Different Heat Tolerance of Two Creeping Bentgrass Cultivars Related to Altered Accumulation of Organic Metabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Measurement of Physiological Parameters
2.3. Extraction, Separation, and Measurement of Leaf Metabolites
2.4. Statistical Analysis
3. Results
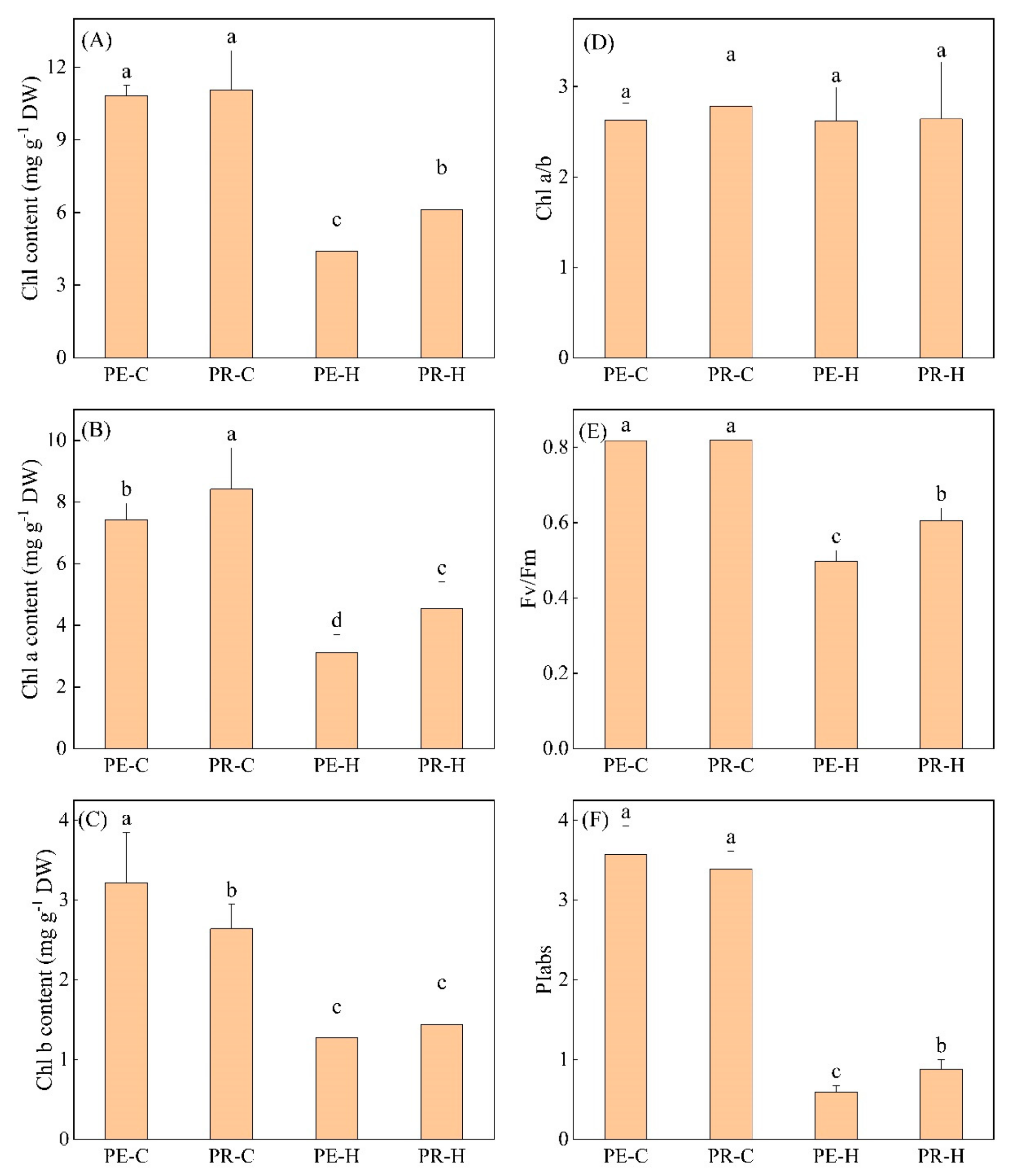
3.1. Physiological Responses of Two Genotypes to High-Temperature Stress
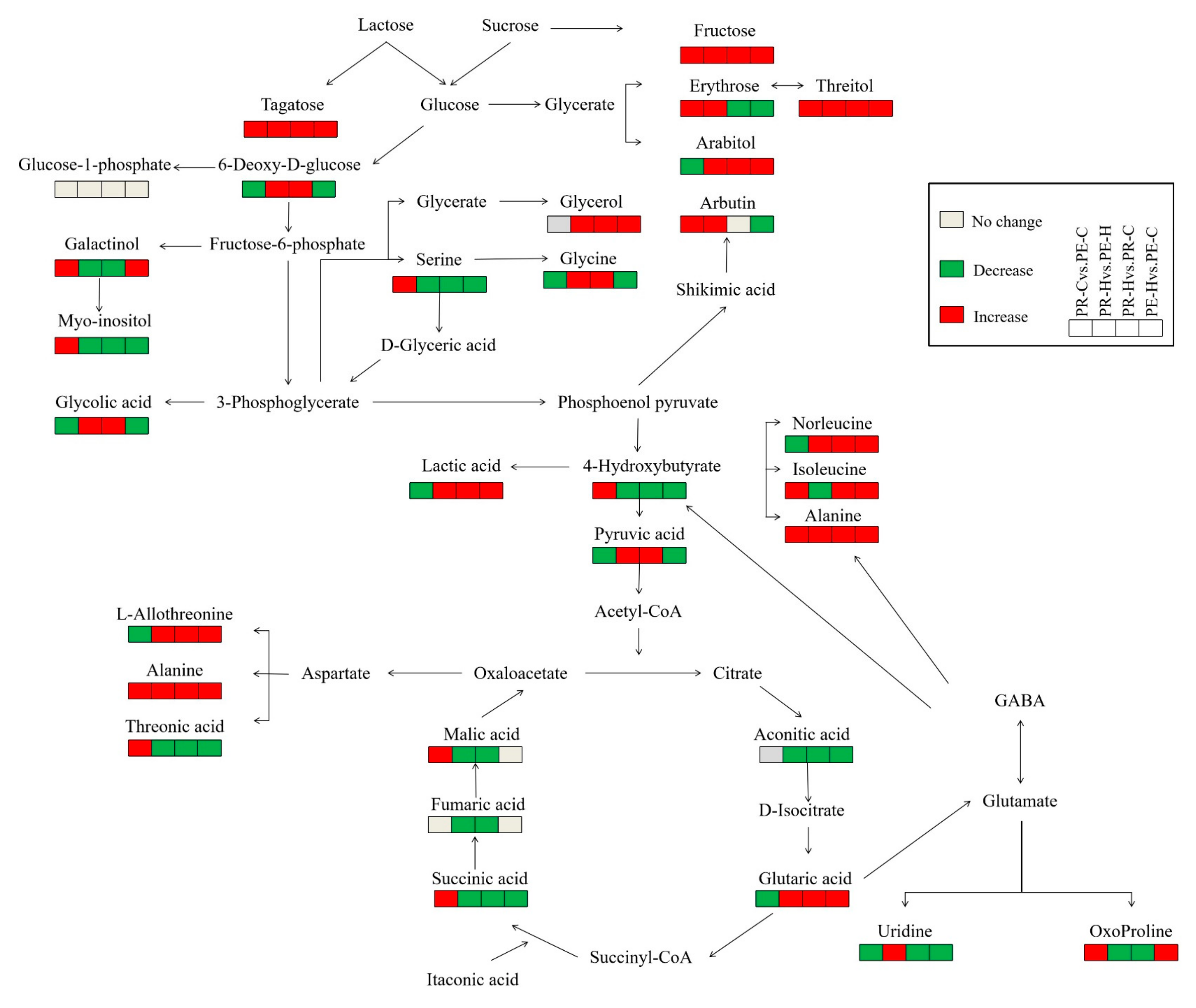
3.2. Change in Metabolomics in the Two Genotypes in Response to Heat Stress
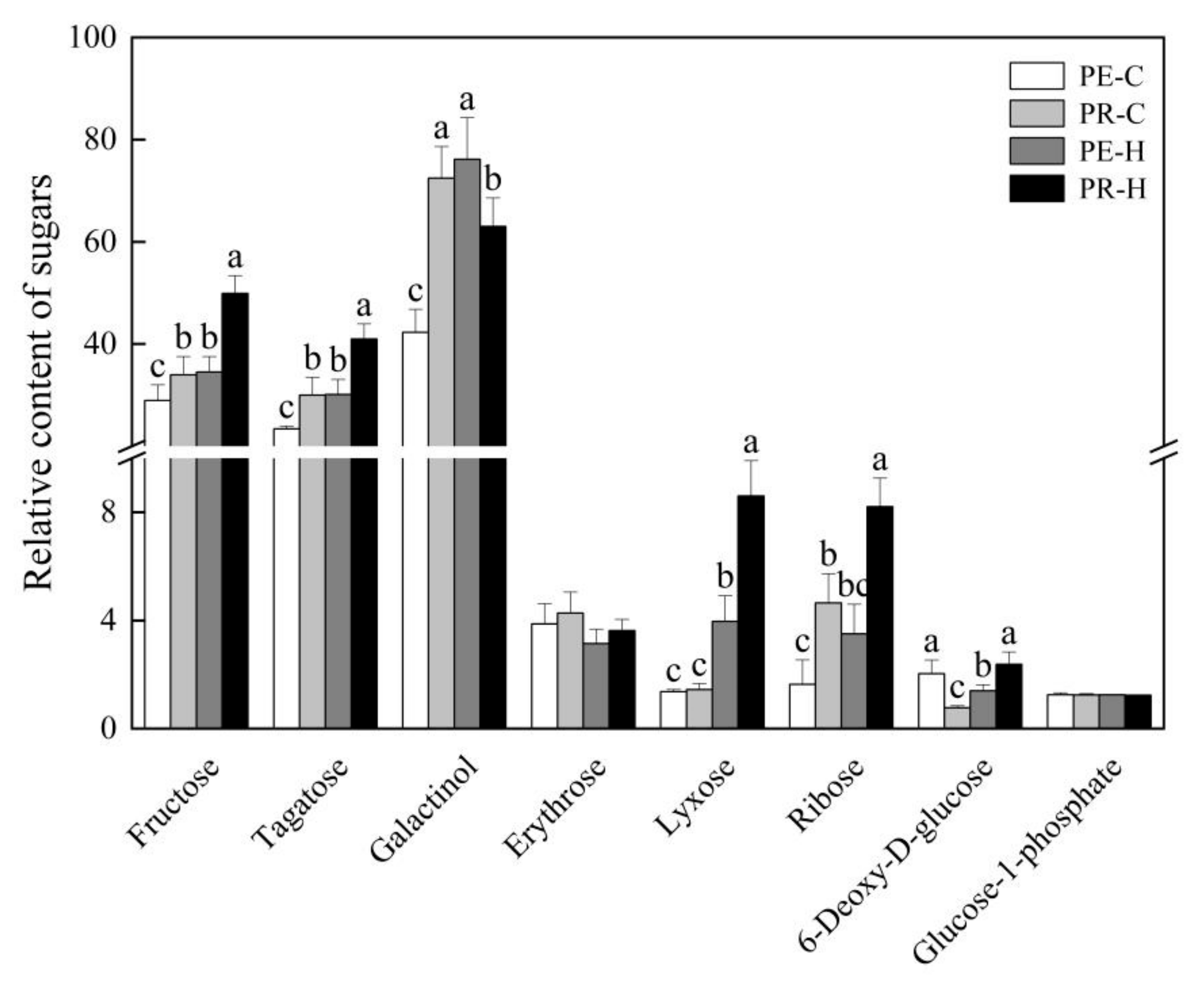
3.3. Changes in the Contents of Carbohydrates and Amino Acids in the Two Genotypes in Response to Heat Stress
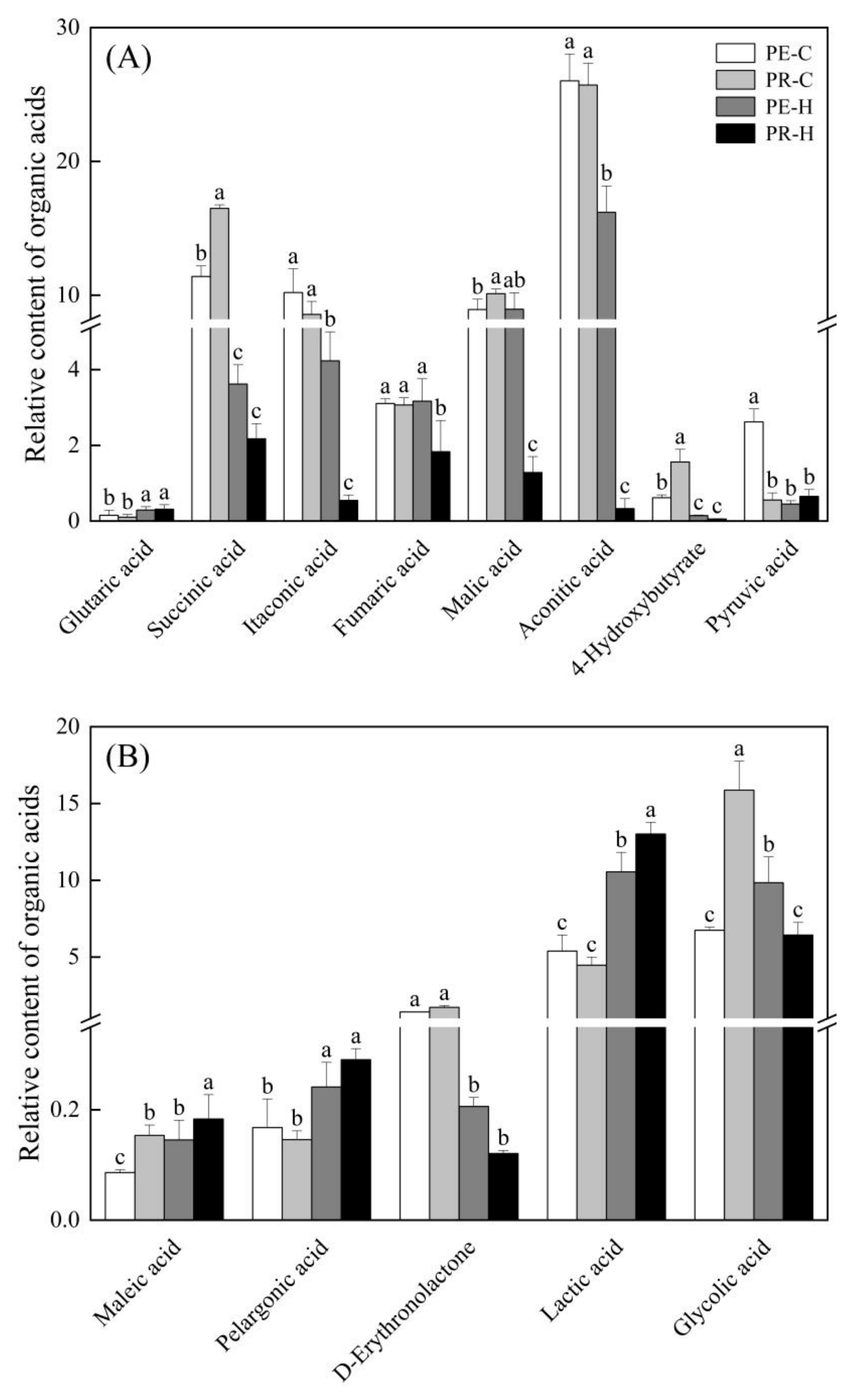
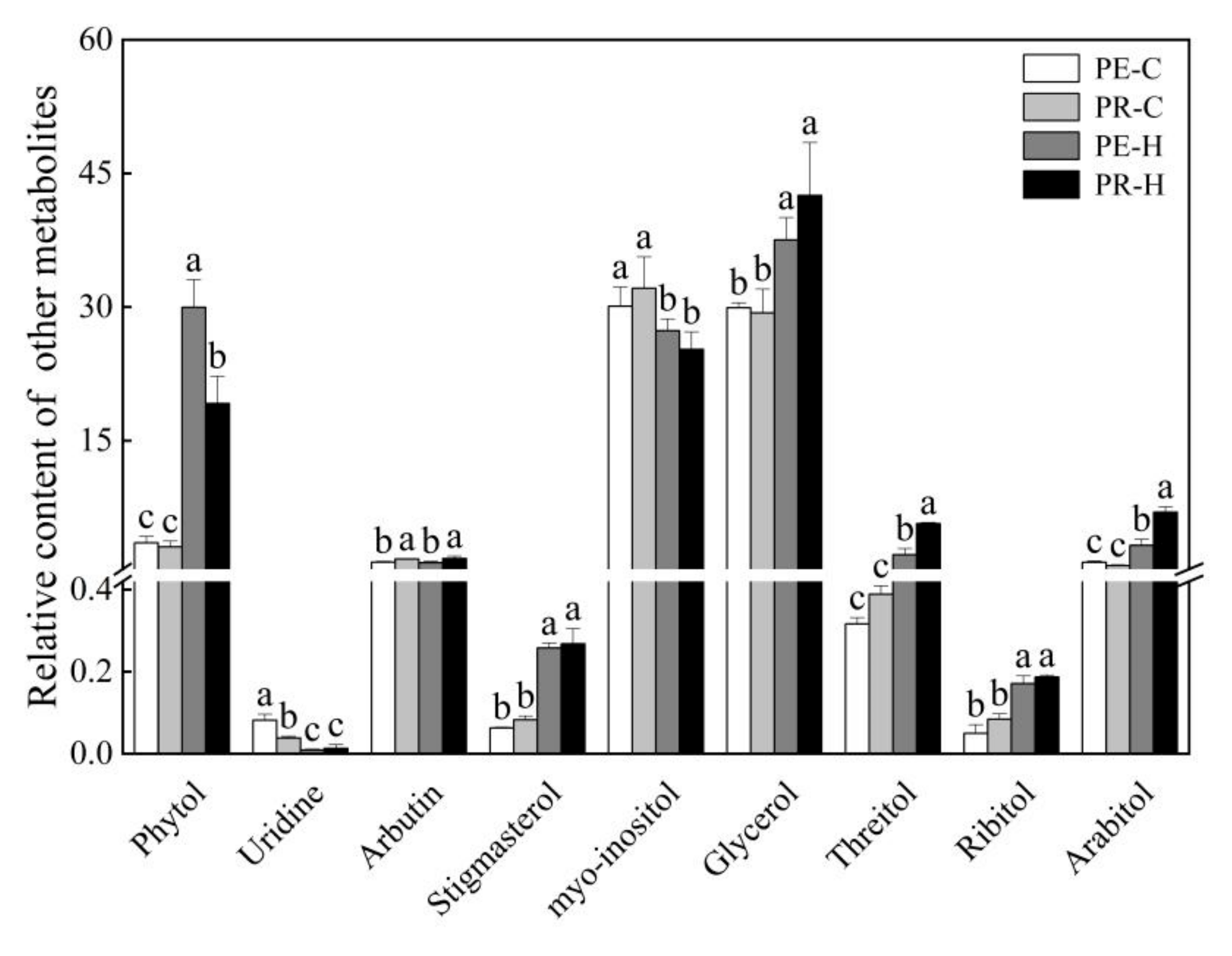
3.4. Changes in the Contents of Organic Acids and Other Metabolites in the Two Genotypes in Response to Heat Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Sun, T.X.; Wang, W.L.; Chan, Z.L. How do cool-season turfgrasses respond to high temperature: Progress and challenges. Grass Res. 2024, 4, e010. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, W.; Cheng, B.; Xu, J.; Han, L.; Peng, Y. Turf quality and physiological responses to summer stress in four creeping bentgrass cultivars in a subtropical zone. Plants 2022, 11, 665. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Qin, B.; He, W.; Chen, Y.; Yin, Y.; Cao, Y.; An, W.; Mu, Z.; Qin, K. Metabolomic and transcriptomic analyses of Lycium barbarum L. under heat stress. Sustainability 2022, 14, 12617. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Chen, H.H.; Murata, N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Biol. 2002, 5, 250–257. [Google Scholar] [CrossRef]
