Abstract
C2H2 zinc finger (C2H2-ZF) transcription factors, characterized by the presence of a conserved ZnF-C2H2 domain, are widespread among plant-pathogenic fungi such as Magnaporthe oryzae, Fusarium graminearum, and Sclerotinia sclerotiorum and have critical roles in the regulation of fungal growth, development, stress adaptation, and secondary metabolism. However, little is known about the presence and roles of C2H2-ZF transcription factors in Setosphaeria turcica (syn. Exserohilum turcicum), the causal agent of northern corn leaf blight. To address this gap, we identified the complete set of C2H2-ZF transcription factors in the S. turcica genome and characterized their structural characteristics, physicochemical properties, and protein–protein interaction network. We then used RNA sequencing to profile their expression dynamics during fungal development and host infection. The 27 S. turcica C2H2-ZF proteins were classified into three major subfamilies and contained six conserved motifs. All 27 genes were transcribed during 5 stages of fungal development, and 24 were expressed during the infection of susceptible maize, suggesting that they function in both fungal growth and pathogenesis. This study represents the first systematic characterization of C2H2-ZF proteins in S. turcica, offering insight into their potential roles in pathogenicity and establishing a foundation for future functional studies of individual family members.
1. Introduction
Northern corn leaf blight (NCLB) is a destructive fungal disease caused by Setosphaeria turcica (syn. Exserohilum turcicum), which primarily infects maize (Zea mays) leaves. The pathogen causes large, yellowish-brown lesions on leaves, severely impairing photosynthesis. This leads to premature plant senescence and poor grain filling, ultimately reducing yields by 30–50% in severe cases [1]. This disease is widespread across maize-growing regions and poses a significant challenge to crop productivity. The average global annual loss can reach several billion dollars [2]. S. turcica exhibits substantial genetic diversity and frequent variation, enabling it to adapt rapidly to environmental changes and host resistance mechanisms [3]. The optimal environmental conditions for S turcica epidemics include temperatures between 20 and 28 °C (with 25 °C being most favorable) and sustained relative humidity ≥90% coupled with leaf wetness duration exceeding 6 h. Under such conditions, the pathogen can rapidly complete its infection cycle, resulting in extensive lesion formation on host plants [4]. Currently, maize breeding has successfully developed varieties carrying the Ht series of disease-resistant genes, which demonstrate good resistance to NCLB. However, with the rapid evolution of the S. turcica and the emergence of new pathogenic races, the traditional single-gene resistance has been gradually overcome, leading to reduced effectiveness of these resistant varieties [5].
The rapid emergence of new physiological species of S. turcica has led to the frequent breakdown of host resistance, making disease management increasingly challenging. Although genetic resistance remains a key strategy, it must be integrated with cultural practices, chemical control, and molecular-based pathogen monitoring for sustainable disease control [6]. Advances in transcriptomics and genomics have provided insights into the regulatory networks that govern S. turcica development and virulence and have enabled the identification of potential genetic targets for disease intervention. Future research on fungal transcription factors, host–pathogen interactions, and novel resistance mechanisms will be critical for the development of durable, broad-spectrum control strategies.
Zinc finger (ZF) proteins are transcription factors with finger-like DNA-binding domains that participate in diverse biological processes, including transcriptional regulation, mRNA transport, chromatin remodeling, protein folding, and maintenance of cellular structure [7]. The first ZF transcription factor was identified and shown to regulate gene transcription in Xenopus laevis oocytes [8], and ZF proteins have subsequently been studied extensively in both plants and animals [9]. ZF proteins are classified into nine subfamilies on the basis of their conserved cysteine (Cys, C) and histidine (His, H) motifs: C2H2, C3H, C3HC4, C2HC5, C4HC3, C2HC, C4, C6, and C8 [10]. Among these, the C2H2-ZF subfamily has been most extensively characterized [11]. They are characterized by a canonical ZF domain, typically consisting of 25–30 amino acids with a conserved Cys-X-Cys-X-His-X-His sequence motif (in which X represents any amino acid sequence) [12].
In plants, C2H2-ZF proteins regulate the expression of genes involved in growth, development, and stress response. For example, the heterologous expression of the Arabidopsis C2H2-ZF gene SZT in tobacco enhances tolerance to cold and high salinity [13,14]. Likewise, fungal C2H2-ZF transcription factors have been shown to modulate development, stress adaptation, and the biosynthesis of secondary metabolites [15]. Examples include the C2H2-ZF transcription factors ScMsn2 and ScMsn4 in Saccharomyces cerevisiae, which regulate stress response pathways [16,17], Cap1p in Candida albicans, which promotes resistance to hydrogen peroxide [18], and CRE1 in Neurospora crassa, which regulates the production and secretion of extracellular enzymes such as endoglucanase [19]. In Acremonium chrysogenum, the C2H2-ZF transcription factor AcStuA suppresses sporulation by downregulating AcbrlA and AcabaA, while also inducing hyphal swelling and modulating cell wall integrity [20,21]. In Aspergillus nidulans, the C2H2-ZF protein BrlA governs sporulation [22], and in Trichoderma reesei, CRE1 directly regulates cellulase secretion [23]. Given the diverse roles of C2H2-ZF proteins in fungal species, investigating the C2H2-ZF family in S. turcica will likely provide insights into the regulatory mechanisms that control its development and pathogenicity, ultimately contributing to a better understanding of fungal virulence and host–fungus interactions.
The physicochemical properties of proteins, including theoretical isoelectric point (pI), instability index, and hydrophobicity, are fundamental determinants of protein structure, function, and practical applications. The pI governs protein solubility and net charge distribution under varying pH conditions, making it essential for protein purification techniques like ion-exchange chromatography [24]. The instability index provides predictive insights into protein degradation kinetics, informing stabilization strategies for vaccine antigens and engineered enzymes in industrial processes. Hydrophobicity values dictate protein folding pathways through hydrophobic core formation and mediate membrane integration of transmembrane proteins. Collectively, these properties enable rational protein engineering by elucidating structure–function relationships, with wide-ranging implications from drug development to agricultural biotechnology. Their quantitative analysis serves as a cornerstone for both fundamental research and translational applications in the life sciences [25].
In previous work, we characterized two C2H2-ZF transcription factors in S. turcica: StMSN2, which regulates fungal growth, pathogenesis, and adaptation to hyperosmotic stress [26]; and StSTE12, which is essential for appressorium development and host penetration [27]. Nonetheless, most studies on C2H2-ZF proteins in phytopathogenic fungi remain limited to individual genes, and no systematic explorations of the entire family have been performed in S. turcica to date. To address this gap, we identified and bioinformatically characterized the complete set of C2H2-ZF transcription factors in the S. turcica genome, predicted their protein–protein interaction network, and examined their expression profiles during fungal development and host infection. This systematic approach provides a comprehensive framework for understanding the roles of C2H2-ZF proteins in the development and pathogenicity of S. turcica, laying the groundwork for functional studies of individual family members.
