Optimizing Nitrogen Fixation in Vicia sativa: The Role of Host Genetic Diversity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Sample and Data Collection of Shoot, Root, and Nodule Parameters
2.3. Statistical Analysis and Additional Software
3. Results
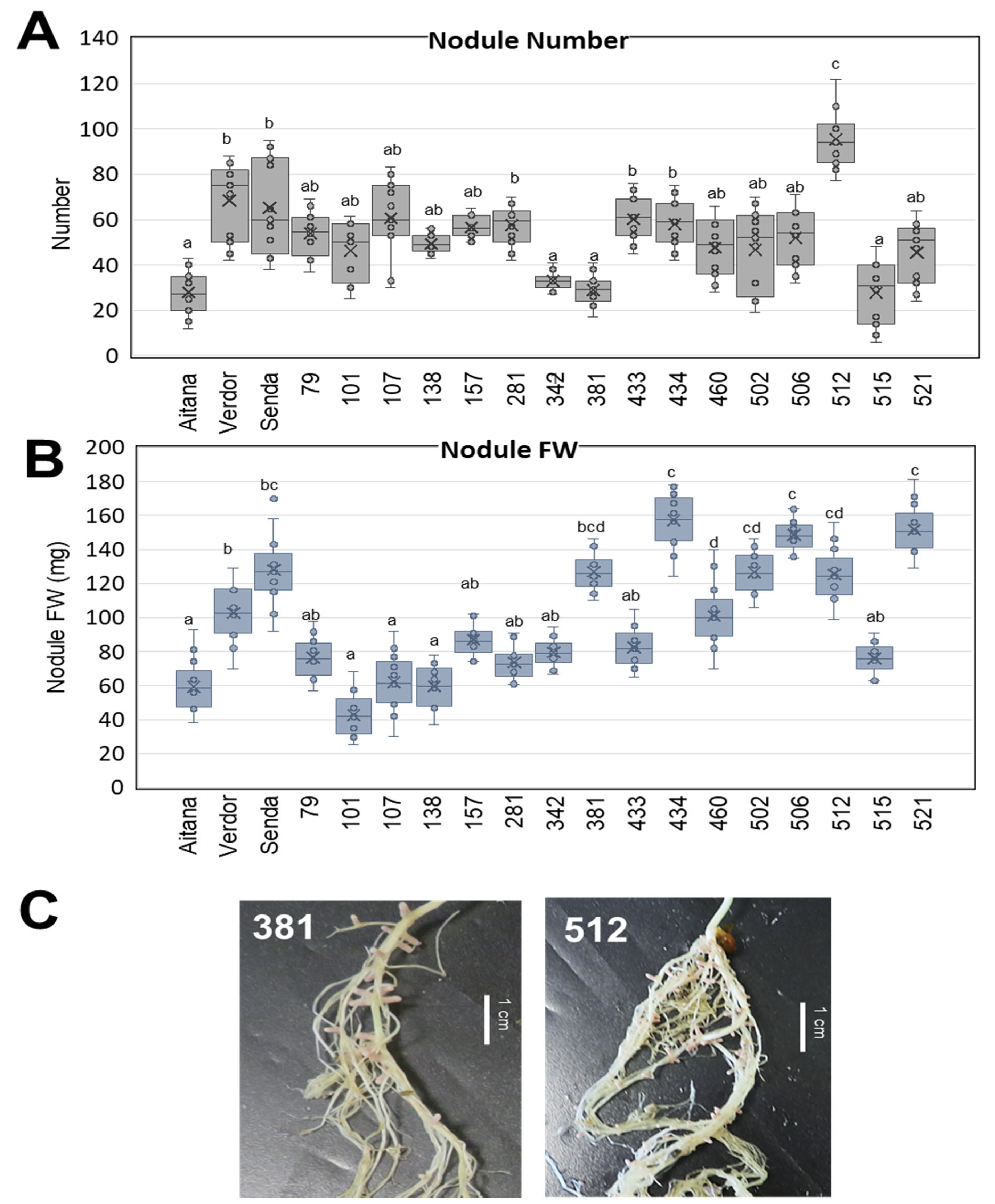
3.1. V. sativa Genotype Impacts on Nodule Formation and Nodule Development
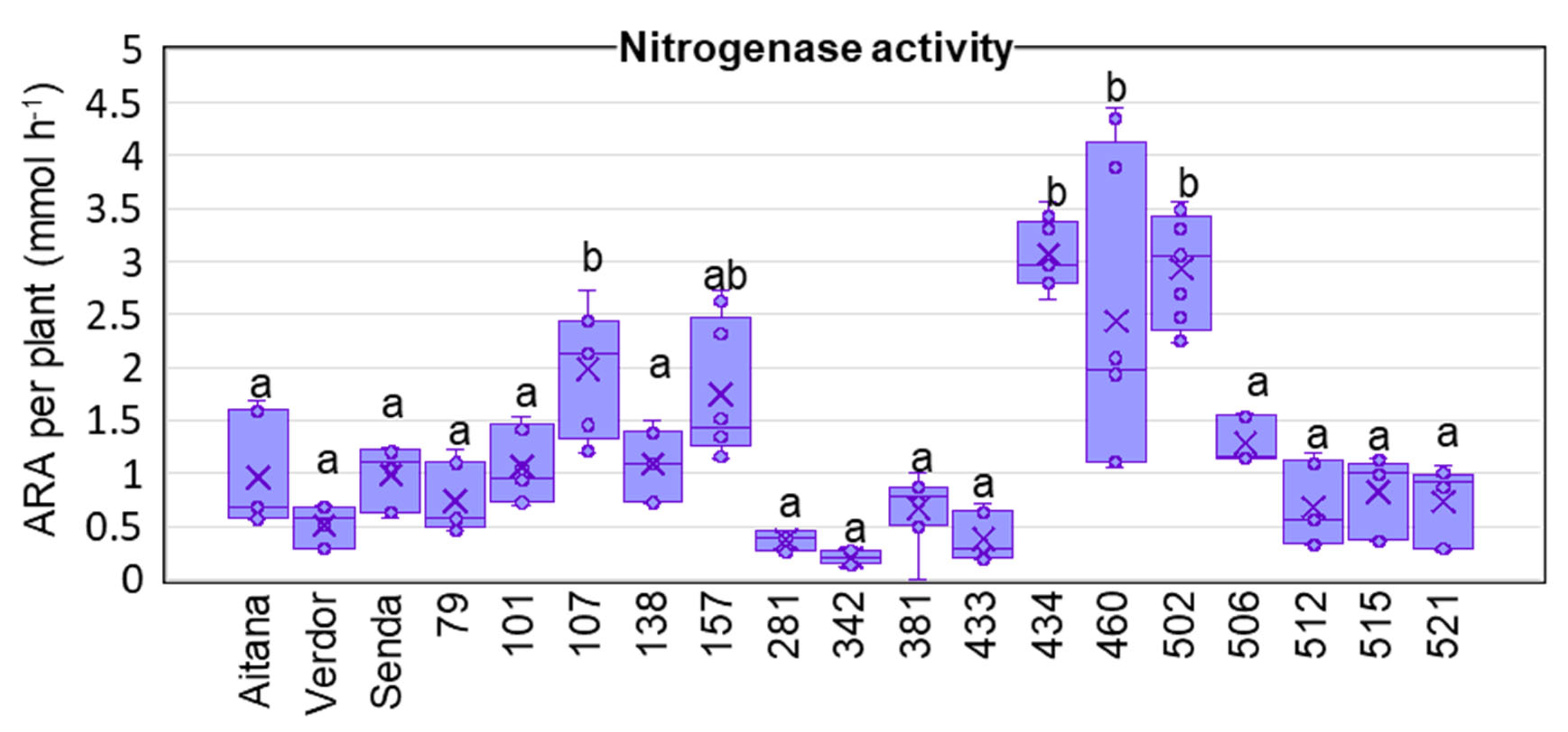
3.2. V. sativa Genotype Impacts on Nodule Nitrogenase Activity
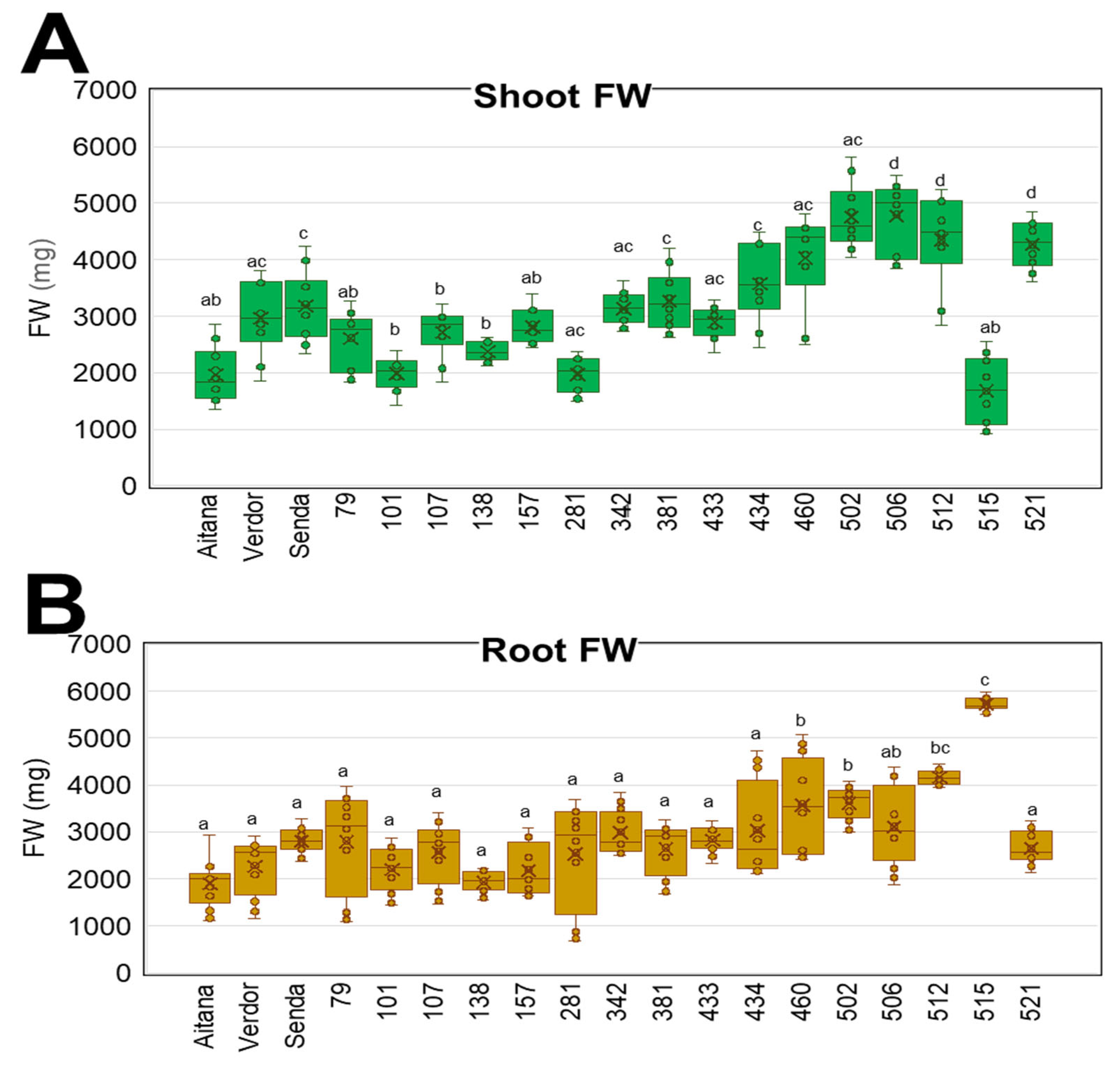
3.3. V. sativa Genotype Impacts on Shoot and Root Biomass
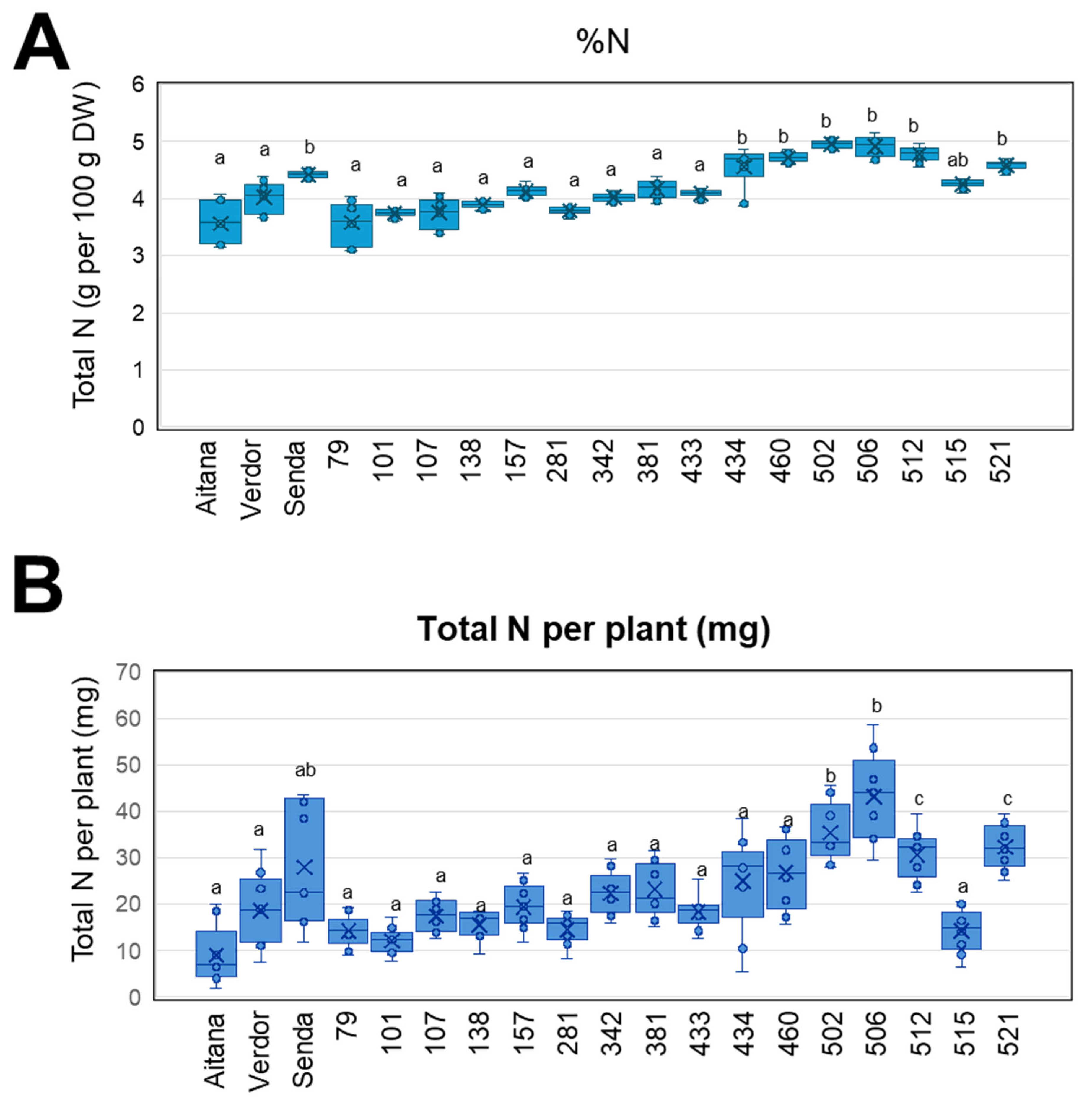
3.4. V. sativa Genotype Impacts on Biological Nitrogen Fixation
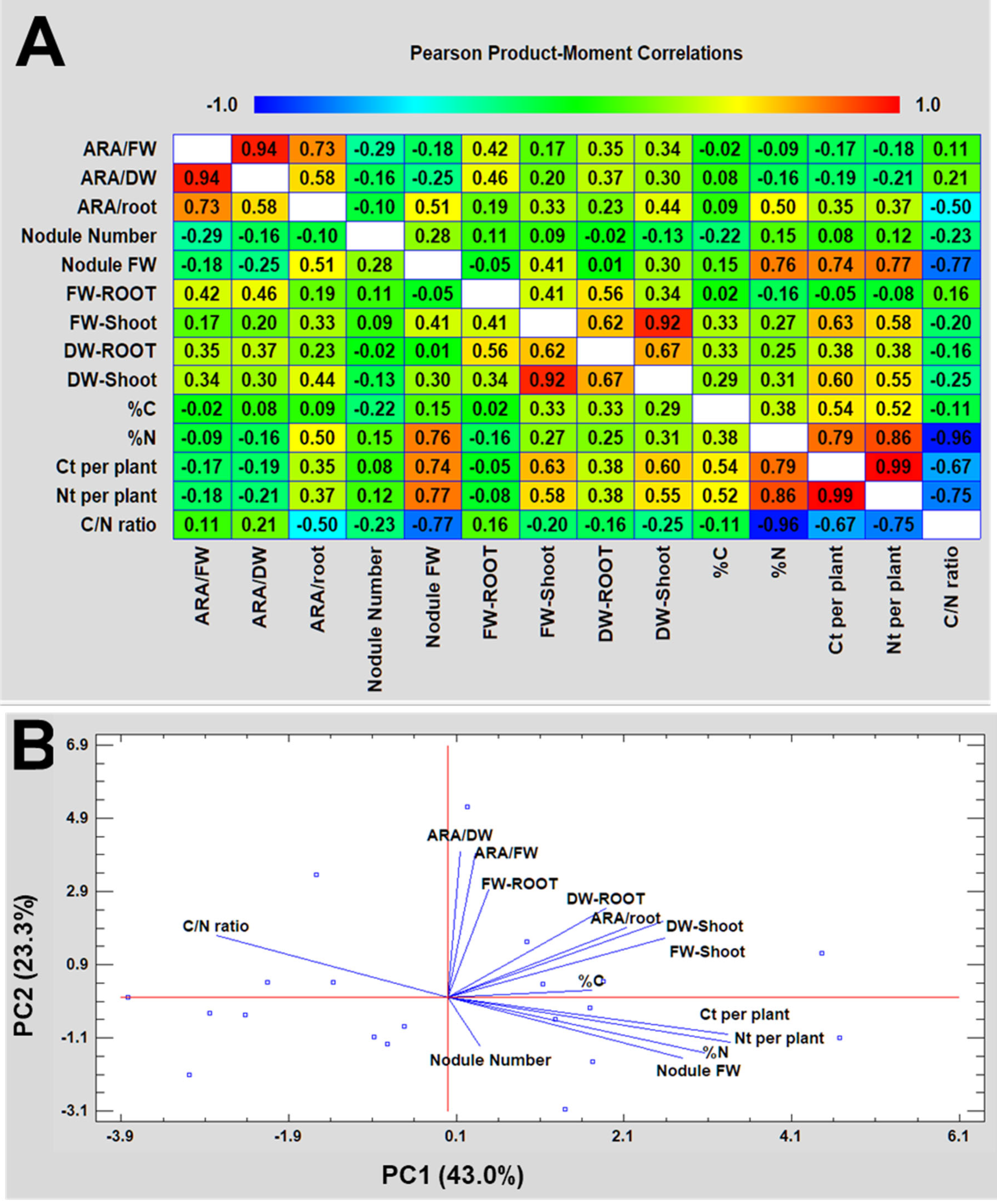
3.5. Parameter Correlation
4. Discussion
Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARA | Acetylene Reduction Assay |
| BNF | Biological Nitrogen Fixation |
| C/N | Carbon/Nitrogen |
| CRF | National Spanish Plant Genetic Resources Center |
| GWAS | Genome-Wide Association Studies |
| NUE | Nitrogen Utilization Efficiency |
| PCA | Principal Component Analysis |
| PC | Principal Component |
| QTL | Quantitative Trait Loci |
References
- Smýkal, P.; Coyne, C.J.; Ambrose, M.J.; Maxted, N.; Schaefer, H.; Blair, M.W.; Berger, J.; Greene, S.L.; Nelson, M.N.; Besharat, N.; et al. Legume Crops Phylogeny and Genetic Diversity for Science and Breeding. Crit. Rev. Plant Sci. 2015, 34, 43–104. [Google Scholar] [CrossRef]
