Exogenous Sugar Alcohols Enhance Peach Seedling Growth via Modulation of Rhizosphere Bacterial Communities
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Growth Conditions
2.2. Treatments for the Pot Experiment
2.3. Collection of Rhizosphere Soil Samples
2.4. Rhizosphere Soil Physicochemical Properties
2.5. Determination of Peach Seedling Growth Indexes
2.6. Determination of Leaf Photosynthetic and Nutrient Index
2.7. DNA Extraction, PCR Amplification and ILLUMINA Miseq Sequencing
2.8. Statistical Analysis
3. Results
3.1. Rhizosphere Soil Physicochemical Properties and Peach Seedling Growth
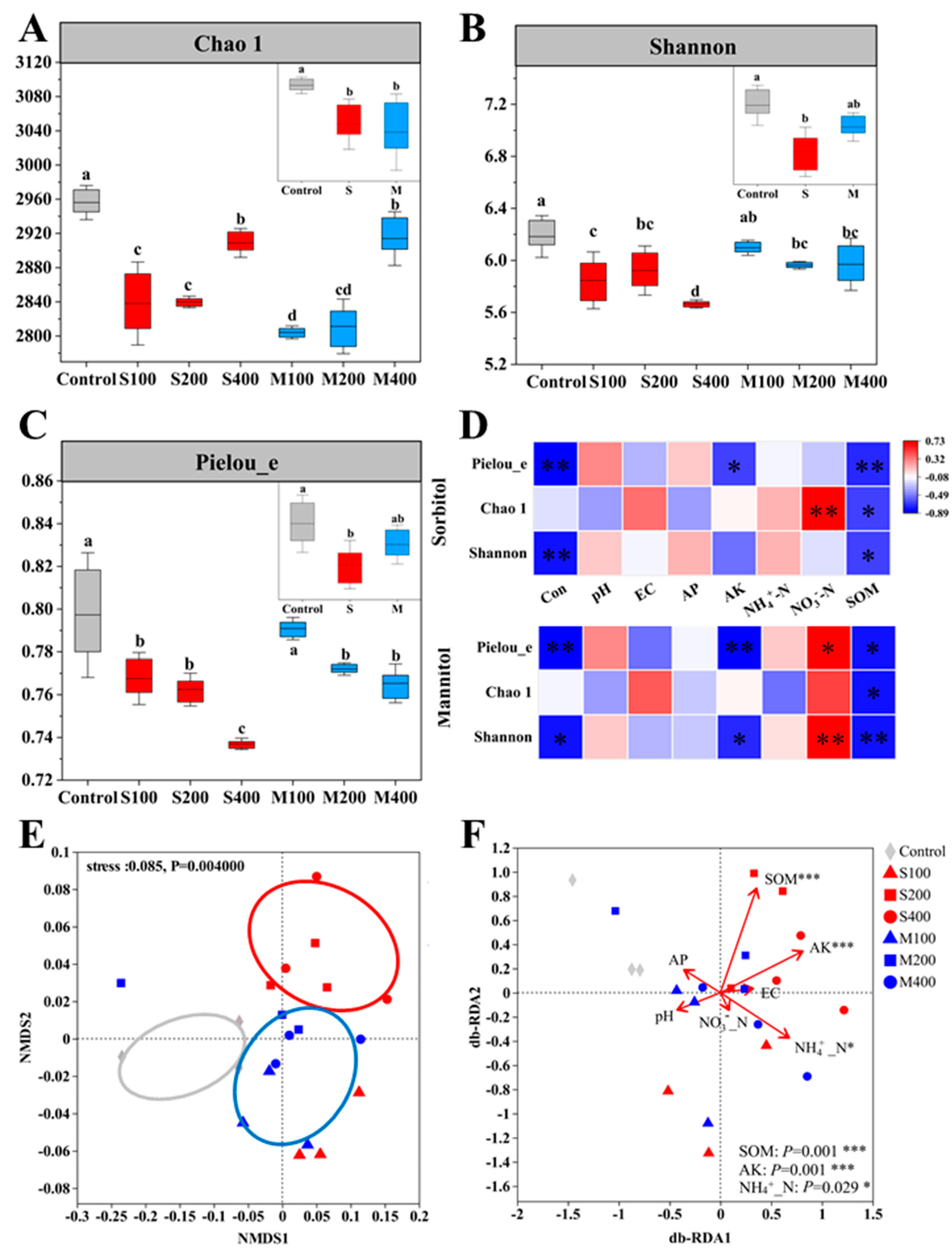
3.2. Diversity of Rhizosphere Bacteria
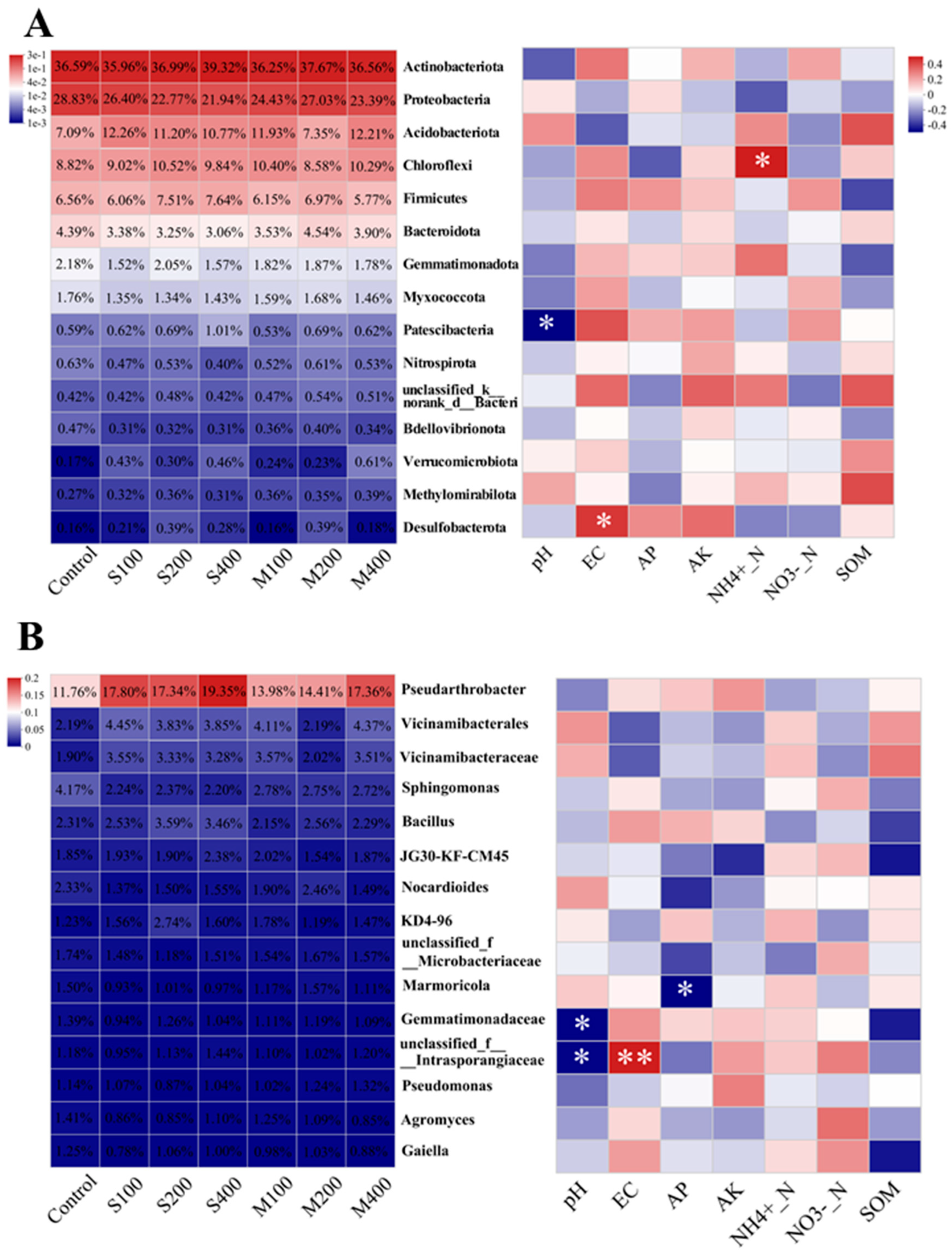
3.3. Rhizosphere Bacterial Composition
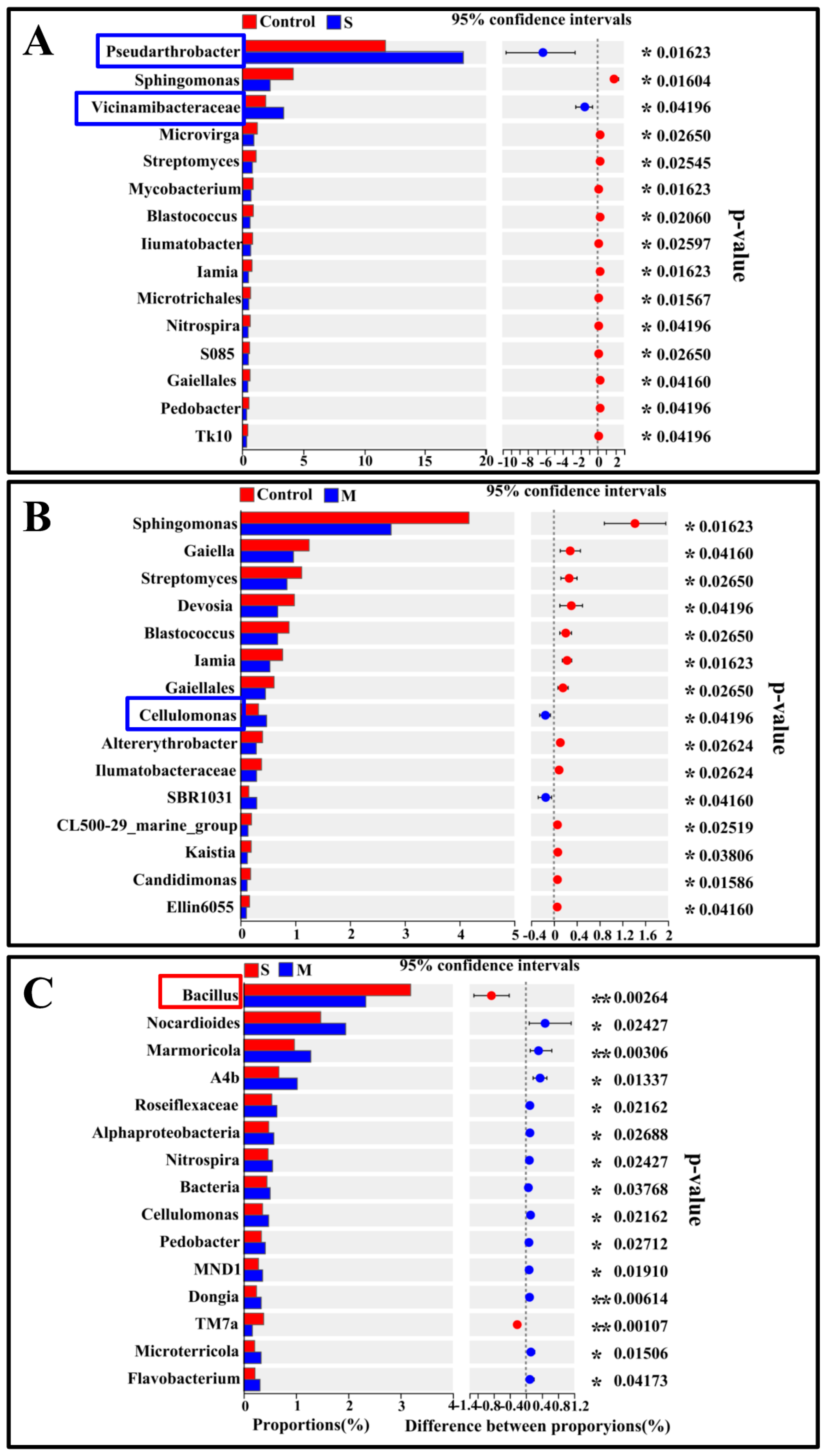
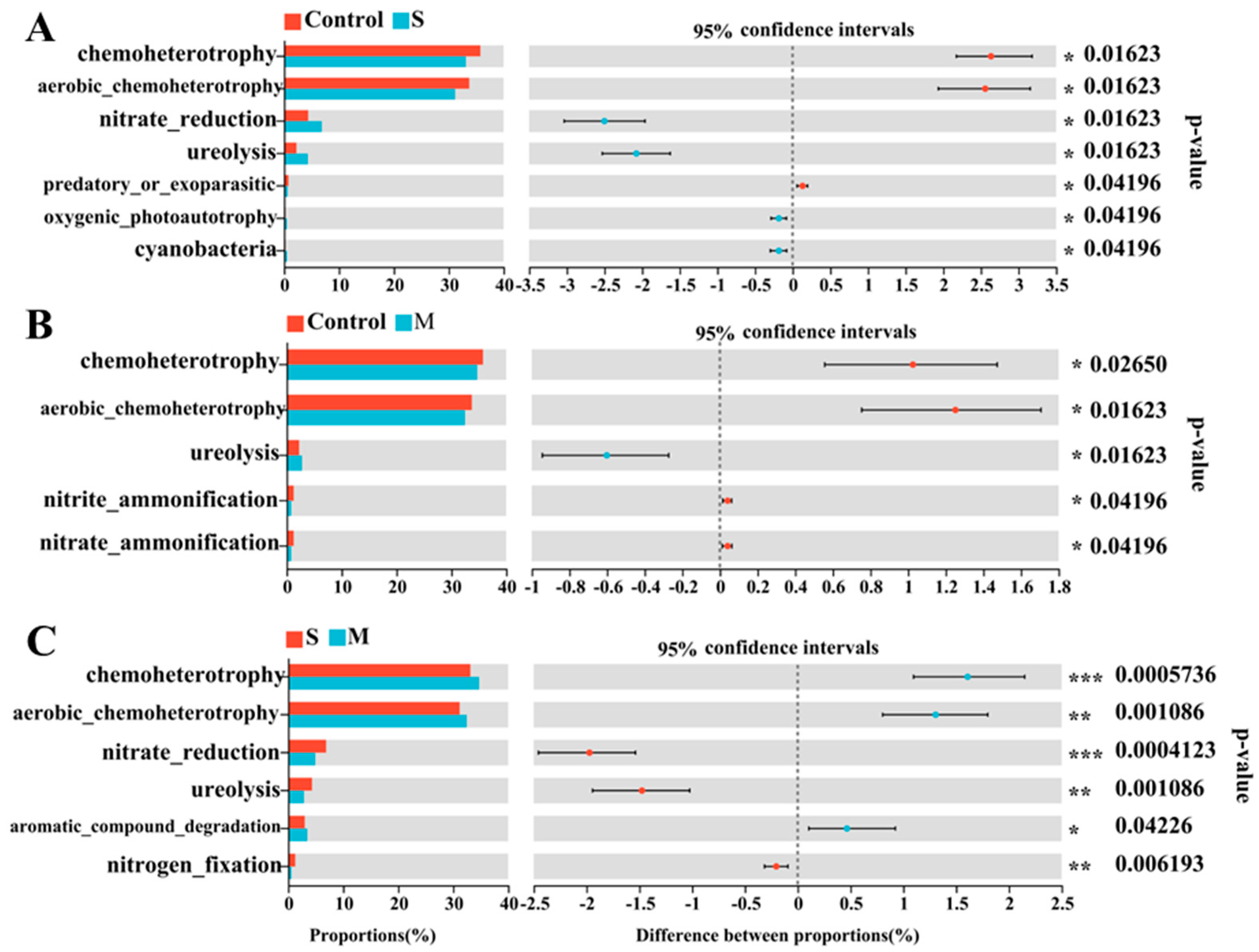
3.4. Difference Analysis and Functional Prediction of Rhizosphere Bacteria
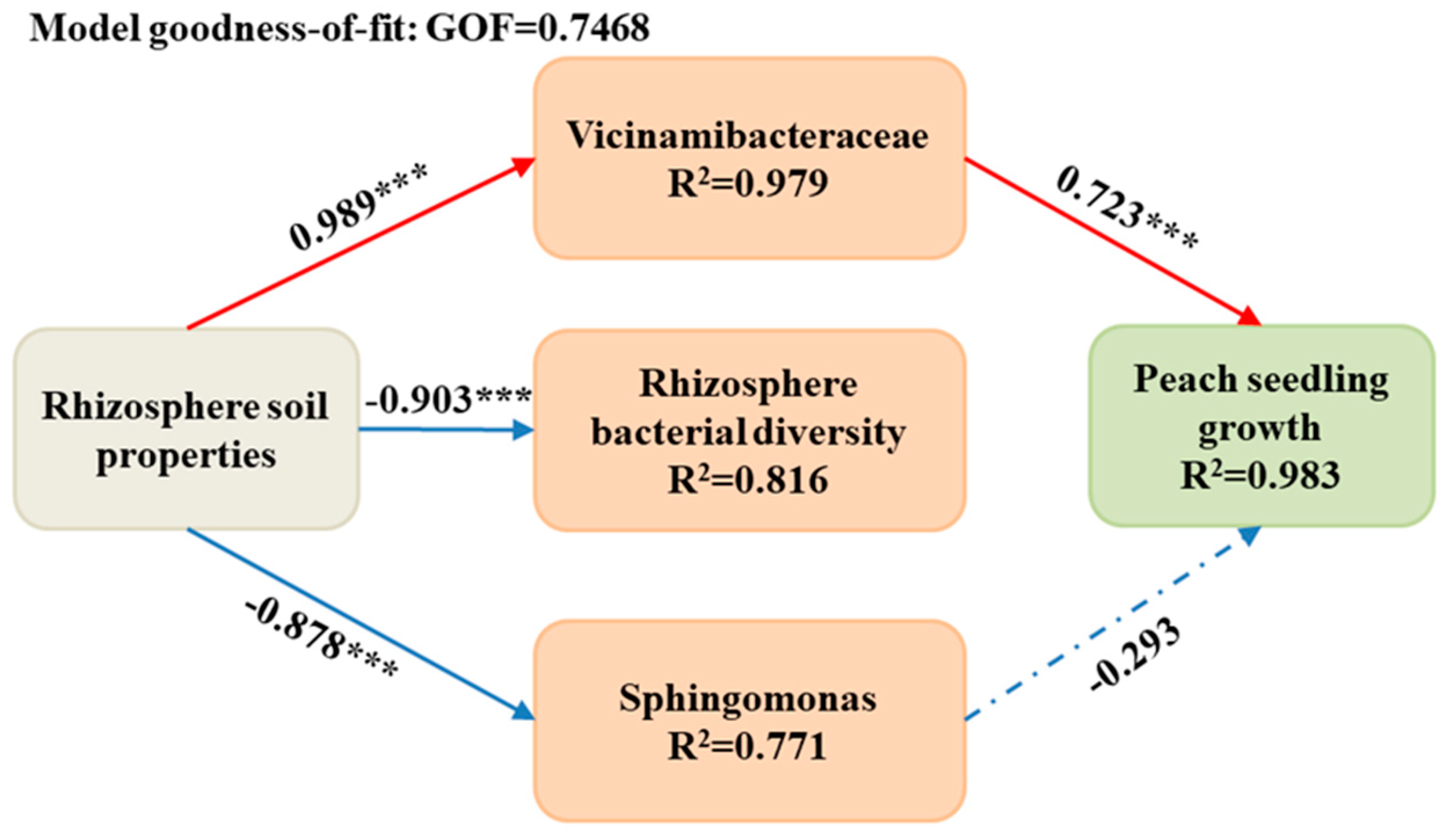
3.5. Correlation Analysis of Rhizosphere Bacteria
4. Discussion
4.1. Sorbitol and Mannitol Altered Rhizosphere Soil Properties and Promoted Peach Seedling Growth
4.2. Sorbitol and Mannitol Assembled Rhizosphere Bacterial Community via Affecting the Rhizosphere Environment of Peach Seedlings
4.3. Sorbitol and Mannitol Promote Peach Seedling Growth Through Affecting the Rhizosphere Bacterial Community Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| S | Sorbitol |
| M | Mannitol |
| S100 | Mixed with 100 mg/kg sorbitol and KH2PO4 |
| S200 | Mixed with 200 mg/kg sorbitol and KH2PO4 |
| S400 | Mixed with 400 mg/kg sorbitol and KH2PO4 |
| M100 | Mixed with 100 mg/kg mannitol and KH2PO4 |
| M200 | Mixed with 200 mg/kg mannitol and KH2PO4 |
| M400 | Mixed with 400 mg/kg mannitol and KH2PO4 |
| SOM | Soil organic matter |
| NO3−-N | Nitrate nitrogen |
| NH4+-N | Ammonium nitrogen |
| AP | Available phosphorus |
| AK | Available potassium |
| EC | Electrical conductivity |
| RA | Root activity |
| TTC | Triphenyl tetrazolium chloride |
| RV | Root volume |
| FRW | Fresh root weight |
| FLW | Leaf weight |
| PW | Plant weight |
| PH | Plant height |
