Contribution of Roots and Shoots of Three Summer Cover Crops to Soil C and N Cycling Post-Termination
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Cover Crop Establishment
2.3. Cover Crop Biomass and Root Shoot Allocation
2.4. Cover Crop Root and Shoot Residue Decomposition Kinetics
3. Results
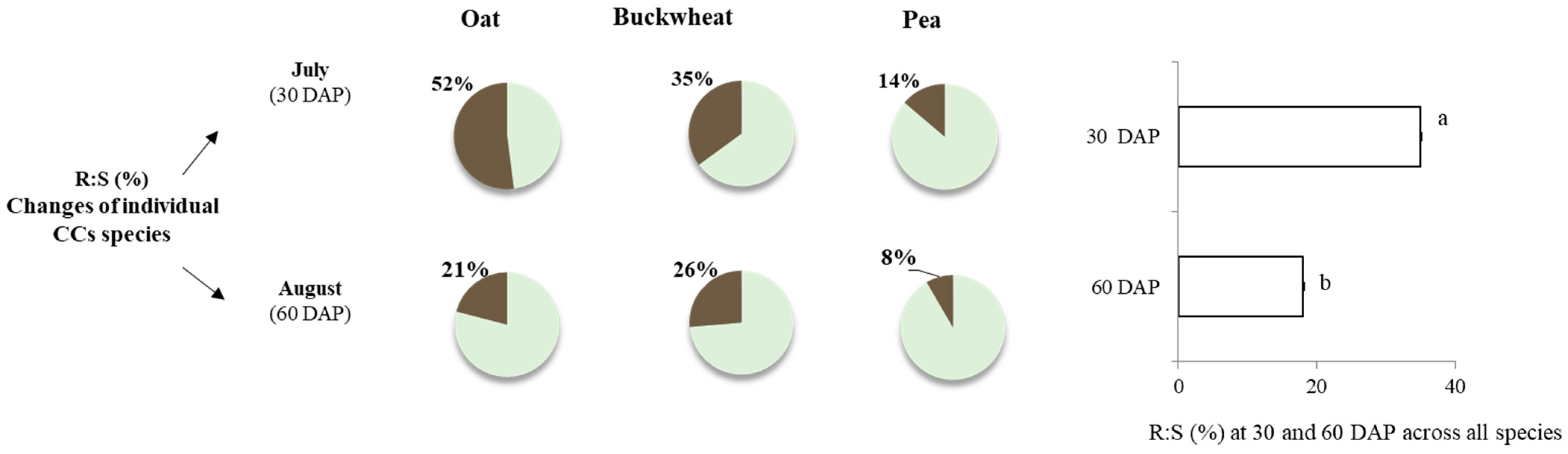
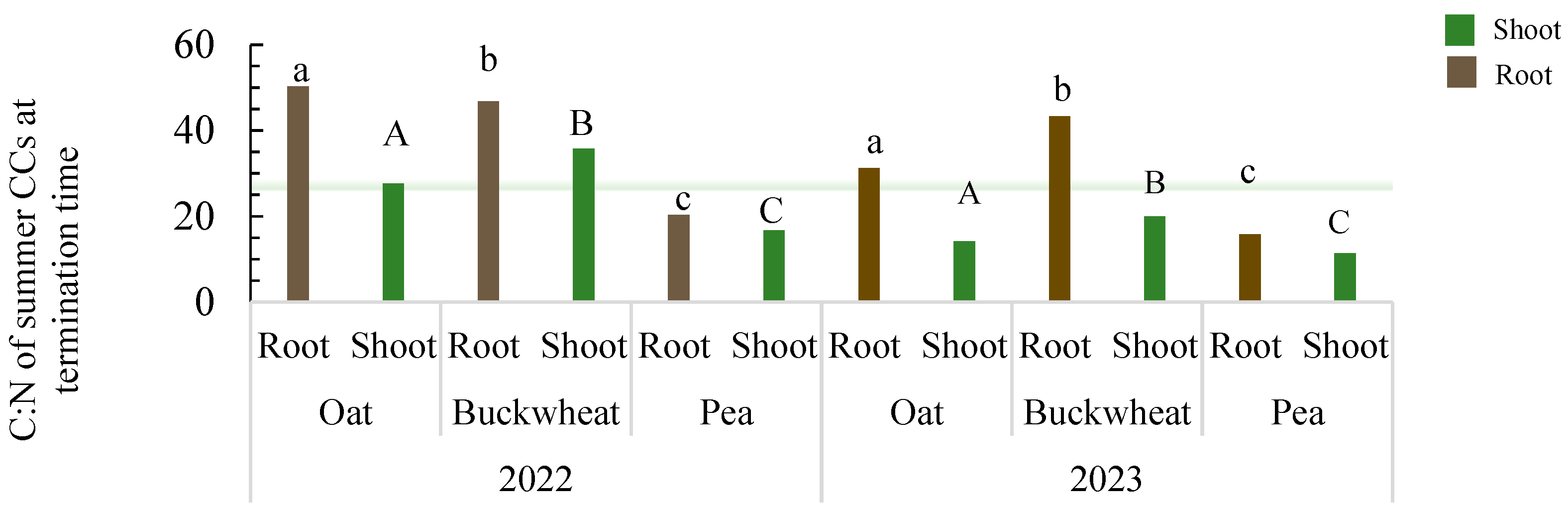
3.1. Cover Crop Biomass, Root and Shoot Assimilate Allocation, C and N Distribution
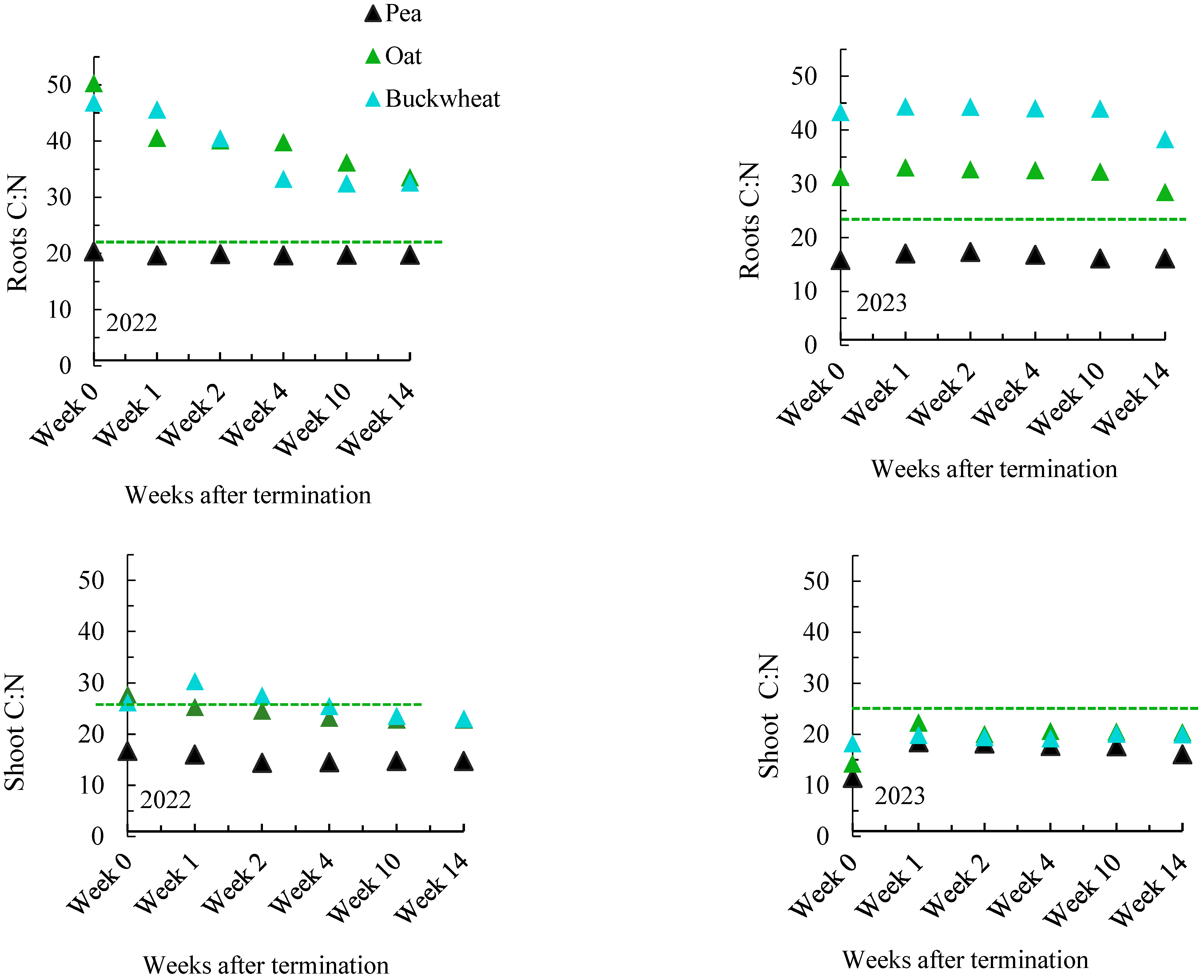
3.1.1. Decomposition Trend of Root and Shoot Residues
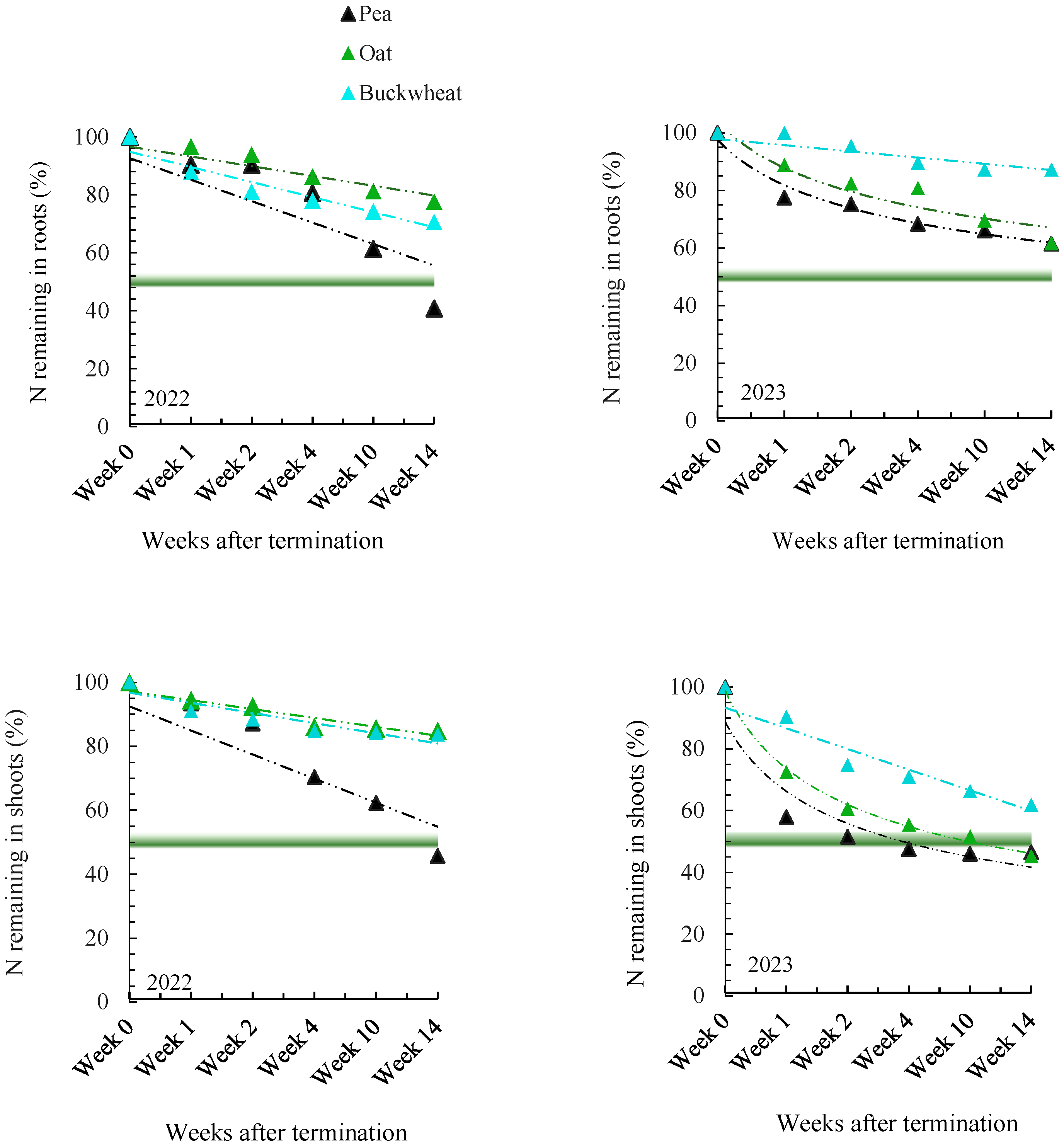
3.1.2. N and C Release from Roots and Shoots of Cover Crop Residues
4. Discussion
4.1. Cover Crop Biomass, Root and Shoot Assimilate Allocation, and C and N Distribution
4.2. Decomposition Trend of Root and Shoot Residues
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lyu, H.; Li, Y.; Wang, Y.; Wang, P.; Shang, Y.; Yang, X.; Wang, F.; Yu, A. Drive soil nitrogen transformation and improve crop nitrogen absorption and utilization—A review of green manure applications. Front. Plant Sci. 2024, 14, 1305600. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bista, P.; Deb, S.K.; Ghimire, R. Simulating cover crops impacts on soil water and nitrogen dynamics and silage yield in the semi-arid Southwestern United States. Agric. Water Manag. 2025, 307, 109246. [Google Scholar] [CrossRef]
- Ahmadnia, F.; Ebadi, A.; Hashemi, M.; Ghavidel, A.; Alebrahim, M.T. Impact of Oat (Avena sativa L.) and Daikon Radish (Raphanus Sativus var. Longipinnatus) Cover Crops on Some Soil Properties. Appl. Soil Res. 2024, 12, 1–17. [Google Scholar]
- Ghorbi, S.; Ebadi, A.; Parmoon, G.; Siller, A.; Hashemi, M. The Use of Faba Bean Cover Crop to Enhance the Sustainability and Resiliency of No-Till Corn Silage Production and Soil Characteristics. Agronomy 2023, 13, 2082. [Google Scholar] [CrossRef]
- Robertson, G.P.; Vitousek, P.M. Nitrogen in agriculture: Balancing the cost of an essential resource. Annu. Rev. Environ. Resour. 2009, 34, 97–125. [Google Scholar] [CrossRef]
- Rees, H.W.; Chow, T.L. Impact of incorporated hay mulch on selected physical, chemical and microbiological properties of a loam soil in Atlantic Canada after five years of continuous potato production. Can. J. Soil Sci. 2005, 85, 161–172. [Google Scholar] [CrossRef]
- Lami, F.; Vuerich, M.; Fabro, M.; Zandigiacomo, P.; Braidot, E.; Petrussa, E.; Barbieri, S.; Volpe, V.; Sigura, M.; Delle Vedove, G.; et al. Sustaining multiple ecosystem functions in agricultural landscapes: Effect of summer cover crops on weed control, soil quality and support to pollinators. Crop Prot. 2024, 184, 106832. [Google Scholar] [CrossRef]
- Weiler, D.A.; Giacomini, S.J.; Recous, S.; Bastos, L.M.; Pilecco, G.E.; Dietrich, G.; Aita, C. Trade-off between C and N recycling and N2O emissions of soils with summer cover crops in subtropical agrosystems. Plant Soil 2018, 433, 213–225. [Google Scholar] [CrossRef]