- Liu, X.; Huang, B. Carbohydrate accumulation in relation to heat stress tolerance in two creeping bentgrass cultivars. J. Am. Soc. Hortic. Sci. 2000, 125, 442–447. [Google Scholar] [CrossRef]
- Rossi, S.; Chapman, C.; Yuan, B.; Huang, B. Glutamate acts as a repressor for heat-induced leaf senescence involving chlorophyll degradation and amino acid metabolism in creeping bentgrass. Grass Res. 2021, 1, 4. [Google Scholar] [CrossRef]
- Jiang, Y. Application of gamma-aminobutyric acid and nitric oxide on turfgrass stress resistance: Current knowledge and perspectives. Grass Res. 2023, 3, 3. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.; Peng, Y.; Huang, B. Metabolic pathways regulated by γ-aminobutyric acid (GABA) contributing to heat tolerance in creeping bentgrass (Agrostis stolonifera). Sci. Rep. 2016, 6, 30338. [Google Scholar] [CrossRef]
- McBride, S.; Rossi, S.; Huang, B. Differential metabolic responses to heat stress associated with interspecific variations in stress tolerance for annual bluegrass and creeping bentgrass. Grass Res. 2024, 4, e013. [Google Scholar] [CrossRef]
- Du, H.; Wang, Z.; Yu, W.; Liu, Y.; Huang, B. Differential metabolic responses of perennial grass Cynodon transvaalensis × Cynodon dactylon (C4) and Poa pratensis (C3) to heat stress. Physiol. Plant. 