| LA | Leaf area |
| SPAD | The relative content of chlorophyll |
| Ci | Intercellular CO2 concentration |
| Gs | Stomatal conductance |
| VPD | Saturated water vapor pressure difference |
| A | Net photosynthetic rate |
| E | Transpiration rate |
| LN | Leaf nitrogen |
| LP | Leaf phosphorus |
| LK | Leaf potassium |
| SEM | Structural equation model |
References
- Xu, L.; Chen, C. Economic situation and development countermeasures of Chinese peach industry. J. Fruit Sci. 2023, 40, 133–143. (In Chinese) [Google Scholar] [CrossRef]
- Li, G.M.; Peng, F.T.; Xiao, Y.S.; Zhang, H.M.; Wang, Z.Y.; Wang, Z.T. Evaluation of soil nutrient status and N, P load risk in peach orchards atmountainous area in mid-Shandong. J. Shandong Agric. Univ. (Nat. Sci.) 2011, 42, 392–400. (In Chinese) [Google Scholar]
- Yan, K.F.; Peng, F.T.; Fang, L.; Li, Y.; Zhang, H.M. Effects of different irrigation and fertilizer methods on soil moisture, nutrient and plant growth in peach orchard. J. Soil Water Conserv. 2013, 27, 160–164+283. [Google Scholar] [CrossRef]
- Yang, G.; Wang, Y.; Wang, S.Q.; Zhao, X. Legume cover with optimal nitrogen management and nitrification inhibitor enhanced net ecosystem economic benefits of peach orchard. Sci. Total Environ. 2023, 873, 162141. [Google Scholar] [CrossRef]
- Li, Y.Y.; Lv, Y.; Peng, F.T.; Xiao, Y.S. Effect of glycine combined with urea on the absorption, transportation and transformation of nitrogen in wild peach seedlings (Amygdalu spersica (L.) Batsch). Sci. Hortic. 2023, 308, 111549. [Google Scholar] [CrossRef]
- Chen, X.S.; Guo, W.W.; Xu, J.; Cong, P.H.; Wang, L.R.; Liu, C.H.; Li, X.Y.; Wu, S.J.; Yao, Y.X.; Chen, X.L. Genetic improvement and promotion of fruit quality of main fruit trees. Sci. Agric. Sin. 2015, 48, 3524–3540. (In Chinese) [Google Scholar] [CrossRef]
- Liu, H.M.; Yu, H.L.; Shao, W.; Xu, B.B.; Zhang, Z.H.; Shi, Z.Y.; Zhao, X.F.; Xu, G.Y.; Yang, J.H.; Si, P. Effects of sorbitol and mannitol combined with NPK on the growth, fruit quality and nutrient absorption of peach. J. Fruit Sci. 2021, 38, 911–921. (In Chinese) [Google Scholar] [CrossRef]
