Castor: A Renewed Oil Crop for the Mediterranean Environment
Abstract
1. Introduction
2. Establishing Castor as an Advantageous Oil Crop in Europe: Economic Overview
2.1. Oil World Scenario
2.1.1. Castor Insights
2.1.2. Castor Challenges Towards the Market
3. Origin and Distribution of the Palmus Christi
4. Biological and Genetic Features
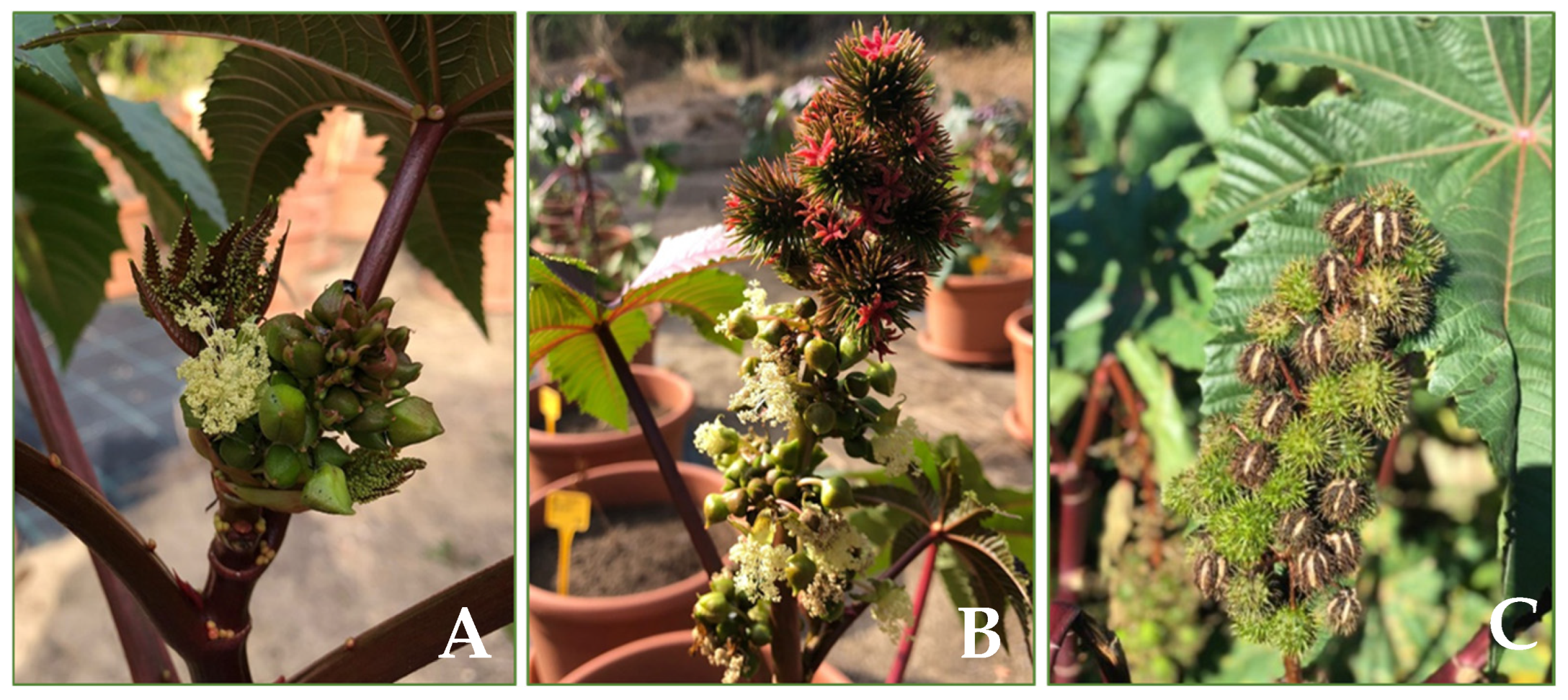
4.1. Botanical Features
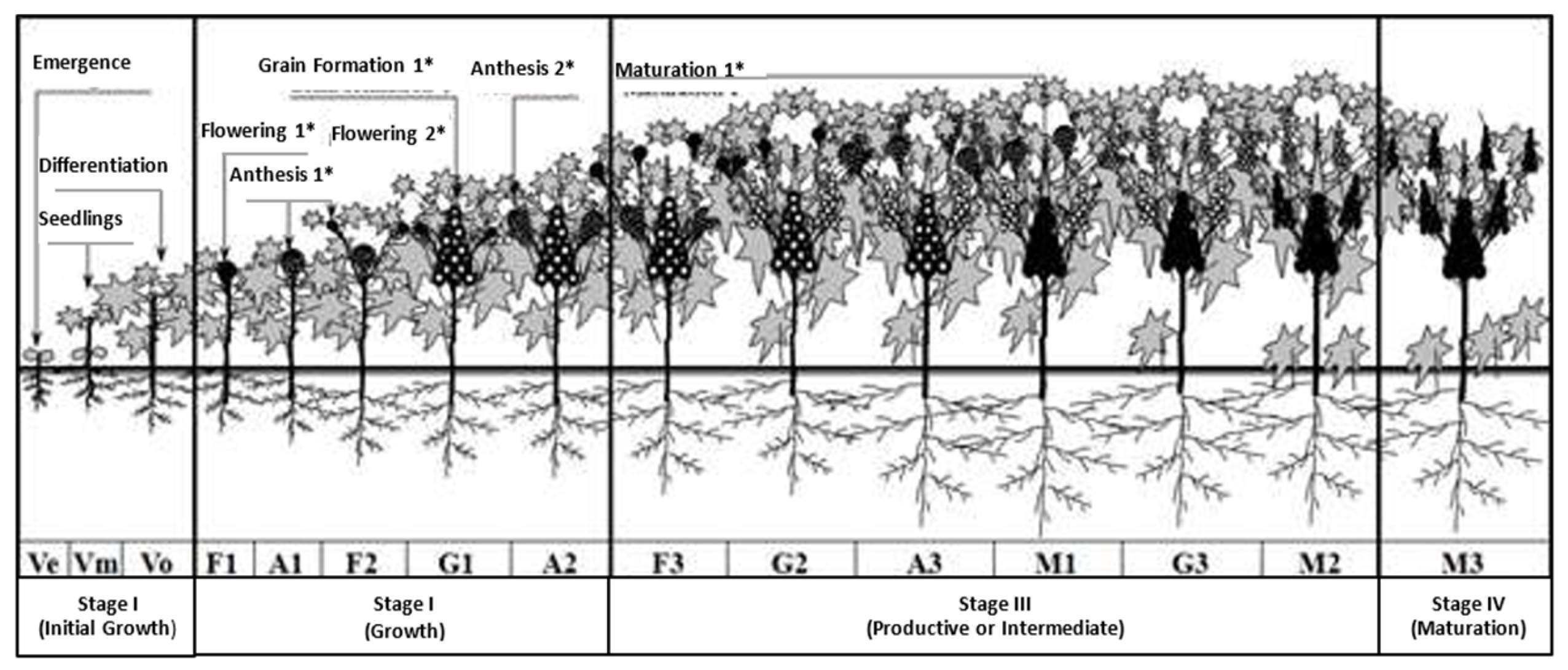
4.2. Phenology
5. Castor Crop Requirements
5.1. Thermal Requirements
5.2. Light Requirements
5.3. Soil Requirements
5.4. Water Requirements
5.5. Nutrients Requirements
6. Breeding and Variety Development
6.1. Dwarf Varieties
6.2. Breeding Improvement
6.3. Castor Response to Abiotic Stresses
7. Agronomic Management in the Mediterranean Environment
7.1. Sowing
7.2. Irrigation
7.3. Fertilization
7.4. Weed Management
7.5. Pests and Diseases
7.6. Harvest
8. The Wide Potentiality of Castor
8.1. Potential Feedstock
8.1.1. Castor Oil
8.1.2. Biofuels
8.1.3. Organic Fertilizer
8.1.4. Medicinal and Pharmaceuticals
8.1.5. Phytoremediation
9. Final Remarks and Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhattarai, H. Renewable Energy and Energy Storage Systems. In Energy Conversion: Methods, Technology and Future Directions; Delve Publishing: New York, NY, USA, 2022; pp. 269–289. [Google Scholar] [CrossRef]
- Cozzi, L.; Gould, T. World Energy Outlook 2021; International Energy Agency (IEA): Paris, France, 2021; pp. 1–386. [Google Scholar]
- Osorio-González, C.S.; Gómez-Falcon, N.; Sandoval-Salas, F.; Saini, R.; Brar, S.K.; Ramírez, A.A. Production of Biodiesel from Castor Oil: A Review. Energies 2020, 13, 2467. [Google Scholar] [CrossRef]
- Alamsyah, Z.; Ratnasari, D.; Elwamendri, E.; Fauzia, G. The Influence of EU Renewable Energy Directive Policy to Indonesian Palm Oil Exports to Some EU Countries. IOP Conf. Ser. Earth Environ. Sci. 2024, 1302, 012149. [Google Scholar] [CrossRef]
- Meijaard, E.; Brooks, T.M.; Carlson, K.M.; Slade, E.M.; Garcia-Ulloa, J.; Gaveau, D.L.A.; Lee, J.S.H.; Santika, T.; Juffe-Bignoli, D.; Struebig, M.J.; et al. The Environmental Impacts of Palm Oil in Context. Nat. Plants 2020, 6, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- European Commission. DIRETTIVA (UE) 2018/2001 DEL PARLAMENTO EUROPEO E DEL CONSIGLIO Dell’11 Dicembre 2018 Sulla Promozione Dell’uso Dell’energia Da Fonti Rinnovabili. Gazz. Uff. dell’Unione Eur. 2018, 2018, 128. [Google Scholar]
