Sufficient access to safe and nutritious food is governed by a variety of complex and interconnected factors. With the global population expected to surpass eight billion, several significant challenges threaten food security, including disease outbreaks [1,2] and geopolitical conflicts that destabilize agricultural systems and supply chains [3]. Additionally, antimicrobial and agrochemical resistance undermines soil biodiversity and critical ecosystem services [4]. Concurrently, extreme weather events—such as prolonged droughts and intense floods—are increasingly eroding crop productivity [5]. Climate change also alters soil microbial communities and nutrient availability [6]. Furthermore, socioeconomic pressures, including rising living costs, exacerbate disparities in access to nutrient-rich diets [7,8]. Collectively, these factors create a complex, interconnected crisis for global food security.
In response, sustainable agricultural practices have emerged as an urgent priority, with microbiome-based innovations offering promising strategies to enhance plant health, mitigate disease, and optimize agricultural resilience [9]. Central to these efforts is the One Health framework [10], which emphasizes the inextricable linkages among human, animal, and environmental health. Within this paradigm, agricultural microbiomes play a pivotal role in co-regulating ecological balance, evolutionary adaptation, and systemic stability. Often overlooked, the complex interactions among soil microbial communities significantly contribute to improved soil health, crop productivity, and ecosystem sustainability (Figure 1). Understanding the intricate mechanisms that underpin plant growth and resilience in the face of these challenges requires a closer look at the soil environment. Plant development is strongly influenced by soil fertility and various microbial interactions that facilitate the solubilization and mobilization of essential nutrients such as phosphorus and nitrogen [11]. The soil microbiome is increasingly recognized as a crucial foundation for ecosystem resilience and functioning, mediating biogeochemical cycles. For example, microbes enhance soil fertility through their role in biogeochemical cycles [12] and contribute to elevated carbon storage in soils with higher plant diversity [13]. Microbial activity, including that of rhizosphere fungi, also impacts soil hydrophobicity and hydraulic function, highlighting the far-reaching consequences of these communities [14,15]. Thus, thriving soil microbial communities are a vital component of the success of regenerative agriculture practices.
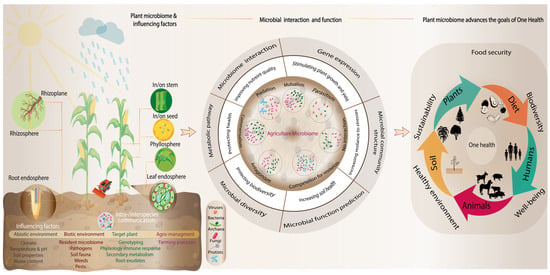
Figure 1.
Microbial communities play critical roles in agricultural ecosystems, with microbes inhabiting various plant-associated niches, engaging in interactions such as mutualism, antagonism, commensalism, and even predation. These microbes (bacteria, viruses, archaea, fungi, and protists) support plant growth and health through improved nutrient acquisition, often driven by complex metabolic pathways, and protection against pathogens. The structure and diversity of these microbial communities, along with associated gene expression patterns, significantly influence plant function, ultimately boosting crop productivity. This richness and complexity are vital for ensuring healthy and productive crops, aligning with the One Health concept, which emphasizes the interconnectedness of human, animal, and environmental health.
However, the delicate balance of these beneficial microbial communities is increasingly threatened by modern intensive agricultural practices—such as monoculture and the excessive use of pesticides—often leading to reduced soil health and increased susceptibility to soil-borne plant diseases, which pose a major threat to global agriculture by causing significant yield losses and economic burdens. Traditional chemical-based control methods are increasingly unsustainable, ineffective, and environmentally harmful [10]. In contrast, microbiome-based solutions offer a sustainable approach to enhancing plant health and reducing disease. Rhizosphere microbial communities achieve this by playing a crucial role in nutrient cycling and soil fertility, with beneficial microbes like nitrogen-fixing rhizobia and arbuscular mycorrhizal fungi (AMF) enhancing nutrient uptake and producing bioactive compounds that stimulate root growth and stress tolerance [11,16]. Furthermore, they act as a natural defense system, suppressing pathogens through antimicrobial production, resource competition, and immune activation [12]. Recent studies, including those on Brachiaria brizantha, have demonstrated the potential of specific plant–microbe systems to improve soil carbon and nitrogen content while stabilizing microbial networks. The microbiome associated with B. brizantha, particularly enriched in taxa such as Nitrosomonadaceae, Sphingomonas, and Gemmatimonas, was shown to enhance nitrogen cycling and increase microbial biomass [13]. These findings underscore the need to integrate microbiome-centered approaches into broader frameworks for sustainable agricultural development. However, it is crucial to view microbes as a single tool within a broader toolbox, where integrating diverse approaches and technologies is essential to solving the complex challenges of modern agriculture.
