1. Introduction
Field crops are very often exposed to unfavorable or even extreme biotic and abiotic factors that adversely affect plant metabolism, growth, and development, as well as yield and quality. Primary food production in the world is, among other things, limited by soil fertility and climate, e.g., weather conditions [
1,
2,
3]. For successful maize production, water and heat are of the utmost importance. Maize is a plant that requires an adequate water supply for physiological development and adequate yield. However, water demand differs across stages of development, being lowest during early development and peaking during the reproductive phase and grain filling. Drought stress, particularly when combined with high temperatures during flowering, pollination, and fertilization, delays silking, causes silk drying, and leads to pollen abortion, which may result in poor fertilization or incomplete grain development [
4,
5]. Heat stress during the late vegetative phase causes maize yield reduction of up to 30%, while during the flowering and lag phase, up to 50% [
6]. When combined with drought, a drastically greater yield loss is possible. In general, insufficient precipitation in combination with high temperatures represents one of the main limiting factors for plant growth and development that are least manageable by agronomic practices and cause agricultural losses of more than 220 billion dollars [
7]. Kim and Lee [
8] state that climate change negatively affects a wide range of physiological and developmental processes: it reduces seed germination and seedling growth, reduces leaf expansion, stunts shoot and root growth, accelerates ripening, reduces plant height, disrupts photosynthesis, affects nutrient absorption, decreases pollination and fertilization, lowers grain yield, etc. Although Croatia, especially its eastern part, belongs to an optimal agroecological maize growing zone, significant year-to-year variations in the average maize yield are often caused primarily by drought and high temperatures in the summer months during maize flowering and fertilization stages or other agrotechnical practices [
9,
10]. Maize is the dominant field crop in Croatia. According to the Croatian Bureau of Statics, around 30% of arable land is occupied with this crop, with an annual production of around 2 million tons. In total cereal production, expressed in terms of quantity, maize accounts for 55% to 60% and contributes around 8% to 10% of the total agricultural output expressed in prices [
11].
Besides the influence of climate, to achieve high yields and quality, maize requires adequate soil fertility, which is very often impaired by a lack or excess of certain mineral elements. Soil acidity is one of the most widespread global constraints to successful production. It is estimated that approximately 50% of all arable soils worldwide have some level of acidity [
12]. In Croatia, about 32% of total agricultural land is acidic, mostly in the eastern region, where the majority of agricultural production takes place [
13,
14]. According to research, two soil types dominate: pseudogley and alluvial soil. Soil acidification is a very slow and long-term natural process of accumulation of H
+ ions in both the liquid and solid phases of the soil. However, this process has been accelerated in recent decades due to anthropogenic activities. In general, low soil fertility in acidic soils is the result of the toxicity of Al, Mn, and Fe and the reduced availability of P, Ca, and Mg. Maize requires at least 16 macro and microelements for normal growth and development through the entire life cycle, which are mainly acquired from the soil [
15]. Microelements, whose role is almost equal to macroelements, are essential, although they are present in very low concentrations in the soil and the plant. Under conditions of excessive soil acidity, the availability of most microelements may increase. However, their uptake and translocation within the plant depend on numerous factors [
16]. According to the literature, there are two main approaches to mitigate the adverse effects of soil acidity. One proposes agrotechnical and/or land improvement measures, such as liming, phosphatization, and targeted fertilization [
17,
18,
19]. The other underlines the genetic variability in maize, suggesting that differences in the sensitivity or tolerance of genotypes to specific soil conditions can be used for better plant adaptation rather than the soil constantly adapting to the plant [
20,
21].
In addition to agroecological factors such as climate and soil that influence the growth and development of maize, the role of genotype is also very important because different genotypes are characterized by the varying ability to absorb available elements in the soil, even under the same agroecological conditions. Selecting a suitable maize genotype for a specific growing region can lead to higher grain yields, better quality as well as increased uptake and accumulation of microelements in the grain [
22,
23].
