A Tillage-Dependent System of Arable Pests: How Soil Condition and Prevailing Climate Influence Pest Occurrence?
Abstract
:1. Introduction
2. The Effects of Physical and Chemical Parameters of the Soils on the Presence of Pests
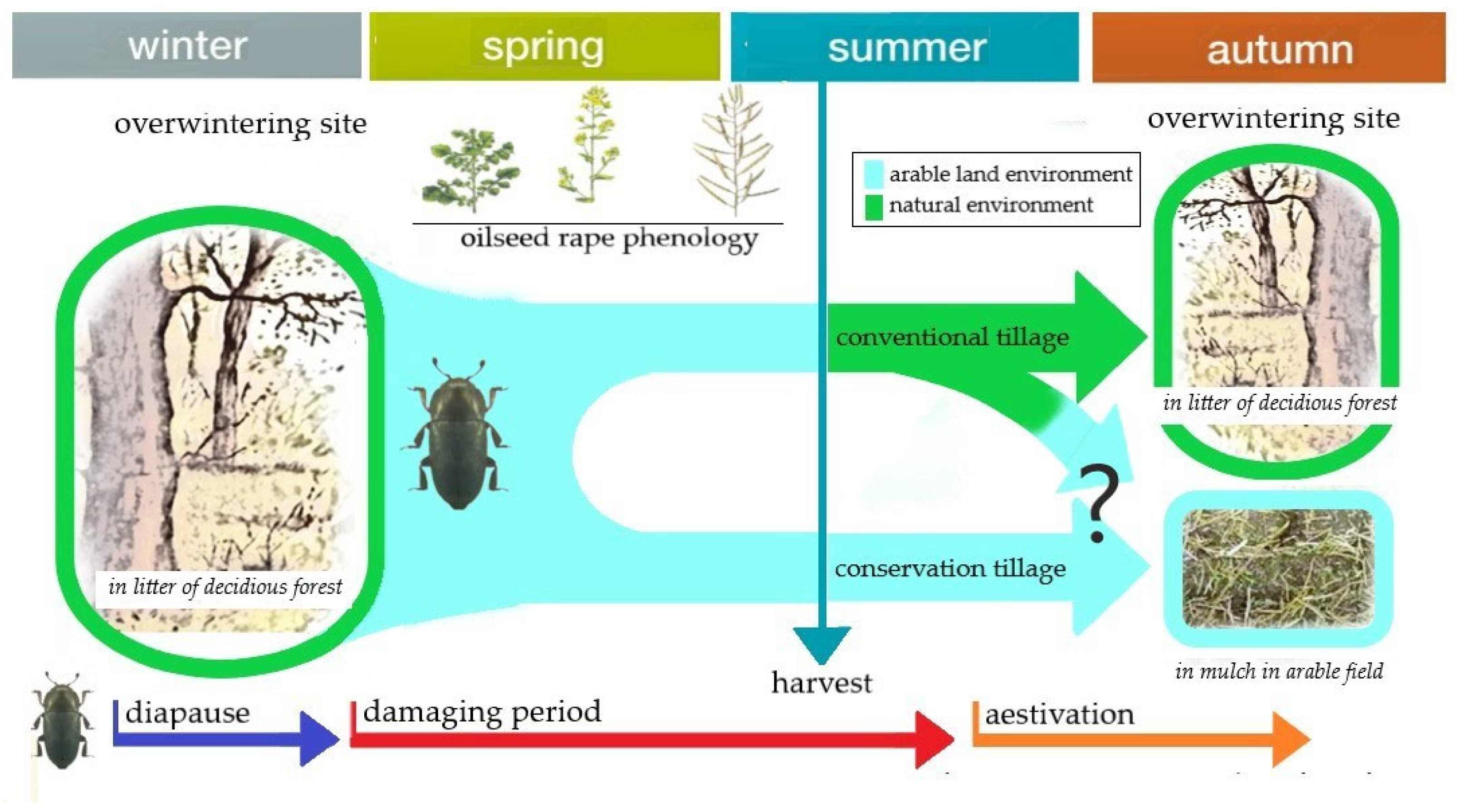
3. Characteristics of Central European Arable Crop Production and Tillage Systems in the Light of the Attachment of Pests to the Soil
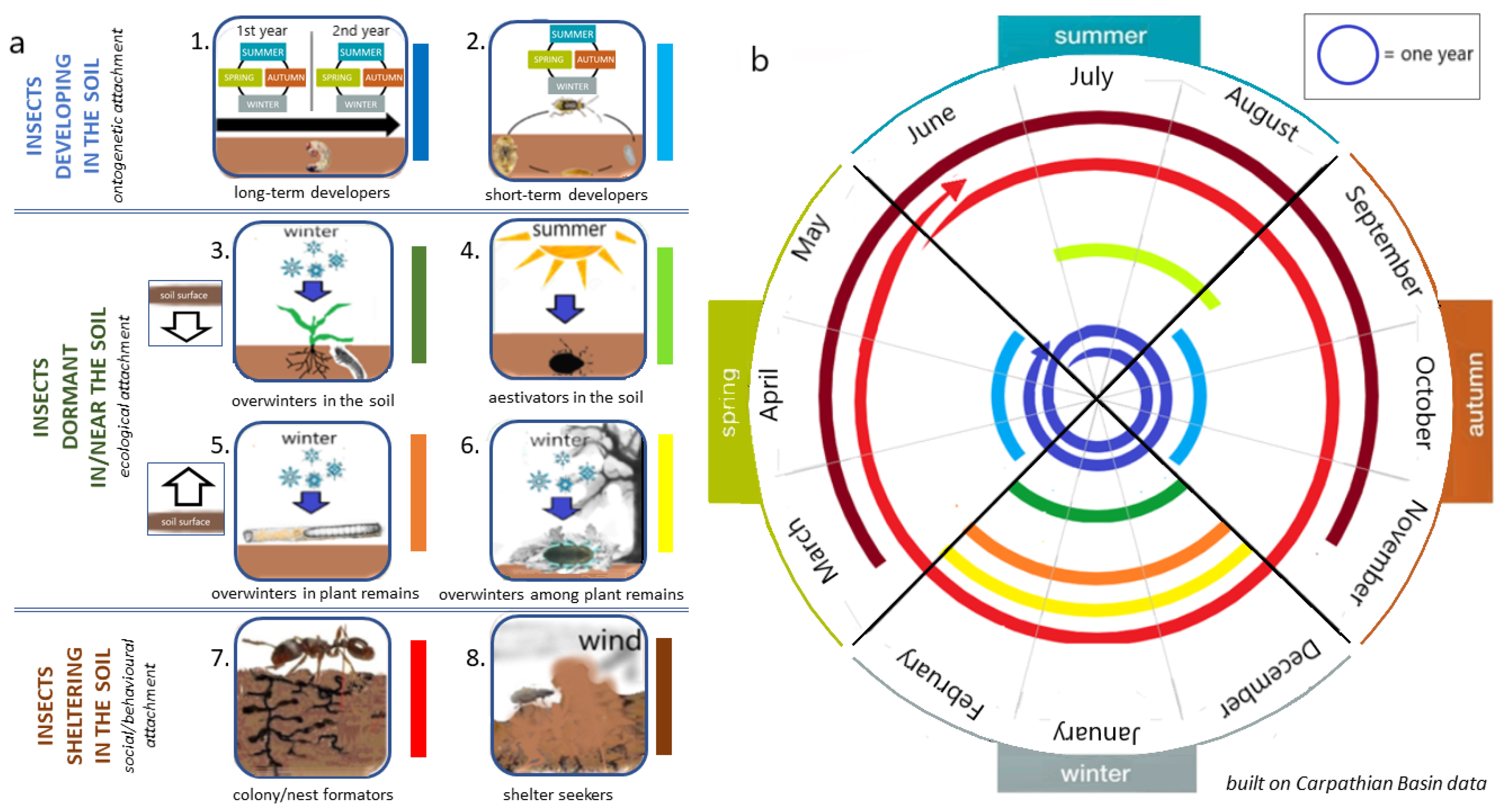
4. Main Groups of Herbivores by Their Attachment to the Soil
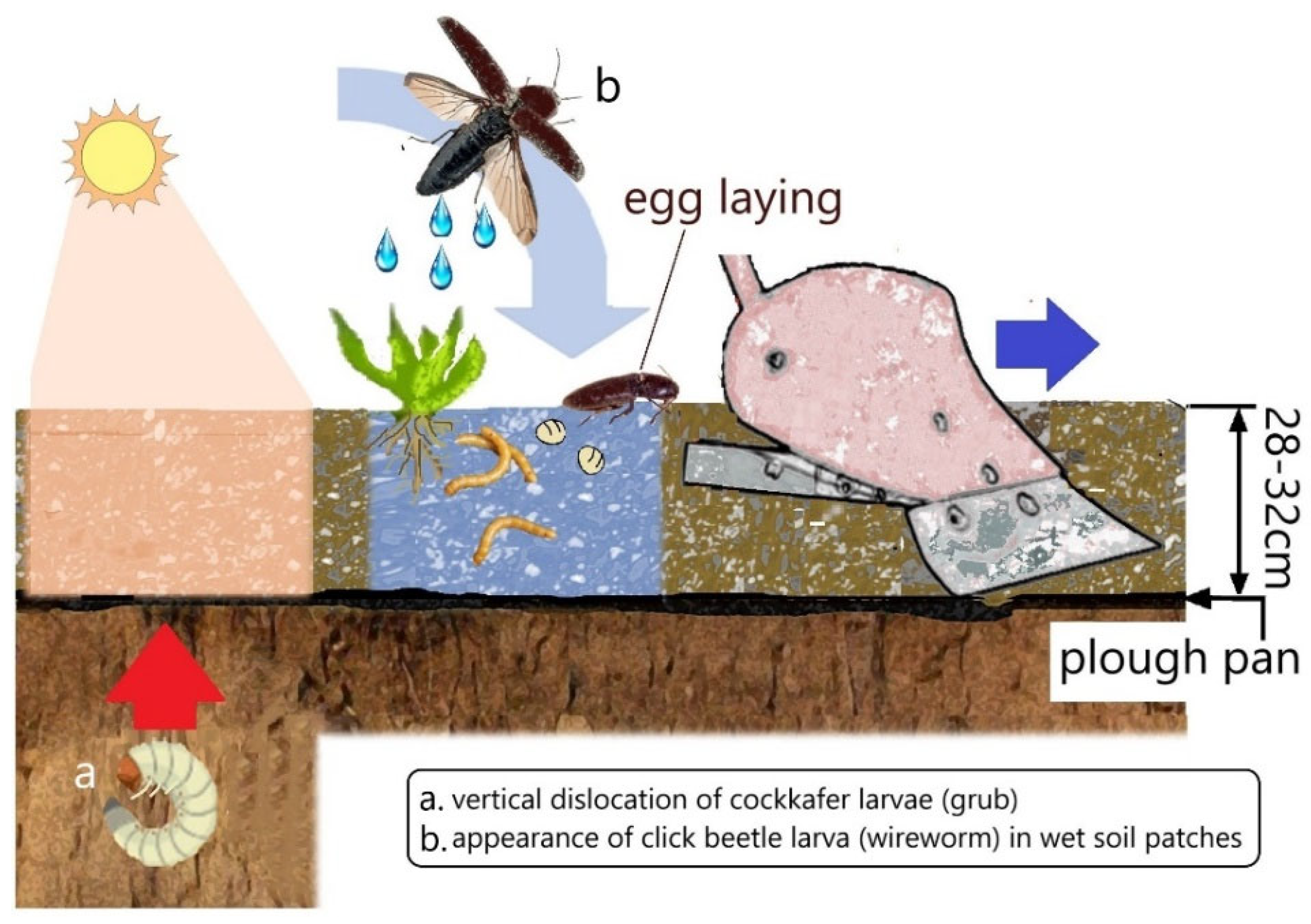
4.1. The Group of Pests That Develop in the Soil for Years—Long-Term Developers
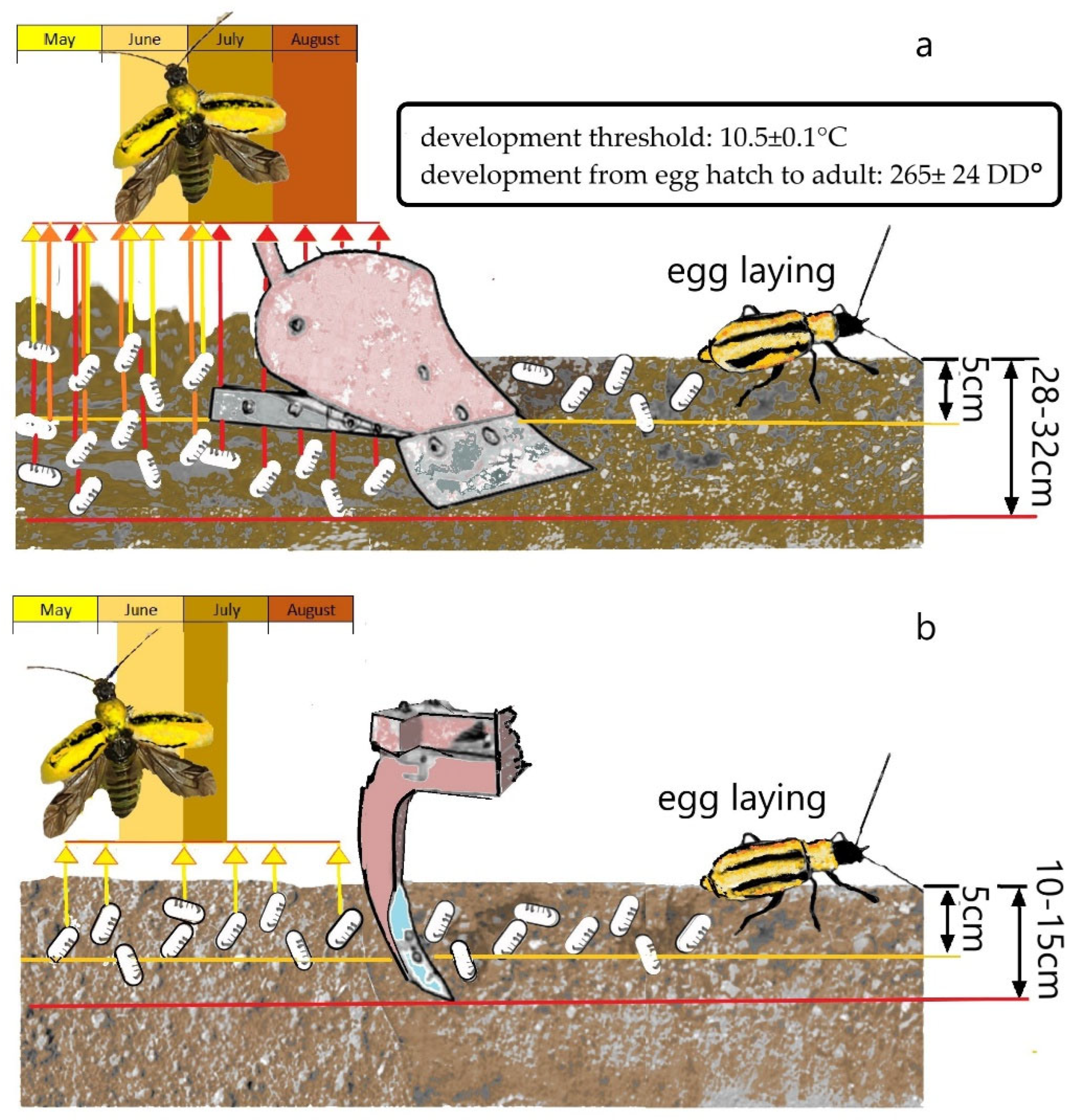
4.2. The Group of Pests Which Developmental Stages Are Soil-Bound—Short-Term Developers
4.3. The Group of Pests That Are Hibernating in the Soil—Overwinters in Soil
4.4. The Group of Pests That Spends the Summer Drought in the Soil—Aestivators in Soil
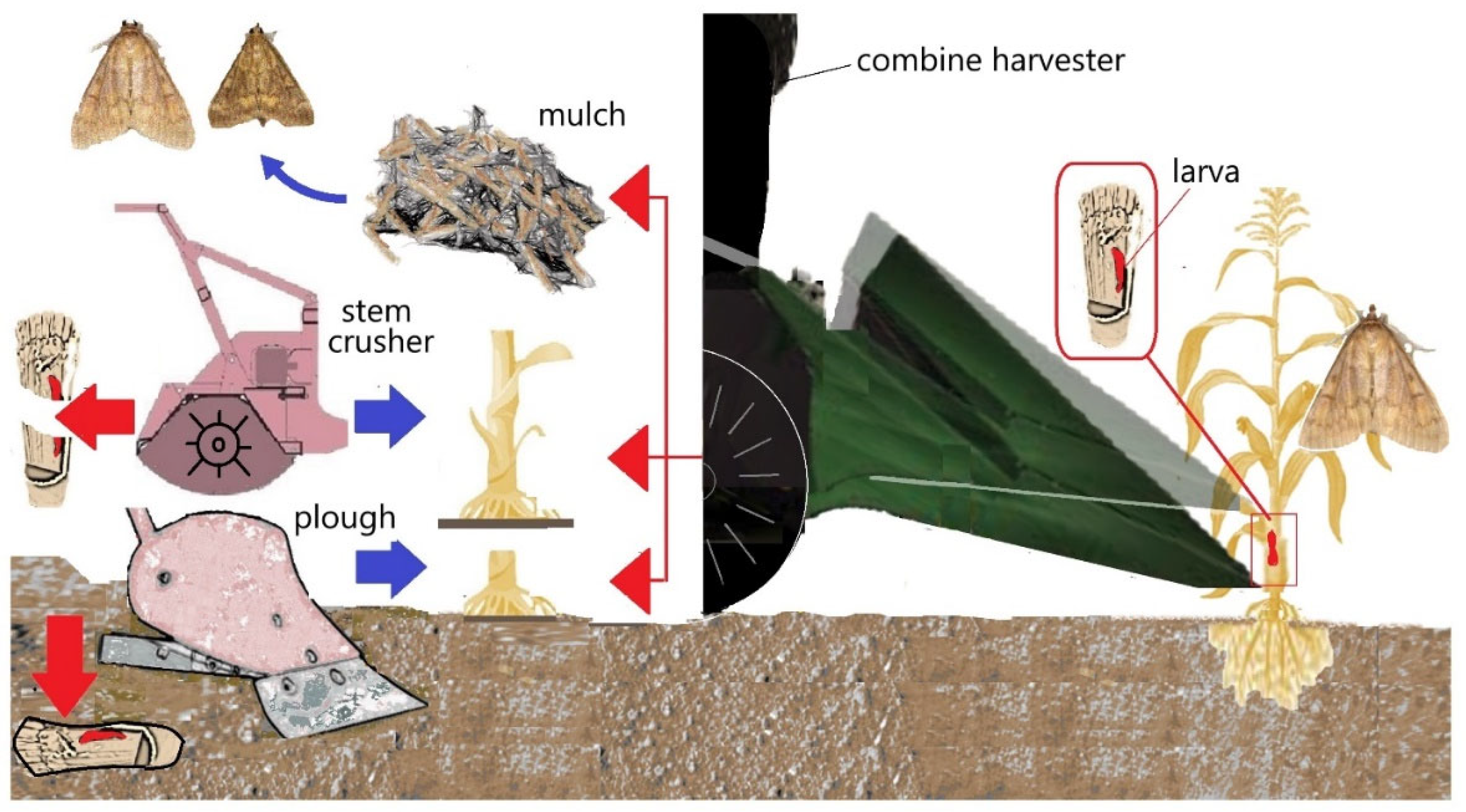
4.5. The Group of Pests Hibernating on the Soil Surface in Plant Tissues—Overwinters in Plant Remains
4.6. The Group of Pests Hibernating on the Soil Surface Among Plant Residues—Overwinters Among Plant Remains
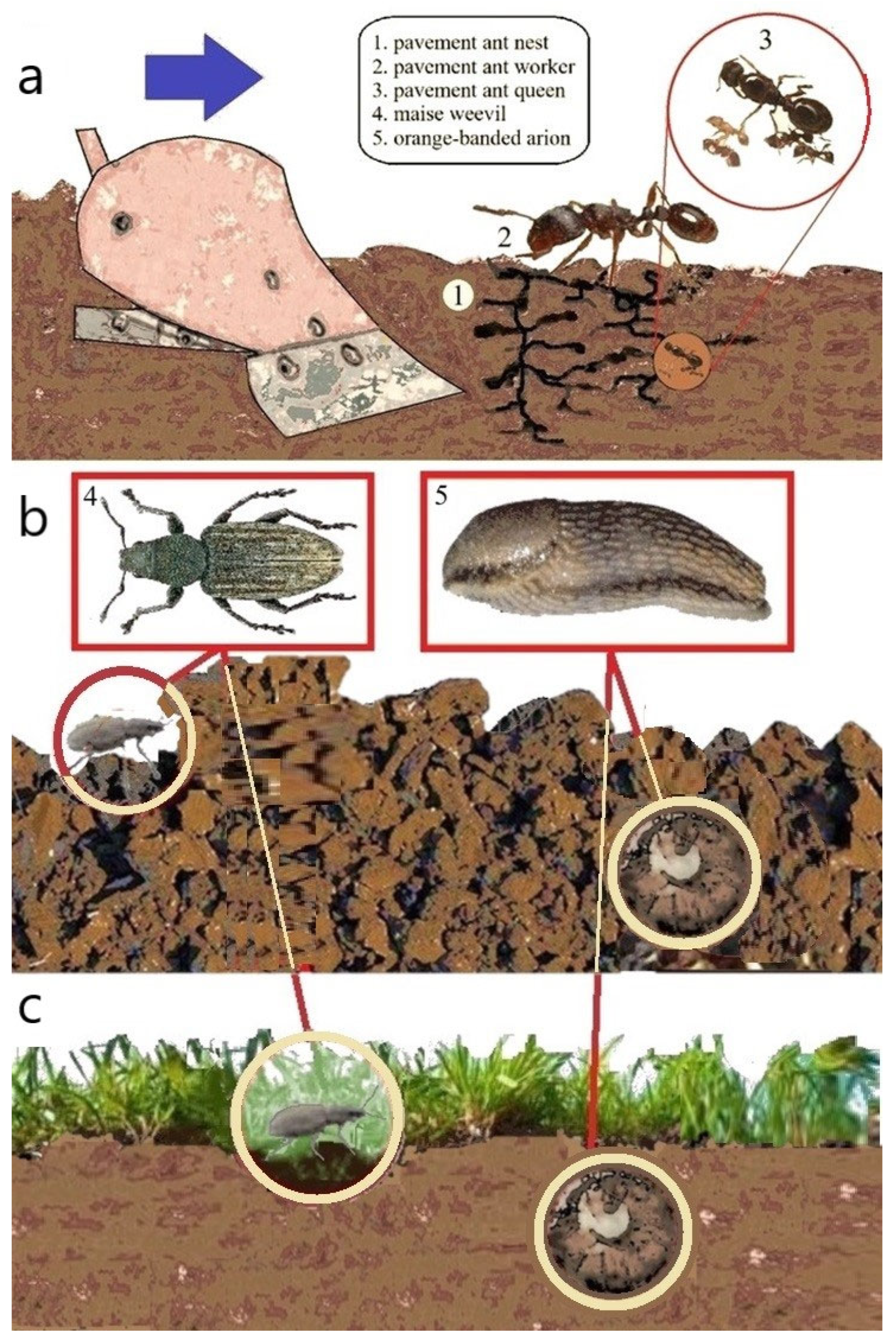
4.7. The Group of Pests That Build Nests and Colonies in the Soil—Colony/Nest Formators
4.8. The Group of Pests That Find Shelter in the Soil Between Soil Clods—Shelter-Seekers
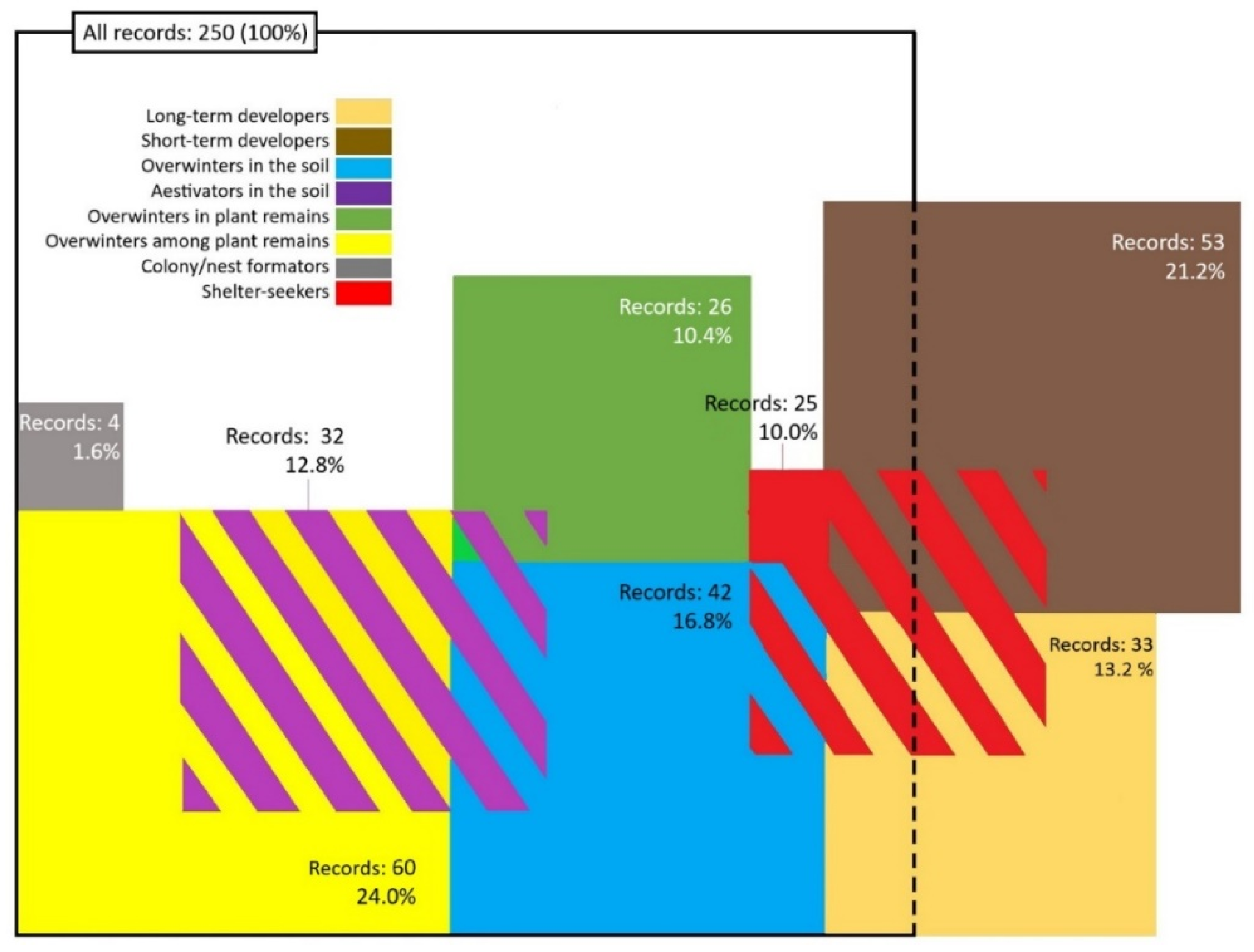
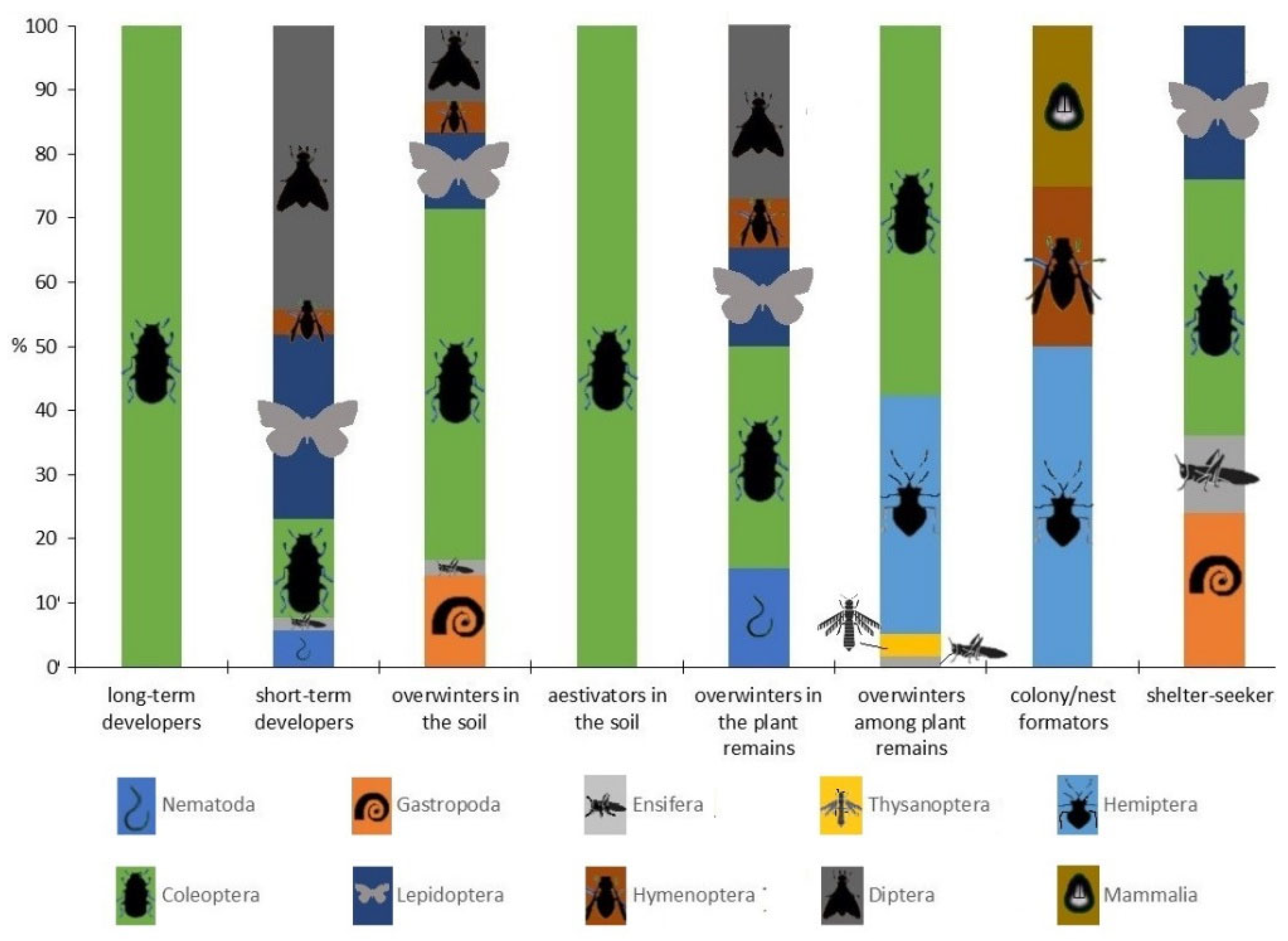
5. Comprehensive Dominance and Taxonomic Analysis of Herbivorous Groups Bound to Soil as a Medium
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coughenour, C.M.; Chamala, S. Conservation Tillage and Cropping Innovation: Constructing the New Culture of Agriculture; Iowa State University Press: Ames, IA, USA, 2000. [Google Scholar]
- Birkás, M.; Bencsik, K.; Stingli, A.; Percze, A. Correlation between moisture and organic matter conservation in soil tillage. Cer. Res. Commun. 2005, 33, 25–28. [Google Scholar] [CrossRef]
- Solodovnikov, A.P.; Denisov, K.E.; Danilov, A.N.; Korsak, V.V.; Pimonov, K.I. Minimizing tillage to preserve the agro-chemical and water-physical properties of southern black soil after vegetative reclamation. Int. J. Mech. Eng. Technol. 2018, 9, 1166–1172. [Google Scholar]
- Khan, N.I.; Malik, A.U.; Umer, F.; Bodla, M.I. Effect of tillage and farmyard manure on physical properties of soil. Int. Res. J. Plant Sci. 2010, 1, 75–82. [Google Scholar]
- Farahani, E.; Emami, H.; Forouhar, M. Effects of tillage systems on soil organic carbon and some soil physical properties. Land Degrad. Dev. 2022, 33, 1307–1320. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, Y.; Li, T.; Chen, L.; Chen, Y.; Sui, P. Changes in soil microbial biomass, diversity, and activity with crop rotation in cropping systems: A global synthesis. Appl. Soil Ecol. 2023, 186, 104815. [Google Scholar] [CrossRef]
- Mikanová, O.; Javůrek, M.; Šimon, T.; Friedlová, M.; Vach, M. The effect of tillage systems on some microbial characteristics. Soil Till. Res. 2009, 105, 72–76. [Google Scholar] [CrossRef]
- Montanyá, I.; Catalán, G.; Tenorio, J.L.; Garcia-Baudin, J.M. Effect of the tillage systems on weed flora composition. Opt. Mediterran. Ser. A 2006, 69, 143–147. [Google Scholar]
- Rodríguez, E.; Fernández-Anero, F.J.; Ruiz, P.; Campos, M. Soil arthropod abundance under conventional and no tillage in a Mediterranean climate. Soil Till. Res. 2006, 85, 229–233. [Google Scholar] [CrossRef]