The critical role of the microbiome across diverse ecosystems, including agriculture, has driven significant advancements in omics technologies, providing unprecedented capabilities for in-depth data analysis. A diverse array of omics approaches, including culturomics, amplicon sequencing, metagenomics, metatranscriptomics, metaproteomics, viromics, and metabolomics, has emerged for studying microbiome [17,18]. The vast datasets generated by these methods necessitate sophisticated bioinformatics tools for effective analysis and interpretation [19,20]. For amplicon sequencing, commonly utilized tools include QIIME2 [21] and USEARCH [22,23], enabling insights into microbial diversity and taxonomy. Metagenomic data analysis frequently employs tools such as Trimmomatic [24] and fastp [25] for quality control, Kraken2 [26] for accurate taxonomic classification, and the HUMAnN3 pipeline [27] for comprehensive functional profiling.
Beyond these core tools, a rich ecosystem of bioinformatics resources has emerged to facilitate comprehensive microbiome analysis. Locally installed pipelines like Culturome [28], EasyAmplicon [29,30], EasyMetagenome [31], and MicrobiomeStatPlots [32] offer tailored solutions for culturome, amplicon, and metagenomic data, including visualization capabilities. Specialized workflows such as MetaProteomeAnalyzer [33] and Notame [34] cater to the unique challenges of metaproteomics and metabolomics data, respectively. Comprehensive online platforms like MicrobiomeAnalyst [35] provide integrated analysis for amplicon, metagenomic, and metabolomic datasets, while viromics benefits from dedicated tools like MGV [36], ViWrap [37], and Hecatomb [38]. Furthermore, specialized online platforms and databases (e.g., iNAP [39], GeNets [40], IPGA [41], EVenn [42], and FoodMicroDB [43]) offer focused resources for specific aspects of microbiome research. The development and application of R language packages [44], along with evolving methods for robust biomarker identification [45], further underscore the dynamism of this field. These collective advances highlight that multi-omics analysis has become a cornerstone in addressing complex biological questions.
The power of these advancements, particularly in metagenomics, has profoundly transformed our ability to explore and harness the intricate microbial diversity within agricultural ecosystems. By enabling the detailed investigation of plant-associated microbiota and their complex interactions, metagenomics provides invaluable insights into microbial functions that hold immense potential for precision agriculture. Recognizing this central role of the microbiome in driving agroecological innovation, we launched the first edition of the Special Issue titled “Metagenomic Analysis for Unveiling Agricultural Microbiome” in Agronomy. This initial endeavor garnered significant attention from the scientific community, leading to the publication of 17 high-quality papers https://www.mdpi.com/journal/agronomy/special_issues/S70BHD8E5H (accessed on 31 December 2023) and accumulating over 40,000 views. Building upon this success and the continued rapid evolution of the field, we initiated the second edition https://www.mdpi.com/journal/agronomy/special_issues/YOQL5J6Y9F (accessed on 30 April 2025). This second edition presents seven original research articles that collectively illustrate the expanding role of metagenomics in agricultural microbiome research, spanning three interrelated thematic areas—plant health and productivity, microbial ecology in agroecosystems, and microbiome contributions to crop quality and environmental adaptation.
