Study on the Effects of Planting Alfalfa (Medicago sativa L.) and Adding Biochar on Soil Fertility in Jujube Orchards
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Experimental Area
2.2. Experimental Design
2.3. Sample Collection
2.4. Indicator Determination Method
2.5. Data Analysis
3. Results
3.1. Effect of Biochar Addition on Alfalfa Growth
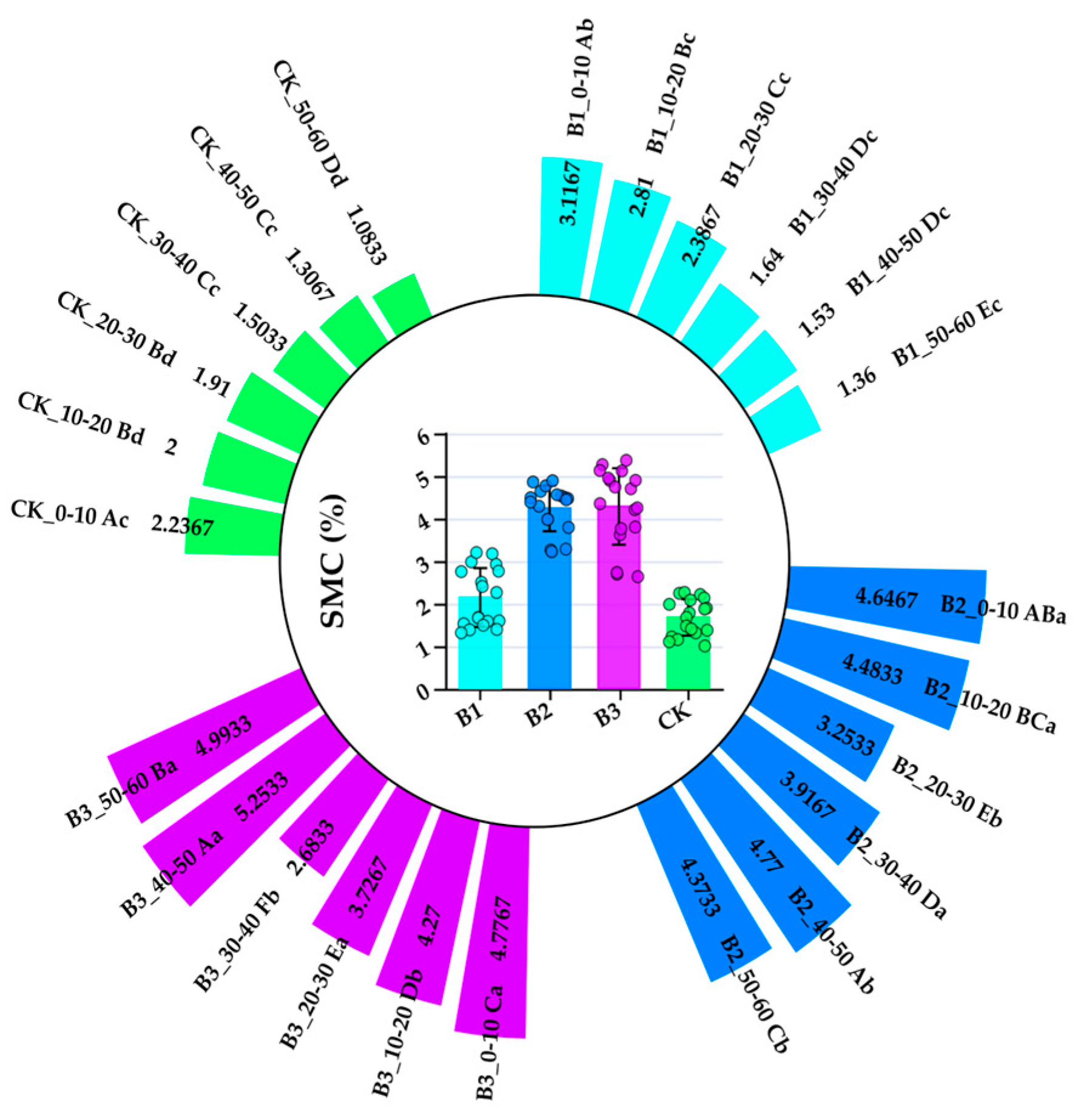
3.2. Effects of Planting Alfalfa and Applying Different Gradients of Biochar on Soil Moisture Content
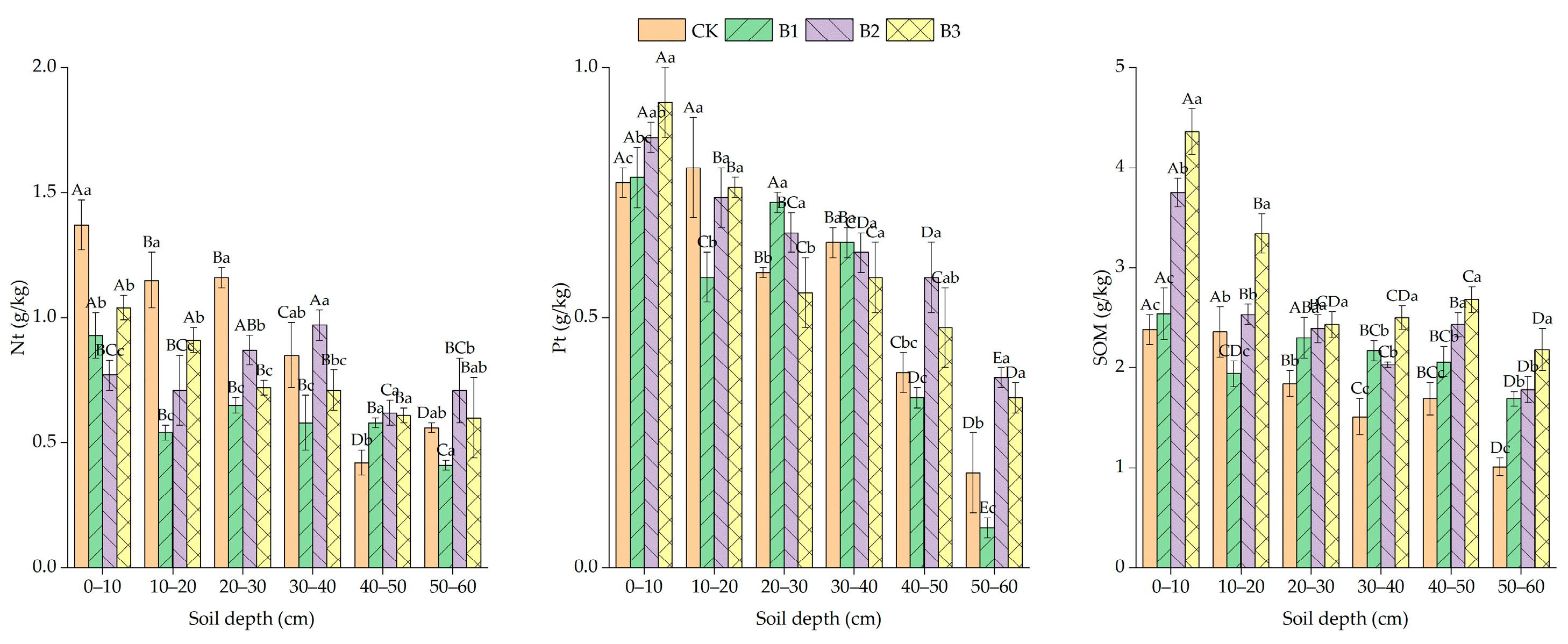
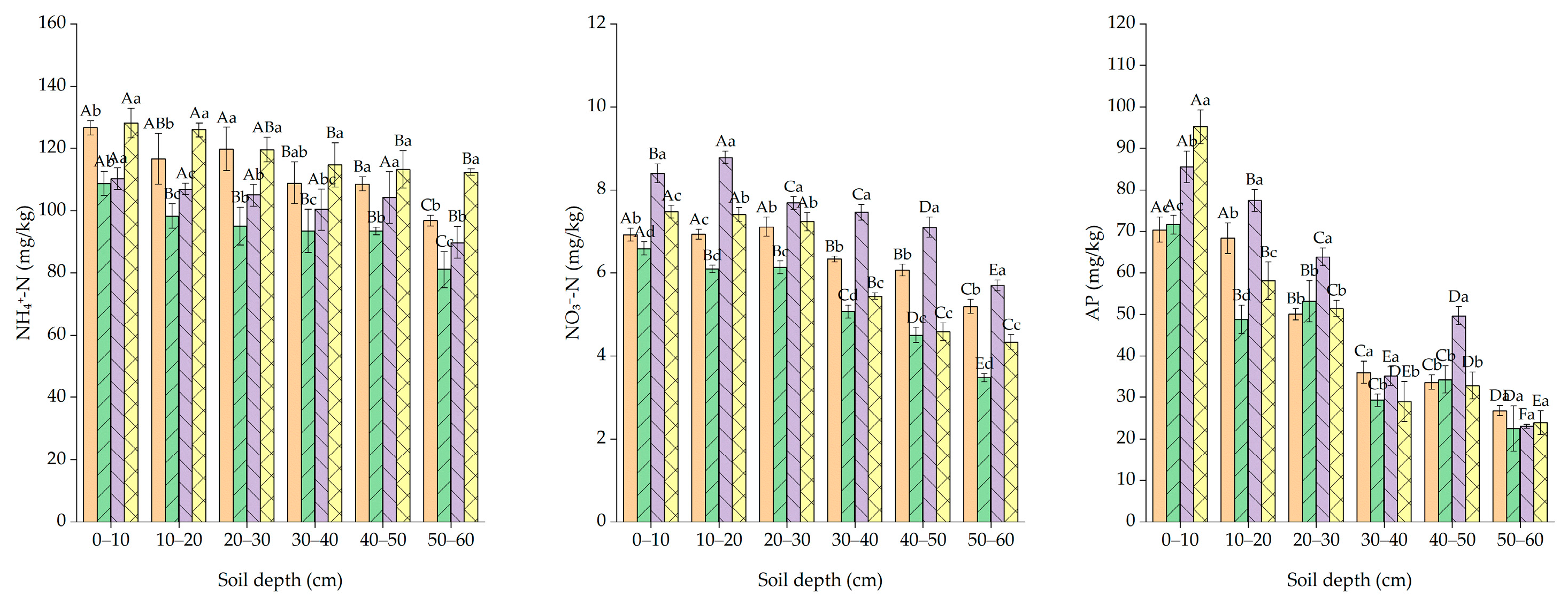
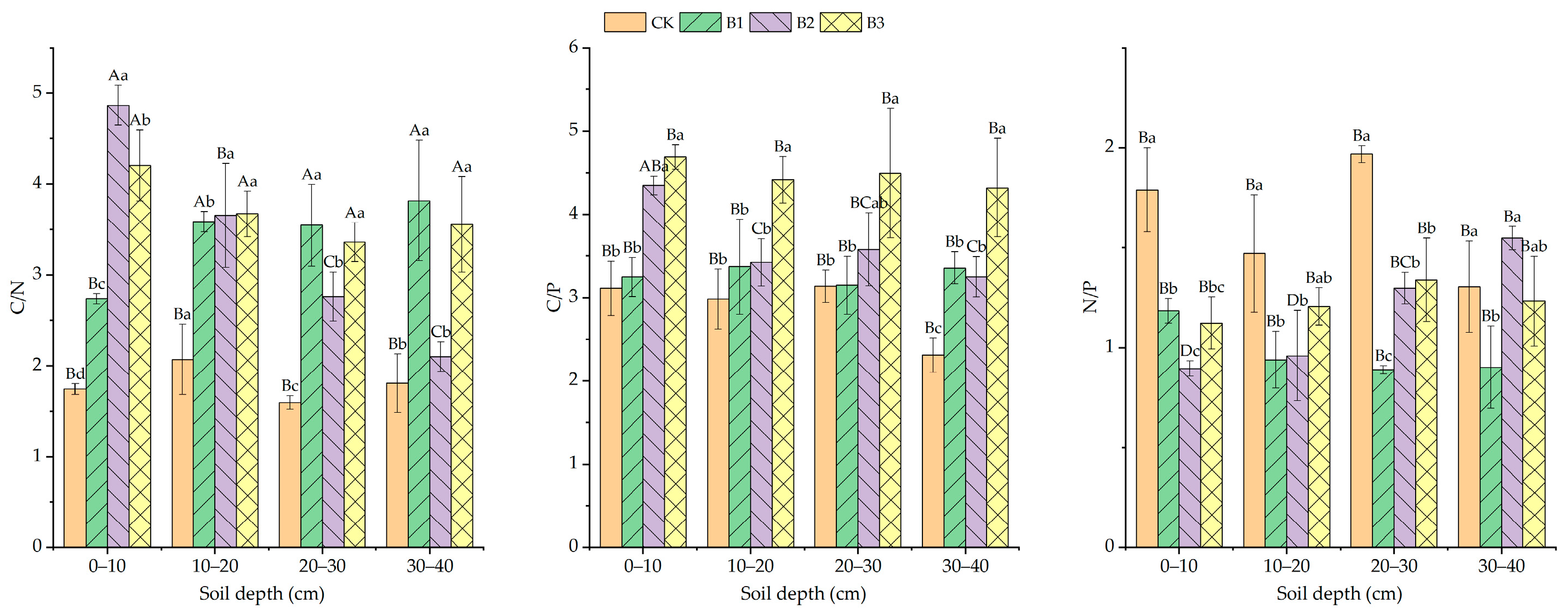
3.3. Effect of Planting Alfalfa with Different Gradients of Biochar on Soil Nutrient Status
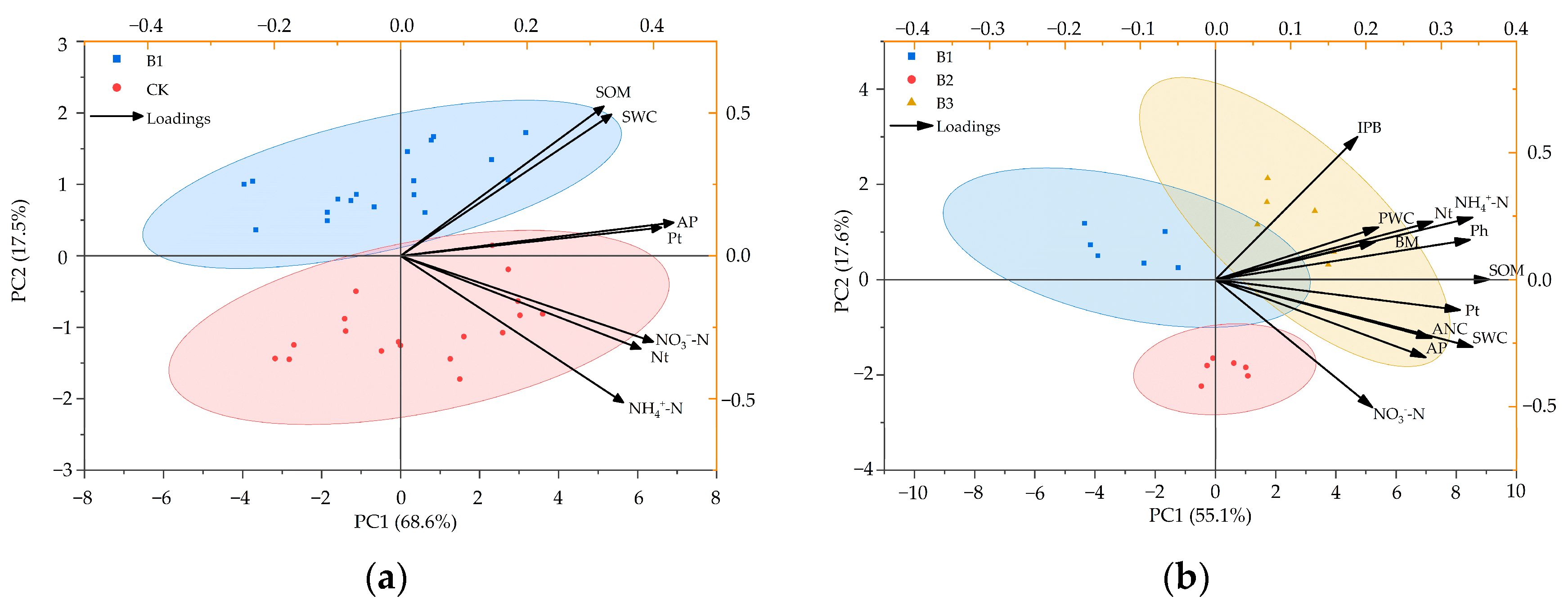
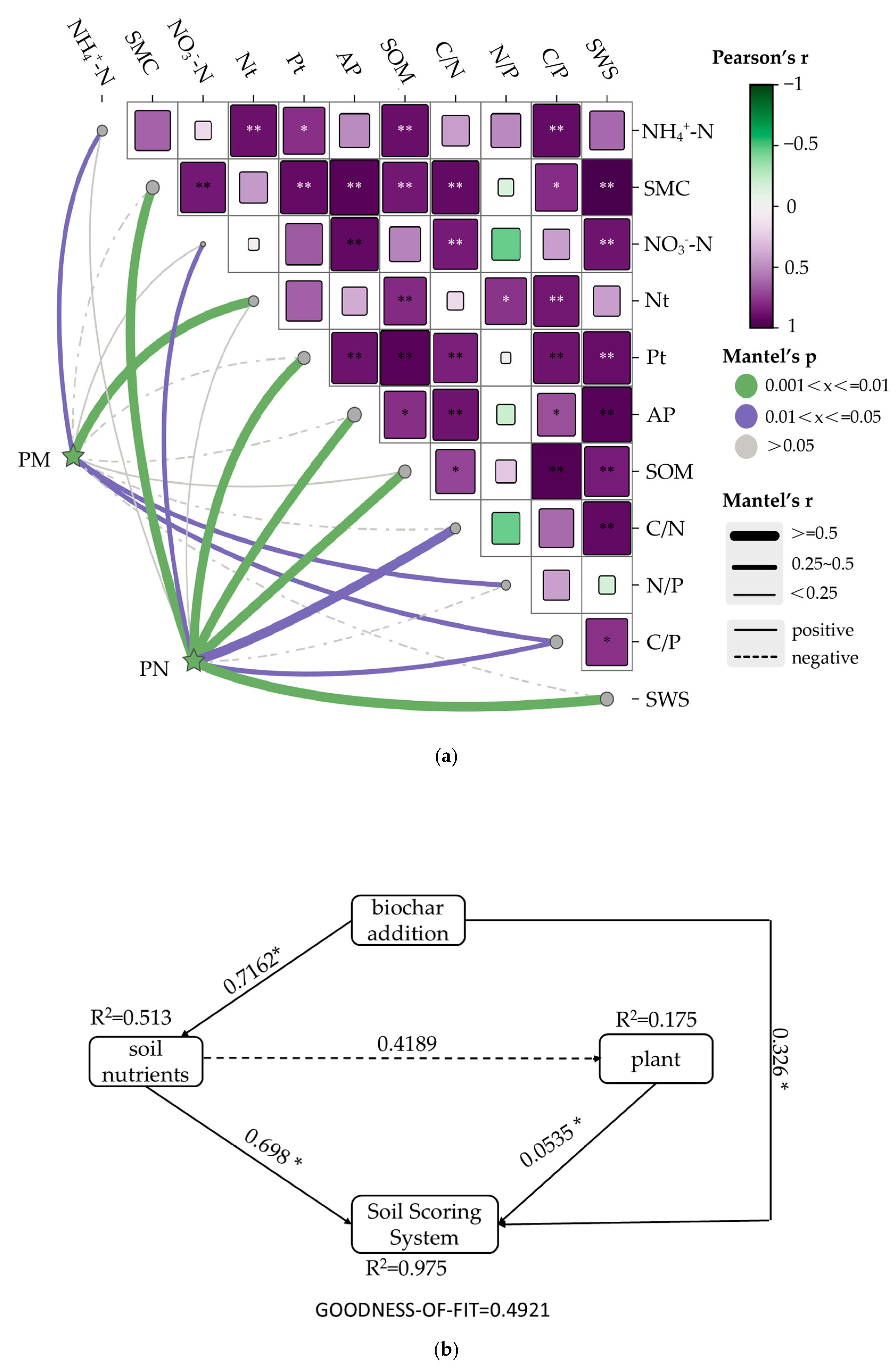
3.4. Principal Component Analysis, Mantel Test, and Partial Least Squares of Plant Growth and Soil Nutrients
4. Discussion
4.1. Effects of Biochar Application on Alfalfa Growth
4.2. Effects of Planting Alfalfa with Different Gradients of Biochar on Soil Water Content
4.3. Effects of Planting Alfalfa with Different Gradients of Biochar on Soil Nutrients
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, J.; Kou, L.; Wang, H.; He, X.; Xiong, Z.; Liu, C.; Cui, H. Carbon Emission Prediction Model and Analysis in the Yellow River Basin Based on a Machine Learning Method. Sustainability 2022, 14, 6153. [Google Scholar] [CrossRef]
- Kai, C.; Asmi, F. Research on Ecologicultural Environment Under the Background of Rural Revitalisation. Ecol. Chem. Eng. 2023, 30, 291–297. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, S.; Li, J. Study on the Spatial-Temporal Pattern and Driving Mechanism of Tourism Eco-Security in the Yellow River Basin. Int. J. Environ. Res. Public Health 2023, 20, 3562. [Google Scholar] [CrossRef]
- Yu, X.; Xie, X.; Meng, S. Modeling the Responses of Water and Sediment Discharge to Climate Change in the Upper Yellow River Basin, China. J. Hydrol. Eng. 2017, 22, 05017026. [Google Scholar] [CrossRef]