- Yu, H.L.; Shao, W.; Xu, G.Y.; Xie, N.; Yang, X.J.; Gao, D.T.; Si, P. Soil amendment with sorbitol and mannitol changes the soil microbial community and its enzymatic activities. J. Soils Sediments 2022, 23, 1857–1876. [Google Scholar] [CrossRef]
- Morandi, B.; Corelli, G.L.; Rieger, M.; Lo, B.R. Carbohydrate availability affects growth and metabolism in peach fruit. Physiol. Plant. 2008, 133, 229–241. [Google Scholar] [CrossRef]
- Li, C.; Meng, D.; Piñeros, M.A.; Mao, Y.; Dandekar, A.M.; Cheng, L. A sugar transporter takes up both hexose and sucrose for sorbitol-modulated in vitro pollen tube growth in apple. Plant Cell 2020, 32, 449–469. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Shiraz, M.; Mir, A.R.; Alam, P.; Hayat, S. Comparative study of stress generated by osmolytes on the growth, photosynthesis and metabolic responses in Nigella sativa. Biocatal. Agric. Biotechnol. 2023, 52, 102818. [Google Scholar] [CrossRef]
- Fan, L.; Zhao, J.G. The current status and prospect of potassium dihydrogen phosphate in China. Phosphate Compd. Fertil. 2006, 21, 34–37. (In Chinese) [Google Scholar]
- Li, T.; Liu, H.H.; Zhang, Y.P.; Chen, Q.Y.; Zuo, B.B. Study on regulation of KH2PO4 on senescence mechanism of the leaves in muskmelon fruiting nodes. J. Hebei Agric. Univ. 2018, 41, 61–66. (In Chinese) [Google Scholar] [CrossRef]
- Qu, Q.; Zhang, Z.; Peijnenburg, W.J.; Liu, W.; Lu, T.; Hu, B.; Chen, J.; Chen, J.; Lin, Z.; Qian, H. Rhizosphere Microbiome Assembly and Its Impact on Plant Growth. J. Agric. Food Chem. 2020, 68, 5024–5038. [Google Scholar] [CrossRef]
- Fu, X.M.; Mao, X.M.; Yang, H.X.; Long, C.R.; Liu, H.M.; Du, Y.X.; Dong, M.C.; Yue, J.Q.; Li, J.X. Review on rhizosphere microorganisms of fruit trees. China Fruits 2021, 217, 5–9. (In Chinese) [Google Scholar] [CrossRef]
- Jones, D.L.; Hinsinger, P. The rhizosphere: Complex by design. Plant Soil 2008, 312, 1–6. [Google Scholar] [CrossRef]