- Sharley, D.J.; Hoffmann, A.A.; Thomson, L.J. The effects of soil tillage on beneficial invertebrates within the vineyard. Agric. For. Entomol. 2008, 10, 233–243. [Google Scholar] [CrossRef]
- Betancur-Corredor, B.; Lang, B.; Russell, D.J. Reducing tillage intensity benefits the soil micro- and mesofauna in a global meta-analysis. Eur. J. Soil. Sci. 2022, 73, e13321. [Google Scholar] [CrossRef]
- Feng, L.; Liang, A.; Gao, Q.; Wu, D. Effects of three different tillage patterns on ground and below-ground dwelling insect assemblage. J. Biobased. Mater. Bioenergy 2023, 17, 53–64. [Google Scholar] [CrossRef]
- Han, H.; Ning, T.Y.; Li, Z. Effects of tillage and weed management on the vertical distribution of microclimate and grain yield in a winter wheat field. Plant Soil Environ. 2013, 59, 201–207. [Google Scholar] [CrossRef]
- Irmak, S.; Kukal, M.S.; Mohammed, A.T.; Djaman, K. Disk-till vs no-till maize evapotranspiration, microclimate, grain yield, production functions, and water productivity. Agric. Water Manag. 2019, 216, 177–195. [Google Scholar] [CrossRef]
- Josa, R.; Hereter, A. Effects of tillage systems in dryland farming on near-surface water content during the late winter period. Soil Till. Res. 2005, 82, 173–183. [Google Scholar] [CrossRef]
- De Almeida, W.S.; Panachuki, E.; De Oliveira, P.T.S.; Da Silva Menezes, R.; Sobrinho, T.A.; De Carvalho, D.F. Effect of soil tillage and vegetal cover on soil water infiltration. Soil Till. Res. 2018, 175, 130–138. [Google Scholar] [CrossRef]
- Wilson, D.I.; Köhler, H.; Cai, L.; Majschak, J.P.; Davidson, J.F. Cleaning of a model food soil from horizontal plates by a moving vertical water jet. Chem. Eng. Sci. 2015, 123, 450–459. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, C.; Fan, J.; Zhang, X.; Chen, K.; Chen, J.; Li, X. Evaluating the effects of subsoiler type and spacing on tillage resistance and soil conservation with DEM simulation and field experiment. Int. J. Agric. Biol. Engin. 2025, 18, 115–123. [Google Scholar] [CrossRef]
- Busari, M.A.; Kukal, S.S.; Amanpreet Kaur, R.; Bhatt, A.K.R.; Dulazi, A.A. Conservation tillage impacts on soil, crop and the environment. Int. Soil Water Conserv. Res. 2015, 3, 119–129. [Google Scholar] [CrossRef]
- Lv, L.; Gao, Z.; Liao, K.; Zhu, Q.; Zhu, J. Impact of conservation tillage on the distribution of soil nutrients with depth. Soil Till. Res. 2023, 225, 105527. [Google Scholar] [CrossRef]
- Wisler, G.C.; Norris, R.F. Interactions between weeds and cultivated plants as related to management of plant pathogens. Weed Sci. 2005, 53, 914–917. [Google Scholar] [CrossRef]
- Nikolić, L.; Šeremešić, S.; Ljevnaić-Mašić, B.; Latković, D.; Konstantinović, B. Weeds and their ecological indicator values in a long-term experiment. Appl. Ecol. Environ. Res. 2020, 18, 47–75. [Google Scholar] [CrossRef]
- Toshova, T.B.; Csonka, É.; Subchev, M.A.; Tóth, M. The seasonal activity of flea beetles in Bulgaria. J. Pest Sci. 2009, 82, 295–303. [Google Scholar] [CrossRef]
- Slavíková, L.; Ibrahim, E.; Alquicer, G.; Tomašechová, J.; Šoltys, K.; Glasa, M.; Kundu, J.K. Weed hosts represent an important reservoir of turnip yellows virus and a possible source of virus introduction into oilseed rape crop. Viruses 2022, 14, 2511. [Google Scholar] [CrossRef] [PubMed]
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2010, 22, 357–365. [Google Scholar] [CrossRef]
- Rezaei, E.E.; Webber, H.; Asseng, S.; Boote, K.; Durand, J.L.; Ewert, F.; Martre, P.; McCarthy, D. Climate change impacts on crop yields. Nat. Rev. Earth. Environ. 2023, 4, 831–846. [Google Scholar] [CrossRef]
