Fine Mapping of Quantitative Trait Loci (QTL) with Resistance to Common Scab in Diploid Potato and Development of Effective Molecular Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Pathogen Inoculation
2.2. Disease Detection and Resistance Evaluation
2.3. DNA Extraction and Marker Development
2.4. Primer Amplification and PCR Product Detection
2.5. Construction of Genetic Linkage Map
2.6. Quantitative Real-Time PCR (qRT-PCR) Analysis
3. Results
3.1. Resistance Evaluation of the Population Materials
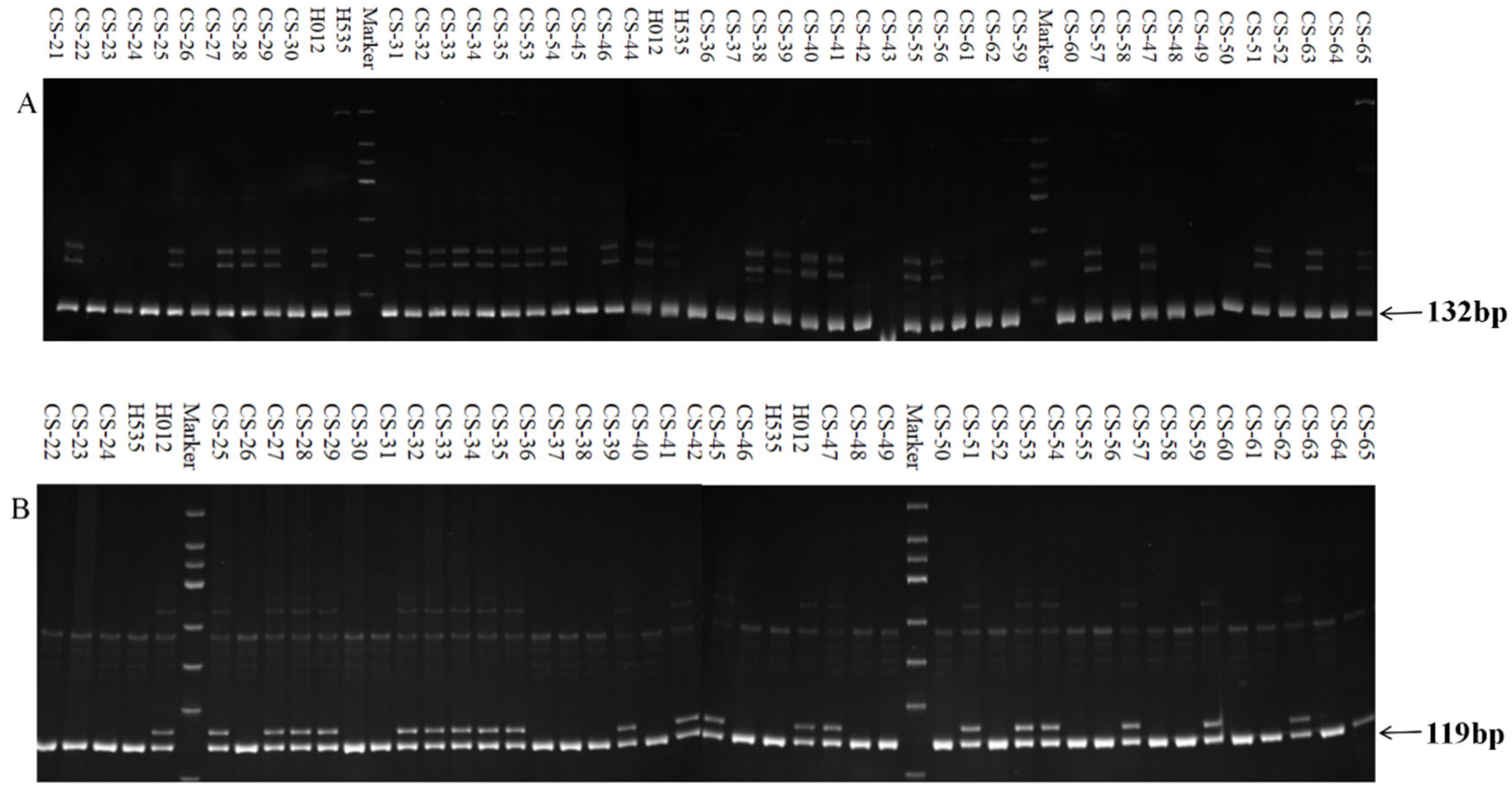
3.2. Development and Identification of Markers
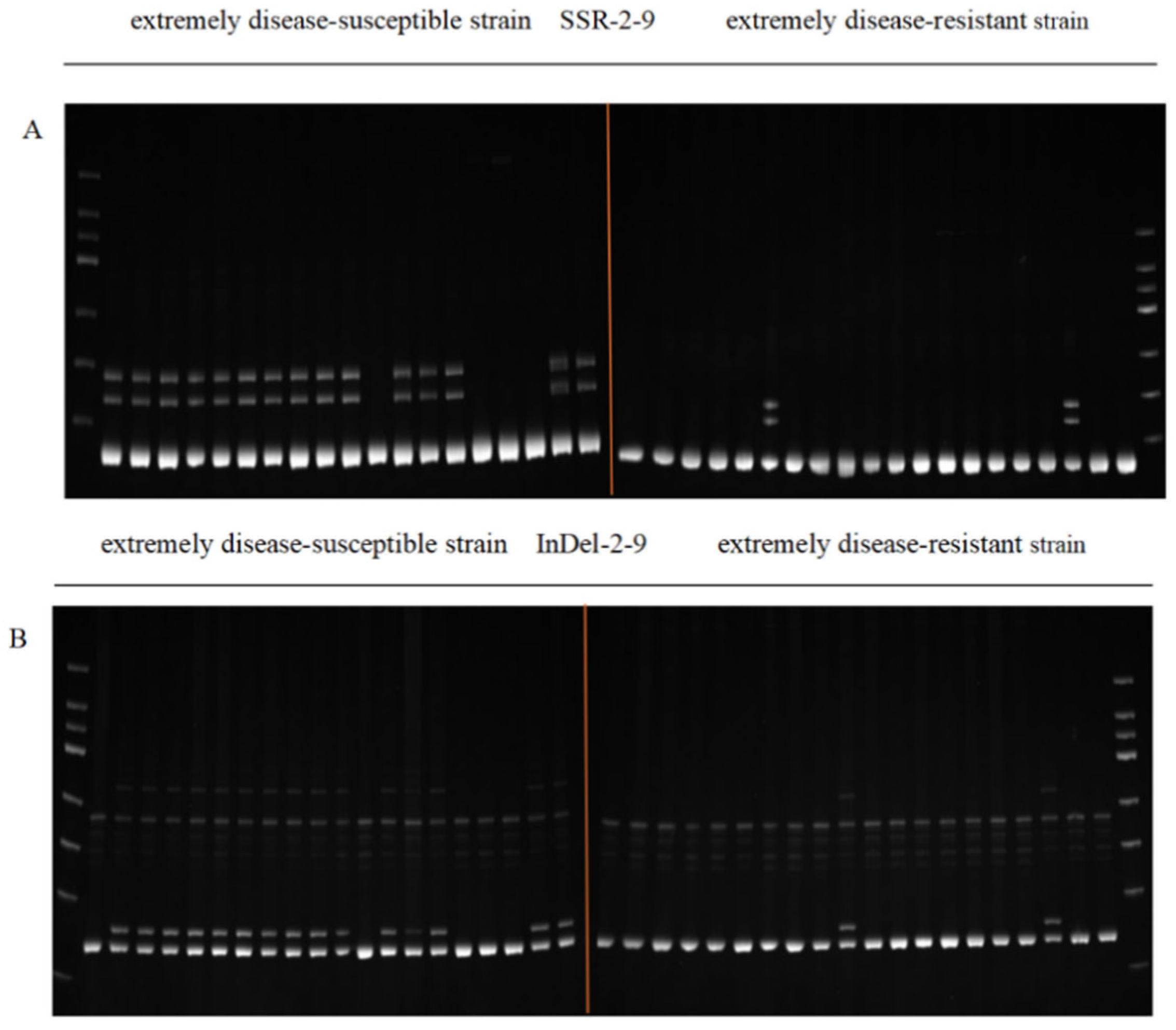
3.3. Marker Fit Validation in the Population
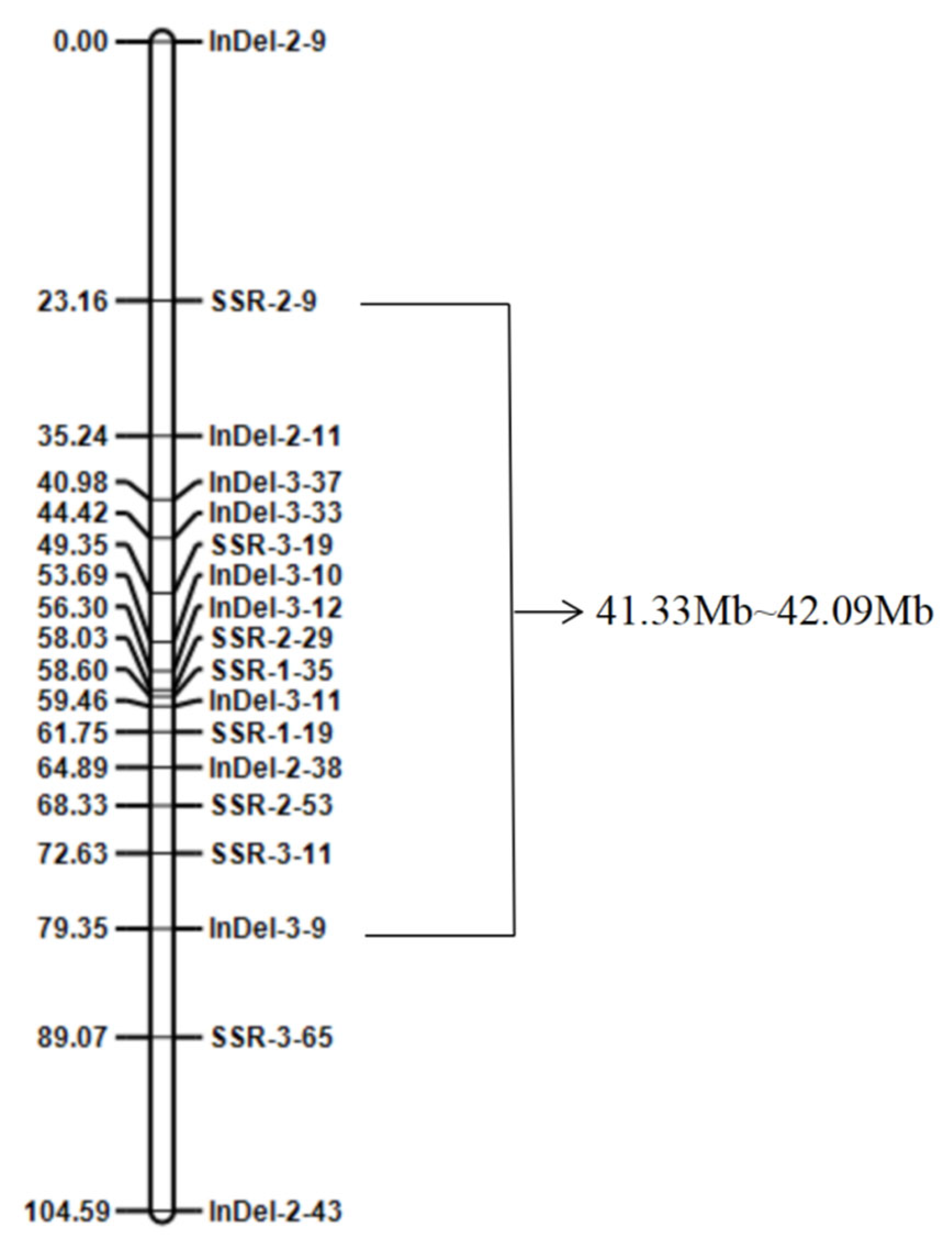
3.4. Construction of the Resistance Genetic Linkage Map
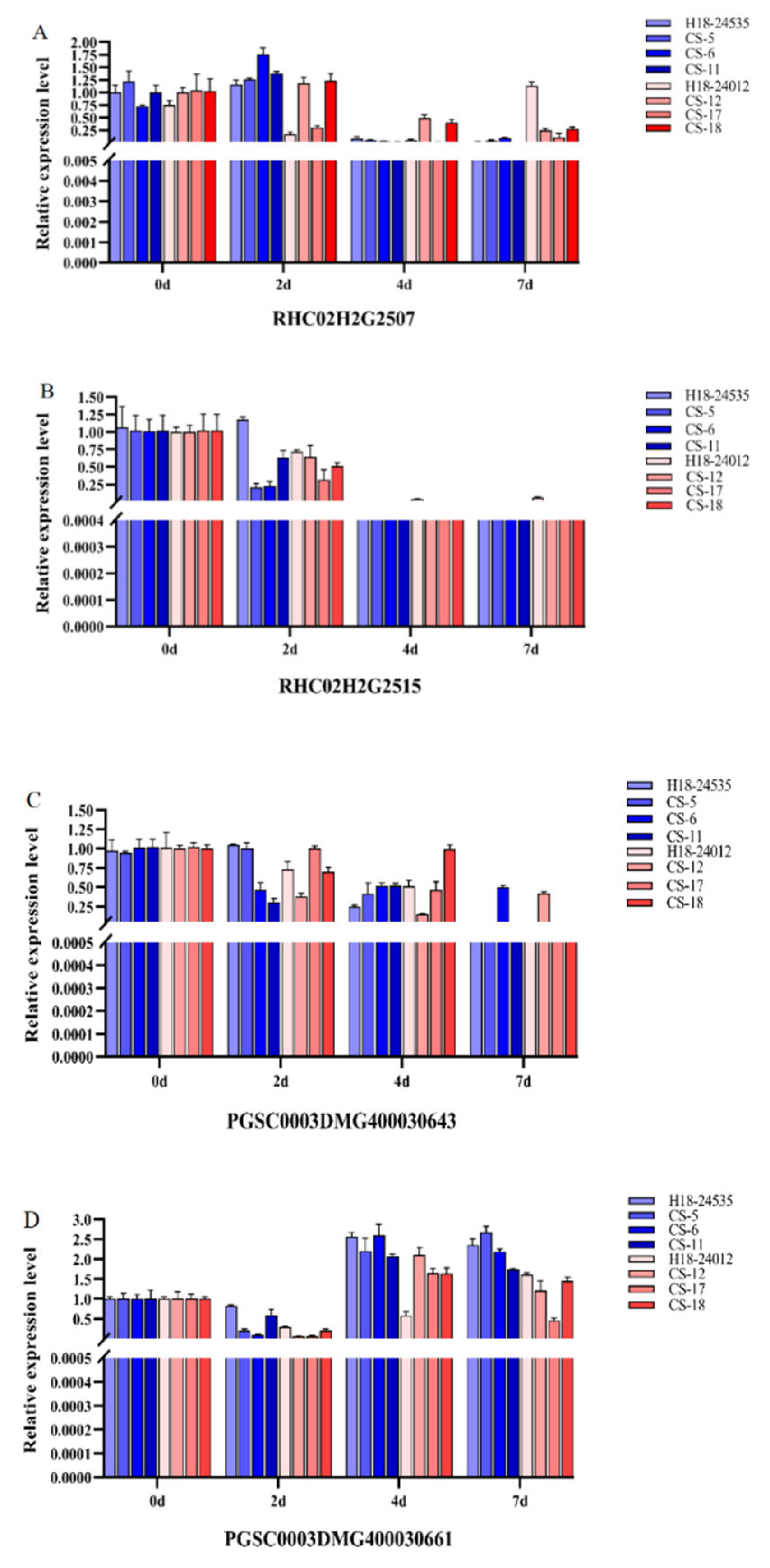
3.5. Expression Level Analysis of Resistance Genes
4. Discussion
4.1. Resistance Gene Mapping
4.2. Candidate Gene Expression Level Analysis
4.3. Candidate Gene Functional Analysis
5. Conclusions
- A total of 214 pairs of SSR primers and 133 pairs of InDel primers were designed within the 38–43 Mb interval of chromosome 2. Through t-tests, 18 markers linked to resistance indentified. The linkage rates of three SSR markers with the disease-resistant trait in extreme materials ranged from 70.0% to 90.0%, and the linkage rates of six InDel markers with the disease-resistant trait were also 70.0–90.0%, which can be used for molecular marker-assisted selection.
