (140.5) followed by G3716 (140.7) and G3744 (142.0) | G37 (154.2) followed by G21151 (151.5) and 3840 (149.7) |
| Maturity time (day) | G3744 (165.7) followed by G3716 (167.0) and G3715 (167.2) | G21151 (180.0) followed by G37 (179.0) and G3673 (177.7) |
| Plant height (cm) | G37 (43.4) followed by G21151 (42.9) and G3653 (40.8) | G3761 (30.5) followed by G3710 (30.7) and G3839 (31.1) |
| Number of branches | G21151 (11.8) followed by G37 (11.8) and G3697 (10.8) | G3715 (7.55) followed by G3710 (7.58) and G3828 (7.92) |
| First pod height (cm) | G21151 (18.9) followed by G37 (17.3) and G3771 (15.3) | G3715 (8.49) followed by G3839 (9.9) and G3662 (10.1) |
| Number of pods | G3840 (104.6) followed by G3673 (99.3) and G3819 (93.8) | G3780 (31.6) followed by G3759 (42.6) and G3716 (43.5) |
| Biological yield (kg ha−1) | G3837 (7109) followed by G3771 (6540) and G3840 (6470) | G3713 (3580) followed by G3839 (3681) and G3743 (3701) |
| Grain yield (kg ha−1) | G3771 (2579) followed by G3658 (2203) and G3673 (2054) | G3839 (743) followed by G3828 (744) and G3713 (862) |
| Harvest index (%) | G3771 (37.3) followed by G35 (36.2) and G3673 (35.3) | G3703 (18.2) followed by G21151 (18.3) and G3828 (19.1) |
| 1000-seed weight (g) | G3771 (54.9) followed by G3780 (53.6) and G3750 (48.4) | G3710 (21.0) followed by G3839 (22.6) and G3828 (22.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceritoglu, M.; Çığ, F.; Erman, M.; Ceritoglu, F. Integrating Multi-Trait Selection Indices for Climate-Resilient Lentils: A Three-Year Evaluation of Earliness and Yield Stability Under Semi-Arid Conditions. Agronomy 2025, 15, 1554. https://doi.org/10.3390/agronomy15071554
Ceritoglu M, Çığ F, Erman M, Ceritoglu F. Integrating Multi-Trait Selection Indices for Climate-Resilient Lentils: A Three-Year Evaluation of Earliness and Yield Stability Under Semi-Arid Conditions. Agronomy. 2025; 15(7):1554. https://doi.org/10.3390/agronomy15071554
Chicago/Turabian StyleCeritoglu, Mustafa, Fatih Çığ, Murat Erman, and Figen Ceritoglu. 2025. "Integrating Multi-Trait Selection Indices for Climate-Resilient Lentils: A Three-Year Evaluation of Earliness and Yield Stability Under Semi-Arid Conditions" Agronomy 15, no. 7: 1554. https://doi.org/10.3390/agronomy15071554
APA StyleCeritoglu, M., Çığ, F., Erman, M., & Ceritoglu, F. (2025). Integrating Multi-Trait Selection Indices for Climate-Resilient Lentils: A Three-Year Evaluation of Earliness and Yield Stability Under Semi-Arid Conditions. Agronomy, 15(7), 1554. https://doi.org/10.3390/agronomy15071554







