Genotype and Environment Shape the Free and Bound Phenolics and Lipid Content in Oat (Avena sativa L.) Grains
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals
2.3. Extraction of Free and Bound Phenolic Compounds
2.4. Estimation of Phenolic Concentration in Various Fractions
2.5. Antioxidant Activity of Phenolic Fractions
2.6. Total Lipid Determination in Oat Caryopses
2.7. Statistical Analysis
3. Results
3.1. Phenolic Compounds
3.1.1. Free Phenolic Content in Oat Cultivars
3.1.2. Level of Bound Phenolic Compounds in Oat Cultivars
3.2. Antioxidant Activity of Phenolic Fractions
3.2.1. Antioxidant Capacity of Free Phenolics
3.2.2. Antioxidant Power of Bound Phenolics
3.3. Lipid Content
3.4. Statistical Evaluation of Environmental and Genetic Factors Affecting Biochemical Parameters in Oat
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, B.L.; Zheng, Z.; Ren, C. Chapter 6—Oat. In Crop Physiology Case Histories for Major Crops, 1st ed.; Sadras, V.O., Calderini, D.F., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 222–248. [Google Scholar]
- Zwer, P.K. Oat: Overview. In Encyclopedia of Food Grains, 2nd ed.; Wrigley, C., Corke, H., Seetharaman, K., Faubion, J., Eds.; Elsevier: Oxford, UK, 2016; Volume 1, pp. 173–183. [Google Scholar] [CrossRef]
- Koroluk, A.; Paczos-Grzęda, E.; Sowa, S.; Boczkowska, M.; Toporowska, J. Diversity of polish oat cultivars with a glance at breeding history and perspectives. Agronomy 2022, 12, 2423. [Google Scholar] [CrossRef]
- Leggett, J.M.; Thomas, H. Oat evolution and cytogenetics. In The Oat Crop. World Crop Series, 1st ed.; Welch, R.W., Ed.; Springer: Dordrecht, The Netherlands, 1995; pp. 120–149. [Google Scholar] [CrossRef]
- Nan, J.; Ling, Y.; An, J.; Wang, T.; Chai, M.; Fu, J.; Wang, G.; Yang, C.; Yang, Y.; Han, B. Genome resequencing reveals independent domestication and breeding improvement of naked oat. GigaScience 2023, 12, giad061. [Google Scholar] [CrossRef]
- Valentine, J. Naked oats. In The Oat Crop. World Crop Series, 1st ed.; Welch, R.W., Ed.; Springer: Dordrecht, The Netherlands, 1995; pp. 504–532. [Google Scholar] [CrossRef]
- Burrows, V.D. Hulless oat development, application, and opportunities. In Oats: Chemistry and Technology, 2nd ed.; Webster, F.H., Wood, P.J., Eds.; American Association of Cereal Chemists, Inc.: Saint Paul, MN, USA, 2011; pp. 31–50. [Google Scholar] [CrossRef]
- Schmitz, E.; Nordberg Karlsso, E.; Adlercreutz, P. Warming weather changes the chemical composition of oat hulls. Plant Biol. 2020, 22, 1086–1091. [Google Scholar] [CrossRef]