- Liu, X.; Qiao, L.; Zhong, Y.; Wan, X.; Xue, W.; Liu, P. Pathways of suspended sediments transported from the Yellow River mouth to the Bohai Sea and Yellow Sea. Estuar. Coast. Shelf Sci. 2020, 236, 106639. [Google Scholar] [CrossRef]
- Bibi, S.; Irshad, M.; Ullah, F.; Mahmood, Q.; Shahzad, M.; Tariq, M.A.U.R.; Hussain, Z.; Mohiuddin, M.; An, P.; Ng, A.W.M.; et al. Phosphorus extractability in relation to soil properties in different fields of fruit orchards under similar ecological conditions of Pakistan. Front. Ecol. Evol. 2023, 10, 1077270. [Google Scholar] [CrossRef]
- Dong, X.; Singh, B.P.; Li, G.; Lin, Q.; Zhao, X. Biochar has little effect on soil dissolved organic carbon pool 5 years after biochar application under field condition. Soil Use Manag. 2019, 35, 466–477. [Google Scholar] [CrossRef]
- Costa, M.C.G.; Freire, A.G.; Lourenço, D.V.; de Sousa, R.R.; de Andrade Feitosa, J.P.; Mota, J.C.A. Hydrogel composed of potassium acrylate, acrylamide, and mineral as soil conditioner under saline conditions. Sci. Agric. 2022, 79, e20200235. [Google Scholar] [CrossRef]
- Chen, L.; Li, D.; Shao, Y.; Adni, J.; Wang, H.; Liu, Y.; Zhang, Y. Comparative Analysis of Soil Microbiome Profiles in the Companion Planting of White Clover and Orchard Grass Using 16S rRNA Gene Sequencing Data. Front. Plant Sci. 2020, 11, 538311. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, F. Nitrogen nutrient supply and greenhouse gas mitigation potentials from cover crops across Chinese croplands. J. Clean. Prod. 2023, 425, 138881. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, Y.; Li, H.; Li, S.; Wang, X.; Chai, H. Difference of Bacterial Community Structure in the Meadow, Maize, and Continuous Cropped Alfalfa in Northeast China. Front. Microbiol. 2022, 13, 794848. [Google Scholar] [CrossRef]
- Chen, L. Fractal Features of Soil Particle Size Distribution in Alfalfa Grassland with Different Planting Years of Sandy area. Soil Water Conserv. China 2014, 10, 54–57. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, H.; Fu, S.; Yao, Q. Variation in Soil Microbial Community Structure Associated with Different Legume Species Is Greater than that Associated with Different Grass Species. Front. Microbiol. 2017, 8, 1007. [Google Scholar] [CrossRef]