- Čarni, A.; Ćuk, M.; Krstonošić, D.; Škvorc, Ž. Study of forage quality of grasslands on the southern margin of the Pannonian Basin. Agronomy 2021, 11, 2132. [Google Scholar] [CrossRef]
- Kladivko, E.J. Tillage systems and soil ecology. Soil Till. Res. 2001, 61, 61–76. [Google Scholar] [CrossRef]
- Lowe, W.H.; Martin, T.E.; Skelly, D.K.; Woods, H.A. Metamorphosis in an era of increasing climate variability. Trends Ecol. Evol. 2021, 36, 360–375. [Google Scholar] [CrossRef]
- Johnson, S.N.; Gregory, P.J.; McNicol, J.W.; Oodally, Y.; Zhang, X.; Murray, P.J. Effects of soil conditions and drought on egg hatching and larval survival of the clover root weevil (Sitona lepidus). Appl. Soil Ecol. 2010, 44, 75–79. [Google Scholar] [CrossRef]
- Luckmann, W.H. Observations on the biology and control of Glischrochilus quadrisignatus. J. Econ. Entomol. 1963, 56, 681–686. [Google Scholar] [CrossRef]
- Bengtsson, G.; Nilsson, E.; Rydén, T.; Wiktorsson, M. Irregular walks and loops combined in small-scale movement of a soil insect: Implications for dispersal biology. J. Theor. Biol. 2004, 231, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Danks, H.V. Insect Dormancy: An Ecological Perspective; Biological Survey of Canada: Ottawa, ON, Canada, 1987. [Google Scholar]
- Lin, P.A.; Paudel, S.; Lai, P.C.; Gc, R.K.; Yang, D.H.; Felton, G.W. Integrating water and insect pest management in agriculture. J. Pest Sci. 2024, 97, 521–538. [Google Scholar] [CrossRef]
- Miles, H.W. Wireworms and the breaking up of grassland. Agric. J. Ministry. Agric. Great. Brit. 1939, 46, 480–488. [Google Scholar]
- Staudacher, K.; Schallhart, N.; Pitterl, P.; Wallinger, C.; Brunner, N.; Landl, M.; Kromp, B.; Glaauninger, J.; Traugott, M. Occurrence of Agriotes wireworms in Austrian agricultural land. J. Pest Sci. 2013, 86, 33–39. [Google Scholar] [CrossRef]
- Levine, E.; Oloumi-Sadeghi, H. Soil moisture effects on the survival of Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae). Environ. Entomol. 1991, 20, 673–678. [Google Scholar]
- Eskafi, F.M.; Fernandez, A. Larval–pupal mortality of Mediterranean fruit fly (Diptera: Tephritidae) from interaction of soil, moisture, and temperature. Environ. Entomol. 1990, 19, 1666–1670. [Google Scholar] [CrossRef]
- Pilz, C.; Wegensteiner, R.; Keller, S. Natural occurrence of insect pathogenic fungi and insect parasitic nematodes in Diabrotica virgifera virgifera populations. BioControl 2008, 53, 353–359. [Google Scholar] [CrossRef]
- Páez, D.J.; Dukic, V.; Dushoff, J.; Fleming-Davies, A.; Dwyer, G. Eco-evolutionary theory and insect outbreaks. Am. Nat. 2017, 189, 616–629. [Google Scholar] [CrossRef]
- Dively, G.P.; Patton, T. An evaluation of cultural and chemical control practices to reduce slug damage in no-till corn. Insects 2022, 13, 277. [Google Scholar] [CrossRef]
- Jasrotia, P.; Kumari, P.; Malik, K.; Kashyap, P.L.; Kumar, S.; Bhardwaj, A.K.; Singh, G.P. Conservation agriculture-based crop management practices impact diversity and population dynamics of the insect-pests and their natural enemies in agroecosystems. Front. Sustain. Food Syst. 2023, 7, 1173048. [Google Scholar] [CrossRef]
- Ugolini, A. Orientation and behavior of soil-dwelling insects in relation to light. J. Insect Behav. 2017, 30, 208–216. [Google Scholar]
- Dowdy, W.W. The influence of temperature on vertical migration of invertebrates inhabiting different soil types. Ecology 1944, 25, 449–460. [Google Scholar] [CrossRef]
