Abstract
The xeno-fungusphere, a novel microbial ecosystem formed by integrating exogenous fungi, indigenous soil microbiota, and electroactive microorganisms within microbial fuel cells (MFCs), offers a transformative approach for agricultural remediation and medicinal plant conservation. By leveraging fungal enzymatic versatility (e.g., laccases, cytochrome P450s) and conductive hyphae, this system achieves dual benefits. First, it enables efficient degradation of recalcitrant agrochemicals, such as haloxyfop-P, with a removal efficiency of 97.9% (vs. 72.4% by fungi alone) and a 27.6% reduction in activation energy. This is driven by a bioelectric field (0.2–0.5 V/cm), which enhances enzymatic activity and accelerates electron transfer. Second, it generates bioelectricity, up to 9.3 μW/cm2, demonstrating real-world applicability. In medicinal plant soils, xeno-fungusphere MFCs restore soil health by stabilizing the pH, enriching dehydrogenase activity, and promoting nutrient cycling, thereby mitigating agrochemical-induced inhibition of secondary metabolite synthesis (e.g., ginsenosides, taxol). Field trials show 97.9% herbicide removal in 60 days, outperforming conventional methods. Innovations, such as adaptive electrodes, engineered strains, and phytoremediation-integrated systems, have been used to address soil and fungal limitations. This technology bridges sustainable agriculture and bioenergy recovery, offering the dual benefits of soil detoxification and enhanced crop quality. Future IoT-enabled monitoring and circular economy integration promise scalable, precision-based applications for global agroecological resilience.
1. Introduction: Concept of Xeno-Fungusphere
Microbial fuel cells (MFCs) are bioelectrochemical systems that harness the metabolic activity of electroactive microorganisms to simultaneously degrade organic pollutants and generate electricity [1,2]. In a typical soil MFC, organic contaminants (e.g., herbicides) are oxidized at the anode by electrogenic bacteria, releasing electrons that flow through an external circuit to the cathode, where oxygen reduction occurs. This process not only accelerates pollutant mineralization, but also creates a directional bioelectric field that enhances microbial interactions [3] and nutrient cycling [4]. The objectives of this study are twofold: (1) to investigate the degradation mechanisms of herbicides (e.g., haloxyfop-P) in fungal-enhanced MFCs, focusing on enzymatic action, electrochemical stimulation, and microbial synergy; and (2) to explore the application potential of this technology in regard to medicinal plant cultivation, analyzing its effects on plant growth and secondary metabolite synthesis, while addressing the practical challenges.
Fungi have emerged as pivotal agents in bioremediation, due to their enzymatic versatility and adaptability to harsh environments [5]. Their extensive hyphal networks enable efficient colonization of contaminated soils, while secreted extracellular enzymes (e.g., laccases, cytochrome P450s (CYPs)) catalyze the breakdown of recalcitrant compounds, such as polycyclic aromatic hydrocarbons (PAHs) [6], azo dyes [7], and halogenated herbicides [8,9]. Notably, fungal mycelia can act as “bioelectrochemical highways”, facilitating electron transfer between spatially separated redox reactions, a trait synergistically aligned with MFC functionality [10].
The contamination of medicinal plant soils poses a critical challenge to global health and agriculture [11,12]. Intensive agrochemical applications, while boosting crop yields, lead to persistent residues in soils, cultivating high-value species like Ophiopogon japonicus [13] and Panax ginseng [14]. For instance, aryloxyphenoxypropionate (AOPP) herbicides, such as haloxyfop-P, are frequently detected in these soils, disrupting plant secondary metabolite synthesis (e.g., taxol and ginsenosides) and posing the risk of bioaccumulation in herbal products [15,16,17]. Conventional remediation methods, including physicochemical adsorption and electrokinetics, often fail to achieve complete detoxification without damaging soil microbiota, a limitation that underscores the need for innovative solutions [18].
The term xeno-fungusphere refers to a dynamic microbial ecosystem formed by the interaction of exogenous fungi with indigenous soil microbiota and electroactive microorganisms within a bioelectrochemical system, such as an MFC (Figure 1). Figure 1 illustrates the bioelectrochemical reactions involving multiple microbial groups, namely algae, archaea, fungi, and bacteria, within the xeno-fungusphere MFC system. This concept integrates fungal bioaugmentation, soil electrochemical dynamics, and pollutant degradation, creating localized “hotspots” of metabolic activity that enhance contaminant removal, while generating bioelectricity. The xeno-fungusphere leverages the functional traits of fungi (e.g., enzymatic versatility, stress tolerance) and synergizes with electrogenic bacteria to drive coupled biogeochemical processes, such as organic pollutant mineralization and nutrient cycling [19].