- Fei, D.; Xiaobing, L.; Hong, W.; Meng, Z.; Ruihua, L.; Li, X. GIS-based assessment of land suitability for alfalfa cultivation: A case study in the dry continental steppes of northern China. Span. J. Agric. Res. 2014, 12, 364–375. [Google Scholar] [CrossRef]
- Parvis, Y.; Reza, E.H.; Jamshid, R.; Morteza, Z.; Mahdi, M.M.; Mahdi, G. Drought stress tolerance in vetch plants (Vicia sp.): Agronomic evidence and physiological signatures. J. Agric. Sci. 2024, 163, 13–26. [Google Scholar]
- Song, K.; Gao, J.; Li, S.; Sun, Y.; Sun, H.; An, B.; Hu, T.; He, X. Experimental and Theoretical Study of the Effects of Rare Earth Elements on Growth and Chlorophyll of Alfalfa (Medicago sativa L.) Seedling. Front. Plant Sci. 2021, 12, 731838. [Google Scholar] [CrossRef]
- Liu, Q.; Wan, W.; Chen, W.; Zhang, C.; Gao, H.; Zhang, J.; Sun, Z.; Li, H. Adaptation strategies of three legumes to soil phosphorus availability in steppes of Inner Mongolia. Plant Soil 2025, 1–18. [Google Scholar] [CrossRef]
- Zheng, X.; Li, X.; Singh, B.P.; Wei, L.; Huang, L.; Huang, Y.; Huang, Q.; Chen, X.; Su, Y.; Liu, Z.; et al. Biochar protects hydrophilic dissolved organic matter against mineralization and enhances its microbial carbon use efficiency. Sci. Total Environ. 2021, 795, 148793. [Google Scholar] [CrossRef]
- Ren, S.; Zhong, J.; Wang, K.; Liu, R.; Feng, H.; Dong, Q.; Yang, Y. Application of biochar in saline soils enhances soil resilience and reduces greenhouse gas emissions in arid irrigation areas. Soil Tillage Res. 2025, 250, 106500. [Google Scholar] [CrossRef]
- Madari, B.E.; Silva, M.A.S.; Carvalho, M.T.M.; Maia, A.H.N.; Petter, F.A.; Santos, J.L.S.; Tsai, S.M.; Leal, W.G.O.; Zeviani, W.M. Properties of a sandy clay loam Haplic Ferralsol and soybean grain yield in a five-year field trial as affected by biochar amendment. Geoderma 2017, 305, 100–112. [Google Scholar] [CrossRef]