- Ling, N.; Wang, T.; Kuzyakov, Y. Rhizosphere bacteriome structure and functions. Nat. Commun. 2022, 13, 836. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-Growth-Promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Lu, L.; Liu, N.; Fan, Z.; Liu, M.; Zhang, X.; Tian, J.; Yu, Y.; Lin, H.; Huang, Y.; Kong, Z. A novel PGPR strain, Streptomyces lasalocidi JCM 3373T, alleviates salt stress and shapes root architecture in soybean by secreting indole-3-carboxaldehyde. Plant Cell Environ. 2024, 47, 1941–1956. [Google Scholar] [CrossRef]
- Qiao, Y.Z.; Wang, Z.D.; Sun, H.; Guo, H.Y.; Song, Y.; Zhang, H.; Ruan, Y.; Xu, Q.C.; Huang, Q.W.; Shen, Q.R.; et al. Synthetic community derived from grafted watermelon rhizosphere provides protection for ungrafted watermelon against via microbial synergistic effects. Microbiome 2024, 12, 101. [Google Scholar] [CrossRef]
- Al-Turki, A.; Murali, M.; Omar, A.F.; Rehan, M.; Sayyed, R.Z. Recent advances in PGPR-mediated resilience toward interactive effects of drought and salt stress in plants. Front. Microbiol. 2023, 14, 1214845. [Google Scholar] [CrossRef] [PubMed]
- Hermans, S.M.; Buckley, H.L.; Case, B.S.; Curran-Cournane, F.; Taylor, M.; Lear, G. Using soil bacterial communities to predict physico-chemical variables and soil quality. Microbiome 2020, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Marasco, R.; Rolli, E.; Fusi, M.; Michoud, G.; Dafonchio, D. Grapevine rootstocks shape underground bacterial microbiome and networking but not potential functionality. Microbiome 2018, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; Xun, M.; Shi, J.Y.; Chen, B.; Cheng, Y.J.; Zhang, W.W.; Yang, H.Q. Root 1-aminocyclopropane-l-carboxylic acid (ACC) and rhizosphere ACC deaminase-producing bacteria affect apple root architecture under soil compaction stress. Plant Soil 2023, 489, 629–643. [Google Scholar] [CrossRef]
- Fanin, N.; Bertrand, I. Aboveground litter quality is a better predictor than belowground microbial communities when estimating carbon mineralization along a land-use gradient. Soil Biol. Biochem. 2016, 94, 48–60. [Google Scholar] [CrossRef]
- David, J.K. A Primer on Partial Least Squares Structural Equation Modeling. Long Range Plan. 2013, 46, 184–185. [Google Scholar] [CrossRef]