- An 18-polymorphic-marker-based genetic linkage map with a total length of 104.59 cm was constructed. The scab resistance gene was mapped between the linkage markers SSR-2-9 and InDel-3-9, corresponding to the physical position within the 41.33–42.09 Mb interval on chromosome 2.
- The expression patterns of genes within the candidate region were verified using quantitative real-time PCR technology. The resistant and susceptible parent materials, as well as some resistant and susceptible progeny materials, were inoculated with the KS28-2 suspension. Samples were collected at four time points, namely 0 d (uninoculated control), 2 d, 4 d, and 7 d, for gene expression analysis. Resistance—related genes with significantly different expression levels between resistant and susceptible individuals were obtained.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dees, W.M.; Wanner, A.L. In Search of Better Management of Potato Common Scab. Potato Res. 2012, 55, 249–268. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Díaz-Cruz, G..; Cheng, Z.; Bignell, D.R.D. Virulence mechanisms of plant-pathogenic Streptomyces species: An updated review. Microbiology 2019, 165, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Khatri, B.B.; Tegg, S.R.; Brown, H.P.; Wilson, C.R. Temporal association of potato tuber development with susceptibility to common scab and Streptomyces scabiei induced responses in the potato periderm. Plant Pathol. 2011, 60, 776–786. [Google Scholar] [CrossRef]
- Bum, S.K.; Michael, G. Streptomyces avermitilis sp. nov., nom. rev., a taxonomic home for the avermectin-producing streptomycetes. Int. J. Syst. Evol. Microbiol. 2002, 52 Pt 6, 2011–2014. [Google Scholar] [CrossRef]
- Wanner, L.A. A survey of genetic variation in Streptomyces isolates causing potato common scab in the United States. Phytopathology 2006, 96, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Emils, S.B.; Gustafsson, N. Scab resistance in potato cultivars. Acta Agric. Scand. 1953, 3, 33–52. [Google Scholar] [CrossRef]
- Faucher, E.; Savard, T.; Beaulieu, C. Characterization of actinomycetes isolated from common scab lesions on potato tubers. Can. J. Plant Pathol. 1992, 14, 197–202. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, W.; Li, Y.; Liu, D.; Liu, Q.; Zhang, T. Identification of Potato Scab Pathogens in China. Sci. Agric. Sin. 2006, 39, 313–318. (In Chinese) [Google Scholar]
- Adams, M.J.; Lapwood, D.H. Studies on the lenticel development, surface microflora and infection by common scab (Streptomyces scabies) of potato tubers growing in wet and dry soils. Ann. Appl. Biol. 1978, 90, 335–343. [Google Scholar] [CrossRef]
- Loria, R.; Coombs, J.; Yoshida, M.; Kers, J.; Bukhalid, R. A paucity of bacterial root diseases: Streptomyces succeeds where others fail. Physiol. Mol. Plant Pathol. 2003, 62, 65–72. [Google Scholar] [CrossRef]
- Garry, G.; Forbes, A.G.; Salas, A.; Santa, M.; Perez, W.; Nelson, R. Genetic diversity and host differentiation among isolates of Phytophthora infestans from cultivated potato and wild solanaceous hosts in Peru. Plant Pathol. 2005, 54, 740–748. [Google Scholar] [CrossRef]