Based on the hypothesis, abiotic stress can be harmful to maize plants, reducing both yield and grain quality, while the combination of two or more abiotic stresses can be unpredictable and may cause severe damage to the plant. Therefore, the aim of this research was to evaluate the effects of stressful abiotic conditions, extreme precipitation, drought, high air temperature, and soil acidity on maize grain yield and quality, as well as to determine the genotype-specific responses of ten maize hybrids under such stressful agroecological conditions.
4. Discussion
The main goal of maize breeding and production is to achieve high and stable yields with the best possible grain quality. However, usually, they are limited by two major factors. These are the genotype, or the total inheritance of an individual, and the environment, which provides more or less favorable conditions for the expression of a particular genotype [
29,
30,
31]. Also, under intense stress conditions, interactions between environment and genotypes are more pronounced and complex because genotypes show different levels of phenotypic expression in different environmental conditions, and the variations in values were extremely large [
32,
33,
34]. In our study, analysis of variance determined the statistical significance of all treatments (environment, genotype, and their interaction) except hybrids for grain yield. Further, very large variations between environments, hybrids, and interactions were found.
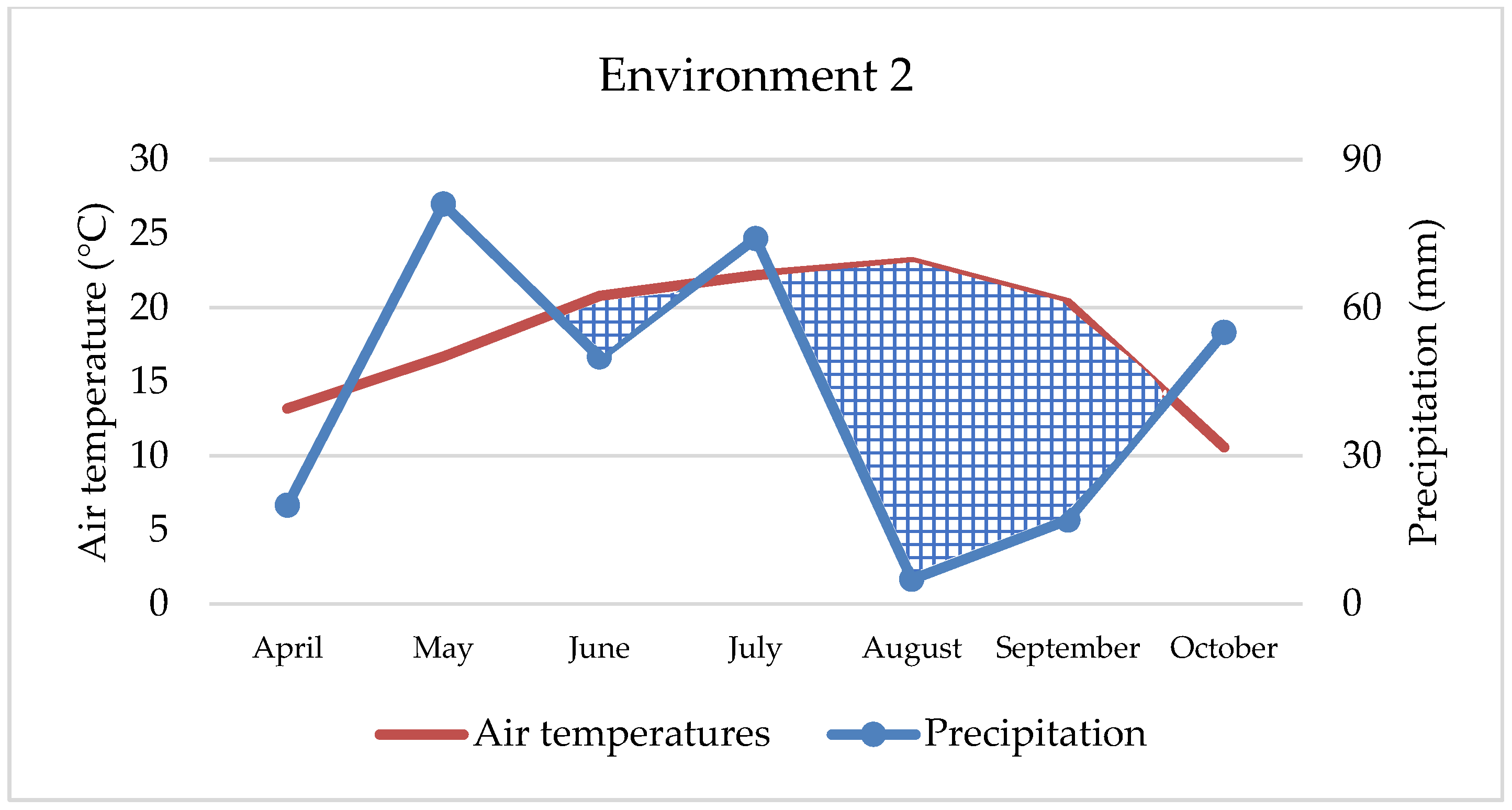