- Dusser, P. The European Energy Policy for 2020–2030 RED II: What Future for Vegetable Oil as a Source of Bioenergy? OCL 2019, 26, 51. [Google Scholar] [CrossRef]
- Mellor, P.; Lord, R.A.; João, E.; Thomas, R.; Hursthouse, A. Identifying Non-Agricultural Marginal Lands as a Route to Sustainable Bioenergy Provision—A Review and Holistic Definition. Renew. Sustain. Energy Rev. 2021, 135, 110220. [Google Scholar] [CrossRef]
- Cafaro, V. Selection of a Ricinus communis L. Genotype and Improvement of the Agronomic Management in Semi-Arid Mediterranean Environment. Ph.D. Thesis, University of Catania, Sicily, Italy, 2023. [Google Scholar]
- Syahir, A.Z.; Zulkifli, N.W.M.; Masjuki, H.H.; Kalam, M.A.; Alabdulkarem, A.; Gulzar, M.; Khuong, L.S.; Harith, M.H. A Review on Bio-Based Lubricants and Their Applications. J. Clean. Prod. 2017, 168, 997–1016. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Ballesteros Plata, D.; Alves Saboya, R.M.; Tavares De Luna, F.M.; Cavalcante, C.L.; Rodríguez-Castellón, E. An Overview of the Biolubricant Production Process: Challenges and Future Perspectives. Processes 2020, 8, 257. [Google Scholar] [CrossRef]
- Quaranta, E. Lubricant Oil Consumption and Opportunities for Oil-Free Turbines in the Hydropower Sector: A European Assessment. Energies 2023, 16, 834. [Google Scholar] [CrossRef]
- Cavelius, P.; Engelhart-Straub, S.; Mehlmer, N.; Lercher, J.; Awad, D.; Brück, T. The Potential of Biofuels from First to Fourth Generation. PLoS Biol. 2023, 21, e3002063. [Google Scholar] [CrossRef]
- Rutz, D.; Janssen, R.; van den Berg, N.; Spekreijse, J.; Reumerman, P.; Matschegg, D.; Bacovsky, D.; Gröngröft, A.; Karampinis, E.; Kourkoumpas, D.; et al. Summary Paper for Policy Makers: Retrofitting Europe’s Industry with Bioenergy. 2019, 24. Available online: https://www.biofit-h2020.eu/files/pdfs/D2.6_BIOFIT%20Summary_2019-11-25b.pdf (accessed on 25 February 2025).
- Industry reports Europe Vegetable Oil Market (2016–2026) 2021. Available online: https://agriculture.ec.europa.eu/system/files/2019-10/agricultural-outlook-report-2016_en_0.pdf (accessed on 25 February 2025).