- Meyer, L.A.; Sullivan, S.M. Bright lights, big city: Influences of ecological light pollution on reciprocal stream-riparian invertebrate fluxes. Ecol. Appl. 2013, 23, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Milosavljević, I.; Esser, A.D.; Crowder, D.W. Effects of environmental and agronomic factors on soil-dwelling pest communities in cereal crops. Agric. Ecosyst. Environ. 2016, 225, 198. [Google Scholar] [CrossRef]
- McColloch, J.W. The reciprocal relation of soil and insects. Ecology 1922, 3, 288–301. [Google Scholar] [CrossRef]
- Payne, D.; Gregory, P.J. The temperature of the soil. In Russell’s Soil Conditions and Plant Growth, 11th ed.; Ylan, W., Ed.; Longman Scientific and Technical: Harlow, UK, 1988; pp. 282–297. [Google Scholar]
- Fidler, J.H. An investigation into the relation between chafer larvae and the physical factors of their soil habitat. J. Anim. Ecol. 1936, 5, 333–347. [Google Scholar] [CrossRef]
- Vandenberg, N.J.; Stager, D.E. Beetle assemblages in loess soils: A case study from the Midwest. Ecol. Entomol. 2013, 38, 321–331. [Google Scholar]
- Hibbard, B.E.; Haeussler, B.J. Relation between soil texture and the mortality of Diabrotica virgifera virgifera in corn. J. Econ. Entomol. 2004, 97, 1094–1113. [Google Scholar]
- Elliott, N.C.; Smith, W.P. The influence of environmental conditions on Zabrus tenebrionides survivorship in various soil types. Ecol. Entomol. 2006, 31, 153–162. [Google Scholar]
- Madarász, B.; Juhos, K.; Ruszkiczay-Rüdiger, Z.; Benke, S.; Jakab, G.; Szalai, Z. Conservation tillage vs conventional tillage: Long-term effects on yields in continental, sub-humid Central Europe, Hungary. Int. J. Agric. Sustain. 2016, 4, 408–427. [Google Scholar] [CrossRef]
- Vígh, E.; Fertő, I.; Fogarasi, J. Impacts of climate on technical efficiency in the Hungarian arable sector. Stud. Agric. Econ. 2018, 120, 41–46. [Google Scholar] [CrossRef]
- Pinke, G. The status of arable plant habitats in Eastern Europe. In The Changing Status of Arable Habitats in Europe; Hurford, C., Wilson, P., Storkey, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 75–87. [Google Scholar] [CrossRef]
- Birkás, M.; Dekemati, I.; Kende, Z.; Radics, Z.; Szemők, A. From the multi-ploughing soil tillage to direct drilling—Progress in soil tillage and soil conservation. Agrokém. Talajt. 2018, 67, 253–268. [Google Scholar] [CrossRef]
- Kovács, G.P.; Simon, B.; Balla, I.; Bozóki, B.; Dekemati, I.; Gyuricza, C.; Percze, A.; Birkás, M. Conservation tillage improves soil quality and crop yield in Hungary. Agronomy 2023, 13, 894. [Google Scholar] [CrossRef]
- Bottlik, L.; Csorba, S.; Gyuricza, C.; Kende, Z.; Birkás, M. Climate challenges and solutions in soil tillage. Appl. Ecol. Environ. Res. 2014, 12, 13–23. [Google Scholar] [CrossRef]
- Birkás, M.; Jug, D.; Kende, Z.; Kisić, I.; Szemők, A. Soil tillage response to the climate threats—Revolution of the classic theories. Agric. Cons. Sci. 2018, 83, 1–9. [Google Scholar]
- Bogunovic, I.; Kovács, G.P.; Dekemati, I.; Kisić, I.; Balla, I.; Birkás, M. Long-term effect of soil conservation tillage on soil water content, penetration resistance, crumb ration and crusted area. Plant Soil Environ. 2019, 65, 442–448. [Google Scholar] [CrossRef]
- Qu, Y.; Pan, C.; Guo, H. Factors affecting the promotion of conservation tillage in black soil—The case of Northeast China. Sustainability 2021, 13, 9563. [Google Scholar] [CrossRef]