- Soumare, A.; Boubekri, K.; Lyamlouli, K.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L. From Isolation of Phosphate Solubilizing Microbes to Their Formulation and Use as Biofertilizers: Status and Needs. Front. Bioeng. Biotechnol. 2020, 7, 425. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.J.; Moustakas, K.; Witek-Krowiak, A. Bio-based fertilizers: A practical approach towards circular economy. Bioresour. Technol. 2019, 295, 122223. [Google Scholar] [CrossRef]
- Dalias, P.; Neocleous, D. Comparative Analysis of the Nitrogen Effect of Common Agricultural Practices and Rotation Systems in a Rainfed Mediterranean Environment. Plants 2017, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Poole, P.; Ramachandran, V.; Terpolilli, J. Rhizobia: From saprophytes to endosymbionts. Nat. Rev. Microbiol. 2018, 16, 291–303. [Google Scholar] [CrossRef]
- Kebede, E. Contribution, Utilization, and Improvement of Legumes-Driven Biological Nitrogen Fixation in Agricultural Systems. Front. Sustain. Food Syst. 2021, 5, 767998. [Google Scholar] [CrossRef]
- Mng’ong’o, M.E.; Ojija, F.; Aloo, B.N. The role of Rhizobia toward food production, food and soil security through microbial agro-input utilization in developing countries. Case Stud. Chem. Environ. Eng. 2023, 8, 100404. [Google Scholar] [CrossRef]