Several contributions focus on the impact of plant-associated microbial communities on crop performance, nutrient acquisition, and disease suppression. Building on the importance of optimizing crop production and resilience, Yao et al. [13] examined two soybean cultivars with contrasting tolerance to continuous cropping and found that the susceptible cultivar accumulated specific rhizosphere metabolites that promoted pathogenic fungi, disrupting microbial balance and weakening resistance. Hao et al. [46] explored the diversity and functions of endophytic bacteria in pea seedlings, identifying multiple strains capable of producing growth-promoting compounds such as IAA and ACC deaminase, reinforcing the potential of these microbes as biofertilizers. Dong et al. [47] investigated the effects of sulfur fertilization on maize yield and soil microbial composition, revealing that rare microbial taxa played a disproportionately important role in mediating crop responses, with different sulfur sources influencing microbial diversity and interaction networks.
Other studies addressed microbial responses to environmental conditions and agricultural management. Zheng et al. [48] analyzed methane emissions from double-season rice paddies under five fertilization regimes and linked emission variability to shifts in methanogenic and methanotrophic microbial populations, along with functional genes such as mcrA and pmoA. Their results suggest that microbiome-based interventions can help mitigate greenhouse gas emissions in rice systems. Zhang et al. [19] conducted a comprehensive survey of fungal communities in rice grains from three major producing regions in China, revealing clear geographic differences in toxigenic fungi distribution driven by environmental and edaphic factors. Their work provides critical insights into microbial risk profiling and food safety management.
Finally, this Issue includes studies that highlight the role of microbiomes in influencing crop adaptation and product quality. Ren et al. [49] integrated volatomics and microbiome analysis to show that vineyard location significantly affected both grape aroma profiles and associated microbial communities, with key bacterial taxa correlating with specific aromatic compounds. Their findings emphasize the influence of microbiomes on crop sensory traits and the expression of terroir. Cheng et al. [50] investigated how plant hormone signaling pathways—specifically strigolactone and karrikin signaling—affected root microbiome assembly in Nicotiana attenuata. They demonstrated that genetic disruption of these pathways altered microbial recruitment in different root zones, providing new evidence of hormonal control over microbiome structure under desert conditions.
Collectively, these studies reflect the diverse and innovative directions in agricultural microbiome research. They demonstrate how metagenomics and multi-omics technologies are being applied to address pressing challenges in crop productivity, environmental resilience, climate adaptation, and food security. By offering both mechanistic insights and practical applications, this Special Issue contributes to advancing microbial solutions for sustainable agriculture. A central challenge remains in translating these valuable insights into practical, sustainable applications. Effective collaboration among research, biotechnology, and agriculture is essential to develop innovative solutions. Future studies should prioritize long-term assessments of microbiome-based interventions and the advancement of analytical tools to fully harness the power of metagenomics.