According to the Croatian Bureau of Statistics [
35], maize yields in the Republic of Croatia have varied greatly over the years. In the period from 2005 to 2023, the average yield was 5.7 t ha
−1 but with extremely large variation from 4.0 t ha
−1 (2010) to 9.0 t ha
−1 (2019). The biggest reasons for the variation are environmental factors, especially precipitation and temperature regimes, having a significant impact. In this study, a relatively high average yield (8.61 t ha
−1) was achieved despite negative environmental influences such as drought, high air temperature, soil acidity, or even excessive rainfall, which may be related to the good genetic material of the tested hybrids. The best yield was achieved on ENV2, which is characterized by neutral soil pH and severe drought during August and September (5 mm and 17 mm, respectively). At the same time, air temperature was higher for 1.8 °C and 3.6 °C. However, in the second and third decade of July, when maize is in the most sensitive phase of development, a small amount of precipitation fell (61 mm), which was sufficient for flowering and grain fertilization. Similar observations of the influence of weather conditions have been made by others [
36,
37,
38,
39]. The authors state that the application of conventional agricultural techniques, good fertilization and liming on acidic soils, and the sowing of drought-tolerant hybrids at optimal times or the application of new sowing technologies can mitigate negative impacts on maize yields. Also, conservation agriculture is one of the solutions for the future due to a whole range of positive advantages [