2. Materials and Methods
2.1. Strains, Plant Materials, and Culture Conditions
The wild-type (WT) S. turcica strain 01-23 was used in this study and is preserved at the Hebei Provincial Key Laboratory of Plant Physiology and Molecular Pathology. It was cultured at 25 °C on potato dextrose agar (PDA) containing 20% potato infusion, 2% glucose, and 1.5% agar and was periodically sub-cultured to maintain viability. S. cerevisiae AH109 competent cells were purchased from Coolaber, Beijing, China. The maize inbred lines B73 were grown under controlled greenhouse conditions as described previously [28].
2.2. Characterization and Physicochemical Profiling of StZF Proteins in S. turcica
Publicly available genome and protein sequences for S. turcica were downloaded from the Joint Genome Institute database (http://genome.jgi-psf.org/Settu1/Settu1.home.html, (accessed on 9 June 2024)). The Hidden Markov model (HMM) profile for the C2H2-ZF protein family (PF00096) was obtained from the Pfam database (http://pfam.xfam.org/, (accessed on 9 June 2024)) [29] and used with the default parameters of HMMER 3.3.2 software (http://hmmer.org/, (accessed on 9 June 2024)) to identify C2H2-ZF transcription factors in the S. turcica protein sequences. The SMART tool (http://smart.embl-heidelberg.de, (accessed on 9 June 2024)) was used to identify known protein domains in the C2H2-ZF family members, and the ExPASy Proteomics Server (http://expasy.org, (accessed on 9 June 2024)) was used to predict their physicochemical properties, including molecular weight, isoelectric point, hydrophilicity, and instability index [30].
2.3. Structural Analysis of the StZF Genes
The coding sequence of each C2H2-ZF gene was aligned to its corresponding DNA sequence using the online tool GSDS 2.0 (http://gsds.cbi.pku.edu.cn, (accessed on 12 June 2024)) to examine its gene structure and the numbers and positions of introns and exons [31].
2.4. Multiple Sequence Alignment and Conserved Motif Analysis of the S. turcica StZF Proteins
The conserved C2H2 domains of the S. turcica C2H2-ZF proteins were aligned with ClustalX2 software (http://www.clustal.org/clustal2/, (accessed on 15 June 2024)), and the resulting multiple sequence alignment was visualized using BioEdit 7.2 (https://thalljiscience.github.io, (accessed on 15 June 2024)). The MEME online tool (http://meme-suite.org, (accessed on 16 June 2024)) was used to identify up to 8 motifs in the C2H2-ZF proteins, and the motifs were then imported as an XML file into TBtools-II2.111 for further editing [32].
2.5. Phylogenetic Analysis of the StZF Proteins
C2H2-ZF transcription factors were first identified in the genomes of M. oryzae and S. cerevisiae using the method outlined in Section 2.2. The protein sequences of all C2H2-ZF transcription factors in S. turcica, M. oryzae, and S. cerevisiae were then imported into ClustalX2 in FASTA format for multiple sequence alignment. The resulting alignment was then imported into MEGA 6.0 software, and a phylogenetic tree of the C2H2-ZF transcription factors was constructed using the neighbor-joining method with 1000 bootstrap replicates [33].
2.6. Prediction of a Protein–Protein Interaction (PPI) Network for the StZF Proteins
Orthology mapping was used to predict the interactions among members of the StZF family. First, homologs of the S. turcica StZF proteins were identified in the S. cerevisiae genome database using BLAST 2.1.0.0 (https://www.yeastgenome.org/, (accessed on 15 November 2024)) [34]. STRING (https://string-db.org, (accessed on 15 November 2024)) was then used to generate a predicted PPI network for the S. turcica StZF proteins based on the known PPIs of S. cerevisiae, with nodes representing proteins and edges representing interactions [35].
2.7. Total RNA Extraction and Real-Time Quantitative PCR (qRT–PCR)
Total RNA was extracted from fungal cultures maintained at 25 °C in complete darkness for 14 days. Following homogenization in liquid nitrogen, RNA isolation was performed using Trizol reagent (Tiangen Biotech, Beijing, China) with subsequent DNaseI treatment to remove genomic DNA contamination. First-strand cDNA was synthesized using the extracted total RNA as a template. Genomic DNA was removed using a commercial reverse transcription kit (M5 Super Plus qPCR RT kit with a gDNA removal step; Mei5 Bioservices, Beijing, China), and the resulting cDNA was stored at −20°C. To confirm the reliability of the RNA-sequencing (RNA-seq) data, we measured the relative expression of randomly selected differentially expressed genes (DEGs) by qRT–PCR. Gene-specific primers (100–250 bp amplicons) were designed using Primer 3.0 and synthesized by Sangon Biotech (Shanghai, China) [36]; all primer sequences are provided in Table S1. The tubulin gene served as an internal reference, and primer concentrations were standardized to 0.2 μM. After amplification with the TransStart Top Green qPCR SuperMix kit (Transgen Biotech, Beijing, China), qPCR was performed on the ABI Prism 7500 Fast Real-Time PCR system, according to the manufacturer’s instructions, and relative gene expression was quantified using the 2−ΔΔCt method [37].
2.8. Yeast Two-Hybrid (Y2H) Assay to Test Protein–Protein Interactions of StZF7, StZF8, and StZF16
Target genes were individually cloned into the pGBKT7 and pGADT7 vectors to generate bait and prey constructs, respectively, and the constructs were transformed into yeast AH109 competent cells (No. CC317, Coolaber, Beijing, China) using the lithium acetate method. First, the bait vector and the empty pGADT7 vector were co-transformed into AH109 yeast and plated on SD/-Leu/-Trp/-His medium. If self-activation was observed, various concentrations of 3-amino-1,2,4-triazole (3-AT) were added to the medium to inhibit self-activation. Bait and prey vectors were then co-transformed into AH109 yeast and grown on SD/-Leu/-Trp and SD/-Leu/-Trp/-His/-Ade plates with an appropriate 3-AT concentration. The plates were inverted and incubated at 28 °C for 3–5 days for observation of colony growth [38]. All experiments were performed in triplicate, and the primers used for Y2H constructs are listed in Table S1.
2.9. Expression of StZF Genes Across Five Developmental Stages in S. turcica
Samples were collected from five developmental stages of S. turcica: mycelium, conidia, germ tubes, appressoria, and penetration pegs. Conidia were obtained by first culturing S. turcica on PDA at 25 °C in the dark for 15 days. Aerial hyphae were removed with sterile cotton swabs, and the underlying hyphae were incubated for an additional 3 days under the same conditions. Conidial suspensions were collected by addition of 8 mL of sterile distilled water to the plates, followed by centrifugation at 12,000× g. Germ tubes, appressoria, and penetration pegs were collected by spreading 5 mL of conidial suspension onto water agar plates overlaid with sterile cellophane, which were then incubated at 25 °C in the dark. Germ tubes were harvested at 4 h post inoculation (hpi), appressoria at 10 hpi, and penetration pegs at 16 hpi. Finally, mycelium was harvested with truncated pipette tips after 7 days of culture on PDA medium at 25 °C in the dark. For each material, a 2 g sample was collected, and all experiments were performed in triplicate. One gram of each sample was sent to Novogene Bioinformatics Technology (Beijing, China) for high-throughput RNA-seq analysis, and the remaining 1 g was used for RNA extraction and qRT–PCR [39].