2011, 141, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Casler, M.D.; Duncan, R.R. Turfgrass Biology, Genetics, and Breeding; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Krishnan, S.; Ma, Y.; Merewitz, E. Leaf trimming and high temperature regulation of phytohormones and polyamines in creeping bentgrass leaves. J. Am. Soc. Hortic. Sci. 2016, 141, 66–75. [Google Scholar] [CrossRef]
- Miller, G.L.; Brotherton, M.A. Creeping bentgrass summer decline as influenced by climatic conditions and cultural practices. Agron. J. 2020, 112, 3500–3512. [Google Scholar] [CrossRef]
- Rossi, S.; Huang, B. Regulatory roles of morphactin on suppressing chlorophyll degradation under heat stress in creeping bentgrass. Grass Res. 2023, 3, 11. [Google Scholar] [CrossRef]
- Li, Q.; Li, R.; He, F.; Yang, Z.; Yu, J. Growth and physiological effects of chitosan on heat tolerance in creeping bentgrass (Agrostis stolonifera). Grass Res. 2022, 2, 1–7. [Google Scholar] [CrossRef]
- Li, Q.; Bian, Y.; Li, R.; Yang, Z.; Liu, N.; Yu, J. Chitosan-enhanced heat tolerance associated with alterations in antioxidant defense system and gene expression in creeping bentgrass. Grass Res. 2023, 3, 7. [Google Scholar] [CrossRef]
- Yang, H.; Yuan, Y.; Liu, X.; Du, Y.; Li, Z. Phytohormonal homeostasis, chloroplast stability, and heat shock transcription pathways related to the adaptability of creeping bentgrass species to heat stress. Protoplasma 2025, 262, 649–665. [Google Scholar] [CrossRef]