- Bekele, B.; Habtemariam, T.; Gemi, Y. Evaluation of conservation tillage methods for soil moisture conservation and maize grain yield in low moisture areas of SNNPR, Ethiopia. Water Cons. Sci. Engin. 2022, 7, 119–130. [Google Scholar] [CrossRef]
- Tauber, M.J.; Tauber, C.A. Quantitative response to day length during diapause in insects. Nature 1973, 244, 296–297. [Google Scholar] [CrossRef]
- Roberts, R.M. Seasonal strategies in insects. N. Z. Entomol. 1978, 6, 350–356. [Google Scholar] [CrossRef]
- Numata, H.; Shintani, Y. Diapause in univoltine and semivoltine life cycles. Ann. Rev. Entomol. 2023, 68, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Bale, J.S.; Hayward, S.A.L. Insect overwintering in a changing climate. J. Exp. Biol. 2010, 213, 980–994. [Google Scholar] [CrossRef] [PubMed]
- Convey, P. Overwintering strategies of terrestrial invertebrates in Antarctica—The significance of flexibility in extremely seasonal environments. Eur. J. Entomol. 2013, 9, 489–505. [Google Scholar]
- Wagenhoff, E.; Blum, R.; Delb, H. Spring phenology of cockchafers, Melolontha spp. (Coleoptera: Scarabaeidae), in forests of South-Western Germany: Results of a 3-year survey on adult emergence, swarming flights, and oogenesis from 2009 to 2011. J. For. Sci. 2014, 60, 154–165. [Google Scholar] [CrossRef]
- Traugott, M.; Benefer, C.M.; Blackshaw, R.P.; van Herk, W.G.; Vernon, R.S. Biology, ecology, and control of elaterid beetles in agricultural land. Ann. Rev. Entomol. 2015, 60, 313–334. [Google Scholar] [CrossRef]
- Nikoukar, A.; Rashed, A. Integrated pest management of wireworms (Coleoptera: Elateridae) and the rhizosphere in agroecosystems. Insects 2022, 13, 769. [Google Scholar] [CrossRef]
- Jakhar, B.L.; Baloda, A.S.; Saini, K.K.; Yadav, T. Evaluation of some insecticides as seed dresser against white grubs in groundnut crop. J. Entomol. Zool. Stud. 2020, 8, 1468–1469. [Google Scholar]
- Berezina, V.M. Effect of hydrothermic soil conditions on the vertical migration of the cockchafer larvae Melolontha hippocastani. Bull. Plant Prot. 1941, 5, 43–56. [Google Scholar]
- Bligaard, J. Damage thresholds for cabbage root fly (Delia radicum L) in cauliflower assessed from pot experiments. Acta Agric. Scand. B 1999, 49, 57–64. [Google Scholar] [CrossRef]
- Berger, H.K. The western corn rootworm (Diabrotica virgifera virgifera), a new maize pest threatening Europe. EPPO Bull. 2001, 31, 411–414. [Google Scholar] [CrossRef]
- Showler, A.T. Water deficit stress-host plant nutrient accumulations and associations with phytophagous arthropods. In Abiotic Stress-Plant Responses and Applications in Agriculture; Vahdati, K., Leslie, C., Eds.; Intechopen: London, UK, 2013; pp. 387–410. [Google Scholar] [CrossRef]
- Kryazheva, A.P.; Ponomareva, E.A.; Sorokina, M.V. Ecological and economic foundations of protection of winter wheat from damage by Zabrus tenebrioides Gz (Coleoptera, Carabidae). Entomol. Obozr. 1978, 57, 713–721. [Google Scholar]
- Schaafsma, A.W.; Whitfield, G.H.; Ellis, C.R. A temperature-dependent model of egg development of the western corn rootworm, Diabrotica virgifera virgifera Leconte (Coleoptera: Chrysomelidae). Can. Entomol. 1991, 123, 1183–1197. [Google Scholar] [CrossRef]
- Gray, M.E.; Tollefson, J.J. Influence of tillage systems on egg populations of western and northern corn rootworms (Coleoptera: Chrysomelidae). J. Kans. Entomol. Soc. 1988, 61, 186–194. [Google Scholar]
- Gray, M.E.; Tollefson, J.J. Emergence of the western and northern corn rootworms (Coleoptera: Chrysomelidae) from four tillage systems. J. Econ. Entomol. 1988, 81, 1398–1403. [Google Scholar] [CrossRef]