2.10. Expression of StZF Genes in S. turcica During Infection of Maize
Maize seeds (B73 cultivar) were sown in pots and grown to the 5–6-leaf stage. A freshly prepared spore suspension was evenly sprayed onto the leaves of three replicate plants of each genotype, and the inoculated plants were maintained under dark and humid conditions for 24 h. Pathogen-inoculated and control maize leaves were collected at three infection stages (0, 24, and 72 h) and stored at −80 °C for subsequent use. All experiments were performed in triplicate. One portion of each sample was sent to Novogene for high-throughput RNA-seq as described above, and the remaining portion was used for RNA extraction and qRT–PCR.
3. Results
3.1. Identification and Physicochemical Analysis of StZF Proteins in S. turcica
We used an HMM profile for the C2H2-ZF protein family to identify 27 candidate C2H2-ZF proteins in S. turcica, which we named StZF1–27 (Table S2) [40]. The online tools PFAM and SMART confirmed that all StZF proteins contained at least one typical ZnF-C2H2 domain. The StZF proteins ranged in size from 69 (StZF10) to 1158 (StZF17) amino acids, with molecular weights from 8.155 kDa (StZF10) to 126.017 kDa (StZF17) and theoretical isoelectric points (pIs) from 5.15 (StZF7) to 10.06 (StZF20). About half (51.9%) of the StZFs had a pI greater than 7, indicating that they were weakly alkaline. All StZFs had an instability index greater than 40, suggesting that they were unstable, and all had negative average hydrophobicity values, indicating that they were hydrophilic.
3.2. Structures of the StZF Genes in S. turcica
Most of the StZF gene sequences contained introns, and there was substantial variation in intron numbers, positions, and lengths among the genes (Figure 1). The number of introns ranged from 0 to 7, although the majority of StZF genes (17) contained 1 or 2 introns. Intron phase, i.e., the position of an intron within a codon, is commonly used to analyze the structural evolution of genes. Here, the StZF genes included 30 exon types: nine 0-0 symmetrical (i.e., exons flanked by two introns both in phase 0), seven 2-2 symmetrical, three 1-1 symmetrical, and 11 asymmetrical.
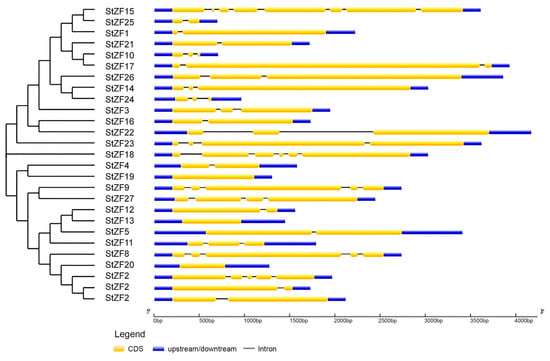
Figure 1.
Gene structural characteristics of the StZF family.
3.3. Conserved Motifs in the StZF Proteins of S. turcica
Multiple sequence alignment revealed that the S. turcica StZF proteins all contained at least one highly conserved ZnF-C2H2 domain (Cys-X-Cys-X-His-X-His). In addition to the conserved cysteine (C) and histidine (H) residues, other highly conserved residues were present in the intervening sequence denoted by X; these included lysine (K), phenylalanine (F), arginine (R), glutamic acid (E), aspartic acid (D), and leucine (L). MEME and TBtools analyses revealed the presence of six conserved motifs, 8–45 amino acids in length, in the StZFs (Figure 2A). Together, Motifs 1–3 formed the complete conserved ZnF-C2H2 domain; Motif 1 showed particularly high conservation, with 21 of its 45 residues highly conserved. On the basis of their phylogenetic relationships, the StZFs could be divided into two major subfamilies that differed in the number of protein motifs. StZFs in subfamily I contained more numerous and diverse domains than those in subfamily II; all six conserved motifs were present in subfamily I, whereas subfamily II lacked Motifs 4 and 5 (Figure 2B). Only StZF18 could not be unambiguously assigned to a subfamily.
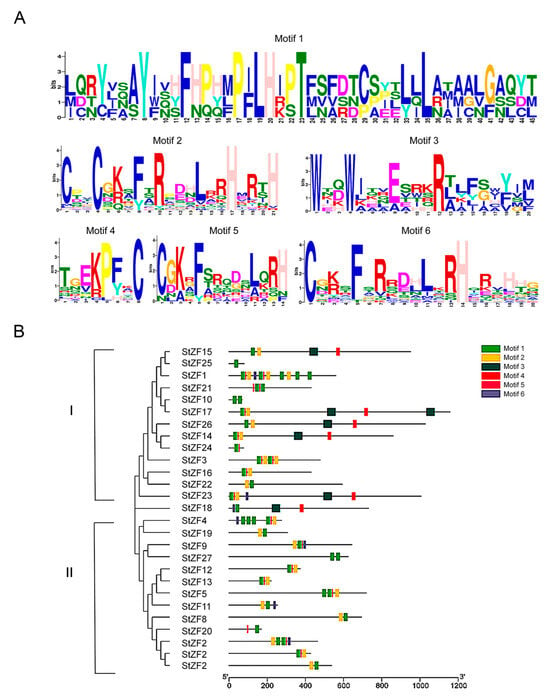
Figure 2.
Multiple sequence alignment and conserved motifs in the StZF family. (A) WebLogo diagrams depicting the six conserved motifs identified in the StZF proteins. (B) A neighbor-joining phylogenetic tree of the StZF proteins, showing their conserved protein motifs. The StZFs could be divided into two major subfamilies on the basis of their phylogenetic relationships.
3.4. Phylogenetic Analysis of the StZF Proteins in S. turcica
The S. cerevisiae serves as a well-established fungal model organism. Phylogenetic analysis reveals a closer evolutionary relationship between M. oryzae and S. turcica. To further explore the evolutionary relationships among C2H2-ZF proteins, we identified 31 C2H2-ZF proteins in S. cerevisiae and 25 in M. oryzae (ScZFs and MoZFs, respectively), then constructed a phylogenetic tree of the C2H2-ZF proteins from S. turcica, S. cerevisiae, and M. oryzae (Figure 3). On the basis of their phylogenetic relationships, the ZF proteins could be divided into three groups: groups I, II, and III. Group I was the largest, with 46 members (14 StZFs, 14 ScZFs, and 18 MoZFs), followed by group II with 34 members (11 StZFs, 11 ScZFs, and 12 MoZFs). Group III contained only 2 StZFs and 1 ScZF. The two plant pathogens, M. oryzae and S. turcica, had similar numbers of C2H2-ZF proteins, and 77.8% of the StZFs clustered in the same clades with MoZFs.