- Zahran, H.H. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev. MMBR 1999, 63, 968–989. [Google Scholar] [CrossRef]
- Streeter, J.G. Nitrate inhibition of legume nodule growth and activity: I. Long term studies with a continuous supply of nitrate. Plant Physiol. 1985, 77, 321–324. [Google Scholar] [CrossRef]
- Sessitsch, A.; Howieson, J.G.; Perret, X.; Antoun, H.; Martínez-Romero, E. Advances in Rhizobium Research. Crit. Rev. Plant Sci. 2002, 21, 323–378. [Google Scholar] [CrossRef]
- Provorov, N.A.; Tikhonovich, I.A. Genetic resources for improving nitrogen fixation in legume-rhizobia symbiosis. Genet. Resour. Crop Evol. 2003, 50, 89–99. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, H. Enacting partner specificity in legume–rhizobia symbioses. aBIOTECH 2024. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Miwa, H.; Obirih-Opareh, J.; Suzaki, T.; Yasuda, M.; Okazaki, S. Novel rhizobia exhibit superior nodulation and biological nitrogen fixation even under high nitrate concentrations. FEMS Microbiol. Ecol. 2020, 96, fiz184. [Google Scholar] [CrossRef]
- Perkus, E.A.; Grossman, J.M.; Pfeiffer, A.; Rogers, M.A.; Rosen, C.J. Exploring Overwintered Cover Crops as a Soil Management Tool in Upper-midwest High Tunnels. HortScience 2022, 57, 171–180. [Google Scholar] [CrossRef]