- Administration, U.S.E.I. Crude Oil Prices Briefly Traded Below $9 in Spring 2020 But Have Since Been Mostly Flat; U.S. Energy Information Administration (EIA): Washington, DC, USA, 2024. [Google Scholar]
- Gasparatos, A.; Stromberg, P.; Takeuchi, K. Sustainability Impacts of First-Generation Biofuels. Anim. Front. 2013, 3, 12–26. [Google Scholar] [CrossRef]
- European Environment Agency. Agriculture Contributes to Climate Change. Agric. Clim. Change 2015, 19, 1–9. Available online: https://www.eea.europa.eu/en/analysis/maps-and-charts/climate-change-and-agriculture (accessed on 25 January 2025).
- Deng, X.; Ma, Y.; Cheng, S.; Jin, Z.; Shi, C.; Liu, J.; Lin, J.; Yan, X. Castor Plant Adaptation to Salinity Stress during Early Seedling Stage by Physiological and Transcriptomic Methods. Agronomy 2023, 13, 693. [Google Scholar] [CrossRef]
- Bunting, E. Oilseed Crops. In Experimental Agriculture; Cambridge University Press: Cambridge, UK, 1983; Volume 20, p. 660. [Google Scholar]
- Yadav, P.; Anjani, K. Assessment of Variation in Castor Genetic Resources for Oil Characteristics. JAOCS J. Am. Oil Chem. Soc. 2017, 94, 611–617. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, S.; Sarma, S.J.; Brar, S.K. A Comprehensive Review of Castor Oil-Derived Renewable and Sustainable Industrial Products. Environ. Prog. Sustain. Energy 2023, 42, e14008. [Google Scholar] [CrossRef]
- Alola, A.A.; Özkan, O.; Obekpa, H.O. Examining the Patterns of Disaggregate Energy Security Risk and Crude Oil Price: The USA Scenario over 1970–2040. Resour. Policy 2023, 82, 103514. [Google Scholar] [CrossRef]
- FAOSTAT Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#data (accessed on 3 November 2024).
- Anastasi, U.; Sortino, O.; Cosentino, S.L.; Patanè, C. Seed Yield and Oil Quality of Perennial Castor Bean in a Mediterranean Environment. Int. J. Plant Prod. 2014, 9, 99–116. [Google Scholar]
- Cafaro, V.; Calcagno, S.; Patanè, C.; Cosentino, S.L. Effects of Sowing Dates and Genotypes of Castor (Ricinus communis L.) on Seed Yield and Oil Content in the South Mediterranean Basin. Agronomy 2023, 13, 2167. [Google Scholar] [CrossRef]
- Suardi, A.; Stefanoni, W.; Latterini, F.; Pari, R.; Lazar, S.; Fernando, A.L.; Palmieri, N. The Economic and Environmental Assessment of Castor Oil Supply Chain. Eur. Biomass Conf. Exhib. Proc. 2021, 1184–1188. [Google Scholar]
- Chen, X.; Zhang, H.; Teng, A.; Zhang, C.; Lei, L.; Ba, Y.; Wang, Z. Photosynthetic Characteristics, Yield and Quality of Sunflower Response to Deficit Irrigation in a Cold and Arid Environment. Front. Plant Sci. 2023, 14, 1280347. [Google Scholar] [CrossRef]
- Izgi, M.N.; Çil, A.; Çil, A.N. Yield and Quality Characteristics of Sunflower (Helianthus annuus L.) Cultivars by Different Sowing Dates. J. Appl. Life Sci. Environ. 2024, 57, 371–394. [Google Scholar] [CrossRef]
- Ravneet, K.; Thallada, B. Potential of Castor Plant (Ricinus communis) for Production of Biofuels, Chemicals, and Value-Added Products. In Waste Biorefinery; Thallada, B., Ashok, P., Eldon, R., Rene, D.C.W.T., Eds.; Springer: Singapore, 2020; pp. 269–310. [Google Scholar]
- Severino, L.S.; Auld, D.L.; Baldanzi, M.; Cândido, M.J.D.; Chen, G.; Crosby, W.; Tan, D.; He, X.; Lakshmamma, P.; Lavanya, C.; et al. A Review on the Challenges for Increased Production of Castor. Agron. J. 2012, 104, 853–880. [Google Scholar] [CrossRef]
- Rasetti-Escargueil, C.; Avril, A. Medical Countermeasures against Ricin Intoxication. Toxins 2023, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Abbes, M.; Montana, M.; Curti, C.; Vanelle, P. Ricin Poisoning: A Review on Contamination Source, Diagnosis, Treatment, Prevention and Reporting of Ricin Poisoning. Toxicon 2021, 195, 86–92. [Google Scholar] [CrossRef]
- Greenfield, R.A.; Brown, B.R.; Hutchins, J.B.; Iandolo, J.J.; Jackson, R.; Slater, L.N.; Bronze, M.S. Microbiological, Biological, and Chemical Weapons of Warfare and Terrorism. Am. J. Med. Sci. 2002, 323, 326–340. [Google Scholar] [CrossRef]
- Landoni, M.; Bertagnon, G.; Ghidoli, M.; Cassani, E.; Adani, F.; Pilu, R. Opportunities and Challenges of Castor Bean (Ricinus communis L.) Genetic Improvement. Agronomy 2023, 13, 2076. [Google Scholar] [CrossRef]
- Scholz, V.; da Silva, J.N. Prospects and Risks of the Use of Castor Oil as a Fuel. Biomass Bioenergy 2008, 32, 95–100. [Google Scholar] [CrossRef]
- Thomas, T.P.; Birney, D.M.; Auld, D.L. Viscosity Reduction of Castor Oil Esters by the Addition of Diesel, Safflower Oil Esters and Additives. Ind. Crops Prod. 2012, 36, 267–270. [Google Scholar] [CrossRef]
- González, R.; Kumar Patel, A.; Stefanoni, W.; Latterini, F.; Malkogiannidis, V.; Salpiggidis, V.; Alexopoulou, E.; Pari, L. Mechanical Harvesting of Castor Bean (Ricinus communis L.) with a Combine Harvester Equipped with Two Different Headers: A Comparison of Working Performance. Energies 2022, 15, 2999. [Google Scholar] [CrossRef]
- Pari, L.; Latterini, F.; Stefanoni, W. Herbaceous Oil Crops, a Review on Mechanical Harvesting State of the Art. Agriculture 2020, 10, 309. [Google Scholar] [CrossRef]
- The Holy Bible, Book of Jonah 4:6-7 Book of Jonah; New International Version. Available online: https://www.biblegateway.com/passage/?search=Jonah+4%3A6-7&version=NIV (accessed on 21 April 2023).