40,
41].
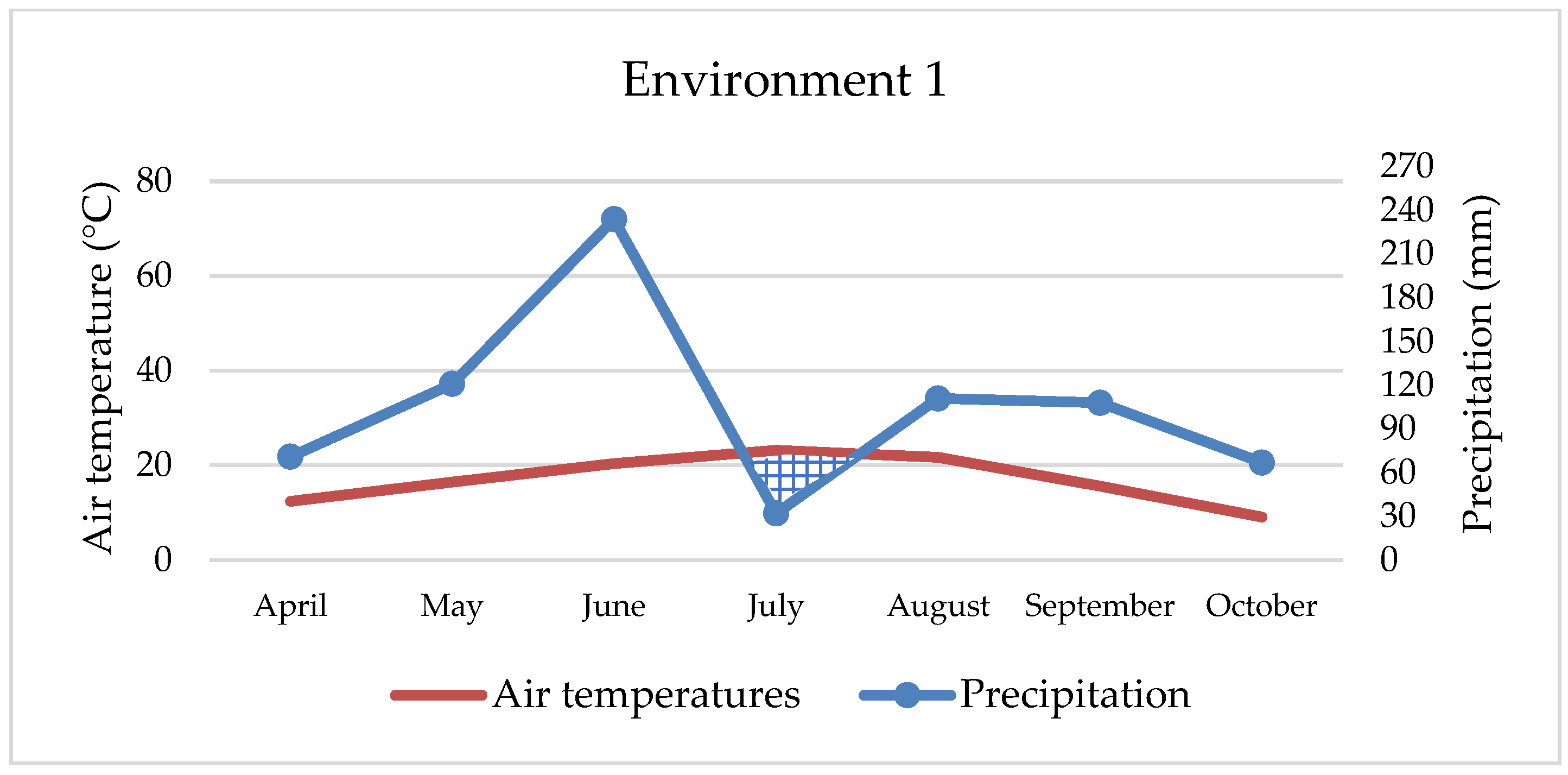
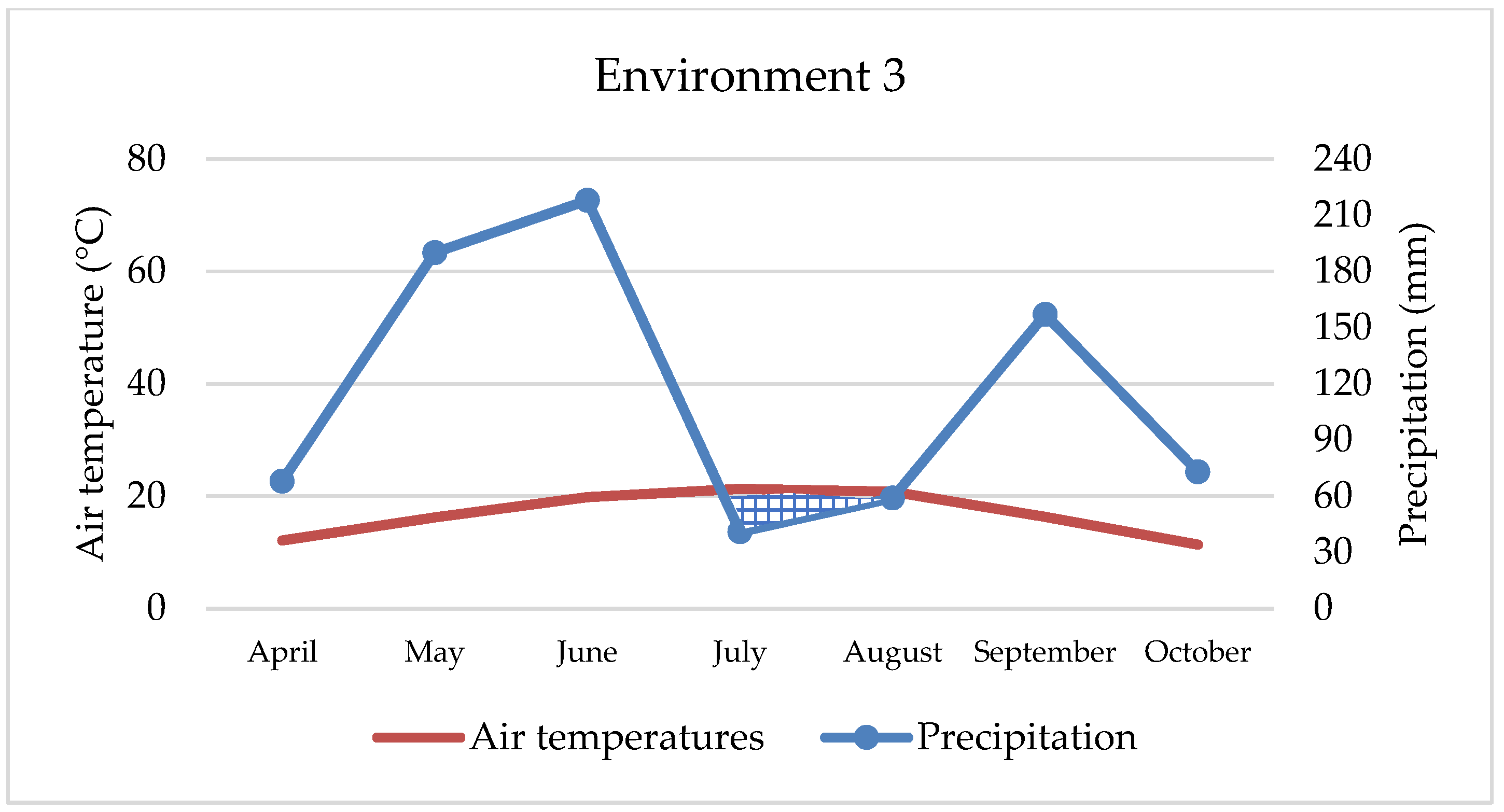
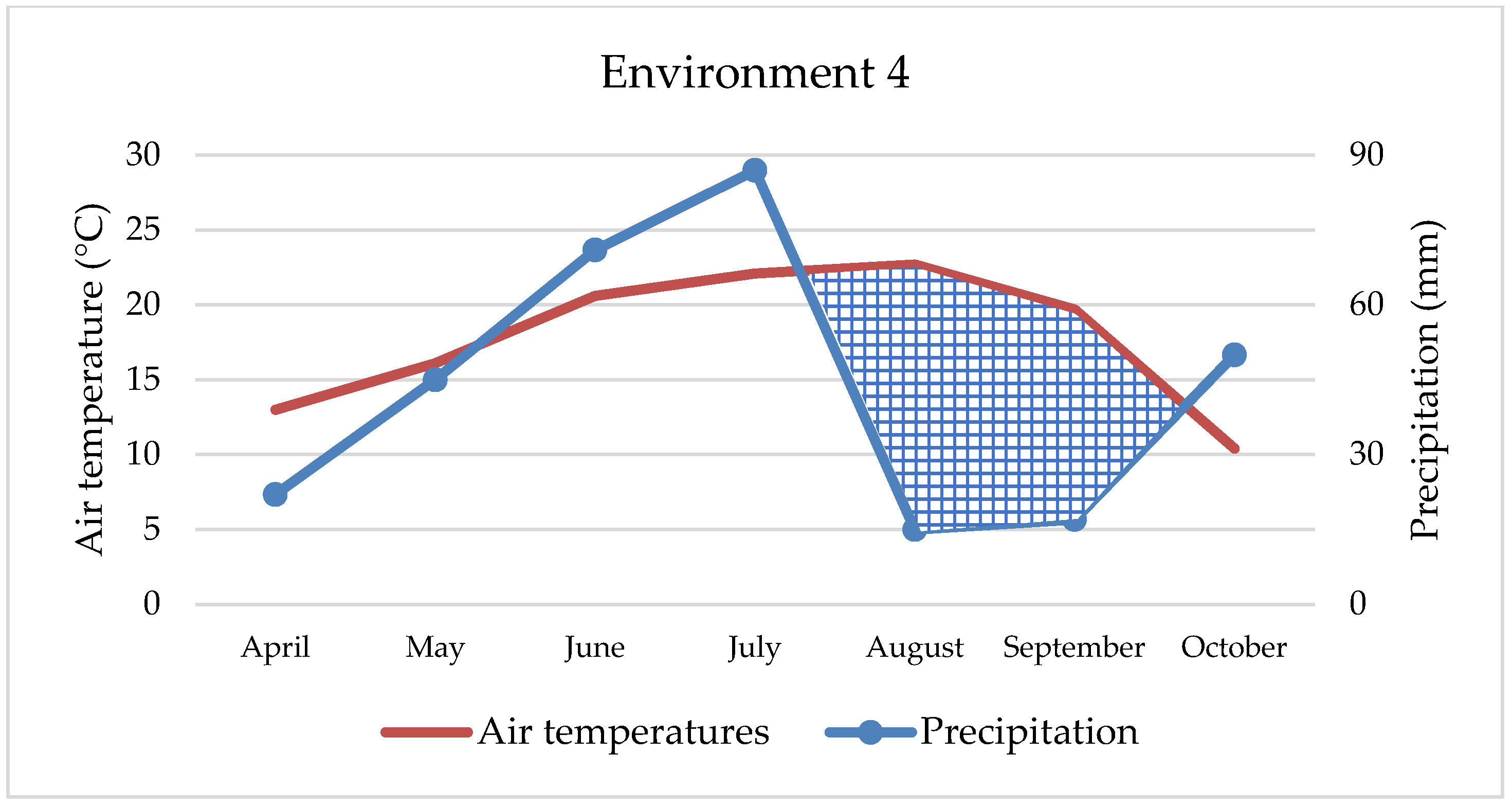
Maize’s water requirements depend primarily on the stage of development, but many other factors, such as weather conditions, soil fertility, photosynthesis intensity, hybrid properties, etc., can also affect water uptake and consumption. Maize needs about 64 mm of water in May, 106 mm in June, 121 mm in July, 124 mm in August, and 63 mm of water in September, while hybrids of later vegetation require a small amount of water in October as well [
42]. Although maize requires large amounts of water, primarily due to its habit, in our study, environments with extreme rainfall (
Figure 1 and
Figure 3) were not the most productive. Thus, ENV1 and ENV3 had lower yields. For example, during May, ENV1 received twice as much precipitation as the observed multi-year average (121 mm and 62 mm, respectively), while in ENV3, the amount was even higher (190 mm and 70 mm), which is not conducive to the normal development of the maize plant. So, although maize requires large amounts of water, in extreme cases and in the early developmental stages, it can have the opposite effect, as observed in our research. Based on data from 1981 to 2016, Li et al. [
43] estimated a yield reduction of up to 34% due to excessive rainfall, mainly in cooler areas and with poorly drained soils, while Markovic et al. [