- Biel, W.; Jacyno, E.; Kawęcka, M. Chemical composition of hulled, dehulled and naked oat grains. S. Afr. J. Anim. Sci. 2014, 44, 189–197. [Google Scholar] [CrossRef]
- Thompson, R.K.; Mustafa, A.F.; McKinnon, J.J.; Maenz, D.; Rossnagel, B. Genotypic differences in chemical composition and ruminal degradability of oat hulls. Can. J. Anim. Sci. 2000, 80, 377–379. [Google Scholar] [CrossRef]
- Yu, P.; McKinnon, J.J.; Christensen, D.A. Improving the nutritional value of oat hulls for ruminant animals with pretreatment of a multienzyme cocktail: In vitro studies. J. Anim. Sci. 2005, 83, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.G.; Cremin, J.D., Jr.; Fahey, G.C., Jr.; Clark, J.H.; Berger, L.L.; Merchen, N.R. Chemically treated oat hulls in diets for dairy heifers and wethers: Effects on intake and digestion. J. Dairy Sci. 1991, 74, 190–201. [Google Scholar] [CrossRef]
- Wróblewska, P.; Hikawczuk, T.; Sierżant, K.; Wiliczkiewicz, A.; Szuba-Trznadel, A. Effect of oat hull as a source of insoluble dietary fibre on changes in the microbial status of gastrointestinal tract in broiler chickens. Animals 2022, 12, 2721. [Google Scholar] [CrossRef]
- Hikawczuk, T.; Szuba-Trznadel, A.; Wróblewska, P.; Wiliczkiewicz, A. Oat hull as a source of lignin-cellulose complex in diets containing wheat or barley and its effect on performance and morphometric measurements of gastrointestinal tract in broiler chickens. Agriculture 2023, 13, 896. [Google Scholar] [CrossRef]
- Leonova, S.; Shelenga, T.; Hamberg, M.; Konarev, A.V.; Loskutov, I.; Carlsson, A.S. Analysis of oil composition in cultivars and wild species of sat (Avena sp.). J. Agric. Food Chem. 2008, 56, 7983–7991. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Penas, E. Health benefits of oat: Current evidence and molecular mechanisms. Curr. Opin. Food Sci. 2017, 14, 26–31. [Google Scholar] [CrossRef]
- Saccomanno, B.; Chambers, A.H.; Hayes, A.; Mackay, I.; McWilliam, S.C.; Trafford, K. Starch granule morphology in oat endosperm. J. Cereal Sci. 2017, 73, 46–54. [Google Scholar] [CrossRef]
- Klose, C.; Arendt, E.K. Proteins in oats; their synthesis and changes during germination: A review. Crit. Rev. Food Sci. Nutr. 2012, 52, 629–639. [Google Scholar] [CrossRef]
- Gell, G.; Kovacs, K.; Veres, G.; Korponay-Szabo, I.R.; Juhasz, A. Characterization of globulin storage proteins of a low prola-min cereal species in relation to celiac disease. Sci. Rep. 2017, 7, 39876. [Google Scholar] [CrossRef]
- Maheshwari, G.; Sowrirajan, S.; Joseph, B. β-Glucan, a dietary fiber in effective prevention of lifestyle diseases—An insight. Bioact. Carbohydr. Diet. Fibre 2019, 19, 100187. [Google Scholar] [CrossRef]