- Dawson, W.R. Studies in Medical History: (A) The Origin of the Herbal. (b) Castor-Oil in Antiquity. Aegyptus 1929, 10, 47–72. [Google Scholar]
- Kole, C.; Rabinowicz, P. Compendium of Plant Genomes the Castor Bean Genome; Springer: Cham, Switzerland, 2018; ISBN 9783319972794. [Google Scholar]
- Watt, G. The Dictionary of the Economic Products of India: Horticultural Index; Government of India: Calcutta, India, 1896. [Google Scholar]
- Gusamo, B.K.; Jimbudo, M. Tree-Borne Oilseed Crops: Jatropha Curcas, Ricinus communis, Anacardium Occidentale and Some Native Trees for Oil Production for Bio-Energy Source in Papua New Guinea. J. Agric. Environ. Sci. 2015, 4, 113–123. [Google Scholar] [CrossRef][Green Version]
- Purseglove, J.W. Castor, Sesame & Safflower By E. A. Weiss London: Leonard Hill Books (1971), pp. 901, £16.00. Exp. Agric. 1972, 8, 282. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Kalam Mohammad Aminul Islam, A.; Yaakob, Z.; Kalam Mohammad Mominul Islam, A. Castor (Ricinus communis): An Underutilized Oil Crop in the South East Asia. In Agroecosystems—Very Complex Environmental Systems; Springer: Singapore, 2021. [Google Scholar]
- Xu, W.; Wu, D.; Yang, T.; Sun, C.; Wang, Z.; Han, B.; Wu, S.; Yu, A.; Chapman, M.A.; Muraguri, S.; et al. Genomic Insights into the Origin, Domestication and Genetic Basis of Agronomic Traits of Castor Bean. Genome. Biol. 2021, 22, 113. [Google Scholar] [CrossRef]
- Xu, W.; Yang, T.; Qiu, L.; Chapman, M.A.; Li, D.Z.; Liu, A. Genomic Analysis Reveals Rich Genetic Variation and Potential Targets of Selection during Domestication of Castor Bean from Perennial Woody Tree to Annual Semi-Woody Crop. Plant Direct 2019, 3, e00173. [Google Scholar] [CrossRef]
- Cheema, N.M.; Azim Malik, M.; Qadir, G.; Zubair Rafique, M.; Nawaz, N. Influence of Temperature and Osmotic Stress on Germination Induction of Different Castor Bean Cultivars. Pakistan J. Bot. 2010, 42, 4035–4041. [Google Scholar]
- Rizzardo, R.A.G.; Milfont, M.O.; Da Silva, E.M.S.; Freitas, B.M. Apis Mellifera Pollination Improves Agronomic Productivity of Anemophilous Castor Bean (Ricinus communis). An. Acad. Bras. Cienc. 2012, 84, 1137–1145. [Google Scholar] [CrossRef]
- Anjani, K. Castor Genetic Resources: A Primary Gene Pool for Exploitation. Ind. Crops Prod. 2012, 35, 1–14. [Google Scholar] [CrossRef]
- Swetman, A. Oilseed Crops, 2nd ed.; Weiss, E.A., Ed.; Ix 364 Pp; Blackwell Science (1999): Oxford, UK, 2000. [Google Scholar]
- Bunting, E.S. Oilseed Crops. By E. A. Weiss. London: Longman (1983), pp. 660, £35. Exp. Agric. 1984, 20, 267–268. [Google Scholar] [CrossRef]
- Simmonds, N.W.; Purseglove, J.W. Tropical Crops. Dicotyledons. J. Ecol. 1969, 57, 846–847. [Google Scholar] [CrossRef]
- Van der Vossen, H.A.M.; Umail, B.E. Plant Resources of South-East Asia; Backhuys Publishers: Leiden, The Netherlands, 2001. [Google Scholar]
- Campbell, D.N. Determining the Agronomic and Physiological Characteristics Of the Castor Plant (Ricinus communis L.): Developing a Sustainable Cropping System for Florida. Master’s Thesis, University of Florida, Gainesville, FL, USA, 2013. Available online: https://ufdcimages.uflib.ufl.edu/UF/E0/04/55/14/00001/CAMPBELL_D.pdf (accessed on 12 March 2023).