- Meinke, L.J.; Sappington, T.W.; Onstad, D.W.; Guillemaud, T.; Miller, N.J.; Komáromi, J.; Levay, N.; Furlan, R.; Kiss, J.; Toth, F. Western corn rootworm (Diabrotica virgifera virgifera LeConte) population dynamics. Agric. For. Entomol. 2009, 11, 29–46. [Google Scholar] [CrossRef]
- Dey, S.; Karmakar, K. Conservation agriculture for the management of insect pests—A review. Int. J. Ecol. Environ. Sci. 2021, 3, 395–400. [Google Scholar]
- Denlinger, D.L. Insect Diapause; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Tauber, M.J.; Tauber, C.A.; Masaki, S. Seasonal Adaptations of Insects; Oxford Univ Press: Oxford, UK, 1986. [Google Scholar]
- Buntin, G.D. Techniques for evaluating yield loss from insects. In Biotic Stress and Yield Loss; Peterson, R.K.D., Higley, L.G., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 37–56. [Google Scholar]
- Battisti, A.; Branco, M.; Mendel, Z. Defoliators in native insect systems of the Mediterranean Basin. Ins. Dis. Mediterr. For. Syst. 2016, 10, 29–45. [Google Scholar] [CrossRef]
- Barbulescu, A.; Voinescu, I.; Sadagorschi, D.; Penescu, A.; Popov, C.; Vasilescu, S. Cruiser 350 FS—A new product for maize and sunflower seed treatment against Tanymecus dilaticollis Gyll. Rom. Agric. Res. 2001, 15, 77–87. [Google Scholar]
- Popov, C.; Barbulescu, A. 50 years of scientific activity in field protection domain against diseases and pests. Ann. NARDI Fund. 2007, 75, 371–404. [Google Scholar]
- Georgescu, E.; Cană, L.; Rîșnoveanu, L. Influence of the sowing data concerning maize leaf weevil (Tanymecus dilaticollis Gyll) attack in atypically climatic conditions from spring period, in South-East of Romania. Lucr. Şti. Ser. Agron. 2019, 62, 39–44. [Google Scholar]
- Short, C.A.; Hahn, D.A. Fat enough for the winter? Does nutritional status affect diapause? J. Ins. Physiol. 2023, 145, 104488. [Google Scholar] [CrossRef] [PubMed]
- Benoit, J.B. Water management by dormant insects: Comparisons between dehydration resistance during summer aestivation and winter diapause. In Aestivation: Progress in Molecular and Subcellular Biology; Navas, C., Carvalho, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 209–229. [Google Scholar] [CrossRef]
- Alford, D.V.; Nilsson, C.; Ulber, B. Insect pests of oilseed rape crops. In Biocontrol of Oilseed Rape Pests; Alford, D.V., Ed.; Blackwell Science: Oxford, UK; Malden, MA, USA, 2003; pp. 9–42. [Google Scholar] [CrossRef]
- Storey, K.B. Life in the slow lane: Molecular mechanisms of estivation. Comp. Biochem. Physiol. A 2002, 133, 733–754. [Google Scholar] [CrossRef] [PubMed]
- Soler, R.S.; Atkinson, R.; Anderson, R.A. Water stress on plants and insect pests: Effects on plant chemistry and insect performance. Insect Sci. 2008, 15, 249–257. [Google Scholar]
- Nguyen, T.T.H. Climate extremes and their effects on pest dynamics: A case study of Spodoptera frugiperda in Europe. Clim. Change 2019, 152, 245–259. [Google Scholar]
- Du Plessis, H.; Schlemmer, M.L.; Van den Berg, J. The effect of temperature on the development of Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae). Insects 2020, 11, 228. [Google Scholar] [CrossRef]
- Hegewald, H.; Wensch-Dorendorf, M.; Sieling, K.; Christen, O. Impacts of break crops and crop rotations on oilseed rape productivity: A review. Eur. J. Agron. 2018, 101, 63–77. [Google Scholar] [CrossRef]
- Pál-Fám, F.; Varga, Z.; Keszthelyi, S. Appearance of the microfungi into corn stalk as a function of the injury of the European corn borer (Ostrinia nubilalis Hbn). Acta Agron. Hung. 2010, 58, 73–79. [Google Scholar] [CrossRef]