- Xu, H.; Yang, H.S.; Xu, Y.; Mao, Z.Q.; Su, H.R. Research progress on rhizosphere environment of fruit trees. J. Shandong Agric. Univ. (Nat. Sci.) 2004, 35, 476–480. (In Chinese) [Google Scholar]
- Ho, S.L.; Chao, Y.C.; Tong, W.F.; Yu, S.M. Sugar coordinately and differentially regulates growth-and stress-related gene expression via a complex signal transduction network and multiple control mechanisms. Plant Physiol. 2001, 125, 877–890. [Google Scholar] [CrossRef]
- Prayogo, C.; Jones, J.E.; Baeyens, J.; Bending, G.D. Impact of biocharon mineralisation of C and N from soil and willow litter and its relationship with microbial community biomass and structure. Biol. Fertil. Soils 2014, 50, 695–702. [Google Scholar] [CrossRef]
- Tonon, T.; Li, Y.; McQueen, M.S. Mannitol biosynthesis in algae: More widespread and diverse than previously thought. New Phytol. 2017, 213, 1573–1579. [Google Scholar] [CrossRef]
- Zhou, Z.; Tran, P.Q.; Kieft, K.; Anantharaman, K. Genome diversification in globally distributed novel marine Proteobacteria is linked to environmental adaptation. ISME J. 2020, 14, 2060–2077. [Google Scholar] [CrossRef] [PubMed]
- Mechri, B.; Tekaya, M.; Attia, F.; Hammami, M.; Chehab, H. Drought stress improved the capacity of Rhizophagus irregularis for inducing the accumulation of oleuropein and mannitol in olive (Olea europaea) roots. Plant Physiol. Biochem. 2020, 156, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.L.; Yang, W.J.; Gao, D.T.; Wei, Z.F.; Yu, H.L. Effects of Sorbitol and Sucrose on Soluble Sugar Content of Peach Fruits and Leaves and Fruits Quality. J. Henan Agric. Sci. 2019, 48, 110–116. (In Chinese) [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Essitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Liu, L.L.; Huang, X.Q.; Zhang, J.B.; Cai, Z.C.; Jiang, K.; Chang, Y.Y. Deciphering the relative importance of soil and plant traits on the development of rhizosphere microbial communities. Soil Biol. Biochem. 2020, 148, 107909. [Google Scholar] [CrossRef]