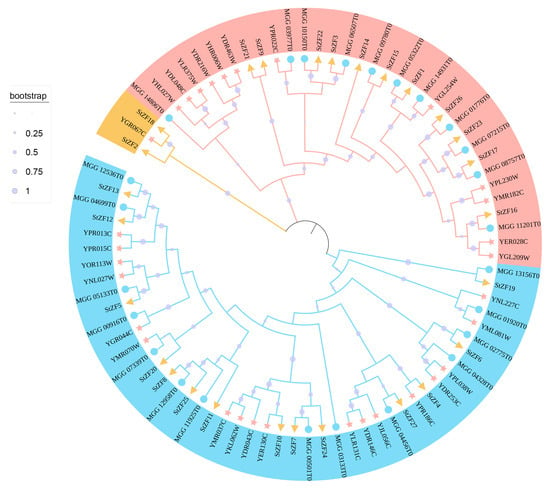
Figure 3.
Neighbor-joining phylogenetic tree of 83 C2H2-ZF proteins from Saccharomyces cerevisiae (pink stars), Magnaporthe oryzae (blue circles), and Setosphaeria turcica (yellow triangles). The sizes of the light purple circles correspond to the branch support values calculated from 1000 bootstrap replicates. The proteins can be divided into 3 groups: group I (blue), group II (red), and group III (yellow).
3.5. Construction and Validation of a PPI Network for the StZF Proteins
To investigate potential interactions among the StZF proteins in S. turcica, we constructed a predicted PPI network based on the interactions of their S. cerevisiae homologs, which we identified by sequence alignment. The predicted S. turcica network comprised 21 proteins with 43 interactions (Figure 4A). To assess the reliability of the network, we performed Y2H assays for interactions among the core node protein StZF8 and two of its predicted interactors, StZF7 and StZF16.
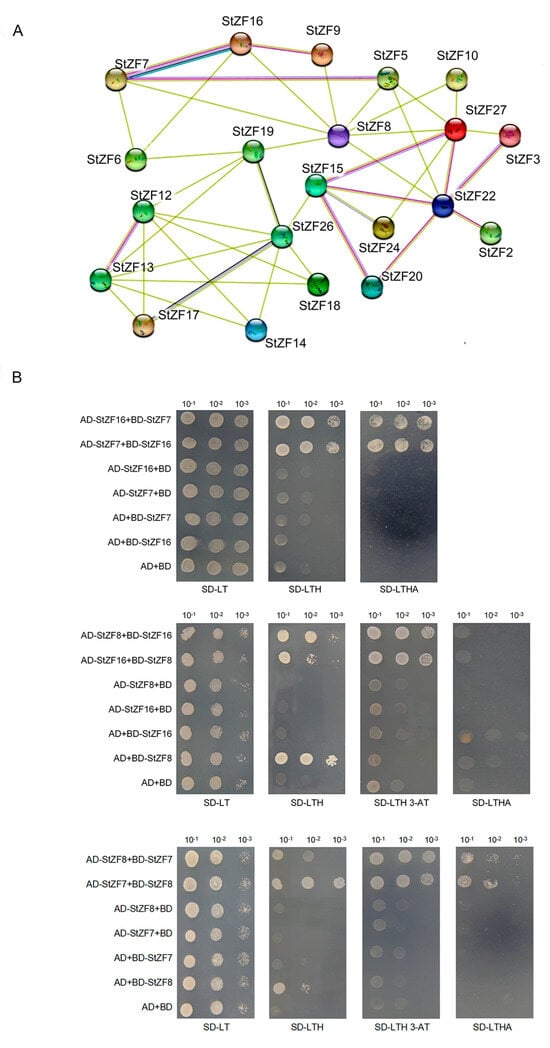
Figure 4.
Construction and validation of a predicted PPI network among the StZF proteins. (A) Predicted interaction network among 21 StZF proteins based on the interactions of their S. cerevisiae homologs. The number of connecting lines between a pair of proteins reflects their predicted interaction strength. (B) Yeast two-hybrid assays were performed to confirm a subset of the predicted StZF interactions. AD and BD represent the yeast vectors pGADT7 and pGBKT7. Co-transformed yeast cells were diluted to 10−1, 10−2, and 10−3, then spotted onto SD/-Trp/-Leu, SD/-Trp/-Leu/-His (+3-AT), and SD/-Trp/-Leu/-His/-Ade media. Growth of the yeast colonies was observed after incubation at 30 °C for 3 days.
Yeast transformed with all experimental and control vector combinations formed white colonies on SD/-Trp/-Leu medium, confirming successful transformation and protein expression. Self-activation was observed for StZF8 on SD/-Trp/-Leu/-His medium, and we therefore added 3-AT to suppress this self-activation in subsequent assays. Yeast transformed with the StZF7 + StZF8, StZF7 + StZF16, and StZF8 + StZF16 vector combinations grew on SD/-Trp/-Leu/-His medium (with added 3-AT as appropriate), confirming the interactions predicted in the PPI network. Yeast transformed with the StZF7 + StZF8 and StZF7 + StZF16 combinations also produced white colonies on SD/-Trp/-Leu/-His/-Ade medium, but no colony growth was observed for the StZF8 + StZF16 combination. These results demonstrated that StZF7 and StZF16, StZF7 and StZF8, and StZF8 and StZF16 interacted directly in the yeast system, although the interaction between StZF8 and StZF16 was relatively weak (Figure 4B). These findings provide support for the predicted interaction network of StZF proteins in S. turcica.
3.6. Expression Patterns of StZF Genes During Growth and Development of S. turcica
Gene expression patterns can provide insight into potential gene functions. We therefore used RNA-seq to measure StZF expression at five developmental stages: mycelium, conidia, germ tube, appressorium, and infection peg (Figure 5A). All 27 StZF genes were expressed at all five developmental stages, although the expression levels of the different StZFs varied within a given stage. For instance, StZF16 exhibited the highest expression in the mycelium, germ tube, appressorium, and infection peg, whereas StZF7 showed the highest expression in conidia. Furthermore, the expression level of the same gene varied across developmental stages. For example, StZF1 expression initially increased from the mycelium to the conidia but decreased in later stages of development, whereas StZF16 expression decreased in the conidia and germ tube but then rose in the appressorium and infection peg. These findings suggest that the StZFs have diverse functions in the growth and development of S. turcica.