- Evren, Y.; Ceyda, O.-K.; Busra, A.; Nur, A.-T.F.; Cagri, G. The regulatory effects of biochar on PSII photochemistry, antioxidant system and nitrogen assimilation in Lemna minor exposed to inorganic pollutants, arsenic and fluoride. J. Environ. Chem. Eng. 2023, 11, 110713. [Google Scholar]
- Dilfuza, E.; Li, L.; Hua, M.; Stephan, W.; Dorothea, B.S. Soil Amendment With Different Maize Biochars Improves Chickpea Growth Under Different Moisture Levels by Improving Symbiotic Performance With Mesorhizobium ciceri and Soil Biochemical Properties to Varying Degrees. Front. Microbiol. 2019, 10, 2423. [Google Scholar]
- Chang, Y.; Rossi, L.; Zotarelli, L.; Gao, B.; Sarkhosh, A. Greenhouse Evaluation of Pinewood Biochar Effects on Nutrient Status and Physiological Performance in Muscadine Grape (Vitis rotundifolia L.). HortScience 2021, 56, 277–285. [Google Scholar] [CrossRef]
- Hale, S.E.; Nurida, N.L.; Jubaedah; Mulder, J.; Sørmo, E.; Silvani, L.; Abiven, S.; Joseph, S.; Taherymoosavi, S.; Cornelissen, G. The effect of biochar, lime and ash on maize yield in a long-term field trial in a Ultisol in the humid tropics. Sci. Total Environ. 2020, 719, 137455. [Google Scholar] [CrossRef] [PubMed]
- Omondi, M.O.; Xia, X.; Nahayo, A.; Liu, X.; Korai, P.K.; Pan, G. Quantification of biochar effects on soil hydrological properties using meta-analysis of literature data. Geoderma 2016, 274, 28–34. [Google Scholar] [CrossRef]
- Rasa, K.; Heikkinen, J.; Hannula, M.; Arstila, K.; Kulju, S.; Hyväluoma, J. How and why does willow biochar increase a clay soil water retention capacity? Biomass Bioenergy 2018, 119, 346–353. [Google Scholar] [CrossRef]
- Blanco-Canqui, M.; Creech, C.F.; Easterly, A.C.; Drijber, R.A.; Jeske, E.S. Does biochar combined with cover crops improve health and productivity of sandy, sloping, and semi-arid soils? Soil Sci. Soc. Am. J. 2024, 88, 1340–1357. [Google Scholar] [CrossRef]