- Li, Z.; Tang, M.; Hassan, M.J.; Zhang, Y.; Han, L.; Peng, Y. Adaptability to high temperature and stay-green genotypes associated with variations in antioxidant, chlorophyll metabolism, and γ-aminobutyric acid accumulation in creeping bentgrass species. Front. Plant Sci. 2021, 12, 750728. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1938, 347, 39. [Google Scholar]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Blum, A.; Ebercon, A. Cell membrane stability as a measure of drought and heat tolerance in wheat 1. Crop Sci. 1981, 21, 43–47. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Barnes, J.D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A.W. A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ. Exp. Bot. 1992, 32, 85–100. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, M.; Qi, H.; Cheng, B.; Hassan, M.J. Foliar application of diethyl aminoethyl hexanoate (DA-6) alleviated summer bentgrass decline and heat damage to creeping bentgrass. Crop Sci. 2024, 64, 1039–1050. [Google Scholar] [CrossRef]
- Qiu, Y.; Su, M.; Liu, Y.; Chen, M.; Gu, J.; Zhang, J.; Jia, W. Application of ethyl chloroformate derivatization for gas chromatography–mass spectrometry based metabonomic profiling. Anal. Chim. Acta 2007, 583, 277–283. [Google Scholar] [CrossRef]
- Jespersen, D.; Zhang, J.; Huang, B. Chlorophyll loss associated with heat-induced senescence in bentgrass. Plant Sci. 2016, 249, 1–12. [Google Scholar] [CrossRef]
- Hassan, M.J.; Najeeb, A.; Liu, S.; Ali, U.; Chandio, W.A.; Li, M.; Liao, Q.; Li, Z. Diethyl aminoethyl hexanoate mitigates drought-stimulated leaf senescence via the regulation of water homeostasis, chlorophyll metabolism, and antioxidant defense in creeping bentgrass. Grass Res. 2025, 5, e004. [Google Scholar] [CrossRef]