- Ruis, S.J.; Blanco-Canqui, H.; Creech, C.F.; Koehler-Cole, K.; Elmore, R.W.; Francis, C.A. Cover Crop Biomass Production in Temperate Agroecozones. Agron. J. 2019, 111, 1535–1551. [Google Scholar] [CrossRef]
- Balkcom, K.S.; Reeves, D.W. Sunn-hemp utilized as a legume cover crop for corn production. Agron. J. 2005, 97, 26–31. [Google Scholar] [CrossRef]
- Bulan, M.T.S.; Stoltenberg, D.E.; Posner, J.L. Buckwheat Species as Summer Cover Crops for Weed Suppression in No-Tillage Vegetable Cropping Systems. Weed Sci. 2015, 63, 690–702. [Google Scholar] [CrossRef]
- Yang, X.; Reynolds, W.D.; Drury, C.F.; Reeb, M.A. Impacts of summer-seeded legume cover crops and termination-tillage method on nitrogen availability to corn in rotation. Front. Soil Sci. 2022, 2, 1082940. [Google Scholar] [CrossRef]
- Bilenky, M.T.; Nair, A.; McDaniel, M.D. Effect of summer cover crops on cabbage yield, weed suppression, and N mineralization in a low input cropping system. Front. Sustain. Food Syst. 2022, 6, 1021639. [Google Scholar] [CrossRef]
- Angst, Š.; Mudrák, O.; Frouz, J.; Jílková, V.; Schnablová, R.; Straková, P.; Veselá, H.; Angst, G. The effect of dead standing (marcescent) biomass on litter decomposition in herbaceous flora is governed by plant functional group. Funct. Ecol. 2024, 38, 1309–1319. [Google Scholar] [CrossRef]
- Follstad Shah, J.J.; Kominoski, J.S.; Ardón, M.; Dodds, W.K.; Gessner, M.O.; Griffiths, N.A.; Hawkins, C.P.; Johnson, S.L.; Lecerf, A.; LeRoy, C.J.; et al. Global synthesis of the temperature sensitivity of leaf litter breakdown in streams and rivers. Glob. Change Biol. 2017, 23, 3064–3075. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Bishal, D.; Regmi, S.; Timilsena, K.; Lamsal, S. Effect of sowing methods and varieties on growth and yield of wheat (Triticum aestivum L.) in Dang, Nepal. Agric. Environ. Sci. 2024, 9, 58–63. [Google Scholar] [CrossRef]
- De Notaris, C.; Peixoto, L.; Mortensen, E.Ø.; Rasmussen, J. Cover crop biomass production as a predictor of nitrogen fertilizer replacement value-legumes secure positive effects. Ecosyst. Environ. 2025, 381, 109446. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Y.; Zhang, B.; Li, L.; Lin, L.; Li, X.; Zeng, Q. The effect of litter decomposition mostly depends on seasonal variation of ultraviolet radiation rather than species in a hyper-arid desert. Front. Environ. Sci. 2024, 12, 1379442. [Google Scholar] [CrossRef]
- Schulz, S.; Brankatschk, R.; Dümig, A.; Kögel-Knabner, I.; Schloter, M.; Zeyer, J. The role of microorganisms at different stages of ecosystem development for soil formation. Biogeosciences 2013, 10, 3983–3996. [Google Scholar] [CrossRef]
- Bergmann, J.; Weigelt, A.; van der Plas, F.; Laughlin, D.C.; Kuyper, T.W.; Guerrero-Ramirez, N.; Valverde-Barrantes, O.J.; Bruelheide, H.; Fresche, G.T.; Iversen, C.M.; et al. The fungal collaboration gradient dominates the root economics space in plants. Sci. Adv. 2020, 6, eaba3756. [Google Scholar] [CrossRef]