- Arif, M.; Ikramullah; Jan, T.; Riaz, M.; Akhtar, K.; Ali, S.; Shah, S.; Jalal, F.; Mian, I.A.; Dawar, K.M.; et al. Biochar and leguminous cover crops as an alternative to summer fallowing for soil organic carbon and nutrient management in the wheat-maize-wheat cropping system under semiarid climate. J. Soils Sediments 2021, 21, 1395–1407. [Google Scholar] [CrossRef]
- Nelson, D.W. Total Carbon, Organic Carbon, and Organic Matter; American Society of Agronomy Inc.: Madison, WI, USA; Soil Science Society of America, Inc.: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Assay of urease activity in soils. Soil Biol. Biochem. 1972, 4, 479–487. [Google Scholar] [CrossRef]
- Bao, S.D. Agrochemical Analysis of Soil; China Agriculture Press: Beijing, China, 2008. [Google Scholar]
- Sheng, M.; Xiong, K.; Wang, L.; Li, X.; Li, R.; Tian, X. Response of soil physical and chemical properties to Rocky desertification succession in South China Karst. Carbonates Evaporites 2018, 33, 15–28. [Google Scholar] [CrossRef]
- David, C.C.; Jacobs, D.J. Principal component analysis: A method for determining the essential dynamics of proteins. Methods Mol. Biol. 2014, 1084, 193–226. [Google Scholar] [CrossRef]
- Giorgio, R. Non-Metric Partial Least Squares. Electron. J. Stat. 2012, 6, 1641–1669. [Google Scholar] [CrossRef]
- Arruda, A.C.S.; da Silva Junkes, B.; Souza, É.S.; Yunes, R.A.; Heinzen, V.E.F. Semi-empirical topological index to predict properties of halogenated aliphatic compounds. J. Chemom. 2008, 22, 186–194. [Google Scholar] [CrossRef]
- Zhang, K.; Li, H.; Zhang, T.; Wang, S.; Liu, L.; Dong, X.; Cong, L.; Song, H.; Wang, A.; Yang, G.; et al. Comprehensive transcriptomic and metabolomic profiling reveals the differences between alfalfa sprouts germinated with or without light exposure. Front. Plant Sci. 2022, 13, 943740. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ma, J.; Wang, X.; Xu, P.; Zheng, S.; Zhao, Y. Effects of Biochar and Sludge on Carbon Storage of Urban Green Roofs. Forests 2018, 9, 413. [Google Scholar] [CrossRef]
- Fan, J.-W.; Yang, X.-W.; Wang, T.; Li, Y.; Zhao, H.; Du, Y.-L. Contrasting Growth, Photosynthesis, Antioxidant Responses and Water Use Efficiency in Two Medicago sativa L. Genotypes under Different Phosphorus and Soil Water Conditions. Agronomy 2020, 10, 1534. [Google Scholar] [CrossRef]