- Hooker, W.J.; Sass, J.E. Histological Aspects of Potato Stem Necrosis Incited by Streptomyces scabies. Am. J. Bot. 1952, 39, 15–19. [Google Scholar] [CrossRef]
- Tegg, S.R.; Wilson, R.C. Relationship of resistance to common scab disease and tolerance to thaxtomin A toxicity within potato cultivars. Eur. J. Plant Pathol. 2010, 128, 143–148. [Google Scholar] [CrossRef]
- Keinath, A.P.; Loria, R. Effects of inoculum density and cultivar resistance on common scab of potato and population dynamics of Streptomyces scabies. Am. Potato J. 1991, 68, 515–524. [Google Scholar] [CrossRef]
- Lazarovits, G.; Hill, J.; Patterson, G.; Conn, K.L.; Crump, N.S. Edaphic soil levels of mineral nutrients, pH, organic matter, and cationic exchange capacity in the geocaulosphere associated with potato common scab. Phytopathology 2007, 97, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.D.; Kinkel, L.L. Inoculum Density and Population Dynamics of Suppressive and Pathogenic Streptomyces Strains and Their Relationship to Biological Control of Potato Scab. Biol. Control 1997, 10, 180–186. [Google Scholar] [CrossRef]
- Pasco, C.; Jouan, B.; Andrivon, D. Resistance of potato genotypes to common scab and netted scab-causing species of Streptomyces. Plant Pathol. 2005, 54, 383–392. [Google Scholar] [CrossRef]
- Yan, M.; Nie, H.; Wang, Y.; Wang, X.; Jarret, R.; Zhao, J.; Wang, H.; Yang, J. Exploring and exploiting genetics and genomics for sweetpotato improvement: Status and perspectives. Plant Commun. 2022, 3, 100332. [Google Scholar] [CrossRef]
- Jacobs, J.M.; Van Eck, H.J.; Arens, P.; Verkerk-Bakker, B.; Te Lintel Hekkert, B.; Bastiaanssen, H.J.; El-Kharbotly, A.; Pereira, A.; Jacobsen, E.; Stiekema, W.J. A genetic map of potato (Solanum tuberosum) integrating molecular markers, including transposons, and classical markers. Theor. Appl. Genet. 1995, 91, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla, C.; Navarro, F.; Vega-Semorile, S.; Palta, J. QTL for pitted scab, hollow heart, and tuber calcium identified in a tetraploid population of potato derived from an Atlantic × Superior cross. Crop Sci. 2021, 61, 1630–1651. [Google Scholar] [CrossRef]
- Murphy, A.M.; De Jong, H. Transmission of resistance to common scab from the diploid to the tetraploid level via 4x-2x crosses in potatoes. Euphytica 1995, 82, 227–233. [Google Scholar] [CrossRef]
- Haynes, K.G.; Wanner, L.A.; Thill, C.A.; Bradeen, J.M.; Novy, R.G.; Whitworth, J.L.; Corsini, D.L.; Vinyard, B.T. Common scab trials of potato varieties and advanced selections at three US locations. Am. J. Potato Res. 2010, 87, 261–276. [Google Scholar] [CrossRef]
- Haynes, K.G.; Goth, R.W.; Young, R.J. Genotype × Environment Interactions for Resistance to Common Scab in Tetrapioid Potato. Crop Sci. 1997, 37, 1163–1167. [Google Scholar] [CrossRef]
- Haynes, K.G.; Christ, B.J.; Burkhart, C.R.; Vinyard, B.T. Heritability of resistance to common scab in diploid potatoes. Am. J. Potato Res. 2009, 86, 165–170. [Google Scholar] [CrossRef]