- Dai, Y.; Yuan, L.; Zhang, S.; Wang, J.; Xie, S.; Zhao, M.; Chen, G.; Sun, R.; Wang, C. Comprehensive evaluation for cold tolerance in wucai (Brassica campestris L.) by the performance index on an absorption basis (PIabs). Agronomy 2019, 9, 61. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, M.; Zeng, W.; Zhang, Y.; Liu, L.; Liu, W.; Peng, Y. Root metabolites remodeling regulated by γ-aminobutyric acid (GABA) improves adaptability to high temperature in creeping bentgrass. Plant Soil 2024, 500, 181–195. [Google Scholar] [CrossRef]
- Bocso, N.S.; Butnariu, M. The biological role of primary and secondary plants metabolites. J. Nutr. Food Process. 2022, 5, 1–7. [Google Scholar]
- Thalmann, M.; Santelia, D. Starch as a determinant of plant fitness under abiotic stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Mizock, B.A. Alterations in carbohydrate metabolism during stress: A review of the literature. Am. J. Med. 1995, 98, 75–84. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiao, D.; Zhang, Z.; Li, Y.; Shi, S.; Yang, Y. Calcium signal regulated carbohydrate metabolism in wheat seedlings under salinity stress. Physiol. Mol. Biol. Plants 2024, 30, 123–136. [Google Scholar] [CrossRef]
- Xalxo, R.; Yadu, B.; Chandra, J.; Chandrakar, V.; Keshavkant, S. Alteration in carbohydrate metabolism modulates thermotolerance of plant under heat stress. Heat Stress Toler. Plants Physiol. Mol. Genet. Perspect. 2020, 23, 77–115. [Google Scholar]