- Ahmet, B.U.G.; Musa, O.M.; Ziya, D.; Nurhans, U. Oil contents and fatty acid composition of oat (Avena sativa L.) seed and oils. J. Agroaliment. Proc. Technol. 2019, 25, 182–186. [Google Scholar]
- Ekholm, P.; Virkki, L.; Ylinen, M.; Johansson, L.; Varo, P. Effects of natural chelating agents on the solubility of some physiologically important mineral elements in oat bran and oat flakes. Cereal Chem. 2000, 77, 562–566. [Google Scholar] [CrossRef]
- Kim, I.S.; Hwang, C.W.; Yang, W.S.; Kim, C.H. Multiple antioxidative and bioactive molecules of oats (Avena sativa L.) in human health. Antioxidants 2021, 10, 1454. [Google Scholar] [CrossRef]
- Collins, F.W. Oat phenolics: Biochemistry and biological functionality. In Oats: Chemistry and Technology, 2nd ed.; Webster, F.H., Wood, P.J., Eds.; American Association of Cereal Chemists, Inc.: Saint Paul, MN, USA, 2011; pp. 157–217. [Google Scholar] [CrossRef]
- Collins, F.W. Oat phenolics: Structure, occurrence, and function. In Oats: Chemistry and Technology; Webster, F.H., Ed.; American Association of Cereal Chemists, Inc.: St. Paul, MN, USA, 1986; pp. 227–295. [Google Scholar]
- Varga, M.; Jójárt, R.; Fónad, P.; Mihály, R.; Palágyi, A. Phenolic composition and antioxidant activity of colored oats. Food Chem. 2018, 268, 153–161. [Google Scholar] [CrossRef]
- Bei, Q.; Liu, Y.; Wang, L.; Chen, G.; Wu, Z. Improving free, conjugated, and bound phenolic fractions in fermented oats (Avena sativa L.) with Monascus anka and their antioxidant activity. J. Funct. Foods 2017, 32, 185–194. [Google Scholar] [CrossRef]
- Krygier, K.; Sosulski, F.; Hogge, L. Free, esterified, and insoluble-bound phenolic acids. 1. Extraction and purification procedure. J. Agric. Food Chem. 1982, 30, 330–334. [Google Scholar] [CrossRef]
- Collins, F.W. Oat phenolics—avenanthramides, novel substituted n-cinnamoylanthranilate alkaloids from oat groats and hulls. J. Agric. Food Chem. 1989, 37, 60–66. [Google Scholar] [CrossRef]
- Antonini, E.; Diamantini, G.; Ninfali, P. The effect of mechanical processing on avenanthramide and phenol levels in two organically grown Italian oat cultivars. J. Food Sci. Technol. 2017, 54, 2279–2287. [Google Scholar] [CrossRef]
- Kulbat, K. The role of phenolic compounds in plant resistance. Biotechnol. Food Sci. 2016, 80, 97–108. [Google Scholar] [CrossRef]
- Kumar, K.; Debnath, P.; Singh, S.; Kumar, N. An overview of plant phenolics and their involvement in abiotic stress tolerance. Stresses 2023, 3, 570–585. [Google Scholar] [CrossRef]
- Bryngelsson, S.; Mannerstedt-Fogelfors, B.; Kamal-Eldin, A.; Andersson, R.; Dimberg, L.H. Lipids and antioxidants in groats and hulls of Swedish oats (Avena sativa L.). J. Sci. Food Agric. 2002, 82, 606–614. [Google Scholar] [CrossRef]