- Vieira, S.; Sikorski, J.; Dietz, S.; Herz, K.; Schrumpf, M.; Bruelheide, H.; Scheel, D.; Friedrich, M.W.; Overmann, J. Drivers of the composition of active rhizosphere bacterial communities in temperate grasslands. ISME J. 2020, 14, 463–475. [Google Scholar] [CrossRef]
- Cao, H.; Jia, M.F.; Xun, M.; Wang, X.S.; Chen, K.; Yang, H.Q. Nitrogen transformation and microbial community structure varied in apple rhizosphere and rhizoplane soils under biochar amendment. J. Soils Sediments 2021, 21, 853–868. [Google Scholar] [CrossRef]
- Wang, M.; Qi, X.; Shi, Y.; Zhao, J.; Ahmad, S.; Akhtar, K.; Chen, B.; Lian, T.; He, B.; Wen, R. Sugarcane straw returning is an approaching technique for the improvement of rhizosphere soil functionality, microbial community, and yield of different sugarcane cultivars. Front. Microbiol. 2023, 14, 1133973. [Google Scholar] [CrossRef]
- Gao, J.L.; Luo, Y.; Wei, Y.L.; Huang, Y.L.; Zhang, H.; He, W.L.; Sheng, H.M.; An, L.Z. Effect of aridity and dune type on rhizosphere soil bacterial communities of Caragana microphylla in desert regions of northern China. PLoS ONE 2019, 14, e0224195. [Google Scholar] [CrossRef]
- Kalinowski, T.; Halden, R.U. Can stress enhance phytoremediation of polychlorinated biphenyls? Environ. Eng. Sci. 2012, 29, 1047–1052. [Google Scholar] [CrossRef]
- Kristensen, J.M.; Singleton, C.; Clegg, L.A.; Petriglieri, F.; Nielsen, P.H. High Diversity and Functional Potential of Undescribed “Acidobacteriota” in Danish Wastewater Treatment Plants. Front. Microbiol. 2021, 12, 643950. [Google Scholar] [CrossRef] [PubMed]
- Huber, K.J.; Overmann, J. Vicinamibacteraceae fam. nov., the first described family within the subdivision 6 Acidobacteria. Int. J. Syst. Evol. Microbiol. 2018, 68, 2331–2334. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nature reviews. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef]
- Orellana, L.H.; Francis, T.B.; Ferraro, M.; Hehemann, J.H.; Fuchs, B.M.; Amann, R.I. Verrucomicrobiota are specialist consumers of sulfated methyl pentoses during diatom blooms. ISME J. 2022, 16, 630–641. [Google Scholar] [CrossRef]
- Zhu, B.L.; Karwautz, C.; Andrei, S.; Klingl, A.; Pernthaler, J.; Lueders, T. A novel Methylomirabilota methanotroph potentially couples methane oxidation to iodate reduction. mLife 2022, 1, 323–328. [Google Scholar] [CrossRef]