- Maina, U.M.; Galadima, I.B.; Gambo, F.M.; Zakaria, D.J. A review on the use of entomopathogenic fungi in the management of insect pests of field crops. J. Entomol. Zool. Stud. 2018, 6, 27–32. [Google Scholar]
- Forcella, F.; Buhler, D.D.; McGiffen, M.E. Pest management and crop residues. In Crops Residue Management; Hatfield, J.L., Stewart, B.A., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 173–190. [Google Scholar]
- Keszthelyi, S.; Binder, A.; Csóka, Á.; Pónya, Z.; Donkó, T. Computer tomography-assisted visualization of the movement triggered by frost in Ostrinia nubilalis overwintering in maize stalks. Physiol. Entomol. 2021, 46, 138–144. [Google Scholar] [CrossRef]
- Andreadis, S.S.; Vryzas, Z.; Papadopoulou-Mourkidou, E.; Savopoulou-Soultani, M. Age-dependent changes in tolerance to cold and accumulation of cryoprotectants in overwintering and non-overwintering larvae of European corn borer Ostrinia nubilalis. Physiol. Entomol. 2008, 33, 365–371. [Google Scholar] [CrossRef]
- Ramm, S.; Voßhenrich, H.H.; Hasler, M.; Reckleben, Y.; Hartung, E. Comparative Analysis of Mechanical In-Field Corn Residue Shredding Methods: Evaluating Particle Size Distribution and Rating of Structural Integrity of Corn Stalk Segments. Agriculture 2024, 14, 263. [Google Scholar] [CrossRef]
- Keszthelyi, S.; Holló, G. Evaluation of influencing factors on the location and displacement of Ostrinia nubilalis larvae in maize stalks measured by computed tomography. J. Plant Prot. Res. 2019, 59, 95–101. [Google Scholar] [CrossRef]
- Schaafsma, A.W.; Meloche, F.; Pitblado, R.E. Effect of mowing corn stalks and tillage on overwintering mortality of European corn borer (Lepidoptera: Pyralidae) in field corn. J. Econ. Entomol. 1996, 89, 1587–1592. [Google Scholar] [CrossRef]
- Hokkanen, H.M.T. Overwintering survival and spring emergence in Meligethes aeneus: Effects of body weight, crowding, and soil treatment with Beauveria bassiana. Entomol. Exp. Appl. 1993, 67, 241–246. [Google Scholar] [CrossRef]
- Sutter, L.; Amato, M.; Jeanneret, P.; Albrecht, M. Overwintering of pollen beetles and their predators in oilseed rape and semi-natural habitats. Agric. Ecosyst. Environ. 2018, 265, 275–281. [Google Scholar] [CrossRef]
- Schaffers, A.P.; Raemakers, I.P.; Sýkora, K.V. Successful overwintering of arthropods in roadside verges. J. Insect Conserv. 2012, 16, 511–522. [Google Scholar] [CrossRef]
- Hahne, H. A Contribution to the biology and control of the beet silphid beetle, Blaps Opaca. Arb. Biol. Reichsanst. Land-u. Forstw. 1930, 17, 499–548. [Google Scholar]
- Aulicky, R.; Tkadlec, E.; Suchomel, J.; Frankova, M.; Heroldová, M.; Stejskal, V. Management of the common vole in the Czech lands: Historical and current perspectives. Agronomy 2022, 12, 1629. [Google Scholar] [CrossRef]
- Vörös, G.; Gallé, L. Ants (Hymenoptera: Formicidae) as primary pests in Hungary: Recent observations. Tiscia 2002, 33, 31–35. [Google Scholar]
- Jacob, J.; Manson, P.; Barfknecht, R.; Fredricks, T. Common vole (Microtus arvalis) ecology and management: Implications for risk assessment of plant protection products. Pest. Manag. Sci. 2014, 70, 869–878. [Google Scholar] [CrossRef]
- Gawałek, M.; Dudek, K.; Ekner-Grzyb, A.; Kwiciński, Z.; Sliwowska, J.H. Ecology of the field cricket (Gryllidae: Orthoptera) in farmland: The importance of livestock grazing. North-West J. Zool. 2014, 10, 432–441. [Google Scholar]