- Power, J.F.; Zachariassen, J.A. Relative Nitrogen Utilization by Legume Cover Crop Species at Three Soil Temperatures. Agron. J. 1993, 85, 134–140. [Google Scholar] [CrossRef]
- Ramírez-Parra, E.; De la Rosa, L. Designing Novel Strategies for Improving Old Legumes: An Overview from Common Vetch. Plants 2023, 12, 1275. [Google Scholar] [CrossRef]
- Parr, M.; Grossman, J.M.; Reberg-Horton, S.C.; Brinton, C.; Crozier, C. Nitrogen Delivery from Legume Cover Crops in No-Till Organic Corn Production. Agron. J. 2011, 103, 1578–1590. [Google Scholar] [CrossRef]
- De la Rosa, L.; López-Román, M.I.; González, J.M.; Zambrana, E.; Marcos-Prado, T.; Ramírez-Parra, E. Common vetch, valuable germplasm for resilient agriculture: Genetic characterization and Spanish core collection development. Front. Plant Sci. 2021, 12, 617873. [Google Scholar] [CrossRef]
- De la Rosa, L.; Zambrana, E.; Ramirez-Parra, E. Molecular bases for drought tolerance in common vetch: Designing new molecular breeding tools. BMC Plant Biol. 2020, 20, 71. [Google Scholar] [CrossRef]
- Brito, B.; Palacios, J.M.; Hidalgo, E.; Imperial, J.; Ruiz-Argüeso, T. Nickel availability to pea (Pisum sativum L.) plants limits hydrogenase activity of Rhizobium leguminosarum bv. viciae bacteroids by affecting the processing of the hydrogenase structural subunits. J. Bacteriol. 1994, 176, 5297–5303. [Google Scholar] [CrossRef]