- Lavergne, S.; Vanasse, A.; Thivierge, M.N.; Halde, C. Pea-based cover crop mixtures have greater plant belowground biomass, but lower plant aboveground biomass than a pure stand of pea. Agric. Ecosyst. Environ. 2021, 322, 107657. [Google Scholar] [CrossRef]
- Amsili, J.P.; Kaye, J.P. Root traits of cover crops and carbon inputs in an organic grain rotation. Agric. Food Syst. 2021, 36, 182–191. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Li, J.; Huang, D. The Utilization and Roles of Nitrogen in Plants. Forests 2024, 15, 1191. [Google Scholar] [CrossRef]
- Wong, K.M.; Griffiths, M.; Moran, A.; Johnston, A.; Liu, A.E.; Sellers, M.A.; Topp, C.N. Cover crop cultivars and species differ in root traits potentially impacting their selection for ecosystem services. Plant Soil 2024, 500, 279–296. [Google Scholar] [CrossRef]
- Kemper, R.; Döring, T.F.; Legner, N.; Meinen, C.; Athmann, M. Oil radish, winter rye and crimson clover: Root and shoot performance in cover crop mixtures. Soil 2023, 506, 157–172. [Google Scholar] [CrossRef]
- Saadat, D.; Siller, A.; Hashemi, M. Phenology, Nitrogen Status, and Yield of Red Clover (Trifolium pretense L.) Affected by Application of Vitamin B12, Humic Acid, and Enriched Biochar. Agronomy 2023, 13, 2885. [Google Scholar] [CrossRef]
- Iwasa, Y.; Roughgarden, J. Shoot/root balance of plants: Optimal growth of a system with many vegetative organs. Popul. Biol. 1984, 25, 78–105. [Google Scholar] [CrossRef]
- Ericsson, T. Growth and shoot: Root ratio of seedlings in relation to nutrient availability. In Nutrient Uptake and Cycling in Forest Ecosystems; Springer: Dordrecht, The Netherlands, 1995; pp. 205–214. [Google Scholar] [CrossRef]
- Palomino, J.; García-Palacios, P.; de Deyn, G.B.; Martínez-García, L.B.; Sánchez-Moreno, S.; Milla, R. Impacts of plant domestication on soil microbial and nematode communities during litter decomposition. Plant Soil 2023, 487, 419–436. [Google Scholar] [CrossRef]
- García-Palacios, P.; Prieto, I.; Ourcival, J.M.; Hättenschwiler, S. Disentangling the Litter Quality and Soil Microbial Contribution to Leaf and Fine Root Litter Decomposition Responses to Reduced Rainfall. Ecosystems 2016, 19, 490–503. [Google Scholar] [CrossRef]
- Talgre, L.; Roostalu, H.; Mäeorg, E.; Lauringson, E. Nitrogen and carbon release during decomposition of roots and shoots of leguminous green manure crops. Agron. Res. 2017, 15, 594–601. [Google Scholar]
- Buyanovsky, G.A.; Wagner, G.H. Carbon transfer in a winter wheat (Triticum aestivum) ecosystem. Biol. Fertil. Soils 1987, 5, 76–82. [Google Scholar] [CrossRef]
- Blenis, P.v.; Chow, P.S.; Stringam, G.R. Effects of burial, stem portion and cultivar on the decomposition of canola straw. Can. J. Plant Sci. 1999, 79, 97–100. [Google Scholar] [CrossRef]
- Etemadi, F.; Hashemi, M.; Zandvakili, O.; Dolatabadian, A.; Sadeghpour, A. Nitrogen Contribution from Winter-Killed Faba Bean Cover Crop to Spring-Sown Sweet Corn in Conventional and No-Till Systems. Agron. J. 2018, 110, 455–462. [Google Scholar] [CrossRef]
- Stewart, C.E.; Moturi, P.; Follett, R.F.; Halvorson, A.D. Lignin biochemistry and soil N determine crop residue decomposition and soil priming. Biogeochemistry 2015, 124, 335–351. [Google Scholar] [CrossRef]