We would like to express our sincere gratitude to all the authors, reviewers, and editorial staff who made this Special Issue possible. We hope these contributions will inspire further research and interdisciplinary collaborations. Looking ahead, we anticipate that future editions will continue to advance the boundaries of agricultural microbiome research, fostering new strategies for resilient, productive, and ecologically sound farming systems.
Author Contributions
Conceptualization, Y.-X.L.; writing—original draft preparation, S.Y.; writing—review and editing, S.Y., Y.W., P.Y. and Y.-X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Natural Science Foundation of China (32470055, U23A20148), the Basic Research Center for Crop Biosafety Sciences (CAAS-BRC-CB-2025-01), and the Agricultural Science and Technology Innovation Program (CAAS-ZDRW202308).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shujaat, N.; Sajid, M.; Khan, F.U.; Nawaz, I. Assessment of fungal diseases and their impact on yield in wheat varieties under fungicide treatment. Plant Prot. 2025, 9, 57–68. [Google Scholar]
- Jones, R.A. Disease pandemics and major epidemics arising from new encounters between indigenous viruses and introduced crops. Viruses 2020, 12, 1388. [Google Scholar] [CrossRef] [PubMed]
- Savary, S.; Akter, S.; Almekinders, C.; Harris, J.; Korsten, L.; Rötter, R.; Waddington, S.; Watson, D. Mapping disruption and resilience mechanisms in food systems. Food Secur. 2020, 12, 695–717. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Zhao, Y.; Zhu, D.; Gillings, M.; Penuelas, J.; Ok, Y.S.; Capon, A.; Banwart, S. Soil biota, antimicrobial resistance and planetary health. Environ. Int. 2019, 131, 105059. [Google Scholar] [CrossRef]
- Sivakumar, M.V. Climate extremes and impacts on agriculture. Agroclimatol. Link. Agric. Clim. 2020, 60, 621–647. [Google Scholar]
- Meena, M.; Yadav, G.; Sonigra, P.; Nagda, A.; Mehta, T.; Swapnil, P.; Harish; Marwal, A.; Kumar, S. Multifarious responses of forest soil microbial community toward climate change. Microb. Ecol. 2023, 86, 49–74. [Google Scholar] [CrossRef]
- Callens, K.; Fontaine, F.; Sanz, Y.; Bogdanski, A.; D ‘Hondt, K.; Lange, L.; Smidt, H.; Van Overbeek, L.; Kostic, T.; Maguin, E. Microbiome-based solutions to address new and existing threats to food security, nutrition, health and agrifood systems’ sustainability. Front. Sustain. Food Syst. 2022, 6, 1047765. [Google Scholar] [CrossRef]
- Oishy, M.N.; Shemonty, N.A.; Fatema, S.I.; Mahbub, S.; Mim, E.L.; Hasan Raisa, M.B.; Anik, A.H. Unravelling the effects of climate change on the soil-plant-atmosphere interactions: A critical review. Soil Environ. Health 2025, 3, 100130. [Google Scholar] [CrossRef]
- Suman, J.; Rakshit, A.; Ogireddy, S.D.; Singh, S.; Gupta, C.; Chandrakala, J. Microbiome as a key player in sustainable agriculture and human health. Front. Soil Sci. 2022, 2, 821589. [Google Scholar] [CrossRef]
- Compant, S.; Cassan, F.; Kostić, T.; Johnson, L.; Brader, G.; Trognitz, F.; Sessitsch, A. Harnessing the plant microbiome for sustainable crop production. Nat. Rev. Microbiol. 2025, 23, 9–23. [Google Scholar] [CrossRef]
- Sessitsch, A.; Wakelin, S.; Schloter, M.; Maguin, E.; Cernava, T.; Champomier-Verges, M.-C.; Charles, T.C.; Cotter, P.D.; Ferrocino, I.; Kriaa, A. Microbiome interconnectedness throughout environments with major consequences for healthy people and a healthy planet. Microbiol. Mol. Biol. Rev. 2023, 87, e00212-22. [Google Scholar] [CrossRef]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.-S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef]
- Yao, X.; He, D.; Zhao, X.; Tan, Z.; Zhao, H.; Xie, F.; Wang, J. Integrated Microbiology and Metabolomics Analysis Reveal How Tolerant Soybean Cultivar Adapt to Continuous Cropping. Agronomy 2025, 15, 468. [Google Scholar] [CrossRef]