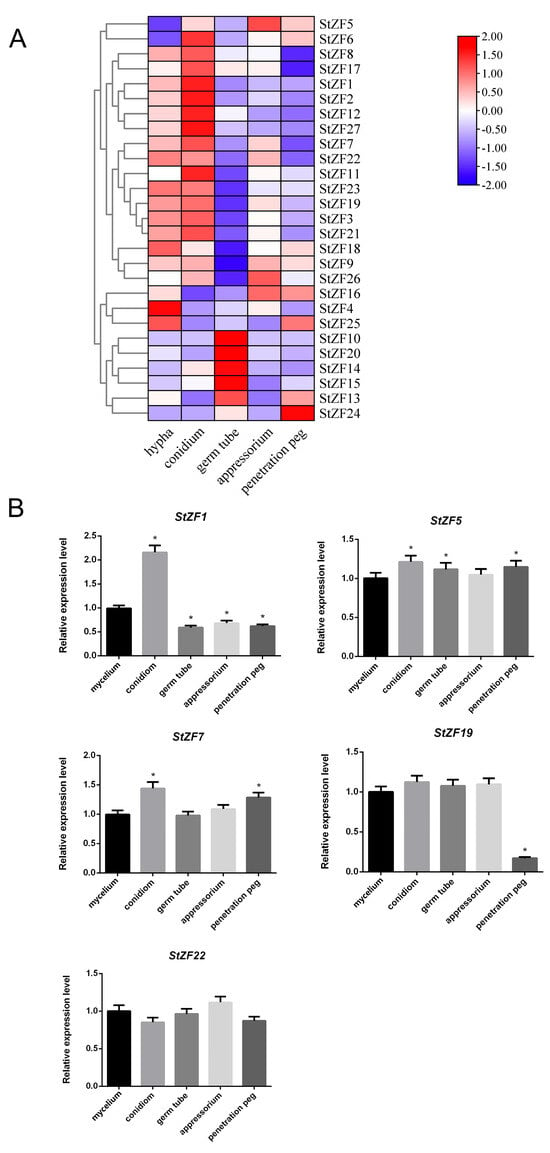
Figure 5.
Expression patterns of the StZF genes at different developmental stages of S. turcica. (A) Heatmap showing the expression levels of 27 StZF genes at five developmental stages as measured by RNA-seq. (B) Relative expression levels of StZF1, StZF5, StZF7, StZF19, and StZF22 at different developmental stages as measured by quantitative real-time PCR. Error bars represent the SD of three replicates. * p < 0.05 (two-tailed Student’s t test).
We randomly selected five StZF genes (StZF1, StZF5, StZF7, StZF19, and StZF22) for qPCR validation. The qPCR results confirmed that these genes were expressed at all five developmental stages, and their expression trends closely matched those observed in the transcriptome data (Figure 5B).
3.7. Expression Patterns of StZF Genes During Infection
To examine the potential roles of StZF genes in S. turcica pathogenesis, we inoculated leaves of the susceptible maize host B73 with a conidial suspension of wild-type S. turcica and analyzed StZF expression levels in the infected host tissue at 0, 24, and 72 h post infection (hpi) (Figure 6A). Three of the StZFs were not expressed during infection (StZF4/24/25). Expression of StZF22, StZF2, and StZF20 was highest at 0 hpi; expression of StZF13 and StZF14 was highest at 72 hpi; and expression of the remaining StZFs was highest at 24 hpi.
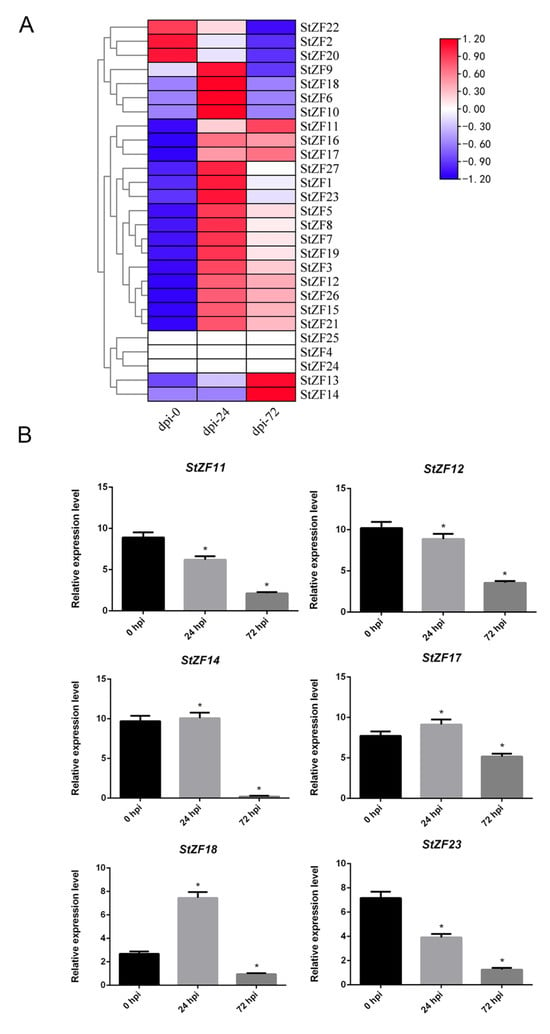
Figure 6.
Expression patterns of the S. turcica StZF genes during infection of a maize B73 host. (A) Heatmap showing the expression levels of 27 StZF genes at 0, 24, and 72 h post infection (hpi), as measured by RNA-seq. (B) Expression levels of StZF11, StZF12, StZF14, StZF17, StZF18, and StZF23 at 0, 24, and 72 hpi, as measured by quantitative real-time PCR. Error bars represent the SD of three replicates. (* p < 0.05, two-tailed Student’s t test).
We randomly selected six genes (StZF11, StZF12, StZF14, StZF17, StZF18, and StZF23) for qPCR validation and measured their expression levels in maize B73 at 0, 24, and 72 hpi. StZF11, StZF12, and StZF23 exhibited similar expression patterns, decreasing slightly at 24 hpi and decreasing markedly at 72 hpi. StZF14, StZF17, and StZF18 were upregulated at 24 hpi and then markedly downregulated at 72 hpi.
4. Discussion
The C2H2-type ZF proteins are a highly conserved family of transcription factors that play crucial roles in multiple biological processes, including DNA/RNA binding, protein–protein interactions, and gene expression regulation [41]. Our whole-genome analysis identified 27 StZF genes in the genome of the fungal pathogen S. turcica, all of which contained this conserved domain.
Comparative genomic analyses have shown that the size of the C2H2 family is correlated with evolutionary complexity [42]. Higher eukaryotes typically contain more C2H2-ZF genes than lower organisms, suggesting that these genes may have important roles in adaptive evolution and developmental regulation [43]. Fungal genomes appear to contain fewer C2H2-type ZF genes than plant genomes: there are 79 in Verticillium dahliae [44] and 30 in Lenzites gibbosa [45] compared with 189 in Oryza sativa [46], 118 in Nicotiana tabacum [47], and 109 in Populus trichocarpa [48]. Here, we identified 27 C2H2-type ZF proteins in S. turcica, 31 in S. cerevisiae, and 25 in M. oryzae, confirming the smaller size of this family in fungi. Phylogenetic analysis revealed that StZFs from S. turcica tended to cluster with MoZFs from M. oryzae in the same evolutionary clades, and C2H2 transcription factors are known to regulate hyphal growth, asexual reproduction, appressorium formation, and pathogenicity in M. oryzae [49]. These findings not only suggest the possibility of conserved pathogenic mechanisms between S. turcica and M. oryzae but also establish a framework for investigating functional diversification and adaptive evolution in filamentous pathogenic fungi.