- Rios, G.F.; de Carvalho, L.G.; da Silva Junior, J.J.; Neto, P.C.; Fraga, A.C. Method and Phenological Characterization of the Stadiums and Phases of the Development of Castor Bean Plants. Afr. J. Agric. Res. 2016, 11, 4488–4497. [Google Scholar] [CrossRef]
- Marin, F.R.; Angelocci, L.R.; Nassif, D.S.P.; Vianna, M.S.; Pilau, F.G.; da Silva, E.H.F.M.; Sobenko, L.R.; Gonçalves, A.O.; Pereira, R.A.A.; Carvalho, K.S. Revisiting the Crop Coefficient–Reference Evapotranspiration Procedure for Improving Irrigation Management. Theor. Appl. Climatol. 2019, 138, 1785–1793. [Google Scholar] [CrossRef]
- Weiss, E.A. Oilseed Crops, 2nd ed.; Blackwell Science: Oxford, UK, 1999; ISBN 0-632-05259-7. [Google Scholar]
- Messina, C.D.; Rotundo, J.; Hammer, G.L.; Gho, C.; Reyes, A.; Fang, Y.; van Oosterom, E.; Borras, L.; Cooper, M. Radiation Use Efficiency Increased over a Century of Maize (Zea Mays L.) Breeding in the US Corn Belt. J. Exp. Bot. 2022, 73, 5503–5513. [Google Scholar] [CrossRef] [PubMed]
- Carrino, L.; Visconti, D.; Fiorentino, N.; Fagnano, M. Biofuel Production with Castor Bean: A Win-Win Strategy for Marginal Land. Agronomy 2020, 10, 1690. [Google Scholar] [CrossRef]
- Salihu, B.Z.; Gana, A.K.; Apuyor, B.O. Castor Oil Plant (Ricinus communis L.): Botany, Ecology and Uses. Int. J. Sci. Res. 2014, 3, 1333–1341. [Google Scholar]
- Severino, L.S.; Auld, D.L. Seed Yield and Yield Components of Castor Influenced by Irrigation. Ind. Crops Prod. 2013, 49, 52–60. [Google Scholar] [CrossRef]
- Falasca, S.L.; Ulberich, A.C.; Ulberich, E. Developing an Agro-Climatic Zoning Model to Determine Potential Production Areas for Castor Bean (Ricinus communis L.). Ind. Crops Prod. 2012, 40, 185–191. [Google Scholar] [CrossRef]
- De Lima, G.S.; Gheyi, H.R.; Nobre, R.G.; Xavier, D.A.; Dos Anjos Soares, L.A.; Cavalcante, L.F.; Dos Santos, J.B. Emergence, Growth, and Flowering of Castor Beans as a Function of the Cationic Composition of Irrigation Water. Semin. Agrar. 2016, 37, 651–664. [Google Scholar] [CrossRef]
- Papazoglou, E.G.; Alexopoulou, E.; Papadopoulos, G.K.; Economou-Antonaka, G. Tolerance to Drought and Water Stress Resistance Mechanism of Castor Bean. Agronomy 2020, 10, 1580. [Google Scholar] [CrossRef]
- Koutroubas, S.D.; Papakosta, D.K.; Doitsinis, A. Water Requirements for Castor Oil Crop (Ricinus communis L.) in a Mediterranean Climate. J. Agron. Crop Sci. 2000, 184, 33–41. [Google Scholar] [CrossRef]
- Patel, J.J.; Patel, D.M.; Patel, J.R.; Damor, R.P. Effect of Farm Yard Manure and Nitrogen on Growth, Yield and Economics of Castor (Ricinus communis L.). Pharma Innov. 2023, 12, 1439–1443. [Google Scholar]
- Zhao, H.; Zhang, C. Analysis on the Research Status and Structure Characteristics of Castor Harvester. In Proceedings of the 2019 IEEE International Conference on Mechatronics and Automation (ICMA), Tianjin, China, 4–7 August 2019; pp. 415–420. [Google Scholar] [CrossRef]
- Baldanzi, M.; Myczkowski, M.L.; Salvini, M.; Macchia, M. Description of 90 Inbred Lines of Castor Plant (Ricinus communis L.). Euphytica 2015, 202, 13–33. [Google Scholar] [CrossRef]
- de Oliveira Neto, S.S.; Ramos, A.R.; da Silva Oliveira, C.E.; da Silva, E.A.A.; Zanotto, M.D. Genetic Divergence and Physiological Quality of Dwarf Castor Bean Lines Seeds. Pesqui. Agropecu. Trop. 2022, 52, 1–18. [Google Scholar] [CrossRef]
- Zanetti, F.; Chieco, C.; Alexopoulou, E.; Vecchi, A.; Bertazza, G.; Monti, A. Comparison of New Castor (Ricinus communis L.) Genotypes in the Mediterranean Area and Possible Valorization of Residual Biomass for Insect Rearing. Ind. Crops Prod. 2017, 107, 581–587. [Google Scholar] [CrossRef]
- Burton, J.D.; Pedersen, M.K.; Coble, H.D. Effect of Cyclanilide on Auxin Activity. J. Plant Growth Regul. 2008, 27, 342–352. [Google Scholar] [CrossRef]
- Oswalt, J.S.; Rieff, J.M.; Severino, L.S.; Auld, D.L.; Bednarz, C.W.; Ritchie, G.L. Plant Height and Seed Yield of Castor (Ricinus communis L.) Sprayed with Growth Retardants and Harvest Aid Chemicals. Ind. Crops Prod. 2014, 61, 272–277. [Google Scholar] [CrossRef]
- Auld, D.L.; Zanotto, M.D.; McKeon, T.; Morris, J. Oil Crops-Handbook of Plant Breeding in Oil Crops-Handbook of Plant Breeding; Springer: New York, NY, USA, 2009; pp. 316–332. [Google Scholar]
- Bertozzo, F.; da Costa Lara, A.C.; Zanotto, M.D. Melhoramento Genético Da Mamona Visando Incremento de Flores Femininas. Bragantia 2011, 70, 271–277. [Google Scholar] [CrossRef]
- Service, F.S.; Simpson, C.E.; Baring, M.R.; Schubert, A.M.; Melouk, H.A.; Black, M.C.; Lopez, Y.; Keim, K.A.; Texas, T. Registrations of Cultivars. Crop Sci. 2002, 2298–2316. Available online: https://www.ars.usda.gov/ARSUserFiles/60701500/Publications/Marshall/MarshallCropSci03.pdf (accessed on 25 February 2025).
- Duran, A.J.F.P.; Lyra, G.P.; Campos Filho, L.E.; Martins, R.H.B.; Bueno, C.; Rossignolo, J.A.; Fiorelli, J. The Use of Castor Oil Resin on Particleboards: A Systematic Performance Review and Life Cycle Assessment. Sustainability 2025, 17, 3609. [Google Scholar] [CrossRef]
- Rojas-Barros, P.; Fernández-Martínez, J.M. Fatty Acid and Tocopherol Accumulation in the Seeds of a High Oleic Acid Castor Mutant. Ind. Crops Prod. 2005, 22, 201–206. [Google Scholar] [CrossRef]
- Rojas-Barros, P.; De Haro, A.; Fernández-Martínez, J.M. Inheritance of High Oleic/Low Ricinoleic Acid Content in the Seed Oil of Castor Mutant OLE-1. Crop Sci. 2005, 45, 157–162. [Google Scholar] [CrossRef]
- Lavanya Cherukupalli, P.; Duraimurugan, P.; Lakshmamma, T.; Manjunatha, K.T.; Ramya, M.; Santhalakshmi Prasad, M.; Senthilvel Senapathy; Vishnuvardhan Reddy, A. Development of Leafhopper and Wilt Resistant Pistillate Line in Castor through Mutation Breeding. In Proceedings of the FAO/IAEA International Symposium on Plant Mutation Breeding and Biotechnology, Vienna, Austria, 27–31 August 2018; Available online: https://conferences.iaea.org/event/145/contributions/5044/ (accessed on 5 June 2024).