- Magnuson, E.; Altshuler, I.; Freyria, N.J.; Leveille, R.J.; Whyte, L.G. Sulfur-cycling chemolithoautotrophic microbial community dominates a cold, anoxic, hypersaline Arctic Spring. Microbiome 2023, 11, 203. [Google Scholar] [CrossRef]
- Kim, S.K.; Kook, M.; Yan, Z.F.; Trinh, H.; Zheng, S.D.; Yang, J.E.; Park, S.Y.; Yi, T.H. Cellulomonas aurantiaca sp. nov., isolated from a soil sample from a tangerine field. Antonie Leeuwenhoek 2019, 112, 1623–1632. [Google Scholar] [CrossRef]
- Fan, K.; Weisenhorn, P.; Gilbert, J.A.; Chu, H. Wheat rhizosphere harbors a less complex and more stable microbial co-occurrence pattern than bulk soil. Soil Biol. Biochem. 2018, 125, 251–260. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, M.; Zhang, W.; Gardea-Torresdey, J.L.; White, J.C.; Ji, R.; Zhao, L. Silver Nanoparticles Alter Soil Microbial Community Compositions and Metabolite Profiles in Unplanted and Cucumber-Planted Soils. Environ. Sci. Technol. 2020, 54, 3334–3342. [Google Scholar] [CrossRef]
- Jones, S.E.; Elliot, M.A. Streptomyces Exploration: Competition, Volatile Communication and New Bacterial Behaviours. Trends Microbiol. 2017, 25, 522–531. [Google Scholar] [CrossRef]

| Soil Parameters | SOM (g/kg) | NO3−-N (mg/kg) | NH4+-N (mg/kg) | AP (mg/kg) | AK (mg/kg) | PH | EC (μg/cm) |
|---|---|---|---|---|---|---|---|
| 5.32 | 11.36 | 20.26 | 108.33 | 537.67 | 6.7 | 320 |
| Treatment | pH | EC (μg/cm) | SOM (%) | NH4+-N (mg/kg) | NO3−-N (mg/kg) | AP (mg/kg) | AK (mg/kg) |
|---|---|---|---|---|---|---|---|
| Control | 6.77 ± 0.06 a | 436.67 ± 40.41 a | 1.29 ± 0.06 c | 12.87 ± 0.72 d | 3.32 ± 0.30 b | 163.38 ± 10.22 ab | 290.32 ± 24.79 c |
| S100 | 6.83 ± 0.06 a | 350.00 ± 20.00 b | 1.37 ± 0.05 b | 9.79 ± 0.71 e | 2.33 ± 0.38 c | 172.88 ± 14.32 a | 289.43 ± 3.15 c |
| S200 | 6.77 ± 0.06 a | 466.67 ± 30.55 a | 1.35 ± 0.05 bc | 14.85 ± 0.43 bc | 2.20 ± 0.52 c | 170.95 ± 7.03 ab | 308.50 ± 3.28 bc |
| S400 | 6.73 ± 0.06 a | 480.00 ± 26.46 a | 1.36 ± 0.05 b | 13.30 ± 0.48 cd | 4.04 ± 0.33 a | 160.16 ± 14.75 ab | 321.47 ± 14.71 b |