- Van Kuijk, M.; Anten, N.P.; Oomen, R.J.; Schieving, F. Stimulating seedling growth in early stages of secondary forest succession: A modeling approach to guide tree liberation. Front. Plant Sci. 2014, 5, 345. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Z.; Shi, S.; He, C. Effects of different crop rotations on alfalfa yield and soil quality in the Jiang-huai area. Acta Prataculturae Sin. 2019, 28, 28–39. [Google Scholar] [CrossRef]
- Streubel, J.D.; Collins, H.P.; Garcia-Perez, M.; Tarara, J.; Granatstein, D.; Kruger, C.E. Influence of Contrasting Biochar Types on Five Soils at Increasing Rates of Application. Soil Sci. Soc. Am. J. 2011, 75, 1402–1413. [Google Scholar] [CrossRef]
- Basso, A.S.; Miguez, F.E.; Laird, D.A.; Horton, R.; Westgate, M. Assessing potential of biochar for increasing water-holding capacity of sandy soils. GCB Bioenergy 2013, 5, 132–143. [Google Scholar] [CrossRef]
- Githinji, L. Effect of biochar application rate on soil physical and hydraulic properties of a sandy loam. Arch. Agron. Soil Sci. 2014, 60, 457–470. [Google Scholar] [CrossRef]
- Yuan, J.-H.; Xu, R.-K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef]
- Laird, D.A.; Fleming, P.; Davis, D.D.; Horton, R.; Wang, B.; Karlen, D.L. Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 2010, 158, 443–449. [Google Scholar] [CrossRef]
- Liu, X.; Elzenga, J.T.M.; Venema, J.H.; Tiedge, K.J. Thriving in a salty future: Morpho-anatomical, physiological, and molecular adaptations to salt stress in alfalfa (Medicago sativa L.) and other crops. Ann. Bot. 2024, 134, 1113–1130. [Google Scholar] [CrossRef]
- Asai, H.; Samson, B.K.; Stephan, H.M.; Songyikhangsuthor, K.; Homma, K.; Kiyono, Y.; Inoue, Y.; Shiraiwa, T.; Horie, T. Biochar amendment techniques for upland rice production in Northern Laos. Field Crops Res. 2008, 111, 81–84. [Google Scholar] [CrossRef]