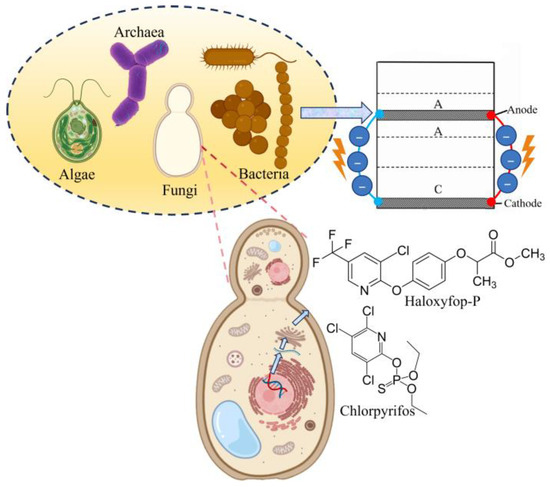
Figure 1.
Xeno-fungusphere in MFC. The cell is connected by two external wires, along which electrons flow from the anode, representing the generation of an electric current. The arrow indicates that these microbial activities are concentrated on the anode surface. A magnified section highlights the central role of fungi, which degrades pollutants (e.g., haloxyfop-P) via secreted enzymes (such as laccase and P450) and conductive hyphae, contributing both to contaminant removal and electron transfer to the anode.
2. Fungal-Augmented MFCs in Agricultural Applications
2.1. Mechanisms of Agrochemical Degradation
Fungal-enhanced MFCs utilize fungi like Myrothecium verrucaria and Talaromyces dalianensis to degrade recalcitrant herbicides (e.g., florpyrauxifen-benzyl and haloxyfop-P). Key mechanisms include:
2.1.1. Enzymatic Action
Fungi play a pivotal role in degrading herbicides, pesticides, and other organic pollutants in soil through the secretion of extracellular enzymes [20,21]. These enzymes, including laccases, CYP monooxygenases, peroxidases, and esterases, catalyze the breakdown of complex chemical structures by cleaving specific bonds, such as C-F, C-Cl, and ester linkages [22] (Figure 2). In the context of MFCs, the bioelectric field generated at the anode enhances fungal enzymatic activity, accelerating pollutant degradation through synergistic electrochemical and biochemical mechanisms [23,24].
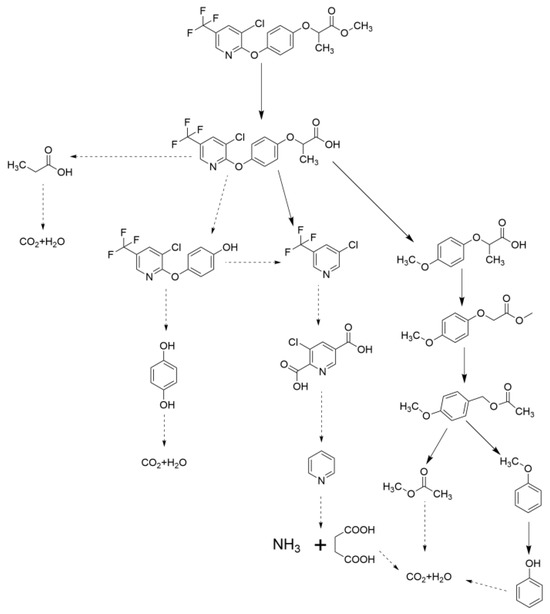
Figure 2.
Inferred degradation pathway of haloxyfop-P in fungi-augmented MFC.
The mechanisms of fungal enzyme action are being elucidated. Laccases, a class of multicopper oxidases, are particularly effective in oxidizing phenolic compounds and aromatic amines found in agrochemicals [25,26]. These enzymes utilize molecular oxygen as an electron acceptor, generating water as a byproduct, while destabilizing pollutant structures. For instance, laccases from Trametes have been shown to degrade triazine herbicides through demethylation and hydroxylation [27], substantially reducing their phytotoxicity. CYP systems, on the other hand, catalyze oxidative reactions targeting halogenated hydrocarbons [28,29]. In M. verrucaria, CYPs mediate the dehalogenation of haloxyfop-P, converting it into less toxic metabolites [19].