| M100 | 6.83 ± 0.11 a | 376.67 ± 23.09 b | 1.39 ± 0.04 ab | 20.17 ± 2.18 a | 2.86 ± 0.43 bc | 155.34 ± 7.73 ab | 287.22 ± 18.11 c |
| M200 | 6.77 ± 0.06 a | 443.33 ± 20.82 a | 1.45 ± 0.05 a | 12.34 ± 0.98 d | 2.37 ± 0.24 c | 174.81 ± 17.44 a | 350.06 ± 15.98 a |
| M400 | 6.73 ± 0.06 a | 450.00 ± 26.46 a | 1.41 ± 0.03 ab | 15.32 ± 0.71 b | 2.55 ± 0.05 c | 149.54 ± 6.30 b | 370.93 ± 16.91 a |
| Treatment | Principal Component Score 1 | Principal Component Score 2 | Principal Component Score 3 | Comprehensive Score | Rank |
|---|---|---|---|---|---|
| Control | −0.32021433 | 0.574044271 | −0.083375812 | 0.581633519 | 6 |
| S100 | 0.520498199 | 0.031939305 | −0.282340818 | 1.0422688 | 2 |
| S200 | 0.452137836 | −0.095817916 | −0.498359967 | 0.539763013 | 7 |
| S400 | 0.499510368 | 0.217532566 | 0.052109883 | 0.713496678 | 5 |
| M100 | 0.134921768 | −0.515345008 | 0.486990538 | 1.364280697 | 1 |
| M200 | 0.340602508 | 0.181277138 | 0.602579732 | 0.735417346 | 4 |
| M400 | 0.195486651 | 0.561095904 | 0.248232531 | 0.868749089 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Li, J.; Shao, W.; Liu, H.; Dong, R.; Xu, G.; Si, P. Exogenous Sugar Alcohols Enhance Peach Seedling Growth via Modulation of Rhizosphere Bacterial Communities. Agronomy 2025, 15, 1548. https://doi.org/10.3390/agronomy15071548
Yu H, Li J, Shao W, Liu H, Dong R, Xu G, Si P. Exogenous Sugar Alcohols Enhance Peach Seedling Growth via Modulation of Rhizosphere Bacterial Communities. Agronomy. 2025; 15(7):1548. https://doi.org/10.3390/agronomy15071548
Chicago/Turabian StyleYu, Huili, Jiaqi Li, Wei Shao, Huimin Liu, Ruiquan Dong, Guoyi Xu, and Peng Si. 2025. "Exogenous Sugar Alcohols Enhance Peach Seedling Growth via Modulation of Rhizosphere Bacterial Communities" Agronomy 15, no. 7: 1548. https://doi.org/10.3390/agronomy15071548
APA StyleYu, H., Li, J., Shao, W., Liu, H., Dong, R., Xu, G., & Si, P. (2025). Exogenous Sugar Alcohols Enhance Peach Seedling Growth via Modulation of Rhizosphere Bacterial Communities. Agronomy, 15(7), 1548. https://doi.org/10.3390/agronomy15071548