- Juneja, S.; Saini, R.; Mukit, A.; Kumar, S. Drought priming modulates ABF, GRFs, related microRNAs and induce metabolic adjustment during heat stress in chickpea. Plant Physiol. Biochem. 2023, 203, 108007. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, B.; Liu, W.; Feng, G.; Zhao, J.; Zhang, L.; Peng, Y. Global metabolites reprogramming induced by spermine contributing to salt tolerance in creeping bentgrass. Int. J. Mol. Sci. 2022, 23, 4472. [Google Scholar] [CrossRef]
- Hu, T.; Liu, S.Q.; Amombo, E.; Fu, J.M. Stress memory induced rearrangements of HSP transcription, photosystem II photochemistry and metabolism of tall fescue (Festuca arundinacea Schreb.) in response to high-temperature stress. Front. Plant Sci. 2015, 6, 403. [Google Scholar] [CrossRef]
- Michailidis, M.; Karagiannis, E.; Tanou, G.; Sarrou, E.; Stavridou, E.; Ganopoulos, I.; Karamanoli, K.; Madesis, P.; Martens, S.; Molassiotis, A. An integrated metabolomic and gene expression analysis identifies heat and calcium metabolic networks underlying postharvest sweet cherry fruit senescence. Planta 2019, 250, 2009–2022. [Google Scholar] [CrossRef]
- Huang, F.; Lei, Y.; Duan, J.; Kang, Y.; Luo, Y.; Ding, D.; Chen, Y.; Li, S. Investigation of heat stress responses and adaptation mechanisms by integrative metabolome and transcriptome analysis in tea plants (Camellia sinensis). Sci. Rep. 2024, 14, 10023. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Q.; Liu, Y.; Chen, J.; Wang, L.; Xiu, Y.; Zheng, H.; Lin, S.; Ling, P.; Tang, M. Combined analysis of transcriptome and metabolome provides insights in response mechanism under heat stress in avocado (Persea americana Mill.). Int. J. Mol. Sci. 2024, 25, 10312. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qiu, S.; Chen, Y.; Li, J.; Xu, T.; Zhong, P.; Shao, X.; Xu, S.; Ma, Z.; Huang, Z.; et al. Integrated transcriptomics and metabolomics provides insights into the Nicotiana tabacum response to heat stress. Front. Plant Sci. 2024, 15, 1425944. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Ali, S.; Zandi, P.; Mehmood, A.; Ullah, S.; Ikram, M.; Ismail, I.; Babar, M.A. Role of sugars, amino acids and organic acids in improving plant abiotic stress tolerance. Pak. J. Bot. 2020, 52, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Song, Z.; Zhao, B.; Zhang, Y.; Wang, R. Physiological and metabolomics responses of Hydrangea macrophylla (Thunb.) Ser. and Hydrangea strigosa Rehd. to lead exposure. Ecotoxicol. Environ. Saf. 2022, 243, 113960. [Google Scholar] [CrossRef]
- Yan, F.; Ma, J.; Peng, M.; Xi, C.; Chang, S.; Yang, Y.; Tian, S.; Zhou, B.; Liu, T. Lactic acid induced defense responses in tobacco against Phytophthora nicotianae. Sci. Rep. 2024, 14, 9338. [Google Scholar] [CrossRef]
- Valitova, J.; Renkova, A.; Beckett, R.; Minibayeva, F. Stigmasterol: An enigmatic plant stress sterol with versatile functions. Int. J. Mol. Sci. 2024, 25, 8122. [Google Scholar] [CrossRef]
- Schaller, H. The role of sterols in plant growth and development. Prog. Lipid Res. 2003, 42, 163–175. [Google Scholar] [CrossRef]
- Piironen, V.; Lindsay, D.G.; Miettinen, T.A.; Toivo, J.; Lampi, A.M. Plant sterols: Biosynthesis, biological function and their importance to human nutrition. J. Sci. Food Agric. 2000, 80, 939–966. [Google Scholar] [CrossRef]