- Guo, M.; Davis, D.; Birchler, J.A. Dosage effects on gene expression in a maize ploidy series. Genetics 1996, 142, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, J.E.; Hackett, C.A.; Pande, B.; Waugh, R.; Bryan, G.J. QTL mapping of yield, agronomic and quality traits in tetraploid potato (Solanum tuberosum subsp. tuberosum). Theor. Appl. Genet. 2008, 116, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Pereira, G.; Mollinari, M.; Qu, X.; Thill, C.; Zeng, Z.-B.; Haynes, K.; Yencho, G.C. Quantitative trait locus mapping for common scab resistance in a tetraploid potato full-sib population. Plant Dis. 2021, 105, 3048–3054. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.R.; Endelman, J.B.; Haynes, K.G.; Jansky, S.H. Quantitative trait loci for resistance to common scab and cold-induced sweetening in diploid potato. Plant Genome 2017, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fofana, B.; Soto-Cerda, B.J.; Zaidi, M.; Main, D.; Fillmore, S. Genome-Wide Genetic Architecture for Common Scab (Streptomyces scabiei L.) Resistance in Diploid Potatoes. Int. J. Mol. Sci. 2025, 26, 1126. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yuan, B.; Zhang, C.; Yao, Q.; He, H.X.; Wang, Q.F.; Liang, J.P.; Li, N.; Zhu, X.; Wang, Z.W. Revealing Key Genes and Pathways in Potato Scab Disease Resistance through Transcriptome Analysis. Agronomy 2024, 14, 291. [Google Scholar] [CrossRef]
- Koizumi, E.; Igarashi, T.; Tsuyama, M.; Ogawa, K.; Kobayashi, A.; Sanetomo, R.; Hosaka, K. Association of Genome-Wide SNP Markers with Resistance to Common Scab of Potato. Am. J. Potato Res. 2021, 98, 149–156. [Google Scholar] [CrossRef]
- Yuan, J.; Bizimungu, B.; Koeyer, D.D.; Rosyara, U.; Wen, Z.; Lagüe, M. Genome-Wide Association Study of Resistance to Potato Common Scab. Potato Res. J. Eur. Assoc. Potato Res. 2020, 63, 253–266. [Google Scholar] [CrossRef]
- Driscoll, J.; Coombs, J.; Hammerschmidt, R.; Kirk, W.; Wanner, L.; Douches, D. Greenhouse and field nursery evaluation for potato common scab tolerance in a tetraploid population. Am. J. Potato Res. 2009, 86, 96–101. [Google Scholar] [CrossRef]
- Goth, R.W.; Haynes, K.G.; Young, R.J.; Wilson, D.R.; Lauer, F.I. Relative resistance of the potato cultivar Krantz to common scab caused by Streptomyces scabies as determined by cluster analysis. Am. J. Potato Res. 1995, 72, 505–511. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, M.; Sun, J.; Zhang, F. Genome-wide genic SSR marker development for the endangered Dongxiang wild rice (Oryza rufipogon). Plant Genet. Resour. 2017, 15, 566–569. [Google Scholar] [CrossRef]
- Li, Q.; Wan, J.-M. SSRHunter: Development of a local searching software for SSR sites. Yi Chuan = Hereditas 2005, 27, 808–810. [Google Scholar]
- Mireille, C.; Sylvie, L.; Thierry, R. Silver staining of proteins in polyacrylamide gels. Nat. Protoc. 2006, 1, 1852–1858. [Google Scholar] [PubMed]
- Ye, M.; Peng, Z.; Tang, D.; Yang, Z.; Li, D.; Xu, Y.; Zhang, C.; Huang, S. Generation of self-compatible diploid potato by knockout of S-RNase. Nat. Plants 2018, 4, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kaiser, N.; Coombs, J.; Felcher, K.; Hammerschmidt, R.; Zuehlke, M.; Buell, C.; Douches, D. Genome-Wide Association Analysis of Common Scab Resistance and Expression Profiling of Tubers in Response to ThaxtominA Treatment Underscore the Complexity of Common Scab Resistance in Tetraploid Potato. Am. J. Potato Res. 2020, 97, 513–522. [Google Scholar] [CrossRef]