- Tien, L.W.; Carlos, M.R.; MacKenzie, M.D. Biochar–manure changes soil carbon mineralization in a Gray Luvisol used for agricultural production. Can. J. Soil Sci. 2022, 102, 225–229. [Google Scholar] [CrossRef]
- Yu, T.; Hao, F.; Gao, K.; Xiong, M.; An, H. Productivity, soil fertility and enzyme activity of mixed forage grasslands improved by alfalfa and nitrogen addition in Horqin Sandy Land, China. Sci. Rep. 2025, 15, 10748. [Google Scholar] [CrossRef]
- He, H.; Peng, Q.; Wang, X.; Fan, C.; Pang, J.; Lambers, H.; Zhang, X. Growth, morphological and physiological responses of alfalfa (Medicago sativa) to phosphorus supply in two alkaline soils. Plant Soil 2017, 416, 565–584. [Google Scholar] [CrossRef]
- Zhang, J.; Lijuan, Z.; Shaojun, Q. Biochar amendment benefits 15N fertilizer retention and rhizosphere N enrichment in a maize-soil system. Geoderma 2022, 412, 115713. [Google Scholar] [CrossRef]
- Nelissen, V.; Rütting, T.; Huygens, D.; Staelens, J.; Ruysschaert, G.; Boeckx, P. Maize biochars accelerate short-term soil nitrogen dynamics in a loamy sand soil. Soil Biol. Biochem. 2012, 55, 20–27. [Google Scholar] [CrossRef]
- Xu, G.; Sun, J.; Shao, H.; Chang, S.X. Biochar had effects on phosphorus sorption and desorption in three soils with differing acidity. Ecol. Eng. 2014, 62, 54–60. [Google Scholar] [CrossRef]
- Du, E.; Terrer, C.; Pellegrini, A.F.A.; Ahlström, A.; van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.; Jackson, R.B. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 2020, 13, 221–226. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, T.-T.; Qian, H.-Y.; Fan, J.-B.; He, Y.-Q.; Sun, B. Nitrogen mineralization as a result of phosphorus supplementation in long-term phosphate deficient soil. Appl. Soil Ecol. 2016, 106, 24–32. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Zhou, C.; Lei, P.; Sootahar, M.K.; Ni, J. Organic and Inorganic Nitrogen forms as Affected by Phosphorus Additions in a Maize–Mustard Cropping System. CLEAN-Soil Air Water 2022, 50, 2100240. [Google Scholar] [CrossRef]