- Emmons, C.L.; Peterson, D.M. Antioxidant activity and phenolic contents of oat groats and hulls. Cereal Chem. 1999, 76, 902–906. [Google Scholar] [CrossRef]
- Emmons, C.L.; Peterson, D.M. Antioxidant activity and phenolic content of oat as affected by cultivar and location. Crop Sci. 2001, 41, 1676–1681. [Google Scholar] [CrossRef]
- Tang, Y.; Li, S.; Yan, J.; Peng, Y.; Weng, W.; Yao, X.; Gao, A.; Cheng, J.; Ruan, J.; Xu, B. Bioactive components and health functions od oat. Food Rev. Int. 2023, 39, 4545–4564. [Google Scholar] [CrossRef]
- Singh, R.; De, S.; Belkheir, A. Avena sativa (oat), a potential neutraceutical and therapeutic agent: An overview. Crit. Rev. Food Sci. Nutr. 2013, 53, 126–144. [Google Scholar] [CrossRef]
- Doehlert, D.C.; McMullen, M.S.; Hammond, J.J. Genotypic and environmental effects on grain yield and quality of oat grown in North Dakota. Crop Sci. 2001, 41, 1066–1072. [Google Scholar] [CrossRef]
- Gorash, A.; Armoniene, R.; Fetch, J.M.; Liatukas, Z.; Danyte, V. Aspects in oat breeding: Nutrition quality, nakedness and disease resistance, challenges and perspectives. Ann. Appl. Biol. 2017, 171, 281–302. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef]
- Hitayezu, R.; Baakdah, M.M.; Kinnin, J.; Henderson, K.; Tsopmo, A. Antioxidant activity, avenanthramide and phenolic acid contents of oat milling fractions. J. Cereal Sci. 2015, 63, 35–40. [Google Scholar] [CrossRef]
- Tong, L.; Liu, L.; Zhong, K.; Wang, Y.; Guo, L.; Zhou, S. Effects of cultivar on phenolic content and antioxidant activity of naked oat in China. J. Integr. Agric. 2014, 13, 1809–1816. [Google Scholar] [CrossRef]
- Chen, C.; Milbury, P.E.; Kwak, H.; Collins, F.W.; Sameul, P.; Blumburg, J.B. Avenanthramides and phenolic acids from oats are bioavailable and act synergistically with vitamin C to enhance hamster and human LDL resistance to oxidation. J. Nutr. 2004, 134, 1459–1466. [Google Scholar] [CrossRef]
- Dimberg, L.H.; Gissen, C.; Nilsson, J. Phenolic compounds in oat grains (Avena sativa L.) grown in conventional and organic systems. AMBIO A J. Hum. Environ. 2005, 34, 331–337. [Google Scholar] [CrossRef]
- Menga, V.; Fares, C.; Troccoli, A.; Cattivelli Land Baiano, A. Effects of genotype, location and baking on the phenolic content and some antioxidant properties of cereal species. Int. J. Food Sci. 2010, 5, 7–16. [Google Scholar] [CrossRef]
- Collins, F.W.; McLachlan, D.C.; Blackwell, B.A. Oat phenolics: Avenalumic acids, a new group of bound phenolic acids from oat groats and hulls. Cereal Chem. 1991, 68, 184–189. [Google Scholar]