- Ntonta, S.; Zengeni, R.; Muchaonyerwa, P.; Chaplot, V. Variability in decomposition rate of sorghum cultivar residues linked to lignin content. Rhizosphere 2024, 29, 100850. [Google Scholar] [CrossRef]
- Chomel, M.; Guittonny-Larchevêque, M.; Fernandez, C.; Gallet, C.; DesRochers, A.; Paré, D.; Jackson, B.G.; Baldy, V. Plant secondary metabolites: A key driver of litter decomposition and soil nutrient cycling. J. Ecol. 2016, 104, 1527–1541. [Google Scholar] [CrossRef]
- Kaštovská, E.; Choma, M.; Angst, G.; Remus, R.; Augustin, J.; Kolb, S.; Wirth, S. Root but not shoot litter fostered the formation of mineral-associated organic matter in eroded arable soils. Soil Tillage Res. 2024, 235, 105871. [Google Scholar] [CrossRef]
- Rossi, L.M.W.; Mao, Z.; Merino-Martín, L.; Roumet, C.; Fort, F.; Taugourdeau, O.; Boukcim, H.; Fourtier, S.; del Rey-Granado, M.; Chevallier, T.; et al. Pathways to persistence: Plant root traits alter carbon accumulation in different soil carbon pools. Plant Soil 2020, 452, 457–478. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Gasser, P. Soil animals alter plant litter diversity effects on decomposition. Acad. Sci. 2005, 102, 1519–1524. [Google Scholar] [CrossRef]
- Wardle, D.A.; Nilsson, M.C.; Zackrisson, O.; Gallet, C. Determinants of litter mixing effects in a Swedish boreal forest. Biochem. 2003, 35, 827–835. [Google Scholar] [CrossRef]
- Ouédraogo, F.; Cornu, J.Y.; Fanin, N.; Janot, N.; Sourzac, M.; Parlanti, E.; Denaix, L. Changes over time in organic matter dynamics and copper solubility in a vineyard soil after incorporation of cover crop residues: Insights from a batch experiment. Chemosphere 2024, 350, 141137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Stockmann, R.; Ng, K.; Ajlouni, S. Revisiting phytate-element interactions: Implications for iron, zinc and calcium bioavailability, with emphasis on legumes. Food Sci. Nutr. 2022, 62, 1696–1712. [Google Scholar] [CrossRef]
- Clark, A. (Ed.) Managing Cover Crops Profitably; Diane Publishing: Collingdale, PA, USA, 2008. [Google Scholar]
- Björkman, T.; Shail, J.W. Using a Buckwheat Cover Crop for Maximum Weed Suppression after Early Vegetables. HortTechnology 2013, 23, 575–580. [Google Scholar] [CrossRef]
- Sainju, U.M.; Lenssen, A.; Caesar-Tonthat, T.; Waddell, J. Tillage and Crop Rotation Effects on Dryland Soil and Residue Carbon and Nitrogen. Soil Sci. Soc. Am. J. 2006, 70, 668–678. [Google Scholar] [CrossRef]
- Sturm, D.J.; Peteinatos, G.; Gerhards, R. Contribution of allelopathic effects to the overall weed suppression by different cover crops. Weed Res. 2018, 58, 331–337. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Mizutani, J.; Hasegawa, K. Allelopathy of oats. II. Allelochemical effect of l-Tryptophan and its concentration in oat root exudates. J. Chem. Ecol. 1994, 20, 315–319. [Google Scholar] [CrossRef]