- Senthil-Kumar, M.; Wang, K.; Mysore, K.S. AtCYP710A1 gene-mediated stigmasterol production plays a role in imparting temperature stress tolerance in Arabidopsis thaliana. Plant Signal. Behav. 2013, 8, e23142. [Google Scholar] [CrossRef]
- Hassan, M.J.; Zhou, M.; Ling, Y.; Li, Z. Diethyl aminoethyl hexanoate ameliorates salt tolerance associated with ion transport, osmotic adjustment, and metabolite reprograming in white clover. BMC Plant Biol. 2024, 24, 950. [Google Scholar] [CrossRef]
- Shu, P.; Wang, Y.; Zhang, L. The effect of α-arbutin on UVB-induced damage and its underlying mechanism. Molecules 2024, 29, 1921. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Zhao, Y.; Li, Z. Different Heat Tolerance of Two Creeping Bentgrass Cultivars Related to Altered Accumulation of Organic Metabolites. Agronomy 2025, 15, 1544. https://doi.org/10.3390/agronomy15071544
Du Y, Zhao Y, Li Z. Different Heat Tolerance of Two Creeping Bentgrass Cultivars Related to Altered Accumulation of Organic Metabolites. Agronomy. 2025; 15(7):1544. https://doi.org/10.3390/agronomy15071544
Chicago/Turabian StyleDu, Yong, Yue Zhao, and Zhou Li. 2025. "Different Heat Tolerance of Two Creeping Bentgrass Cultivars Related to Altered Accumulation of Organic Metabolites" Agronomy 15, no. 7: 1544. https://doi.org/10.3390/agronomy15071544
APA StyleDu, Y., Zhao, Y., & Li, Z. (2025). Different Heat Tolerance of Two Creeping Bentgrass Cultivars Related to Altered Accumulation of Organic Metabolites. Agronomy, 15(7), 1544. https://doi.org/10.3390/agronomy15071544