- Jin, H.; Quan, K.; He, Q.; Kwok, L.-Y.; Ma, T.; Li, Y.; Zhao, F.; You, L.; Zhang, H.; Sun, Z. A high-quality genome compendium of the human gut microbiome of Inner Mongolians. Nat. Microbiol. 2023, 8, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Carlino, N.; Blanco-Míguez, A.; Punčochář, M.; Mengoni, C.; Pinto, F.; Tatti, A.; Manghi, P.; Armanini, F.; Avagliano, M.; Barcenilla, C. Unexplored microbial diversity from 2,500 food metagenomes and links with the human microbiome. Cell 2024, 187, 5775–5795.e15. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jiang, S.; Jiang, C.; Wu, C.; Gao, M.; Wang, Q. A review of root exudates and rhizosphere microbiome for crop production. Environ. Sci. Pollut. Res. 2021, 28, 54497–54510. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, G.; Jiang, S.; Liu, Y.X. Wekemo Bioincloud: A user-friendly platform for meta-omics data analyses. iMeta 2024, 3, 175. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.L.; Chen, T.; Lan, C.; Gan, R.Y.; Yu, J.; Zhao, F.; Liu, Y.X. iMetaOmics: Advancing human and environmental health through integrated meta-omics. iMetaOmics 2024, 1, 21. [Google Scholar] [CrossRef]
- Zhang, F.; Cao, Z.; Zhao, X.; Yan, Q.; Guan, M.; Chen, M.; Lin, X. Microbiome Analysis Revealed the Effects of Environmental Factors on the Presence of Toxigenic Fungi and Toxin Production in Rice Grains. Agronomy 2024, 14, 1681. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, Z.; Luo, C.; Wei, K.; Li, X.; Mu, X.; Duan, M.; Zhu, C.; Jin, L.; He, X. MultiPrime: A reliable and efficient tool for targeted next-generation sequencing. iMeta 2023, 2, 143. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Accuracy of taxonomy prediction for 16S rRNA and fungal ITS sequences. PeerJ 2018, 6, 4652. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, Y.X.; Li, X. USEARCH 12: Open-source software for sequencing analysis in bioinformatics and microbiome. iMeta 2024, 3, 236. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Wood, D.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Beghini, F.; McIver, L.J.; Blanco-Míguez, A.; Dubois, L.; Asnicar, F.; Maharjan, S.; Mailyan, A.; Manghi, P.; Scholz, M.; Thomas, A.M. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. eLife 2021, 10, 65088. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.-X.; Guo, X.; Qin, Y.; Garrido-Oter, R.; Schulze-Lefert, P.; Bai, Y. High-throughput cultivation and identification of bacteria from the plant root microbiota. Nat. Protoc. 2021, 16, 988–1012. [Google Scholar] [CrossRef]
- Yousuf, S.; Luo, H.; Zeng, M.; Chen, L.; Ma, T.; Li, X.; Zheng, M.; Zhou, X.; Chen, L.; Xi, J. Unveiling microbial communities with EasyAmplicon: A user-centric guide to perform amplicon sequencing data analysis. iMetaOmics 2024, 1, 42. [Google Scholar] [CrossRef]
- Liu, Y.X.; Chen, L.; Ma, T.; Li, X.; Zheng, M.; Zhou, X.; Chen, L.; Qian, X.; Xi, J.; Lu, H. EasyAmplicon: An easy-to-use, open-source, reproducible, and community-based pipeline for amplicon data analysis in microbiome research. iMeta 2023, 2, 83. [Google Scholar] [CrossRef]
- Bai, D.; Chen, T.; Xun, J.; Ma, C.; Luo, H.; Yang, H.; Cao, C.; Cao, X.; Cui, J.; Deng, Y.P.; et al. EasyMetagenome: A user-friendly and flexible pipeline for shotgun metagenomic analysis in microbiome research. iMeta 2025, 4, 70001. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Ma, C.; Xun, J.; Luo, H.; Yang, H.; Lyu, H.; Zhu, Z.; Gai, A.; Yousuf, S.; Peng, K. MicrobiomeStatPlots: Microbiome statistics plotting gallery for meta-omics and bioinformatics. iMeta 2025, 4, 70002. [Google Scholar] [CrossRef]
- Schiebenhoefer, H.; Schallert, K.; Renard, B.Y.; Trappe, K.; Schmid, E.; Benndorf, D.; Riedel, K.; Muth, T.; Fuchs, S. A complete and flexible workflow for metaproteomics data analysis based on MetaProteomeAnalyzer and Prophane. Nat. Protoc. 2020, 15, 3212–3239. [Google Scholar] [CrossRef] [PubMed]