- Livesley, S.J.; Stacey, C.L.; Gregory, P.J.; Buresh, R.J. Sieve size effects on root length and biomass measurements of maize (Zea mays) and Grevillea robusta. Plant Soil 1999, 207, 183–193. [Google Scholar] [CrossRef]
- Amato, M.; Pardo, A. Root length and biomass losses during sample preparation with different screen mesh sizes. Plant Soil 1994, 161, 299–303. [Google Scholar] [CrossRef]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Novelli, L.E.; Caviglia, O.P.; Piñeiro, G. Increased cropping intensity improves crop residue inputs to the soil and aggregate-associated soil organic carbon stocks. Soil Tillage Res. 2017, 165, 128–136. [Google Scholar] [CrossRef]
- Smychkovich, A.; Glaze-Corcoran, S.; Keiser, A.; Hashemi, M. Assessing the root and shoot composition, decomposition, carbon contribution and nitrogen mineralization trends of single species and mixed cover crops. Field Crops Res. 2025, 327, 109902. [Google Scholar] [CrossRef]
- Almeida, L.F.; Correndo, A.A.; Hefley, T.; Hintz, G.; Prasad, P.V.V.; Licht, M.; Casteel, S.; Singh, M.; Naeve, S.; Bais, J.; et al. Assessing the influence of environmental drivers on soybean seed yield and nitrogen fixation estimates and uncertainties in the United States. European Eur. J. Agron. 2025, 162, 127428. [Google Scholar] [CrossRef]
- Huddell, A.; Needelman, B.; Law, E.P.; Ackroyd, V.J.; Bagavathiannan, M.v.; Bradley, K.; Davis, A.S.; Evans, J.A.; Everman, W.J.; Flessner, M.; et al. Early-season biomass and weather enable robust cereal rye cover crop biomass predictions. Agric. Environ. Lett. 2024, 9, e20121. [Google Scholar] [CrossRef]
- Duiker, S.W. Establishment and termination dates affect fall-established cover crops. Agron. J. 2014, 106, 670–678. [Google Scholar] [CrossRef]
- Puget, P.; Drinkwater, L.E. Short-Term Dynamics of Root- and Shoot-Derived Carbon from a Leguminous Green Manure. Soil Sci. Soc. Am. J. 2001, 65, 771–779. [Google Scholar] [CrossRef]
- Williams, M.A.; Myrold, D.D.; Bottomley, P.J. Carbon flow from 13C-labeled straw and root residues into the phospholipid fatty acids of a soil microbial community under field conditions. Soil Biol. Biochem. 2006, 38, 759–768. [Google Scholar] [CrossRef]
- Van der Werf, A. Growth analysis and photoassimilate partitioning. In Photoassimilate Distribution in Plants and Crops: Source–Sink Relationships; Routledge: London, UK, 1996; pp. 1–20. [Google Scholar]
- Amanullah. Wheat and Rye Differ in Drymatter Partitioning, Shoot-Root Ratio and Water Use Efficiency Under Organic and Inorganic Soils. Plant Nutr. 2014, 37, 1885–1897. [Google Scholar] [CrossRef]
- Kurz, W.A.; Beukema, S.J.; Apps, M.J. Estimation of root biomass and dynamics for the carbon budget model of the Canadian forest sector. Can. J. For. Res. 1996, 26, 1973–1979. [Google Scholar] [CrossRef]
- Snowdon, P.; Eamus, D.; Gibbons, P.; Khanna, P.; Keith, H.; Raison, J.; Kirschbaum, M. Synthesis of Allometrics, Review of Root Biomass and Design of Future Woody Biomass Sampling Strategies; The Lead Commonwealth Agency on Greenhouse Matters: Canberra, Australia, 2002. Available online: https://www.dcceew.gov.au/ (accessed on 6 June 2025).