- Martinez-Miguel, G.; Trevino-Ramírez, J.E.; Urias-Orona, V.; Zavala-García, F.; Ninno-Medina, G. Contribution of bound phenolic compounds to the total phenol content and antioxidant capacity of oat (Avena sativa) grain fractions. Can. J. Plant Sci. 2018, 98, 1–4. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldivar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Pihlava, J.M.; Hellstrom, J. Contents of phenolic acids, alkyl- and alkenylresorcinols, and avenanthramides in commercial grain products. J. Agric. Food Chem. 2005, 53, 8290–8295. [Google Scholar] [CrossRef]
- Kovacova, M.; Malinova, E. Ferulic and coumaric acids, total phenolic compounds and their correlation in selected oat genotypes. Czech J. Food Sci. 2007, 25, 325–332. [Google Scholar] [CrossRef]
- Faulds, C.B.; Williamson, G. Review: The role of hydroxycinnamates in the plant cell wall. J. Sci. Food Agric. 1999, 79, 393–395. [Google Scholar] [CrossRef]
- Rao, R.S.P.; Muralikrishna, G. Non-starch polysaccharides-phenolic acid complexes from native and germinated cereals and millet. Food Chem. 2004, 84, 527–531. [Google Scholar] [CrossRef]
- Brindzova, L.; Certik, M.; Rapta, P.; Zalibera, M.; Mikulajova, A.; Takacsova, M. Antioxidant activity, β-glucan and lipid contents of oat varieties. Czech J. Food Sci. 2008, 26, 163–173. [Google Scholar] [CrossRef]
- Hodzic, Z.; Pasalic, H.; Memisevic, A.; Srabovic, M.; Saletovic, M.; Poljakovic, M. The influence of total phenols content on antioxidant capacity in the whole grain extracts. Eur. J. Sci. Res. 2009, 28, 471–477. [Google Scholar]
- Givens, D.I.; Davies, T.W.; Laverick, R.M. Effect of variety, nitrogen fertilizer and various agronomic factors on the nutritive value of husked and naked oats grain. Anim. Feed. Sci. Technol. 2004, 113, 169–181. [Google Scholar] [CrossRef]
- Kourimska, L.; Sabolova, M.; Horcicka, P.; Rys, S.; Bozik, M. Lipid content, fatty acid profile, and nutritional value of new oat cultivars. J. Cereal Sci. 2018, 84, 44–48. [Google Scholar] [CrossRef]
- Khawula, S.; Gokul, A.; Niekerk, L.A.; Basson, G.; Keyster, M.; Badiwe, M.; Klein, A.; Nkomo, M. Insights into the effects of hydroxycinnamic acid and its secondary metabolites as antioxidants for oxidative stress and plant growth under environmental stresses. Curr. Issues Mol. Biol. 2024, 46, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Multari, S.; Pihlava, J.M.; Ollennu-Chuasam, P.; Hietaniemi, V.; Yang, B.; Suomela, J.P. Identification and quantification of avenanthramides and free and bound phenolic acids in eight cultivars of husked oat (Avena sativa L.) from Finland. J. Agric. Food Chem. 2018, 66, 2900–2908. [Google Scholar] [CrossRef]