44] report a 7.6% yield reduction under the same conditions. In this research, yield was lower by 15.2% and 28.4% in extreme wet conditions compared to the best yield conditions (ENV2). In contrast to all other environments, ENV 4 achieved statistically the lowest maize yield (5.51 t ha
−1) due to the negative effect of double abiotic stress, reduced fertility, i.e., pronounced soil acidity as well as extreme drought, especially during April, August, and September (22 mm, 15 mm, and 17 mm, respectively) and higher air temperatures in all months. In such challenging conditions, only two hybrids had yields slightly more than 6 t ha and three hybrids less than 5 t ha (
Table 4), which is an extremely low yield for maize growing conditions in eastern Croatia. Therefore, the maize response to different abiotic stress or their combination depends on the interaction of stresses [
45]. For example, in our study, maize growing on acid soil in combination with extreme precipitation achieved better yield (8.08 t ha
−1) than growing on the same soil and drought conditions (5.51 t ha
−1). Depending on the abiotic outdoor environment, maize hybrids react reversibly or irreversibly, and the process is always complex and dynamic [
46]. Over the last 40 years, maize breeding has increased grain yield, and one possible reason is that green, photosynthetically active leaves remain on the stem longer, which increases and prolongs the accumulation of nutrients in the grain [
47]. Furthermore, plant architecture between hybrids, such as physiological metabolisms, photosynthesis characteristics, and stomatal morphologies of leaves, have a significant impact on maize yield under abiotic stress conditions [
48]. In conducted research, hybrids showed high yield and great yield variability, although no statistical significance was determined between them. The highest-yielding hybrids (HYB4 and HYB5) were not always the best in the tested environments (
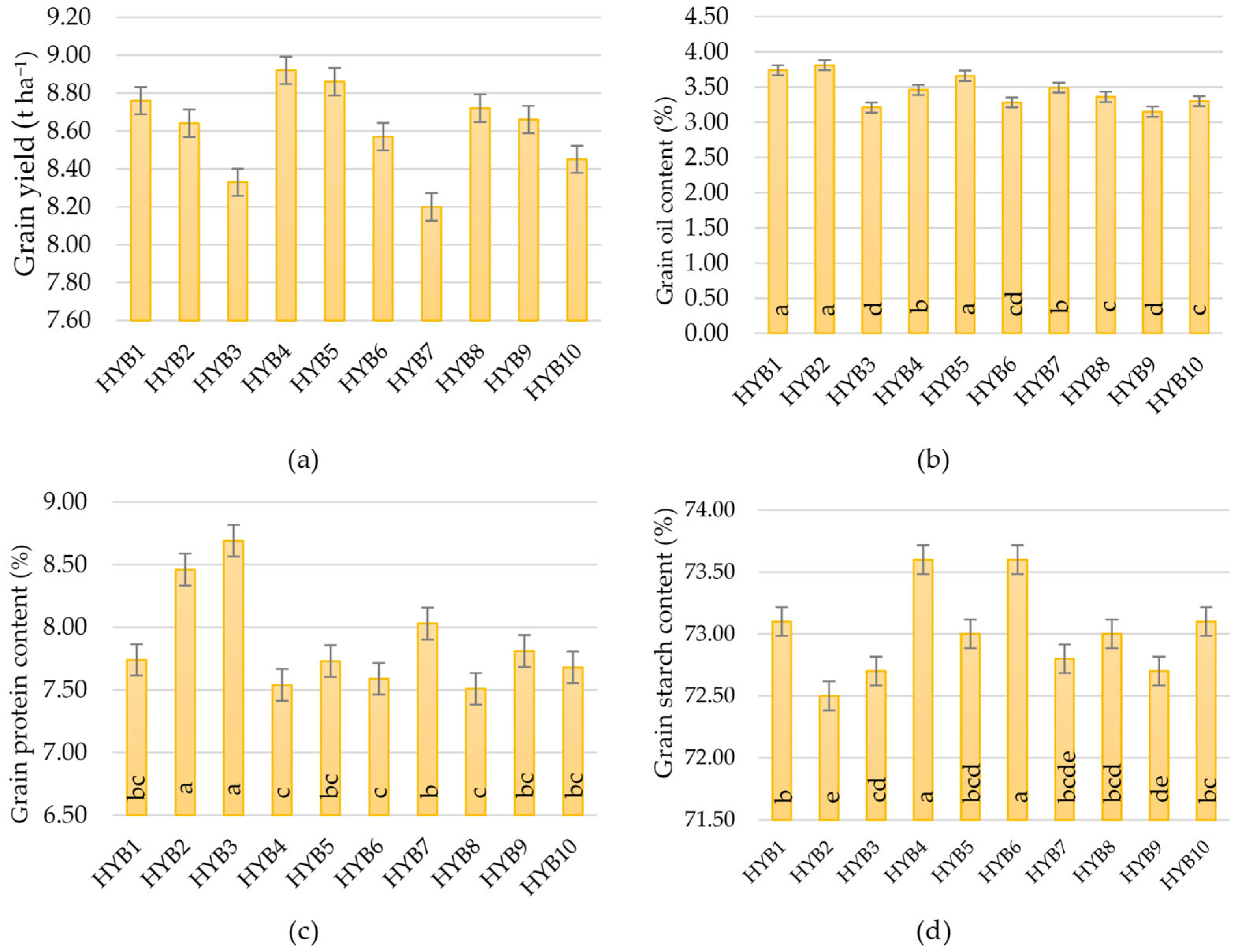
Figure 6), but they showed a certain stability. Also, they showed good results in extremely rainy and dry environments, as well as in acidic soil. Such hybrids should be strongly considered when planning to grow across different environments, especially under abiotic stresses. In any case, the difference between the highest and lowest-yielding hybrid was 720 kg ha. Although the seed owner (Agricultural Institute Osijek) emphasizes the advantage of HYB5, HYB6, and HYB7 as a high-yield, stable hybrid across the environments, in our research, only HYB5 has shown relatively high value across all four stress environments. In addition, we cannot underline the advantage of the early and late maturity groups of maize hybrids in the context of yield because, from each group, some hybrids were excellent thought environments. The above indicates that sowing maize of different vegetation maturity groups and different specific characteristics is the best option for achieving high yields because there is no ideal hybrid that is totally resistant to abiotic stress. In terms of the best hybrids for wet or dry areas, it is not possible to draw a clear line. However, HYB4 and HYB10 may be a good choice for wetter areas, while for drier growing areas, we can only recommend HYB5.