- Bai, L.; Cheng, Y.; She, J.; He, Z.; Liu, H.; Zhang, G.; Cao, R.; Chen, Y. Development of an Efficient Protoplast Isolation and Transfection System for Castor Bean (Ricinus communis L.). Plant Cell Tissue Organ Cult. 2020, 143, 457–464. [Google Scholar] [CrossRef]
- Xu, W.; Chen, Z.; Ahmed, N.; Han, B.; Cui, Q.; Liu, A. Genome-Wide Identification, Evolutionary Analysis, and Stress Responses of the GRAS Gene Family in Castor Beans. Int. J. Mol. Sci. 2016, 17, 1004. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Wu, D.; Zhang, Y.; Li, D.Z.; Xu, W.; Liu, A. Epigenetic Regulation of Seed-Specific Gene Expression by DNA Methylation Valleys in Castor Bean. BMC Biol. 2022, 20, 57. [Google Scholar] [CrossRef]
- Omprakash, S. Identification of Sources, Mechanisms of Resistance and Management of Major Insect Pests on Sesame (Sesamum indicum L.). Master’s Thesis, Professor Jayashankar Telangana State Agricultural University, Hyderabad, India, 2021. Available online: https://krishikosh.egranth.ac.in/handle/1/5810204863 (accessed on 5 June 2023).
- Silva, D.F.; Queiroga, V.P.; Silva, J.G.; Silva, M.G.; Nascimento, J.A. Cultivo da Mamoneira sob Diferentes Tipos de Águas Residuárias e de Abastecimento e Níveis de Água no Solo. Rev. Caatinga 1983, 26, 11–21. Available online: https://periodicos.ufersa.edu.br/caatinga/article/view/2795 (accessed on 5 June 2024).
- Cafaro, V.; Alexopoulou, E.; Cosentino, S.L.; Patanè, C. Assessment of Germination Response to Salinity Stress in Castor through the Hydrotime Model. Agronomy 2023, 13, 2783. [Google Scholar] [CrossRef]
- Thatikunta, R. In Vitro Screening of Castor Genotypes for Stress Tolerance Seed Physiology View Project Horticulture View Project. J. Oilseeds Res. 2001, 18, 292–293. [Google Scholar]
- Sausen, T.L.; Rosa, L.M.G. Growth and Limitations to Carbon Assimilation in Ricinus communis (Euphorbiaceae) under Soil Water Stress Conditions. Acta Bot. Bras 2010, 648–654. [Google Scholar] [CrossRef]
- de Sousa Lopes, L.; Prisco, J.T.; Gomes-Filho, E. Inducing Salt Tolerance in Castor Bean through Seed Priming. Aust. J. Crop Sci. 2018, 12, 943–953. [Google Scholar] [CrossRef]
- Zheng, J.; Suhono, G.B.F.; Li, Y.; Jiang, M.Y.; Chen, Y.; Siddique, K.H.M. Salt-tolerance in Castor Bean (Ricinus communis L.) Is Associated with Thicker Roots and Better Tissue K+/Na+ Distribution. Agriculture 2021, 11, 821. [Google Scholar] [CrossRef]
- Pinheiro, H.A.; Silva, J.V.; Endres, L.; Ferreira, V.M.; de Albuquerque Câmara, C.; Cabral, F.F.; Oliveira, J.F.; de Carvalho, L.W.T.; dos Santos, J.M.; dos Santos Filho, B.G. Leaf Gas Exchange, Chloroplastic Pigments and Dry Matter Accumulation in Castor Bean (Ricinus communis L.) Seedlings Subjected to Salt Stress Conditions. Ind. Crops Prod. 2008, 27, 385–392. [Google Scholar] [CrossRef]
- Abishek, R.; Santhi, R.; Maragatham, S.; Venkatachalam, S.R.; Uma, D.; Lakshmanan, A. Response of Hybrid Castor Under Different Fertility Gradient: Correlation Between Castor Yield and Normalized Difference Vegetation Index (NDVI) Under Inductive Cum Targeted Yield Model on an Alfisol. Commun. Soil Sci. Plant Anal. 2023, 54, 1816–1831. [Google Scholar] [CrossRef]
- Thakkar, K.; Kachhwaha, S.S.; Kodgire, P. Enhanced Castor Seed Oil Extraction Assisted by the Synergistic Effect of Ultrasound and Microwave: Impact on Extraction Effectiveness and Oil Quality. Chem. Eng. Process. Process Intensif. 2023, 185, 109307. [Google Scholar] [CrossRef]
- Singh, P.; Choudhary, K.K.; Chaudhary, N.; Gupta, S.; Sahu, M.; Tejaswini, B.; Sarkar, S. Salt Stress Resilience in Plants Mediated through Osmolyte Accumulation and Its Crosstalk Mechanism with Phytohormones. Front. Plant Sci. 2022, 13, 1006617. [Google Scholar] [CrossRef]
- Han, P.; Li, S.; Yao, K.; Geng, H.; Liu, J.; Wang, Y.; Lin, J. Integrated Metabolomic and Transcriptomic Strategies to Reveal Adaptive Mechanisms in Castor Plant during Germination Stage under Alkali Stress. Environ. Exp. Bot. 2022, 203, 105031. [Google Scholar] [CrossRef]
- Cafaro, V.; Alexopoulou, E.; Cosentino, S.L. Germination Response of Different Castor Bean Genotypes to Temperature for Early and Late Sowing Adaptation in the Mediterranean Regions. Agriculture 2023, 13, 1569. [Google Scholar] [CrossRef]
- Ribeiro, P.R.; Zanotti, R.F.; Deflers, C.; Fernandez, L.G.; de Castro, R.D.; Ligterink, W.; Hilhorst, H.W. Effect of Temperature on Biomass Allocation in Seedlings of Two Contrasting Genotypes of the Oilseed Crop Ricinus communis. J. Plant Physiol. 2015, 185, 31–39. [Google Scholar] [CrossRef]
- Ribeiro, P.R.; Fernandez, L.G.; de Castro, R.D.; Ligterink, W.; Hilhorst, H.W.M. Physiological and Biochemical Responses of Ricinus communis Seedlings to Different Temperatures: A Metabolomics Approach. BMC Plant Biol. 2014, 14, 223. [Google Scholar] [CrossRef]