A PPI network is a graphical representation of the interactions among multiple proteins [50]. Such networks can provide insight into complex biological processes within cells, reveal functional modules and signaling pathways in organisms, and enable the prediction of new biological phenomena or drug targets [51]. A homology mapping approach has been used to predict protein interactions in studies of M. oryzae [52], and we also used this method to construct a predicted PPI network for the StZF proteins in S. turcica. To provide initial confirmation of the predicted network interactions, we performed Y2H experiments and supplemented our analysis with transcriptome expression data. Our results demonstrated that two strongly interacting genes, StZF7 and StZF16, exhibited high expression levels, whereas two weakly interacting genes, StZF8 and StZF16, exhibited high and low expression, respectively. These findings provide evidence for the accuracy of the constructed PPI network and also suggest a potential link between the strength of protein interactions and gene expression patterns.
C2H2-ZF transcription factors play a critical role in regulating fungal pathogenicity. Twenty-two C2H2-ZF proteins have been identified as key regulators of fungal virulence in M. oryzae, with the VRF1 gene specifically influencing appressorium structure and the expression of pathogenicity-related genes [49]. Likewise, the PSCZF1 gene is essential for pathogenicity in Phytophthora sojae, as PSCZF1 mutants lose their ability to infect soybean hosts [53]. In this study, we identified 24 StZFs that were expressed in S. turcica during maize infection. Three were upregulated during early infection, four during late infection, and the remaining 17 during the middle infection stage. Their stage-specific expression patterns suggest that these StZFs may regulate distinct phases of host colonization. Notably, we observed discrepancies between RNA-seq and qPCR quantification results. We addressed this issue by investigating potential biological and technical factors contributing to these discrepancies. These include post-transcriptional regulation, where variations in mRNA stability or translational efficiency may result in transient expression patterns that are not fully captured by transcriptome sequencing. Differences in experimental conditions, such as sample preparation, biological replicates, or time-point sampling between the expression analysis and transcriptome datasets, may also contribute to the observed discrepancies. Functional redundancy or specialization may also explain the divergent expression kinetics, possibly indicating that different ZF proteins play distinct roles in stress responses—some in early defense signaling and others in later-stage regulatory pathways. The early expression of PlCZF1 is crucial for establishing infection in Peronophythora litchii, [54] consistent with our observation of early-upregulated StZFs in S. turcica.
Fungal C2H2-ZF transcription factors play crucial roles in gene-expression regulation, growth and development, stress responses, and secondary metabolism. Ste12-like factors are a special class of fungal C2H2 transcription factors that were initially identified as regulatory targets of the Fus3 mitogen-activated protein kinase cascade involved in mating [55]. In filamentous fungi, Ste12-like proteins play a critical role in development and pathogenicity [56]. Studies have shown that the C2H2-ZF transcription factor Ste12 regulates various cellular processes in C. albicans, including cell-wall biosynthesis, filamentation, and invasive growth [57]. Previous studies in our laboratory found that the mutation of StMSN2 (StZF7) affects the growth, development, pathogenicity, and hyperosmotic stress adaptation of the pathogen [26], whereas StSTE12 (StZF8) participates in the development of appressoria and the infection process [27]. Transcriptomic analysis of pathogen growth, development, and infection processes revealed that StZF7 and StZF16 are highly expressed, and may therefore play key regulatory roles, during these stages. C2H2-ZF proteins in pathogenic fungi serve as critical regulatory targets during fungal infection, and their characterization can help to characterize pathogen infection mechanisms and advance sustainable disease management strategies [58]. In future research, we will focus on the knockout of StZF16 to clarify the roles of these highly expressed StZF genes in the growth, development, and pathogenicity of S. turcica.
5. Conclusions
We identified 27 members of the C2H2-ZF protein family in S. turcica. These proteins were characterized by the presence of six conserved motifs, with motifs 1, 2, and 3 likely being the core sequences essential for structural and functional integrity. Using a homology-based mapping approach, we constructed a PPI network that included 21 members of the StZF family, and we performed Y2H assays to confirm a number of the predicted interactions. Analysis of gene expression revealed that the StZF genes are expressed throughout the growth, development, and pathogenic infection processes of S. turcica. Our findings provide a comprehensive annotation of the C2H2-ZF proteins in S. turcica, laying a foundation for further exploration of their roles in pathogenicity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15061434/s1, Table S1: List of primers; Table S2: Names and physicochemical properties of the StZF proteins.
Author Contributions
Conceptualization, H.J., Q.Z. and P.L.; methodology, M.L., X.L., Z.L. and X.G.; formal analysis, X.L., X.G., J.D., Z.L. and S.G.; investigation, X.L., H.J., Q.Z., Z.L. and P.L.; writing—original draft preparation, H.J., P.L. and Q.Z.; writing—review and editing, H.J., P.L., Q.Z. and Y.L.; supervision, M.L., S.G. and Y.L.; project administration, M.L., J.D. and X.G.; funding acquisition, S.G. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the the National Key Research and Development Program of China (2023YFD1401500), Natural Science Foundation of Hebei (C2023204100), the Basic Research Projects of Universities in Hebei Province Funded by Shijiazhuang (241791197A), Hebei Provincial Central Leading Local Science and Technology Development Fund Project (236Z6507G), the S&T Program of Hebei (24466301D and 23567601H), and the National Natural Science Foundation of China (32402304).
Data Availability Statement
The RNA-seq data are available under accession number PRJNA1185298 at the NCBI Sequence Read Archive (http://www.ncbi.nlm.nih.gov/sra, (accessed on 12 November 2024)).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study, writing of the manuscript, or decision to publish the results.