- Aerts, R.; Bakker, C.; de Caluwe, H. Root turnover as determinant of the cycling of C, N, and P in a dry heathland ecosystem. Biogeochemistry 1992, 15, 175–190. [Google Scholar] [CrossRef]
- Jain, S. Adaptive strategies: Polymorphism, plasticity, and homeostasis. In Topics in Plant Population Biology; Columbia University Press: New York, NY, USA, 1979; pp. 160–187. [Google Scholar]
- Berg, B.; McClaugherty, C. Decomposition and ecosystem function. In Plant Litter; Springer: Berlin/Heidelberg, Germany, 2003; pp. 203–237. [Google Scholar] [CrossRef]
- Pane, C.; Celano, G.; Piccolo, A.; Villecco, D.; Spaccini, R.; Palese, A.M.; Zaccardelli, M. Effects of on-farm composted tomato residues on soil biological activity and yields in a tomato cropping system. Chem. Biol. Technol. Agric. 2015, 2, 4. [Google Scholar] [CrossRef]
- Xu, H.; Wang, M.; You, C.; Tan, B.; Xu, L.; Li, H.; Zhang, L.; Wang, L.; Liu, S.; Hou, G.; et al. Warming effects on C:N:P stoichiometry and nutrient limitation in terrestrial ecosystems. Soil Tillage Res. 2024, 235, 105896. [Google Scholar] [CrossRef]
- Melillo, J.M.; Frey, S.D.; DeAngelis, K.M.; Werner, W.J.; Bernard, M.J.; Bowles, F.P.; Pold, G.; Knorr, M.A.; Grandy, A.S. Long-term pattern and magnitude of soil carbon feedback to the climate system in a warming world. Science 2017, 358, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Justes, E.; Mary, B.; Nicolardot, B. Quantifying and modelling C and N mineralization kinetics of catch crop residues in soil: Parameterization of the residue decomposition module of STICS model for mature and non mature residues. Plant Soil 2009, 325, 171–185. [Google Scholar] [CrossRef]
- Trinsoutrot, I.; Recous, S.; Bentz, B.; Linères, M.; Chèneby, D.; Nicolardot, B. Biochemical Quality of Crop Residues and Carbon and Nitrogen Mineralization Kinetics under Nonlimiting Nitrogen Conditions. Soil Sci. Soc. Am. J. 2000, 64, 918–926. [Google Scholar] [CrossRef]
- Ghahremani, S.; Ebadi, A.; Tobeh, A.; Hashemi, M.; Sedghi, M.; Gholipoouri, A.; Barker, A.v. Short-Term Impact of Monocultured and Mixed Cover Crops on Soil Properties, Weed Suppression, and Lettuce Yield. Commun. Soil Sci. Plant Anal. 2021, 52, 406–415. [Google Scholar] [CrossRef]
- Patel, S.; Singh, A.; Sawyer, J.E.; Lundvall, J.P. Root and shoot biomass and nutrient composition of winter rye cover crop following corn and soybean. Agrosyst. Geosci. Environ. 2024, 7, e70017. [Google Scholar] [CrossRef]
- Jani, A.D.; Grossman, J.M.; Smyth, T.J.; Hu, S. Influence of soil inorganic nitrogen and root diameter size on legume cover crop root decomposition and nitrogen release. Plant Soil 2015, 393, 57–68. [Google Scholar] [CrossRef]
- Doran, J.W.; Smith, M.S. Role of Cover Crops in the Nitrogen Cycle. In Cover Crops for Clean Water; Har grove, W.L., Ed.; Soil and Water Conservation Society: Ankeny, IA, USA, 1991; pp. 85–90. Available online: https://www.scirp.org/reference/referencespapers?referenceid=2338314 (accessed on 26 May 2025).