- Foster, E.; Fogle, E.; Cotrufo, M.F. Sorption to Biochar Impacts β-Glucosidase and Phosphatase Enzyme Activities. Agriculture 2018, 8, 158. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, Y.; Xiang, Y.; Zhang, R.; Lu, H. Responses of soil microbial community structure changes and activities to biochar addition: A meta-analysis. Sci. Total Environ. 2018, 643, 926–935. [Google Scholar] [CrossRef]
| Treatment | Ph (m) | IPB (g·plant−1) | PWC (%) | BM (g·m−2) | ANC (mg·plant−1) |
|---|---|---|---|---|---|
| B1 | 0.52 ± 0.02 c | 23 ± 0.95 b | 63.58 ± 2.84 a | 1361.76 ± 133.34 a | 150.48 ± 13.91 b |
| B2 | 0.59 ± 0.02 b | 21.23 ± 1.19 b | 64.58 ± 3.41 a | 1441.95 ± 154.34 a | 196.9 ± 14.66 a |
| B3 | 0.67 ± 0.03 a | 26.76 ± 1.82 a | 68.37 ± 1.94 a | 1602.55 ± 186.02 a | 200.79 ± 24.11 a |
| Treatment | PC1 | PC2 | Comprehensive Score | Comprehensive Rank |
|---|---|---|---|---|
| CK | 2.63 | 0.67 | 2.23 | 1 |
| B1 | 2.19 | 0.56 | 1.86 | 2 |
| Treatment | PC1 | PC2 | Comprehensive Score | Comprehensive Rank |
|---|---|---|---|---|
| B1 | 1.31 | 0.61 | 1.14 | 3 |
| B2 | 2.85 | 3.00 | 2.89 | 2 |
| B3 | 3.82 | 2.88 | 3.60 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, T.; Liang, S.; Liu, C.; Liu, S.; Sun, L. Study on the Effects of Planting Alfalfa (Medicago sativa L.) and Adding Biochar on Soil Fertility in Jujube Orchards. Agronomy 2025, 15, 1462. https://doi.org/10.3390/agronomy15061462
Jing T, Liang S, Liu C, Liu S, Sun L. Study on the Effects of Planting Alfalfa (Medicago sativa L.) and Adding Biochar on Soil Fertility in Jujube Orchards. Agronomy. 2025; 15(6):1462. https://doi.org/10.3390/agronomy15061462
Chicago/Turabian StyleJing, Tingrui, Shuang Liang, Chubo Liu, Shipeng Liu, and Luanzi Sun. 2025. "Study on the Effects of Planting Alfalfa (Medicago sativa L.) and Adding Biochar on Soil Fertility in Jujube Orchards" Agronomy 15, no. 6: 1462. https://doi.org/10.3390/agronomy15061462
APA StyleJing, T., Liang, S., Liu, C., Liu, S., & Sun, L. (2025). Study on the Effects of Planting Alfalfa (Medicago sativa L.) and Adding Biochar on Soil Fertility in Jujube Orchards. Agronomy, 15(6), 1462. https://doi.org/10.3390/agronomy15061462