- Álvarez-Aragón, R.; Palacios, J.M.; Ramírez-Parra, E. Rhizobial symbiosis promotes drought tolerance in Vicia sativa and Pisum sativum. Environ. Exp. Bot. 2023, 208, 105268. [Google Scholar] [CrossRef]
- Ruiz-Argüeso, T.; Hanus, J.; Evans, H.J. Hydrogen production and uptake by pea nodules as affected by strains of Rhizobium leguminosarum. Arch. Microbiol. 1978, 116, 113–118. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.; Zhu, H. Genetic and molecular mechanisms underlying symbiotic specificity in legume-rhizobium interactions. Front. Plant Sci. 2018, 9, 313. [Google Scholar] [CrossRef]
- Andrews, M.; James, E.K.; Sprent, J.I.; Boddey, R.M.; Gross, E.; Dos Reis, F.B., Jr. Nitrogen fixation in legumes and actinorhizal plants in natural ecosystems: Values obtained using 15N natural abundance. Plant Ecol. Divers. 2011, 4, 131–140. [Google Scholar] [CrossRef]
- Roscher, C.; Thein, S.; Weigelt, A.; Temperton, V.M.; Buchmann, N.; Schulze, E.-D. N2 fixation and performance of 12 legume species in a 6-year grassland biodiversity experiment. Plant Soil 2011, 341, 333–348. [Google Scholar] [CrossRef]
- Terpolilli, J.J.; Hood, G.A.; Poole, P.S. What determines the efficiency of N2-fixing Rhizobium-legume symbioses? Adv. Microb. Physiol. 2012, 60, 325–389. [Google Scholar] [CrossRef] [PubMed]
- Catroux, G.; Hartmann, A.; Revellin, C. Trends in rhizobial inoculant production and use. Plant Soil 2001, 230, 21–30. [Google Scholar] [CrossRef]