- Barański, M.; Lacko-Bartosova, M.; Rembiałkowska, E.; Lacko-Bartosovs, L. The effect of species and cultivation year on phenolic acids content in ancient wheat. Agronomy 2020, 10, 673. [Google Scholar] [CrossRef]
- Kosik, T.; Lacko-Bartosova, M.; Kobida, L. Free phenol content and antioxidant activity of winter wheat in sustainable farming system. J. Microbiol. Biotech. Food Sci. 2014, 3, 247–249. [Google Scholar]
- Ortiz-Robledo, F.; Villanueva-Fierro, I.; Dave Oomah, B.; Lares-Asef, I.; Proal-Nájera, J.B.; Návar-Chaidez, J.J. Avenanthramides and nutritional components of four mexican oat (Avena sativa L.) varieties. Agrociencia 2013, 47, 225–232. [Google Scholar]
- Jimenez-Pulido, I.J.; Daniel, R.; Perez, J.; Martínez-Villaluenga, C.; De Luis, D.; Martín Diana, A.B. Impact of Protein Content on the Antioxidants, Anti-Inflammatory Properties and Glycemic Index of Wheat and Wheat Bran. Foods 2022, 11, 2049. [Google Scholar] [CrossRef]
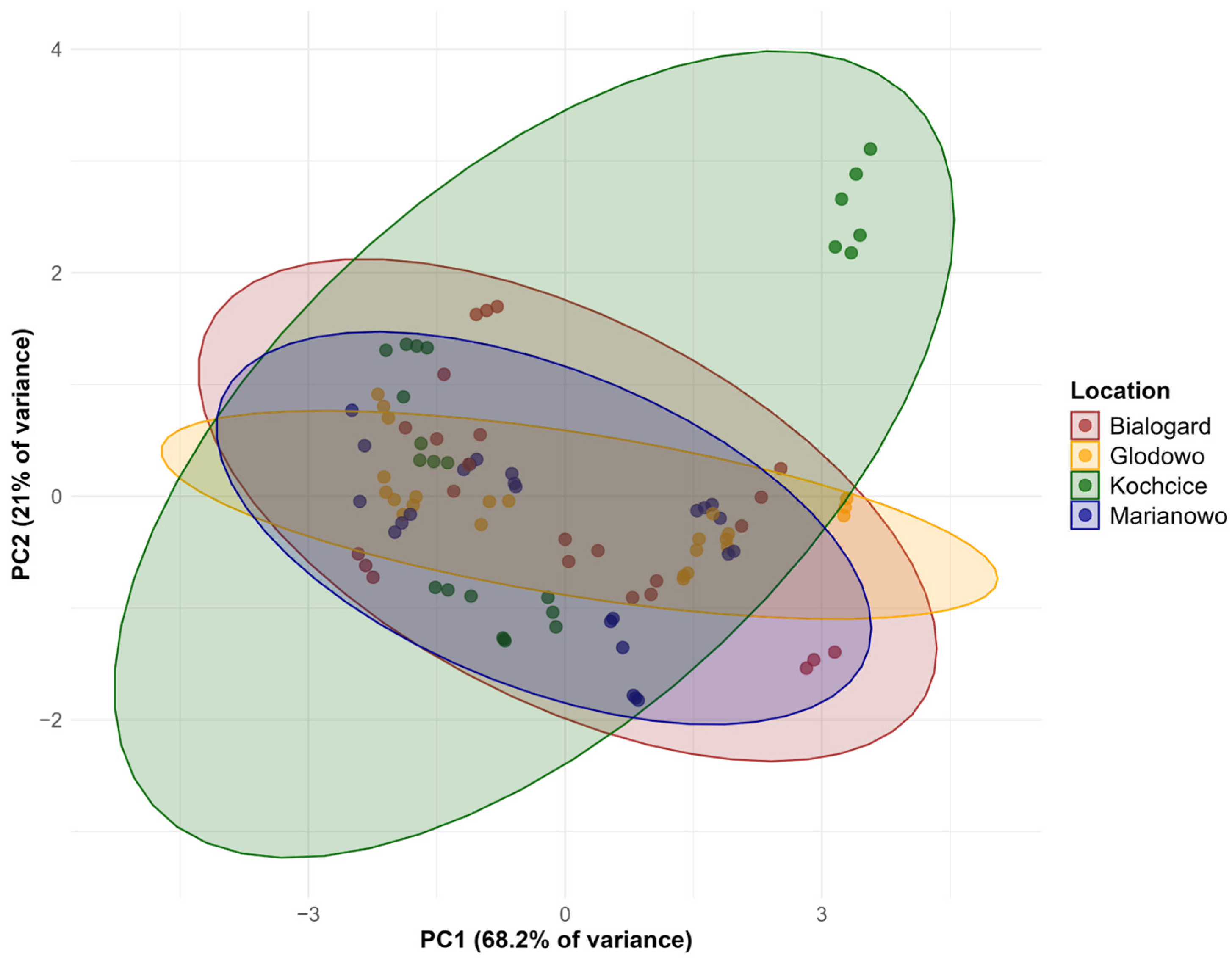
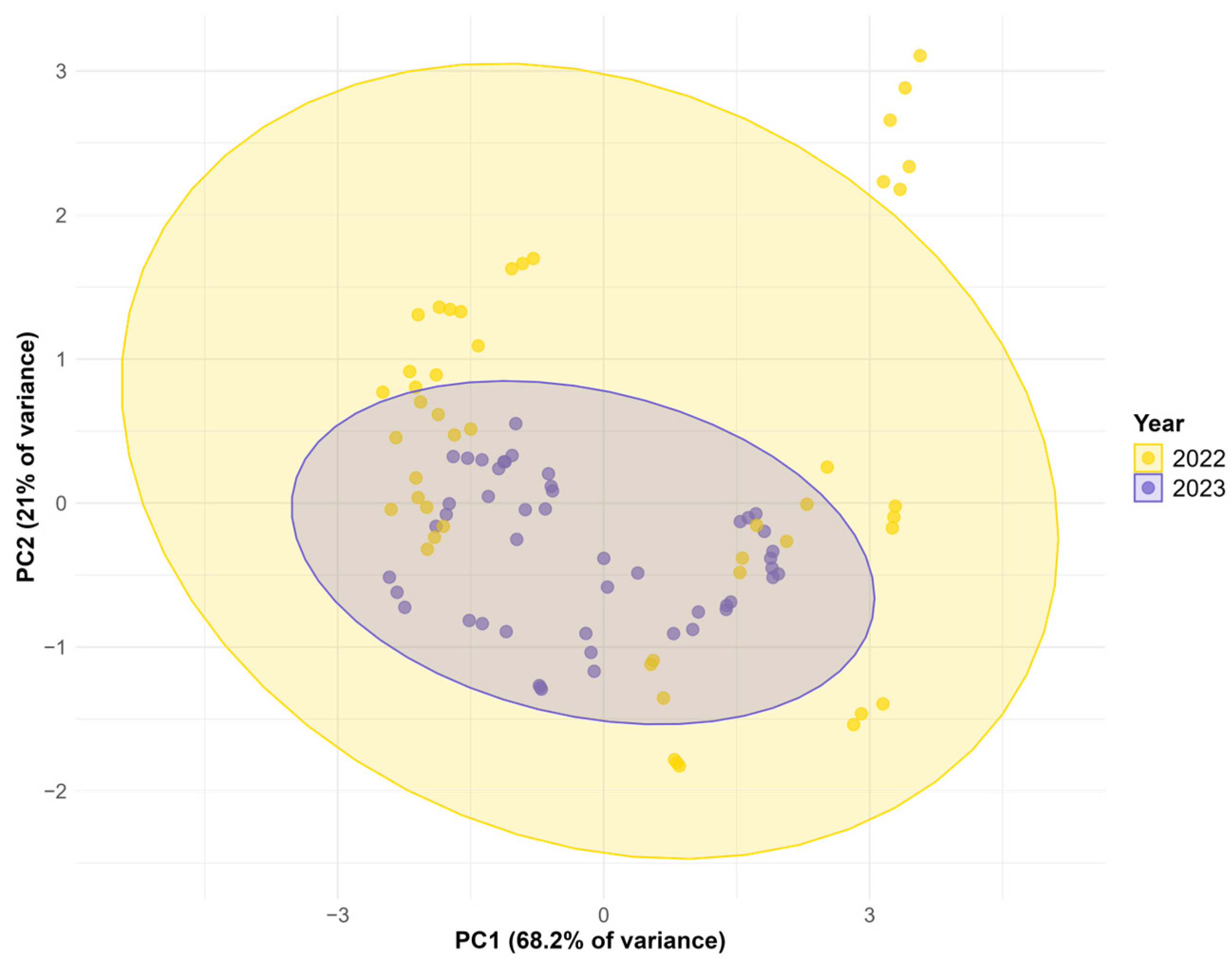
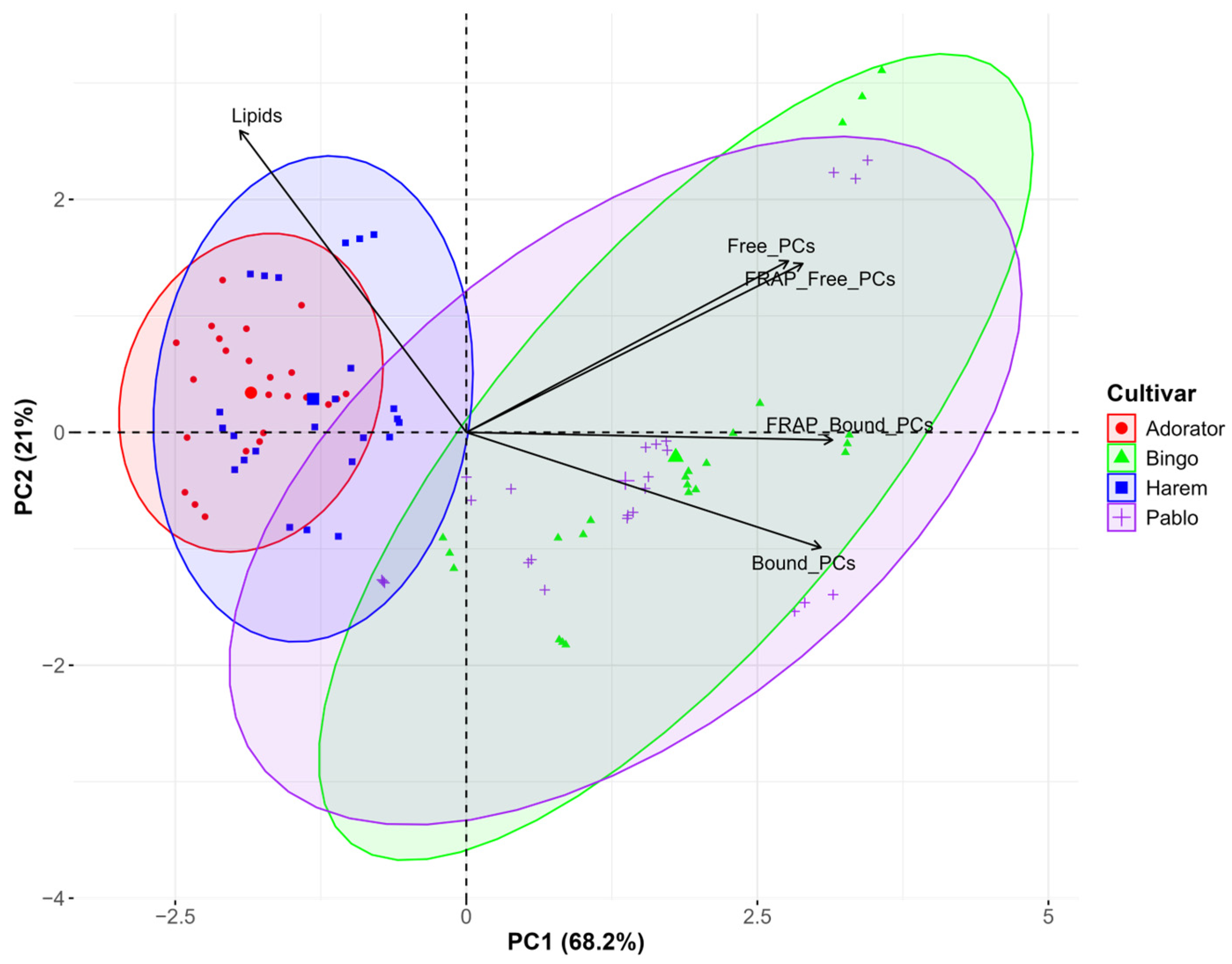
| Cultivar | Year | Concentration of Free Phenolic Compounds [mg kg−1 DW] | |||
|---|---|---|---|---|---|
| Growth Location | |||||
| Białogard | Głodowo | Kochcice | Marianowo | ||
| Adorator | 2022 | 153.30 ± 14.84 hijk | 161.38 ± 1.98 hij | 124.46 ± 1.38 lmno | 113.50 ±2.82 mnop |
| 2023 | 90.77 ± 3.80 p | 101.60 ± 8.33 op | 95.91 ± 13.81 p | 111.85 ± 1.38 nop | |
| MHR Harem | 2022 | 194.44 ± 2.85 def | 137.26 ± 3.21 jklmn | 132.81 ± 3.57 klmn | 114.82 ± 3.36 mnop |
| 2023 | 148.34 ± 9.99 ijkl | 139.84 ± 11.30 jklm | 117.60 ± 1.12 mnop | 124.99 ± 2.14 lmno | |
| Bingo | 2022 | 172.68 ± 12.80 fghi | 211.85 ± 7.29 cd | 338.13 ± 4.86 a | 163.99 ± 1.92 ghij |
| 2023 | 171.46 ± 14.43 fghi | 174.05 ± 1.34 fghi | 125.56 ± 4.24 klmno | 174.88 ± 1.52 fghi | |
| Pablo | 2022 | 237.99 ± 19.38 bc | 253.45 ± 1.37 b | 363.58 ± 19.88 a | 170.94 ± 0.48 fghi |
| 2023 | 208.17 ± 4.13 de | 198.33 ± 5.73 def | 190.02 ± 16.80 defg | 180.59 ± 3.85 efgh | |
| Cultivar | Year | Concentration of Bound Phenolic Compounds [mg kg−1 DW] | |||
|---|---|---|---|---|---|
| Growth Location | |||||
| Białogard | Głodowo | Kochcice | Marianowo | ||
| Adorator | 2022 | 580.87 ± 37.70 kl | 87.59 ± 7.25 m | 450.51 ± 5.01 kl | 409.63 ± 37.47 l |
| 2023 | 509.69 ± 31.51 kl | 483.29 ± 50.81 kl | 530.61 ± 0.98 kl | 867.87 ± 94.17 i | |
| MHR Harem | 2022 | 487.26 ± 12.32 kl | 129.12 ± 14.17 m | 644.42 ± 37.15 jk | 447.92 ± 2.07 kl |
| 2023 | 606.35 ± 25.71 kl | 824.30 ± 32.61 ij | 625.03 ± 65.77 jk | 1113.80 ± 82.33 h | |