- Kavdir, Y.; Smucker, A.J.M. Soil aggregate sequestration of cover crop root and shoot-derived nitrogen. Plant Soil 2005, 272, 263–276. [Google Scholar] [CrossRef]
- Gardner, M.; Sarrantonio, M. Cover Crop Root Composition and Density in a Long-Term Vegetable Cropping System Trial. Sustain. Agric. 2012, 36, 719–737. [Google Scholar] [CrossRef]
- Hansen, V.; Eriksen, J.; Jensen, L.S.; Thorup-Kristensen, K.; Magid, J. Towards integrated cover crop management: N, P and S release from aboveground and belowground residues. Agric. Ecosyst. Environ. 2021, 313, 107392. [Google Scholar] [CrossRef]
- Adamczyk, B.; Simon, J.; Kitunen, V.; Adamczyk, S.; Smolander, A. Tannins and Their Complex Interaction with Different Organic Nitrogen Compounds and Enzymes: Old Paradigms versus Recent Advances. ChemistryOpen. 2017, 6, 610. [Google Scholar] [CrossRef] [PubMed]







| pH | Macronutrients (mg kg−1) | Micronutrients (mg kg−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P | K | Ca | Mg | S | B | Mn | Zn | Cu | Fe | ||
| 2022 | 6.6 | 13.4 | 80 | 569 | 75 | 5.6 | 0 | 1.2 | 0.9 | 1.3 | 2.1 |
| 2023 | 6.8 | 19.7 | 110 | 757 | 89 | 6.8 | 0.1 | 2.7 | 0.5 | 1.0 | 1.6 |
| Cover Crop | Seedling Rate kg ha−1 | Type | Root Characteristic | Allelopathy Potential |
|---|---|---|---|---|
| Fagopyrum esculentum Buckwheat | 67 | broadleaf/non-legume-non-grass | Dense, fibrous | Reported [47] |
| Pisum sativum L. Pea | 67 | broadleaf/legume | Tap root | Not observed |
| Avena sativa L. Oat | 100 | grass | Dense, fibrous | Reported [48] |
| Activity Description | (2023) | (2023) |
|---|---|---|
| CC seeding date | 23 June | 10 July |
| Biomass measurement | July–August | July–August |
| Cover crops termination | 23 August | 21 August |
| CC growth duration | 60 days | 42 days |
| Litter bag placement | 29 August | 26 August |
| CC litter bag study duration | 100 days | 100 days |
| Year | Cover Crop Species | Plant Part | Biomass (kg ha−1) | Biomass Allocation (%) of Total Biomass | Nutrient Yield (kg ha−1) N C | Nutrient Released (kg ha−1 After 14 Weeks) N C | ||
|---|---|---|---|---|---|---|---|---|
| 2022 | Oat | Root | 1393 ± 0.1 b * | 19.49 | 6.15 ± 0.01 a | 309.6 ± 0.01 a | 1.30 ± 0.1 | 81.0 ± 2.9 |
| Shoot | 6478 ± 0.1 B | 80.51 | 59.4 ± 0.03 A | 1631.1 ± 0.01 A | 32.00 ± 0.7 | 204.7 ± 2.1 | ||
| Buckwheat | Root | 1992 ± 0.5 c | 17.95 | 8.7 ± 0.01 b | 410.0 ± 0.02 b | 3.06 ± 0.09 | 99.1 ± 0.8 | |
| Shoot | 7027 ± 0.7 C | 82.05 | 54.4 ± 0.04 B | 1999.2 ± 0.01 B | 14.81 ± 0.17 | 442.9 ± 0.8 | ||
| Pea | Root | 205 ± 0.1 a | 8.08 | 2.81 ± 0.02 c | 57.1 ± 0.02 c | 1.37 ± 0.4 | 9.2 ± 6.8 | |
| Shoot | 2567 ± 0.9 A | 91.93 | 44.9 ± 0.05 C | 751.3 ± 0.01 C | 13.63 ± 0.1 | 190.2 ± 2.5 | ||
| 2023 | Oat | Root | 382 ± 58.1 a | 19.49 | 4.7 ± 0.7 a | 148.4 ± 22.7 a | 1.30 ± 0.07 | 81.0 ± 1.1 |
| Shoot | 1416 ± 25.3 A | 80.51 | 40.4 ± 7.2 A | 586.4 ± 10.3 A | 32.0 ± 0.07 | 204.7 ± 1.7 | ||
| Buckwheat | Root | 362 ± 41.1 a | 17.95 | 3.1 ± 0.3 a | 134.90 ± 15.3 a | 3.06 ± 0.04 | 99.1 ± 3.8 | |
| Shoot | 2261 ± 46.6 A | 82.05 | 50 ± 10.27 A | 999.7 ± 20.5 A | 14.81 ± 0.3 | 442.9 ±1.07 | ||
| Pea | Root | 111 ± 12.7 b | 8.08 | 2.9 ± 0.3 a | 45.9 ± 5.3 b | 1.37 ± 0.05 | 9.2 ± 3.0 | |
| Shoot | 1160 ± 52.63 A | 91.93 | 42.8 ± 1.9 A | 490.50 ± 22.2 A | 13.63 ± 0.15 | 190.2 ± 0.7 | ||
| Plant Part | Year | Residue Species | Mean ± SE (Log-Transformed) | Equation | RMSE | R2 |
|---|---|---|---|---|---|---|
| Roots | 2022 | Buckwheat | 4.24 ± 0.17 b | y = −0.14x + 4.59 * | 0.016 | 0.99 |
| Oat | 4.28 ± 0.04 b | y = −0.25x + 4.58 * | 0.006 | 0.99 | ||