- Jug, D.; Brmez, M.; Ivezic, M.; Stipesevic, B.; Stosic, M. Effect of different tillage systems on populations of common voles (Microtus arvalis Pallas, 1778). Cer. Res. Commun. 2008, 36, 923–926. [Google Scholar]
- Roos, D.; Saldaña, C.C.; Arroyo, B.; Mougeot, F.; Luque-Larena, J.J.; Lambin, X. Unintentional effects of environmentally friendly farming practices: Arising conflicts between zero-tillage and a crop pest, the common vole (Microtus arvalis). Agric. Ecosyst. Environ. 2019, 272, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, R.J.; Hein, G.L.; Blankenship, E.E.; Purrington, F.F.; Wilson, R.G.; Bradshaw, J.D. Comparing the effects of two tillage operations on beneficial epigeal arthropod communities and their associated ecosystem services in sugar beets. J. Econ. Entomol. 2018, 111, 2617–2631. [Google Scholar] [CrossRef]
- Vincent, C.; Hallman, G.; Panneton, B.; Fleurat-Lessard, F. Management of agricultural insects with physical control methods. Ann. Rev. Entomol. 2003, 48, 261–281. [Google Scholar] [CrossRef]
- Lima, M.; Storey, J.M.; Storey, K.B. Antioxidant defenses and metabolic depression: The hypothesis of preparation for oxidative stress in land snails. Comp. Biochem. Physiol. B 1998, 120, 437–448. [Google Scholar] [CrossRef]
- Şereflişan, H.; Duysak, Ö. Hibernation period in some land snail species (Gastropoda: Helicidae), Epiphragmal structure and hypometabolic behaviours. Turk. J. Agric. Food Sci. Technol. 2021, 9, 166–171. [Google Scholar] [CrossRef]
- Rowen, E.K.; Regan, K.H.; Barbercheck, M.E.; Tooker, J.F. Is tillage beneficial or detrimental for insect and slug management? A meta-analysis. Agric. Ecosyst. Environ. 2020, 294, 106849. [Google Scholar] [CrossRef]
- Bayer Crop Science. Slug Control, Bayer Expert Guide—Bayer Crop Science UK. Available online: https://cropscience.bayer.co.uk/mediafile/260326/2808-bcs-xg-slugs-2012-v2.pdf (accessed on 18 July 2019).
- Chandel, R.S.; Verma, K.S.; Rana, A.; Sanjta, S.; Badiyala, A.; Vashisth, S.; Baloda, A.S. The ecology and management of cutworms in India. Orient. Ins. 2022, 56, 245–270. [Google Scholar] [CrossRef]
- Sherlock, P.L.; Bowden, J.; Digby, P.G.N. Studies of elemental composition as a biological marker in insects IV The influence of soil type and host-plant on elemental composition of Agrotis segetum (Denis & Schiffermüller) (Lepidoptera: Noctuidae). Bull. Entom. Res. 1985, 75, 675–687. [Google Scholar] [CrossRef]
- Boiteau, G.; MacKinley, P. Dispersal of adult Colorado potato beetles (Coleoptera: Chrysomelidae) on plant models. Can. Entomol. 2013, 145, 20–28. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keszthelyi, S.; Tóth, Z.; Balog, A. A Tillage-Dependent System of Arable Pests: How Soil Condition and Prevailing Climate Influence Pest Occurrence? Agronomy 2025, 15, 1454. https://doi.org/10.3390/agronomy15061454
Keszthelyi S, Tóth Z, Balog A. A Tillage-Dependent System of Arable Pests: How Soil Condition and Prevailing Climate Influence Pest Occurrence? Agronomy. 2025; 15(6):1454. https://doi.org/10.3390/agronomy15061454
Chicago/Turabian StyleKeszthelyi, Sándor, Zoltán Tóth, and Adalbert Balog. 2025. "A Tillage-Dependent System of Arable Pests: How Soil Condition and Prevailing Climate Influence Pest Occurrence?" Agronomy 15, no. 6: 1454. https://doi.org/10.3390/agronomy15061454
APA StyleKeszthelyi, S., Tóth, Z., & Balog, A. (2025). A Tillage-Dependent System of Arable Pests: How Soil Condition and Prevailing Climate Influence Pest Occurrence? Agronomy, 15(6), 1454. https://doi.org/10.3390/agronomy15061454