| Bingo | 2022 | 3483.28 ± 153.94 a | 2404.21 ± 58.25 cde | 2312.40 ± 60.34 e | 2441.32 ± 63.95 cde |
| 2023 | 1423.43 ± 48.99 g | 2562.19 ± 3.98 c | 1473.12 ± 67.63 g | 2359.97 ± 147.49 cde | |
| Pablo | 2022 | 1844.99 ± 148.29 f | 2554.90 ± 44.49 cd | 2030.39 ± 51.79 f | 2418.77 ± 24.09 cde |
| 2023 | 1308.65 ± 96.91 gh | 2358.09 ± 32.84 de | 1151.35 ± 14.67 h | 2889.33 ± 18.85 b | |
| Cultivar | Year | Antioxidant Activity of Free Phenolic Compounds [mmol kg−1 DW] | |||
|---|---|---|---|---|---|
| Growth Location | |||||
| Białogard | Głodowo | Kochcice | Marianowo | ||
| Adorator | 2022 | 1.12 ± 0.15 jklm | 1.34 ± 0.04 hijk | 1.20 ± 0.05 ijklm | 0.95 ± 0.10 m |
| 2023 | 0.56 ± 0.06 n | 1.11 ± 0.01 jklm | 1.60 ± 0.01 gh | 1.38 ± 0.08 hij | |
| MHR Harem | 2022 | 1.96 ± 0.09 ef | 1.26 ± 0.02 ijkl | 1.31 ± 0.07 hijkl | 1.01 ± 0.08 lm |
| 2023 | 1.23 ± 0.20 ijklm | 1.34 ± 0.10 hijk | 1.06 ± 0.17 klm | 1.46 ± 0.09 hi | |
| Bingo | 2022 | 2.23 ± 0.03 cde | 2.07 ± 0.04 def | 3.02 ± 0.06 a | 1.25 ± 0.02 ijklm |
| 2023 | 1.10 ± 0.06 jklm | 1.81 ± 0.04 fg | 0.94 ± 0.02 m | 2.39 ± 0.15 bc | |
| Pablo | 2022 | 2.26 ± 0.19 cde | 2.67 ± 0.07 b | 3.22 ± 0.10 a | 1.18 ± 0.00 ijklm |
| 2023 | 1.44 ± 0.19 hi | 2.31 ± 0.02 cd | 1.11 ± 0.16 jklm | 2.26 ± 0.06 cde | |
| Cultivar | Year | Antioxidant Activity of Bound Phenolic Compounds [mmol kg−1 DW] | |||
|---|---|---|---|---|---|
| Growth Location | |||||
| Białogard | Głodowo | Kochcice | Marianowo | ||
| Adorator | 2022 | 6.43 ± 0.11 k | 0.83 ± 0.01 n | 6.65 ± 0.23 jk | 3.45 ± 0.26 m |
| 2023 | 5.51 ± 0.28 l | 6.57 ± 0.24 k | 6.41 ± 0.05 k | 9.43 ± 0.09 f | |
| MHR Harem | 2022 | 5.12 ± 0.13 l | 1.28 ± 0.20 n | 7.39 ± 0.03 ij | 4.83 ± 0.14 l |
| 2023 | 8.27 ± 0.27 gh | 8.34 ± 0.06 g | 6.45 ± 0.01 k | 10.81 ± 0.27 de | |
| Bingo | 2022 | 17.47 ± 0.44 a | 11.13 ± 0.86 d | 16.81 ± 0.35 ab | 10.25 ± 0.00 e |
| 2023 | 13.11 ± 0.18 c | 13.05 ± 0.17 c | 7.72 ± 0.01 ghi | 12.91 ± 0.17 c | |
| Pablo | 2022 | 16.57 ± 0.14 b | 17.50 ± 0.10 a | 16.83 ± 0.11 ab | 10.14 ± 0.02 ef |
| 2023 | 13.18 ± 0.11 c | 12.80 ± 0.02 c | 7.59 ± 0.06 hi | 12.95 ± 0.02 c | |
| Cultivar | Year | Lipid Content [%] | |||
|---|---|---|---|---|---|
| Growth Location | |||||
| Białogard | Głodowo | Kochcice | Marianowo | ||
| Adorator | 2022 | 11.24 ± 0.98 bc | 10.11 ± 0.32 cdefgh | 11.62 ± 1.23 ab | 10.87 ± 0.99 bcde |
| 2023 | 9.34 ± 0.34 fghi | 9.63 ± 0.12 efgh | 9.97 ± 0.31 cdefgh | 10.32 ± 0.08 bcdefg | |
| MHR Harem | 2022 | 11.10 ± 0.10 bcd | 8.73 ± 0.32 hijk | 12.67 ± 0.20 a | 9.08 ± 0.02 fghi |
| 2023 | 9.69 ± 0.14 defgh | 8.95 ± 0.52 ghij | 7.47 ± 0.39 klm | 9.72 ± 0.76 defgh | |