- Loake, J.G. Molecular aspects of plant disease resistance. Annual Plant Reviews, Volume 34. Ann. Bot. 2009, 104, v. [Google Scholar] [CrossRef]
- Tameling, W.I.; Elzinga, S.D.; Darmin, P.S.; Vossen, J.H.; Takken, F.L.; Haring, M.A.; Cornelissen, B.J.C. The tomato R gene products I-2 and MI-1 are functional ATP binding proteins with ATPase activity. Plant Cell 2002, 14, 2929–2939. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.U.; Song, W.Y.; Hong, D.; Ko, D.; Yamaoka, Y.; Jang, S.; Yim, S.; Lee, E.; Khare, D.; Kim, K.; et al. Plant ABC transporters enable many unique aspects of a terrestrial plant's lifestyle. Mol. Plant 2016, 9, 338–355. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, J.; Han, L.; Li, Z.G.; Zhang, Z. Comprehensive analysis of tandem amino acid repeats from ten angiosperm genomes. BMC Genom. 2011, 12, 632. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wang, Z.; Cheng, D.; Chen, X.; Chen, Y.; Ma, Z. The ATP-binding protein FgArb1 is essential for penetration, infectious and normal growth of Fusarium graminearum. New Phytol. 2018, 219, 1447–1466. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.D.; Moreira, L.M.; Kapp, D.; Frosch, S.C.; Pühler, A.; Perlic, A.M. The nodulin vfENOD18 is an ATP-binding protein in infected cells of Vicia faba L. nodules. Plant Mol. Biol. 2001, 47, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yang, Y.; Yuan, Y.; Zhang, B.; Liu, T.; Shao, Z.; Li, Y.; Yang, P.; An, J.; Cao, Y. MsABCG1, ATP-Binding Cassette G transporter from Medicago Sativa, improves drought tolerance in transgenic Nicotiana Tabacum. Physiol. Plant. 2024, 176, e14446. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, X.; Yu, S.; Liu, J.; Jiang, L.; Lu, X.; Liu, Y.; Zhang, J.; Li, X.; Zhang, S. The maize ATP-binding cassette (ABC) transporter ZmMRPA6 confers cold and salt stress tolerance in plants. Plant Cell Rep. 2023, 43, 13. [Google Scholar] [CrossRef] [PubMed]
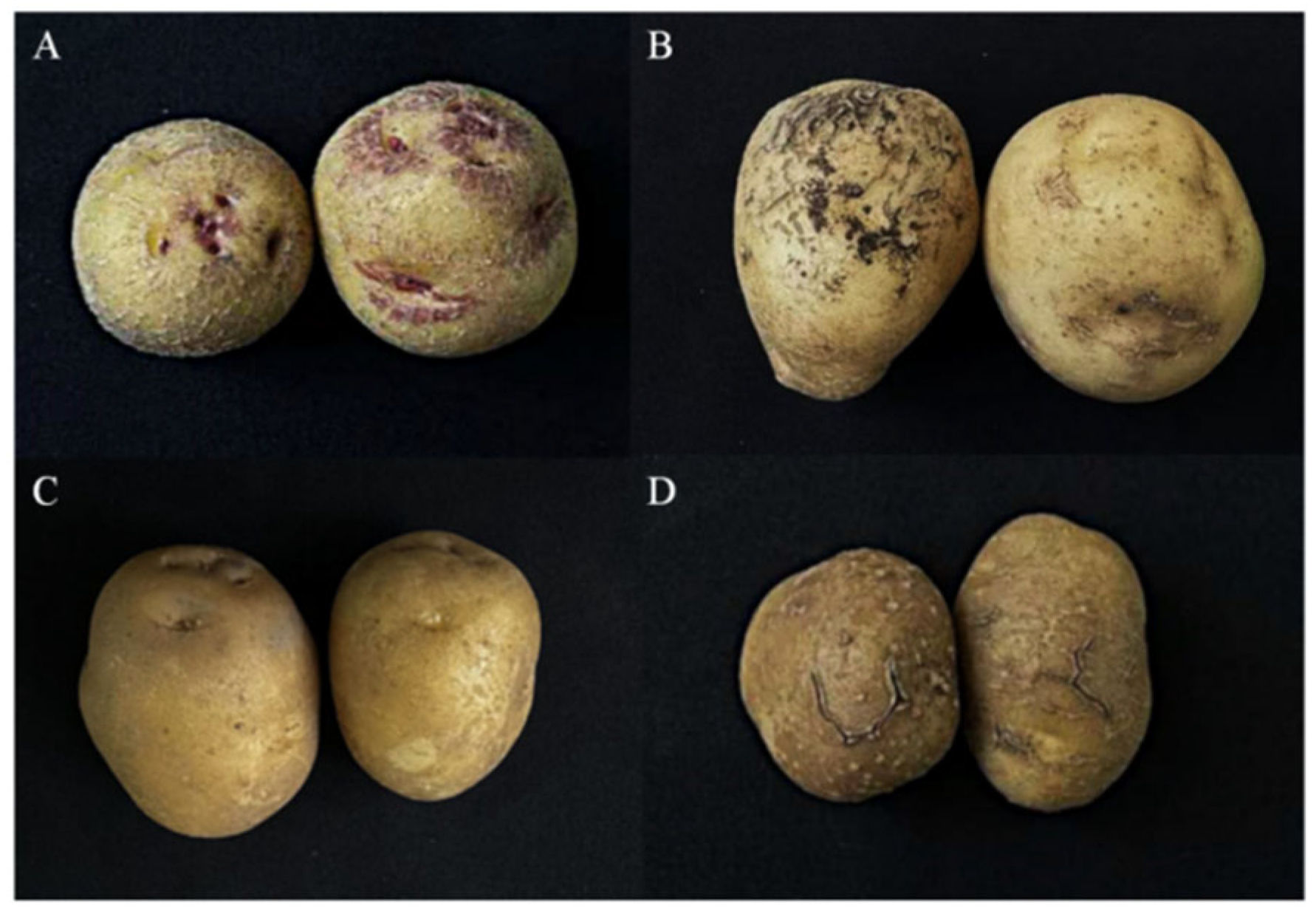
| Trait | H535 Means | H012 Means | F1 Means | F1 Minimum | F1 Maximum |
|---|---|---|---|---|---|
| Incidence rate (%) | 10.00% | 83.33% | 34.58% | 5.00% | 100.00% |
| DSI | 10 | 80 | 40 | 0 | 90 |
| RL | HR | HS | I | HR | HS |
| NO. | Primer | Primer Sequence (5′-3′) | Tm (°C) | Size (bp) |
|---|---|---|---|---|
| 1 | SSR-1-19 | F: AAAGCAGGTAATTGGGCACCTT | 55.0 | 173 |
| R: AGGCAAAGACGCATTGATAGGG | ||||
| 2 | SSR-1-35 | F: AAGAGGTATGCCACATCAACTT | 53.8 | 104 |
| R: AGTGTTACTTGGAGTCTCAACC | ||||
| 3 | SSR-2-9 | F: GATTCAATCGACAGCACGGTAA | 54.0 | 132 |
| R: CCTAGGGATTAGGGAATCAGGT | ||||
| 4 | SSR-2-29 | F: TATACGTGGGCCATTGGTCATC | 54.5 | 182 |
| R: GAGGTCGGTCAGAAGTACAAGT | ||||
| 5 | SSR-2-53 | F: CGAGGTAGTGGCAAGGTCTG | 55.5 | 213 |
| R: GGCATGGCACGAAGTTTCAAA | ||||
| 6 | SSR-3-11 | F: CGAACCCACAACTCTAGCATGA | 55.0 | 173 |
| R: CGCTCCGTCACTGCCAATAG | ||||
| 7 | SSR-3-19 | F: ATCAAGAACATTGGGCAACTCA | 54.0 | 188 |
| R: TACAGGCTCACAGCTTACTCAC | ||||
| 8 | SSR-3-65 | F: CCCATAAGGCCATAACCAAACG | 53.5 | 144 |
| R: CTTCTTCTGCATTCTCTGTCCT | ||||
| 9 | InDel-2-9 | F: TCCCTATGCTTCTCAGATATGGT | 54.0 | 119 |
| R: GGGAGAATTTCGTAGCCACATC | ||||
| 10 | InDel-2-11 | F: CGAATTTCCTTGTGCCGTGATAA | 55.0 | 201 |