| Pea | 4.13 ± 0.03 a | y = 0.008x2 − 0.57x + 4.59 * | 0.032 | 0.99 | ||
| 2023 | Buckwheat | 4.35 ± 0.03 b | y = −0.11x + 4.57 * | 0.054 | 0.95 | |
| Oat | 4.32 ± 0.03 b | y = −0.12x + 4.59 * | 0.011 | 0.99 | ||
| Pea | 4.00 ± 0.02 a | y = 0.076x2 − 0.39x + 4.59 * | 0.022 | 0.99 | ||
| Shoots | 2022 | Buckwheat | 4.30 ± 0.03 C | y = −0.13x + 4.57 * | 0.053 | 0.97 |
| Oat | 4.37 ± 0.01 B | y = −0.10x + 4.59 * | 0.038 | 0.98 | ||
| Pea | 4.18 ± 0.03 A | y = 0.057x2 − 0.31x + 4.58 * | 0.039 | 0.9 | ||
| 2023 | Buckwheat | 4.20 ± 0.03 B | y = −0.27x + 4.62 * | 0.060 | 0.98 | |
| Oat | 3.97 ± 0.02 AB | y = −0.39x + 4.59 * | 0.020 | 0.99 | ||
| Pea | 3.82 ± 0.08 A | y = 0.107x2 − 0.61x + 4.60 * | 0.058 | 0.99 |
| Plant Part | Year | Residue Species | Mean ± SE (Log Transformed) | Equation | RMSE | R2 |
|---|---|---|---|---|---|---|
| Roots | 2022 | Buckwheat | 4.39 ± 0.01 c | y = −0.14x + 4.59 * | 0.017 | 0.99 |
| Oat | 4.48 ± 0.01 b | y = −0.02x + 4.60 * | 0.006 | 0.99 | ||
| Pea | 4.30 ± 0.01 a | y = −0.057x + 4.58 * | 0.032 | 0.99 | ||
| 2023 | Buckwheat | 4.45 ± 0.01 b | y = −0.07x + 4.59 * | 0.051 | 0.93 | |
| Oat | 4.28 ± 0.05 a | y = 0.01096x2 − 0.11x + 4.59 * | 0.006 | 0.99 | ||
| Pea | 4.32 ± 0.03 a | y = 0.005x2 − 0.08x + 4.58 * | 0.028 | 0.99 | ||
| Shoots | 2022 | Buckwheat | 4.48 ± 0.05 B | y = −0.11x + 4.60 * | 0.012 | 0.99 |
| Oat | 4.50 ± 0.01 B | y = −0.05x + 4.59 * | 0.011 | 0.96 | ||
| Pea | 4.30 ± 0.01 A | y = −0.08x + 4.60 * | 0.034 | 0.99 | ||
| 2023 | Buckwheat | 4.33 ± 0.01 B | y = −0.18x + 4.61 * | 0.044 | 0.97 | |
| Oat | 3.98 ± 0.02 A | y = 0.0946x2 − 0.47x + 4.56 * | 0.084 | 0.97 | ||
| Pea | 4.02 ± 0.02 A | y = 0.11x2 + 0.51x + 4.55 * | 0.095 | 0.95 |
| Plant Part | Year | Residue Species | Mean ± SE (Log-Transformed) | Equation | RMSE | R2 |
|---|---|---|---|---|---|---|
| Root | 2022 | Buckwheat | 4.19 ± 0.03 b | y = −0.18x + 4.57 * | 0.066 | 0.97 |
| Oat | 4.50 ± 0.03 b | y = −0.10x + 4.561 * | 0.035 | 0.90 | ||
| Pea | 4.42 ± 0.02 a | y = −0.06x + 4.58 * | 0.063 | 0.91 | ||
| 2023 | Buckwheat | 4.52 ± 0.17 a | y = −0.04x + 4.59 * | 0.00677 | 0.99 | |
| Oat | 4.39± 0.04 b | y = −0.09x + 4.60 * | 0.01954 | 0.99 | ||
| Pea | 4.56 ± 0.03 a | y = −0.056x + 4.59 | 0.01295 | 0.96 | ||
| Shoots | 2022 | Buckwheat | 4.55 ± 0.17 A | y = −0.03x + 4.59 * | 0.00867 | 0.97 |
| Oat | 4.54 ± 0.04 B | y = −0.04x + 4.60 * | 0.02423 | 0.91 | ||
| Pea | 4.43 ± 0.03 A | y = −0.76x + 4.67 * | 0.42677 | 0.51 | ||
| 2023 | Buckwheat | 4.48± 0.03 A | y = −0.21x + 4.61 * | 0.060 | 0.85 | |
| Oat | 4.48 ± 0.02 A | y = −0.09x + 4.61 * | 0.04964 | 0.85 | ||
| Pea | 4.48 ± 0.08 A | y = −0.14x + 4.60 * | 0.06534 | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saadat, D.; Hashemi, M.; Herbert, S.; Siller, A. Contribution of Roots and Shoots of Three Summer Cover Crops to Soil C and N Cycling Post-Termination. Agronomy 2025, 15, 1467. https://doi.org/10.3390/agronomy15061467
Saadat D, Hashemi M, Herbert S, Siller A. Contribution of Roots and Shoots of Three Summer Cover Crops to Soil C and N Cycling Post-Termination. Agronomy. 2025; 15(6):1467. https://doi.org/10.3390/agronomy15061467
Chicago/Turabian StyleSaadat, Dorna, Masoud Hashemi, Stephen Herbert, and Artie Siller. 2025. "Contribution of Roots and Shoots of Three Summer Cover Crops to Soil C and N Cycling Post-Termination" Agronomy 15, no. 6: 1467. https://doi.org/10.3390/agronomy15061467
APA StyleSaadat, D., Hashemi, M., Herbert, S., & Siller, A. (2025). Contribution of Roots and Shoots of Three Summer Cover Crops to Soil C and N Cycling Post-Termination. Agronomy, 15(6), 1467. https://doi.org/10.3390/agronomy15061467