In the context of maize farming, yield is always the dominant trait, while quality is given less importance. However, despite its relatively low protein and oil content, maize is consumed in larger quantities by livestock [
49]. The highest protein percentage was recorded in ENV4, which is characterized by double abiotic stress. This is in line with other research, which shows that under any form of stress, plants accumulate a greater amount of protein [
50,
51]. In both environments where extreme rainfall was recorded, protein content was lower, especially in ENV3, which was exposed to excessive rainfall and an acid soil condition. In the context of tested hybrids, only two (HYB3 and HYB4) show statistically higher values (8.46% and 8.69%). The lowest protein content in the grain was achieved by HYB8 (7.51%), although no statistically significant difference was found with the other six hybrids. This indicates that a larger number of hybrids in the study achieved slightly lower values. Although Nehe et al. [
52] claim that environment has the greatest influence on protein content, in our case, the influence of environment and genotype, i.e., hybrid, was almost equal.
Oil content in our research was under the influence of environment, hybrid, and interactions. Higher values were determined in environments with neutral soil pH, regardless of precipitation amounts and temperature (ENV1 and ENV2), which indicates that maize cultivation for the specific purpose of oil production should be on soils that have an almost neutral pH reaction. As with the protein content, the hybrids of earlier vegetation (HYB1 and HYB2) showed statistically higher values of oil content compared to all other hybrids.
In contrast to all other properties, starch content proved to be the most stable, as the variation among environments was only 0.81%, and among hybrids, 1.5%. Even in the interaction of environment and hybrids, the value was only 3.2%. Therefore, no matter under what abiotic stress conditions the tested hybrids are grown, they will show very uniform values. Very similar results were obtained by Wang et al. [
53]. Based on the analysis of five maize hybrids and four environments, the deviation between environments was 0.92%, 0.56%, and 0.50%. In any case, the conclusions of other authors are contradictory. Some claim that abiotic stress (e.g., drought) significantly reduces starch content and increases amino acids [
54], while others state that drought increases starch content [
55]. The abiotic stress factors assessed in this study represent the greatest threat to maize production today—thus, this research provides useful insights into better understanding the outcomes of genotype–environment interactions in some modern maize hybrids. The results could be used in breeding programs to enhance overall productivity, especially in the context of climate change.