- Neto, V.G.; Ligterink, W.; Hilhorst, H.W.M.; Santos, I.D.; Teixeira, C.R.; Santos, E.E.; Loureiro, M.B.; Takahashi, D.; Fernandez, L.G.; Ribeiro, P.R.; et al. Resilience of Ricinus communis L. to High Temperatures during Germination and Seedling Growth Resulting from Efficient Superoxide Dismutase Modulation. Rev. Bras. Bot. 2024, 47, 311–324. [Google Scholar] [CrossRef]
- Patanè, C.; Cosentino, S.L.; Corinzia, S.A.; Testa, G.; Sortino, O.; Scordia, D. Photothermal Zoning of Castor (Ricinus communis L.) Growing Season in the Semi-Arid Mediterranean Area. Ind. Crops Prod. 2019, 142, 111837. [Google Scholar] [CrossRef]
- Sortino, O.; Cosentino, L.; Sidella, S. Ricino (Ricinus communis L.). In Lo Sviluppo Delle Colture Energetiche in Italia; Consiglio per la Ricerca e la sperimentazione in Agricoltura (CRA): Roma, Italy, 2011; pp. 89–98. ISBN 9788490225370. [Google Scholar]
- Calcagno, S.; Piccitto, A.; Patan, C.; Cosentino, S.L. Effects of Nitrogen Fertilization and Soil Water Content on Seed and Oil Yield in Perennial Castor in a Mediterranean Environment. Agronomy 2023, 13, 1070. [Google Scholar] [CrossRef]
- Reza Sadeghi-Bakhtavari, A.; Hazrati, S. Growth, Yield, and Fatty Acids as Affected by Water-Deficit and Foliar Application of Nitrogen, Phosphorus, and Sulfur in Castor Bean. J. Plant Nutr. 2020, 35, 453–468. [Google Scholar] [CrossRef]
- de Araújo Nascimento, D.; dos Santos Brito, A.; da Silva, L.M.N.; Peixouto, L.S.; Cotrim, V.F. Water Use Efficiency of Castor Bean under Semi-Arid Conditions of Brazil. Agric. Water Manag. 2022, 260, 107278. [Google Scholar] [CrossRef]
- Shah, S.K. Review on Foliar Application of Plant Nutrients on Castor Review on Foliar Application of Plant Nutrients On castor. J. Food. Agric. Res. 2023, 3, 85–100. [Google Scholar]
- Lima, R.L.S.; Severino, L.S.; Sampaio, L.R.; Sofiatti, V.; Gomes, J.A.; Beltrão, N.E.M. Blends of Castor Meal and Castor Husks for Optimized Use as Organic Fertilizer. Ind. Crops Prod. 2011, 33, 364–368. [Google Scholar] [CrossRef]
- Costa, A.G.F.; Sofiatti, V.; Maciel, C.D.D.G.; Poletine, J.P.; Sousa, J.I.D. Weed Management Strategies for Castor Bean Crops. Acta Sci. Agron. 2014, 36, 137–145. [Google Scholar] [CrossRef]
- Vitorino, H.S.; Martins, D.; Costa, S.Í.A.; Marques, R.P.; de Souza, G.S.F.; de Campos, C.F. Eficiência de Herbicidas No Controle de Plantas Daninhas Latifoliadas Em Mamona. Arq. Inst. Biol. 2012, 79, 129–133. [Google Scholar] [CrossRef]
- Reddy, V.D.; Rao, P.N.; Poduri, N.; Rao, K.V. Pests and Pathogens: Management Strategies; CRC Press: Boca Raton, FL, USA, 2011; ISBN 978-0-415-66576-6. [Google Scholar]
- Gahukar, R.T. Management of Pests and Diseases of Castor (Ricinus communis L.) in India: Current Status and Future Perspective. Arch. Phytopathol. Plant Prot. 2018, 51, 956–978. [Google Scholar] [CrossRef]
- Jos, D. Gray Mold of Castor: A Review. Plant Pathol. 2012, 11, 15–25. [Google Scholar] [CrossRef][Green Version]
- Mubofu, E.B. Castor Oil as a Potential Renewable Resource for the Production of Functional Materials. Sustain. Chem. Process. 2016, 4, 11. [Google Scholar] [CrossRef]
- Chan, A.P.; Crabtree, J.; Zhao, Q.; Lorenzi, H.; Orvis, J.; Puiu, D.; Melake-Berhan, A.; Jones, K.M.; Redman, J.; Chen, G.; et al. Draft Genome Sequence of the Oilseed Species Ricinus communis. Nat. Biotechnol. 2010, 28, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.C.; da Silveira Alves, F.G.; Salles, H.O.; Pompeu, R.C.F.F.; Ludke, J.V.; Severino, L.S.; Cândido, M.J.D. The Industrial Process of Solvent Extraction of Castor Oil Reduces the Toxicity of the Meal. Ind. Crop. Prod. 2022, 181, 114800. [Google Scholar] [CrossRef]
- Khalaf, M.; Abdel-Fadeel, W.; Hashish, H.M.A.; Wapet, D.E.M.; Mahmoud, M.M.; Elhady, S.A.; Esmail, M.F.C. Experimental Investigation of Different Extraction Methods for Producing Biofuel from Jatropha Seeds and Castor Seeds. Int. J. Energy Res. 2023, 2023, 1780536. [Google Scholar] [CrossRef]
- Nde, D.B.; Anuanwen, C.F. Optimization Methods for the Extraction of Vegetable Oils: A Review. Processes 2020, 8, 209. [Google Scholar] [CrossRef]
- Marchetti, J.M.; Miguel, V.U.; Errazu, A.F. Possible Methods for Biodiesel Production. Renew. Sustain. Energy Rev. 2007, 11, 1300–1311. [Google Scholar] [CrossRef]
- Keera, S.T.; El Sabagh, S.M.; Taman, A.R. Castor Oil Biodiesel Production and Optimization. Egypt. J. Pet. 2018, 27, 979–984. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Nguyen, M.L.; Su, C.H.; Ong, H.C.; Juan, H.Y.; Wu, S.J. Bio-Derived Catalysts: A Current Trend of Catalysts Used in Biodiesel Production. Catalysts 2021, 11, 812. [Google Scholar] [CrossRef]