- Burghardt Liana, T.; Epstein, B.; Hoge, M.; Trujillo Diana, I.; Tiffin, P. Host-Associated Rhizobial Fitness: Dependence on Nitrogen, Density, Community Complexity, and Legume Genotype. Appl. Environ. Microbiol. 2022, 88, e00526-00522. [Google Scholar] [CrossRef] [PubMed]
- Gleridou, A.; Giannopoulos, G.; Polidoros, A.N.; Mylona, P.V. Lentil Landrace Seed Origin and Genotype Affects Rhizosphere Microbiome. Agronomy 2023, 13, 2910. [Google Scholar] [CrossRef]
- Lyu, X.; Sun, C.; Lin, T.; Wang, X.; Li, S.; Zhao, S.; Gong, Z.; Wei, Z.; Yan, C.; Ma, C. Systemic regulation of soybean nodulation and nitrogen fixation by nitrogen via isoflavones. Front. Plant Sci. 2022, 13, 968496. [Google Scholar] [CrossRef]
- Maren, L.F. Widespread fitness alignment in the legume–rhizobium symbiosis. New Phytol. 2012, 194, 1096–1111. [Google Scholar]
- Kurdali, F.; Kalifa, K.; Al-Shamma, M. Cultivar differences in nitrogen assimilation, partitioning and mobilization in rain-fed grown lentil. Field Crops Res. 1997, 54, 235–243. [Google Scholar] [CrossRef]
- Iqbal, N.; Sadras, V.O.; Denison, R.F.; Zhou, Y.; Denton, M.D. Clade-dependent effects of drought on nitrogen fixation and its components—Number, size, and activity of nodules in legumes. Field Crops Res. 2022, 284, 108586. [Google Scholar] [CrossRef]