| Bingo | 2022 | 5.23 ± 0.03 o | 6.85 ± 0.23 lmn | 9.91 ± 0.29 cdefgh | 7.14 ± 0.65 lmn |
| 2023 | 8.11 ± 0.33 ijkl | 7.15 ± 0.03 lmn | 7.08 ± 0.06 lmn | 7.61 ± 0.29 jklm | |
| Pablo | 2022 | 6.54 ± 0.11 mno | 5.88 ± 0.07 no | 10.46 ± 0.03 bcdef | 5.23 ± 0.05 o |
| 2023 | 5.79 ± 0.01 no | 6.53 ± 0.06 mno | 6.00 ± 0.34 no | 7.07 ± 0.60 lmn | |
| Main Effect | Free PCs | Bound PCs | FRAP Free PCs | FRAP Bound PCs | Lipids |
|---|---|---|---|---|---|
| mg kg−1 DW | mg kg−1 DW | mmol kg−1 DW | mmol kg−1 DW | % DW | |
| Location (F1) | |||||
| Białogard | 172.14 b | 1280.56 c | 1.49 b | 10.71 a | 8.38 b |
| Głodowo | 172.22 b | 1425.46 b | 1.74 a | 8.94 c | 7.98 c |
| Kochcice | 186.01 a | 1152.23 d | 1.68 a | 9.48 b | 9.40 a |
| Marianowo | 144.44 c | 1618.58 a | 1.48 b | 9.35 b | 8.38 b |
| Year (F2) | |||||
| 2022 | 190.29 a | 1420.47 a | 1.75 a | 9.54 b | 8.92 a |
| 2023 | 147.12 b | 1317.94 b | 1.44 b | 9.70 a | 8.15 b |
| Cultivar (F3) | |||||
| Adorator | 119.10 d | 490.01 d | 1.16 d | 5.66 d | 10.39 a |
| MHR Harem | 138.76 c | 609.78 c | 1.33 c | 6.57 c | 9.68 b |
| Bingo | 191.57 b | 2307.49 a | 1.85 b | 12.81 b | 7.39 c |
| Pablo | 225.38 a | 2069.56 b | 2.06 a | 13.45 a | 6.69 d |
| p-value F1 | p < 0.001 | p < 0.001 | p < 0,001 | p < 0.001 | p < 0.001 |
| p-value F2 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| p-value F3 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| Effect size (η2G)-F1 | 0.82 | 0.92 | 0.67 | 0.92 | 0.68 |
| Effect size (η2G)-F2 | 0.90 | 0.50 | 0.79 | 0.14 | 0.53 |
| Effect size (η2G)-F3 | 0.97 | 1.0 | 0.96 | 1.0 | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnucka, E.G.; Kulczycki, G.; Bujak, H.; Pietr, S.J. Genotype and Environment Shape the Free and Bound Phenolics and Lipid Content in Oat (Avena sativa L.) Grains. Agronomy 2025, 15, 1400. https://doi.org/10.3390/agronomy15061400
Magnucka EG, Kulczycki G, Bujak H, Pietr SJ. Genotype and Environment Shape the Free and Bound Phenolics and Lipid Content in Oat (Avena sativa L.) Grains. Agronomy. 2025; 15(6):1400. https://doi.org/10.3390/agronomy15061400
Chicago/Turabian StyleMagnucka, Elżbieta G., Grzegorz Kulczycki, Henryk Bujak, and Stanisław J. Pietr. 2025. "Genotype and Environment Shape the Free and Bound Phenolics and Lipid Content in Oat (Avena sativa L.) Grains" Agronomy 15, no. 6: 1400. https://doi.org/10.3390/agronomy15061400
APA StyleMagnucka, E. G., Kulczycki, G., Bujak, H., & Pietr, S. J. (2025). Genotype and Environment Shape the Free and Bound Phenolics and Lipid Content in Oat (Avena sativa L.) Grains. Agronomy, 15(6), 1400. https://doi.org/10.3390/agronomy15061400