| R: CGAACCAGTGTGGAGCAGTC | ||||
| 11 | InDel-2-29 | F: CGATGGTTCTGTTCTGTGTTTCT | 54.0 | 206 |
| R: TCCTACTCACTAATGGCCTACTC | ||||
| 12 | InDel-2-43 | F: TTCTAGGGTGACAAGTGATAAGC | 53.0 | 170 |
| R: GGAAAGAAGTAAGATCATGCCAG | ||||
| 13 | InDel-3-9 | F: CCACTCGTACTCGCTACTGTAAC | 55.0 | 114 |
| R: TGCCATTAGACGGAGAGAAGTTG | ||||
| 14 | InDel-3-10 | F: TTGAAGCCCTCTTGATCGTGTT | 54.0 | 186 |
| R: GATGAATCGTCACAACTGGAGAC | ||||
| 15 | InDel-3-11 | F: TCCGACGCTTAATCAGTGTTCC | 55.0 | 124 |
| R: TCTTACCATTTCAGACCACCAGG | ||||
| 16 | InDel-3-12 | F: GGCCTCCTCTACTTATCTCACAA | 54.5 | 167 |
| R: TTTGGTTTGCGGTTTCACTCAG | ||||
| 17 | InDel-3-33 | F: ACCTTCTTTCCCTTTGCTACTCA | 54.0 | 153 |
| R: TTCAATCGAAGCTGCTCTGTCA | ||||
| 18 | InDel-3-37 | F: ACTAGCAGAGCGAGCAGAGT | 54.0 | 119 |
| R: GCAGCTATGAACGCCACAAG |
| Primer | Chromosome | Compatibility with Disease Resistance Traits | Degree of Conformity of Sensitive Traits |
|---|---|---|---|
| SSR-1-19 | 2 | 55.0 | 53.3 |
| SSR-1-35 | 2 | 65.0 | 70.0 |
| SSR-2-9 | 2 | 90.0 | 80.0 |
| SSR-2-29 | 2 | 70.0 | 80.0 |
| SSR-2-53 | 2 | 50.0 | 63.3 |
| SSR-3-11 | 2 | 75.0 | 66.7 |
| SSR-3-19 | 2 | 55.0 | 70.0 |
| SSR-3-65 | 2 | 60.0 | 73.3 |
| InDel-2-9 | 2 | 90.0 | 76.7 |
| InDel-2-11 | 2 | 70.0 | 60.0 |
| InDel-2-38 | 2 | 60.0 | 70.0 |
| InDel-2-43 | 2 | 65.0 | 70.0 |
| InDel-3-9 | 2 | 80.0 | 86.7 |
| InDel-3-10 | 2 | 55.0 | 63.3 |
| InDel-3-11 | 2 | 80.0 | 60.0 |
| InDel-3-12 | 2 | 85.0 | 73.3 |
| InDel-3-33 | 2 | 60.0 | 50.0 |
| InDel-3-37 | 2 | 75.0 | 66.7 |
| Number | ID | Description | Star | End |
|---|---|---|---|---|
| 1 | RHC02H2G2507 | ATP-binding protein | 41463591 | 41468173 |
| 2 | RHC02H2G2515 | Splicing factor, arginine/serine-rich | 41537409 | 41546211 |
| 3 | PGSC0003DMG400030643 | AAR2 protein family | 41772830 | 41772830 |
| 4 | PGSC0003DMG401026400 | CRS1 | 41432417 | 41438525 |
| 5 | PGSC0003DMG400026379 | ATP-binding protein | 41527660 | 41530068 |
| 6 | PGSC0003DMG400030658 | Symbiosis receptor-like kinase | 41983220 | 41988281 |
| 7 | PGSC0003DMG400030661 | ATP-binding protein | 42020694 | 42021506 |
| 8 | PGSC0003DMG400030625 | Receptor protein kinase CLAVATA1 | 42176629 | 42180273 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, G.; Jin, G. Fine Mapping of Quantitative Trait Loci (QTL) with Resistance to Common Scab in Diploid Potato and Development of Effective Molecular Markers. Agronomy 2025, 15, 1527. https://doi.org/10.3390/agronomy15071527
Wu G, Jin G. Fine Mapping of Quantitative Trait Loci (QTL) with Resistance to Common Scab in Diploid Potato and Development of Effective Molecular Markers. Agronomy. 2025; 15(7):1527. https://doi.org/10.3390/agronomy15071527
Chicago/Turabian StyleWu, Guoqiang, and Guanghui Jin. 2025. "Fine Mapping of Quantitative Trait Loci (QTL) with Resistance to Common Scab in Diploid Potato and Development of Effective Molecular Markers" Agronomy 15, no. 7: 1527. https://doi.org/10.3390/agronomy15071527
APA StyleWu, G., & Jin, G. (2025). Fine Mapping of Quantitative Trait Loci (QTL) with Resistance to Common Scab in Diploid Potato and Development of Effective Molecular Markers. Agronomy, 15(7), 1527. https://doi.org/10.3390/agronomy15071527