References
- Fang, Y.L.; Zhou, Y.Y.; Li, X.; Gao, Y.; Wang, D.L.; Liu, M.J.; Zhang, Z.J. Histological characterization of the early-stage infection events of Setosphaeria turcica in maize. Plant Pathol. 2021, 71, 251–261. [Google Scholar] [CrossRef]
- Mideros, S.X.; Chung, C.-L.; Wiesner-Hanks, T.; Poland, J.A.; Wu, D.; Fialko, A.A.; Turgeon, B.G.; Nelson, R.J. Determinants of virulence and in vitro development colocalize on a genetic map of Setosphaeria turcica. Phytopathology 2018, 108, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Huang, S.-Y.; Hernandez, A.G.; Adhikari, P.; Jamann, T.M.; Mideros, S.X. Genomic regions associated with virulence in Setosphaeria turcica identified by linkage mapping in a biparental population. Fungal Genet. Biol. 2022, 159, 103655. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, K.; Guo, X.; Turgeon, B.G.; Dong, J. A genome resource of Setosphaeria turcica, causal agent of Northern Leaf Blight of Maize. Phytopathology 2020, 110, 2014–2016. [Google Scholar] [CrossRef]
- Galiano-Carneiro, A.L.; Miedaner, T. Genetics of Resistance and Pathogenicity in the maize/Setosphaeria turcica pathosystem and implications for breeding. Front. Plant Sci. 2017, 8, 1490. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Liu, B.; He, S.; Gao, Z. Analysis of physiological races and genetic diversity of Setosphaeria turcica (Luttr.) K.J. Leonard & Suggs from different regions of China. Can. J. Plant Pathol. 2020, 42, 396–407. [Google Scholar]
- Cassandri, M.; Smirnov, A.; Novelli, F.; Pitolli, C.; Agostini, M.; Malewicz, M.; Melino, G.; Raschellà, G. Zinc-finger proteins in health and disease. Cell Death Discov. 2017, 3, 17071. [Google Scholar] [CrossRef]
- Laurent, A.; Masse, J.; Omilli, F.; Deschamps, S.; Richard-Parpaillon, L.; Chartrain, I.; Pellerin, I. ZFPIP/Zfp462 is maternally required for proper early Xenopus laevis development. Dev. Biol. 2009, 327, 169–176. [Google Scholar] [CrossRef]
- Moulick, D.; Bhutia, K.L.; Sarkar, S.; Roy, A.; Mishra, U.N.; Pramanick, B.; Maitra, S.; Shankar, T.; Hazra, S.; Skalicky, M.; et al. The intertwining of Zn-finger motifs and abiotic stress tolerance in plants: Current status and future prospects. Front. Plant Sci. 2023, 13, 1083960. [Google Scholar] [CrossRef]
- Laity, J.H.; Lee, B.M.; Wright, P.E. Zinc finger proteins: New insights into structural and functional diversity. Curr. Opin. Struct. Biol. 2001, 11, 39–46. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.Y.; Jia, X.M.; Cao, Y.B.; Gao, P.H.; Fu, X.P.; Ying, K.; Chen, W.S.; Jiang, Y.Y. Cap1p is involved in multiple pathways of oxidative stress response in Candida albicans. Free Radic. Biol. Med. 2006, 40, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.D.; Zhang, Y.; Liu, J.; Xie, C.H. Novel potato C2H2-type zinc finger protein gene, StZFP1, which responds to biotic and abiotic stress, plays a role in salt tolerance. Plant Biol. 2010, 12, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xie, R.; Hu, Y.; Du, L.; Wang, F.; Zhao, X.; Liu, D. A C2H2-type zinc finger protein TaZFP8-5B negatively regulates disease resistance. BMC Plant Biol. 2024, 24, 1116. [Google Scholar] [CrossRef]
- Li, W.T.; He, M.; Wang, J.; Wang, Y.P. Zinc finger protein (ZFP) in plants-a review. Plant Omics 2013, 6, 474. [Google Scholar]
- Wang, K.; Ding, Y.; Cai, C.; Chen, Z.; Zhu, C. The role of C2H2 zinc finger proteins in plant responses to abiotic stresses. Physiol. Plant. 2018, 165, 690–700. [Google Scholar] [CrossRef]
- Nicholls, S.; Straffon, M.; Enjalbert, B.; Nantel, A.; Macaskill, S.; Whiteway, M.; Brown, A.J.P. Msn2- and Msn4-like transcription factors play no obvious roles in the stress responses of the fungal pathogen. Eukaryot. Cell 2004, 3, 1111–1123. [Google Scholar] [CrossRef]
- Watanabe, D.; Wu, H.; Noguchi, C.; Zhou, Y.; Akao, T.; Shimoi, H. Enhancement of the initial rate of ethanol fermentation due to dysfunction of yeast stress response components Msn2p and/or Msn4p. Appl. Environ. Microbiol. 2011, 77, 934–941. [Google Scholar] [CrossRef]
- Dai, B.D.; Wang, Y.; Zhao, L.X.; Li, D.D.; Li, M.B.; Cao, Y.B.; Jiang, Y.Y. Cap1p attenuates the apoptosis of Candida albicans. FEBS J. 2013, 280, 2633–2643. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Higuchi, M.; Hashimoto, Y.; Seki, M.; Kobayashi, M.; Kato, T.; Tabata, S.; Shinozaki, K.; Kakimoto, T. Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature 2001, 409, 1060–1063. [Google Scholar] [CrossRef]
- Hu, P.; Wang, Y.; Zhou, J.; Pan, Y.; Liu, G. AcstuA, which encodes an APSES transcription regulator, is involved in conidiation, cephalosporin biosynthesis and cell wall integrity of Acremonium chrysogenum. Fungal Genet. Biol. 2015, 83, 26–40. [Google Scholar] [CrossRef]
- Guan, F.; Pan, Y.; Li, J.; Liu, G. A GATA-type transcription factor AcAREB for nitrogen metabolism is involved in regulation of cephalosporin biosynthesis in Acremonium chrysogenum. Sci. China Life Sci. 2017, 60, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.G.; Xu, S.Y.; Ying, S.H.; Feng, M.G. Roles of BrlA and AbaA in mediating asexual and insect pathogenic lifecycles of metarhizium robertsii. J. Fungi 2022, 8, 1110. [Google Scholar] [CrossRef] [PubMed]
- Cziferszky, A.; Mach, R.L.; Kubicek, C.P. Phosphorylation positively regulates DNA binding of the carbon catabolite repressor Cre1 of Hypocrea jecorina (Trichoderma reesei). J. Biol. Chem. 2002, 277, 14688–14694. [Google Scholar] [CrossRef]
- Verfaillie, D.; Janssen, F.; Van Royen, G.; Wouters, A.G.B. A systematic study of the impact of the isoelectric precipitation process on the physical properties and protein composition of soy protein isolates. Food Res. Int. 2023, 163, 112177. [Google Scholar] [CrossRef]
- De, R.; Mahata, M.K.; Kim, K.T. Structure-Based Varieties of Polymeric Nanocarriers and Influences of Their Physicochemical Properties on Drug Delivery Profiles. Adv. Sci. 2022, 9, 10. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, X.; Li, M.; Si, H.; Zhou, Q.; Liu, X.; Fan, Y.; Zhang, X.; Han, J.; Gu, S.; et al. Effect of osmotic stress on the growth, development and pathogenicity of Setosphaeria turcica. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.Q.; Li, P.; Wu, M.; Hao, Z.M.; Gong, X.D.; Zhang, X.Y.; Tian, L.; Zhang, P.; Wang, Y.; Cao, Z.Y.; et al. StSTE12 is required for the pathogenicity of Setosphaeria turcica by regulating appressorium development and penetration. Microbiol. Res. 2014, 169, 817–823. [Google Scholar] [CrossRef]