- Allito, B.B.; Ewusi-Mensah, N.; Logah, V.; Hunegnaw, D.K. Legume-rhizobium specificity effect on nodulation, biomass production and partitioning of faba bean (Vicia faba L.). Sci. Rep. 2021, 11, 3678. [Google Scholar] [CrossRef] [PubMed]
- Voisin, A.S.; Salon, C.; Jeudy, C.; Warembourg, F.R. Symbiotic N2 fixation activity in relation to C economy of Pisum sativum L. as a function of plant phenology. J. Exp. Bot. 2003, 54, 2733–2744. [Google Scholar] [CrossRef] [PubMed]
- Barsch, A.; Tellström, V.; Patschkowski, T.; Küster, H.; Niehaus, K. Metabolite profiles of nodulated alfalfa plants indicate that distinct stages of nodule organogenesis are accompanied by global physiological adaptations. Mol. Plant-Microbe Interact. MPMI 2006, 19, 998–1013. [Google Scholar] [CrossRef]
- Tang, J.; Li, W.; Wei, T.; Huang, R. Patterns and Mechanisms of Legume Responses to Nitrogen Enrichment: A Global Meta-Analysis. Plants 2024, 13, 3244. [Google Scholar] [CrossRef]
- Zhang, P.; Dumroese, R.K.; Pinto, J.R. Organic or Inorganic Nitrogen and Rhizobia Inoculation Provide Synergistic Growth Response of a Leguminous Forb and Tree. Front. Plant Sci. 2019, 10, 1308. [Google Scholar] [CrossRef]
- Li, P.; Teng, C.; Zhang, J.; Liu, Y.; Wu, X.; He, T. Characterization of drought stress-mitigating Rhizobium from faba bean (Vicia faba L.) in the Chinese Qinghai-Tibet Plateau. Front. Microbiol. 2023, 14, 1212996. [Google Scholar] [CrossRef]