- Klåvus, A.; Kokla, M.; Noerman, S.; Koistinen, V.M.; Tuomainen, M.; Zarei, I.; Meuronen, T.; Häkkinen, M.R.; Rummukainen, S.; Farizah Babu, A. “Notame”: Workflow for non-targeted LC–MS metabolic profiling. Metabolites 2020, 10, 135. [Google Scholar] [CrossRef]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef]
- Nayfach, S.; Páez-Espino, D.; Call, L.; Low, S.J.; Sberro, H.; Ivanova, N.N.; Proal, A.D.; Fischbach, M.A.; Bhatt, A.S.; Hugenholtz, P. Metagenomic compendium of 189,680 DNA viruses from the human gut microbiome. Nat. Microbiol. 2021, 6, 960–970. [Google Scholar] [CrossRef]
- Zhou, Z.; Martin, C.; Kosmopoulos, J.C.; Anantharaman, K. ViWrap: A modular pipeline to identify, bin, classify, and predict viral–host relationships for viruses from metagenomes. iMeta 2023, 2, 118. [Google Scholar] [CrossRef] [PubMed]
- Roach, M.J.; Beecroft, S.J.; Mihindukulasuriya, K.A.; Wang, L.; Paredes, A.; Cárdenas, L.A.C.; Henry-Cocks, K.; Lima, L.F.O.; Dinsdale, E.A.; Edwards, R.A. Hecatomb: An integrated software platform for viral metagenomics. GigaScience 2024, 13, giae020. [Google Scholar] [CrossRef]
- Peng, X.; Feng, K.; Yang, X.; He, Q.; Zhao, B.; Li, T.; Wang, S.; Deng, Y. iNAP 2.0: Harnessing metabolic complementarity in microbial network analysis. iMeta 2024, 3, 235. [Google Scholar] [CrossRef]
- Li, T.; Kim, A.; Rosenbluh, J.; Horn, H.; Greenfeld, L.; An, D.; Zimmer, A.; Liberzon, A.; Bistline, J.; Natoli, T. GeNets: A unified web platform for network-based genomic analyses. Nat. Methods 2018, 15, 543–546. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Y.; Fan, G.; Sun, D.; Zhang, X.; Yu, Z.; Wang, J.; Wu, L.; Shi, W.; Ma, J. IPGA: A handy integrated prokaryotes genome and pan-genome analysis web service. iMeta 2022, 1, 55. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chen, T.; Liu, Y.X.; Huang, L. Visualizing set relationships: EVenn’s comprehensive approach to Venn diagrams. iMeta 2024, 3, 184. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lyu, H.; Yang, H.; Ju, Z.; Ma, C.; Hou, H.; Wang, Y.; Zhou, Y.; Gao, Y.; Yang, J. FoodMicroDB: A microbiome database for composition and time-series research in food. iMetaOmics 2024, 1, 40. [Google Scholar] [CrossRef]
- Wen, T.; Xie, P.; Yang, S.; Niu, G.; Liu, X.; Ding, Z.; Xue, C.; Liu, Y.X.; Shen, Q.; Yuan, J. ggClusterNet: An R package for microbiome network analysis and modularity-based multiple network layouts. iMeta 2022, 1, 32. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Y.; Li, B.; Gong, Y. A novel multi-class classification model for schizophrenia, bipolar disorder and healthy controls using comprehensive transcriptomic data. Comput. Biol. Med. 2022, 148, 105956. [Google Scholar] [CrossRef]
- Hao, J.; Liu, Q.; Song, F.; Cui, X.; Liu, L.; Fu, L.; Zhang, S.; Wu, X.; Zhang, X. Community Diversity of Endophytic Bacteria in the Leaves and Roots of Pea Seedlings. Agronomy 2024, 14, 2030. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, B.; Hou, W.; Zhou, X.; Gao, Q. Differential Effects of Sulfur Fertilization on Soil Microbial Communities and Maize Yield Enhancement. Agronomy 2024, 14, 2251. [Google Scholar] [CrossRef]
- Zheng, J.; Lu, Y.; Xu, P.; Xie, K.; Zhou, C.; Li, Y.; Geng, H.; Wang, Q.; Gu, W. Insights into the Driving Factors of Methane Emission from Double-Season Rice Field Under Different Fertilization Practices in South China. Agronomy 2024, 14, 2767. [Google Scholar] [CrossRef]
- Ren, R.; Zeng, M.; Liu, Y.; Shi, J.; Wan, Z.; Wang, M.; Zhang, S.; Zhang, Z.; Zeng, Q. Grape Endophytic Microbial Community Structures and Berry Volatile Components Response to the Variation of Vineyard Sites. Agronomy 2024, 14, 2186. [Google Scholar] [CrossRef]
- Li, L.; Gupta, A.; Zhu, C.; Xu, K.; Watanabe, Y.; Tanaka, M.; Seki, M.; Mochida, K.; Kanno, Y.; Seo, M.; et al. Strigolactone and karrikin receptors regulate phytohormone biosynthetic and catabolic processes. Plant Cell Rep. 2025, 44, 60. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).