- Lv, R.; Liu, Y.; Gong, X.; Han, J.; Gu, S.; Dong, J. Expression and purification of the transcription factor StMsn2 from Setosphaeria turcica in Escherichia coli. Electron. J. Biotechnol. 2019, 40, 65–70. [Google Scholar] [CrossRef]
- Finn, R.D.; Bateman, A.; Clements, J.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Heger, A.; Hetherington, K.; Holm, L.; Mistry, J.; et al. Pfam: The protein families database. Nucleic Acids Res. 2014, 42, D222–D230. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Chen, C.J.; Wu, Y.; Li, J.W.; Wang, X.; Zeng, Z.H.; Xu, J.; Liu, Y.L.; Feng, J.T.; Chen, H.; He, Y.H.; et al. TBtools-II: A ‘one for all, all for one’ bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.M.; Hong, E.L.; Amundsen, C.; Balakrishnan, R.; Binkley, G.; Chan, E.T.; Christie, K.R.; Costanzo, M.C.; Dwight, S.S.; Engel, S.R.; et al. Saccharomyces Genome Database: The genomics resource of budding yeast. Nucleic Acids Res. 2011, 40, D700–D705. [Google Scholar] [CrossRef] [PubMed]
- Lei, D.; Lin, R.; Yin, C.; Li, P.; Zheng, A. Global protein–protein interaction network of rice sheath blight pathogen. J. Proteome Res. 2014, 13, 3277–3293. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- He, W.; Li, T.; Xiong, B.; Shen, L.; Chen, P. The role and mechanism of BmsPLA2-1-1 in the IMD pathway in silkworm, Bomybx mori. Int. J. Biol. Macromol. 2024, 283, 137297. [Google Scholar] [CrossRef]
- Gong, X.; Han, D.; Zhang, L.; Yin, G.; Yang, J.; Jia, H.; Cao, Z.; Dong, J.; Liu, Y.; Gu, S. Comprehensive analysis of the LysM protein family and functional characterization of the key LysM effector StLysM1, which modulates plant immunity in Setosphaeria turcica1. J. Integr. Agric. 2025, 24, 1860–1874. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the Expasy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Sun, Z.; Liu, R.; Guo, B.; Huang, K.; Wang, L.; Han, Y.; Li, H.; Hou, S. Ectopic expression of GmZAT4, a putative C2H2-type zinc finger protein, enhances PEG and NaCl stress tolerances in Arabidopsis thaliana. 3 Biotech 2019, 9, 166. [Google Scholar] [CrossRef]
- Kim, S.; Choi, K.; Park, C.; Hwang, H.J.; Lee, I. Suppressor of frigida4, encoding a C2H2-type zinc finger protein, represses flowering by transcriptional activation of Arabidopsis flowering locus c. Plant Cell 2006, 18, 2985–2998. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Maruyama, K.; Sakuma, Y.; Meshi, T.; Iwabuchi, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis Cys2/His2-type zinc-finger proteins function as transcription repressors under drought, cold, and high-salinity stress conditions. Plant Physiol. 2004, 136, 2734–2746. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Wang, Y.; Deng, C.; Hu, R.; Tian, C. Phylogenic analysis revealed an expanded C2H2-homeobox subfamily and expression profiles of C2H2 zinc finger gene family in Verticillium dahliae. Gene 2015, 562, 169–179. [Google Scholar] [CrossRef]
- Zhang, J.; Chi, Y.; Li, S.; Zhang, J.; Chen, J. Expression and analysis of zinc finger family gene in Lenzites gibbosa. J. For. Res. 2019, 31, 1889–1898. [Google Scholar] [CrossRef]
- Wu, S.; Chen, Y.; Li, J.; Fu, C.; Luo, X.; Wang, J.; Wan, X.; Huang, K.; Zhou, H.; Xie, G.; et al. Genome-wide analysis of the C2H2-type zinc finger protein family in rice (Oryza sativa) and the role of OsC2H2.35 in cold stress response. Plant Stress 2025, 15, 100772. [Google Scholar] [CrossRef]
- Shi, X.; Gu, Y.; Dai, T.; Wu, Y.; Wu, P.; Xu, Y.; Chen, F. Regulation of trichome development in tobacco by JcZFP8, a C2H2 zinc finger protein gene from Jatropha curcas L. Gene 2018, 658, 47–53. [Google Scholar] [CrossRef]
- Gourcilleau, D.; Lenne, C.; Armenise, C.; Moulia, B.; Julien, J.L.; Bronner, G.; Leblanc-Fournier, N. Phylogenetic study of plant q-type C2H2 zinc finger proteins and expression analysis of poplar genes in response to osmotic, cold and mechanical stresses. DNA Res. 2011, 18, 77–92. [Google Scholar] [CrossRef]
- Cao, H.; Huang, P.; Zhang, L.; Shi, Y.; Sun, D.; Yan, Y.; Liu, X.; Dong, B.; Chen, G.; Snyder, J.H.; et al. Characterization of 47 Cys2-His2 zinc finger proteins required for the development and pathogenicity of the rice blast fungus Magnaporthe oryzae. New Phytol. 2016, 211, 1035–1051. [Google Scholar] [CrossRef]
- Wodak, S.J.; Vlasblom, J.; Turinsky, A.L.; Pu, S. Protein–protein interaction networks: The puzzling riches. Curr. Opin. Struct. Biol. 2013, 23, 941–953. [Google Scholar] [CrossRef]
- Vazquez, A.; Flammini, A.; Maritan, A.; Vespignani, A. Global protein function prediction from protein-protein interaction networks. Nat. Biotechnol. 2003, 21, 697–700. [Google Scholar] [CrossRef]
- Luthfi, M.; Piapukiew, J.; Pandey, R.B.; Sompornpisut, P. Comparative omics analysis for novel target discovery in plant pathogens: A case study for Magnaporthe oryzae. Plant Pathol. 2023, 73, 564–577. [Google Scholar] [CrossRef]
- Wang, Y.; Dou, D.; Wang, X.; Li, A.; Sheng, Y.; Hua, C.; Cheng, B.; Chen, X.; Zheng, X.; Wang, Y. The PSCZF1 gene encoding a C2H2 zinc finger protein is required for growth, development and pathogenesis in Phytophthora sojae. Microb. Pathog. 2009, 47, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Situ, J.; Guan, T.; Dou, Z.; Kong, G.; Jiang, Z.; Xi, P. A C2H2 zinc finger protein PlCZF1 is necessary for oospore development and virulence in Peronophythora litchii. Int. J. Mol. Sci. 2022, 23, 2733. [Google Scholar] [CrossRef] [PubMed]
- Esch, R.K.; Wang, Y.; Errede, B. Pheromone-induced degradation of Ste12 contributes to signal attenuation and the specificity of developmental fate. Eukaryot. Cell 2006, 5, 2147–2160. [Google Scholar] [CrossRef]
- Hoi, J.W.S.; Dumas, B. Ste12 and Ste12-like proteins, fungal transcription factors regulating development and pathogenicity. Eukaryot. Cell 2010, 9, 480–485. [Google Scholar]
- Chang, Y.C.; Penoyer, L.A.; Kwon-Chung, K.J. The second STE12 homologue of Cryptococcus neoformans is MATa-specific and plays an important role in virulence. Proc. Natl. Acad. Sci. USA 2001, 98, 3258–3263. [Google Scholar] [CrossRef]
- Choi, J.; Kim, Y.; Kim, S.; Park, J.; Lee, Y.-H. MoCRZ1, a gene encoding a calcineurin-responsive transcription factor, regulates fungal growth and pathogenicity of Magnaporthe oryzae. Fungal Genet. Biol. 2009, 46, 243–254. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).