- Hami, A.; El Attar, I.; Mghazli, N.; Ennajeh, S.; Ait-Ouakrim, E.H.; Bennis, M.; Oulghazi, S.; Badaoui, B.; Aurag, J.; Sbabou, L.; et al. Enhancing drought tolerance in Pisum sativum and Vicia faba through interspecific interactions with a mixed inoculum of Rhizobium laguerreae and non-host beneficial rhizobacteria. Front. Plant Sci. 2025, 16, 1528923. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Román, M.I.; Castaño-Herrero, C.; De la Rosa, L.; Ramírez-Parra, E. Optimizing Nitrogen Fixation in Vicia sativa: The Role of Host Genetic Diversity. Agronomy 2025, 15, 1479. https://doi.org/10.3390/agronomy15061479
López-Román MI, Castaño-Herrero C, De la Rosa L, Ramírez-Parra E. Optimizing Nitrogen Fixation in Vicia sativa: The Role of Host Genetic Diversity. Agronomy. 2025; 15(6):1479. https://doi.org/10.3390/agronomy15061479
Chicago/Turabian StyleLópez-Román, María Isabel, Cristina Castaño-Herrero, Lucía De la Rosa, and Elena Ramírez-Parra. 2025. "Optimizing Nitrogen Fixation in Vicia sativa: The Role of Host Genetic Diversity" Agronomy 15, no. 6: 1479. https://doi.org/10.3390/agronomy15061479
APA StyleLópez-Román, M. I., Castaño-Herrero, C., De la Rosa, L., & Ramírez-Parra, E. (2025). Optimizing Nitrogen Fixation in Vicia sativa: The Role of Host Genetic Diversity. Agronomy, 15(6), 1479. https://doi.org/10.3390/agronomy15061479