- Bauddh, K.; Singh, K.; Singh, B.; Singh, R.P. Ricinus communis: A Robust Plant for Bio-Energy and Phytoremediation of Toxic Metals from Contaminated Soil. Ecol. Eng. 2015, 84, 640–652. [Google Scholar] [CrossRef]
- Adesanya, V.O.; Cadena, E.; Scott, S.A.; Smith, A.G. Life Cycle Assessment on Microalgal Biodiesel Production Using a Hybrid Cultivation System. Bioresour. Technol. 2014, 163, 343–355. [Google Scholar] [CrossRef]
- Albuquerque, M.C.G.; Machado, Y.L.; Torres, A.E.B.; Azevedo, D.C.S.; Cavalcante, C.L.; Firmiano, L.R.; Parente, E.J.S. Properties of Biodiesel Oils Formulated Using Different Biomass Sources and Their Blends. Renew. Energy 2009, 34, 857–859. [Google Scholar] [CrossRef]
- Diamante, L.M.; Lan, T. Absolute Viscosities of Vegetable Oils at Different Temperatures and Shear Rate Range of 64.5 to 4835 s–1. J. Food Process. 2014, 2014, 234583. [Google Scholar] [CrossRef]
- Talavera-Prieto, N.M.C.; Ferreira, A.G.M.; Portugal, A.T.G.; Egas, A.P.V. Viscosity of Cottonseed Oil and Biodiesel. J. Chem. Eng. Data 2019, 64, 1166–1176. [Google Scholar] [CrossRef]
- Igbax, S.I.; Ogwu, E.A.; Fadeyi, O.A. Effect of Vegetable Oil Viscosity on Biodiesel. Fuel 2022, 90, 1751–1761. [Google Scholar]
- Uddin, M.N.; Techato, K.; Taweekun, J.; Rahman, M.M.; Rasul, M.G.; Mahlia, T.M.I.; Ashrafur, S.M. An Overview of Recent Developments in Biomass Pyrolysis Technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef]
- Balat, M. Production of Biodiesel from Vegetable Oils: A Survey. Energy Sources Part A Recover. Util. Environ. Eff. 2007, 29, 895–913. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Syazmi, Z.A.H.S.; Mofijur, M.; Abas, A.E.P.; Bilad, M.R.; Ong, H.C.; Silitonga, A.S. Patent Landscape Review on Biodiesel Production: Technology Updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- de Oliveira, A.S.; Campos, J.M.S.; Oliveira, M.R.C.; Brito, A.F.; Filho, S.C.V.; Detmann, E.; Valadares, R.F.D.; de Souza, S.M.; Machado, O.L.T. Nutrient Digestibility, Nitrogen Metabolism and Hepatic Function of Sheep Fed Diets Containing Solvent or Expeller Castorseed Meal Treated with Calcium Hydroxide. Anim. Feed Sci. Technol. 2010, 158, 15–28. [Google Scholar] [CrossRef]
- Islam, T.; Bakshi, H.; Sam, S.; Sharma, E.; Hameed, B.; Rathore, B.; Gupta, A.; Ahirwar, S.; Sharma, M. Assessment of Antibacterial Potential of Leaves of Ricinus communis Against Pathogenic and Dermatophytic Bacteria. Int. J. Pharma Res. Dev. Online 2010, 1, 1–7. [Google Scholar]
- Li, J.; Chen, M.; Yang, X.; Zhang, L. Effect and Mechanisms of Soil Functional Groups in Bacterial-Enhanced Cadmium Contaminated Soil Phytoremediation. Environ. Technol. Innov. 2024, 33, 103531. [Google Scholar] [CrossRef]
- Ilavarasan, R.; Mallika, M.; Venkataraman, S. Anti-Inflammatory and Free Radical Scavenging Activity of Ricinus communis Root Extract. J. Ethnopharmacol. 2006, 103, 478–480. [Google Scholar] [CrossRef]
- Almeida, R.N.; Almeida, R.N.; Navarro, D.S.; Barbosa-Filho, J.M. Plants with Central Analgesic Activity. Phytomedicine 2001, 8, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Ladda, P.L.; Magdum, C.S. Evaluation of Anti-Tubercular Activity of Ricinus communis Linn. By Proportion, Nra and Bact/Alert Methods. Int. J. Pharm. Pharm. Sci. 2012, 4, 474–478. [Google Scholar]
- Reserved, A.R. Anti-Fertility Activity of Methanol Extracts of Three Different Seed Varieties of Ricinus communis Linn. Niger. J. Pharm. Sci. 2007, 6, 78–83. [Google Scholar]
- Saier, M.H.; Trevors, J.T. Phytoremediation. Water. Air. Soil Pollut. 2010, 205, 61–63. [Google Scholar] [CrossRef]
- Gibbs, H.K.; Salmon, J.M. Mapping the World’s Degraded Lands. Appl. Geogr. 2015, 57, 12–21. [Google Scholar] [CrossRef]
- Tripathi, S.; Sharma, P.; Purchase, D.; Chandra, R. Distillery Wastewater Detoxification and Management through Phytoremediation Employing Ricinus communis L. Bioresour. Technol. 2021, 333, 125192. [Google Scholar] [CrossRef]

| Crop | Oil Content (%) |
|---|---|
| Castor bean | 35–65 |
| Palm | 30–60 |
| Rapeseed | 30–50 |
| Sunflower | 25–48 |
| Soybean | 15–22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cafaro, V.; Testa, G.; Patanè, C. Castor: A Renewed Oil Crop for the Mediterranean Environment. Agronomy 2025, 15, 1402. https://doi.org/10.3390/agronomy15061402
Cafaro V, Testa G, Patanè C. Castor: A Renewed Oil Crop for the Mediterranean Environment. Agronomy. 2025; 15(6):1402. https://doi.org/10.3390/agronomy15061402
Chicago/Turabian StyleCafaro, Valeria, Giorgio Testa, and Cristina Patanè. 2025. "Castor: A Renewed Oil Crop for the Mediterranean Environment" Agronomy 15, no. 6: 1402. https://doi.org/10.3390/agronomy15061402
APA StyleCafaro, V., Testa, G., & Patanè, C. (2025). Castor: A Renewed Oil Crop for the Mediterranean Environment. Agronomy, 15(6), 1402. https://doi.org/10.3390/agronomy15061402