Esterases are critical for hydrolyzing ester bonds in synthetic pyrethroids and organophosphate pesticides [30], as well as herbicides [31]. For example, esterases from epiphytic yeasts participate in the biodegradation of chlorpyrifos [32]. Aspergillus hydrolyze the ester linkages in chlorpyrifos, yielding 3,5,6-trichloro-2-pyridinol [33], which is further mineralized by soil bacteria. Peroxidases, including lignin peroxidase and manganese peroxidase, generate free radicals that non-specifically attack aromatic rings in PAHs and azo dyes [7,34]. Phanerochaete chrysosporium employs these enzymes to degrade benzo[a]pyrene, a carcinogenic PAH, into CO2 and water via oxidative cleavage [35]. The lignin peroxidase of fungi could be responsible for the degradation of herbicide pretilachlor and its major metabolite [36].
The electrochemical enhancement of enzymatic activity justifies the combinatorial use of the xeno-fungusphere and MFCs [19]. The bioelectric field in MFCs (typically 0.2–0.5 V/cm) significantly enhances fungal enzymatic efficiency through multiple pathways. First, the electric field polarizes enzyme molecules, altering their conformational stability and increasing their substrate-binding affinity. After electric field treatment, the conductivity and complex dielectric constant of the enzyme solution increases [37], which could alter the electron transfer between the enzyme’s active site and redox mediators, thus changing the catalytic turnover rate. Second, the anode serves as an electron sink, diverting electrons generated during enzymatic oxidation away from competitive pathways, thereby reducing thermodynamic barriers. For instance, in Talaromyces dalianensis-augmented MFCs, the activation energy (Ea) for haloxyfop-P degradation decreased from 58 kJ/mol to 42 kJ/mol, enabling complete mineralization within 60 days [22], demonstrating that bioelectric fields reduce Ea via laccase conformational polarization [38]. In a composting system, when an electric field was coupled with an Fe anode, the crude fiber decomposition and humic acid formation were accelerated [39], partially due to the upregulations of carbohydrate-active enzymes under the electric stimulation.
Additionally, the bioelectric field stimulates fungal hyphal elongation and branching [40], expanding the spatial distribution of enzyme-secreting structures. This increases the contact between enzymes and pollutants, particularly in heterogeneous soil matrices. The primary constraint for MFC systems is scaling them up from the laboratory to practical applications [41], and the enzymatic actions in field trials warrant further studies.
The broad-spectrum degradation capabilities of fungi are favored [5]. The enzymatic versatility of fungi extends beyond herbicides to insecticides, fungicides, and industrial pollutants [24,42,43]. For example, in a soil MFC, the species associated with metolachlor degradation belonged to Mortierella, Kernia, Chaetomium, and Trichosporon [44]. Similarly, the basic extracellular oxidoreductases, for e.g., laccases, manganese peroxidases, and lignin peroxidases, of white rot fungus, Sporotrichum pruinosum, showed enhanced degradation activities under bioelectrochemical conditions [45].
While fungal enzymes exhibit remarkable adaptability, their activity is influenced by the soil pH, temperature, and co-contaminants [46,47,48]. Optimizing MFC operational parameters (e.g., electrode materials, voltage gradients) to sustain enzymatic performance in field conditions remains a priority [49]. Advances in genetic engineering, such as codon optimization, molecular chaperone-assisted expression, and atmospheric and room-temperature plasma (ARTP) mutagenesis, in Aspergillus, offer promising solutions to boost the catalytic efficiency of degrading enzymes [50].
2.1.2. Electrochemical Stimulation
Analogous to its effect on bacteria [51], the bioelectric field in MFCs enhances fungal metabolic activity and accelerates electron transfer, reducing degradation Ea. The bioelectric field generated in MFCs plays a pivotal role in enhancing fungal-mediated herbicide degradation by stimulating metabolic activity, optimizing electron transfer pathways, and reducing the Ea required for pollutant breakdown [19]. This electrochemical stimulation synergizes with fungal enzymatic systems, creating a dynamic environment that accelerates bioremediation, while generating bioelectricity. Below, we elaborate on the mechanisms and experimental evidence supporting this phenomenon.
Firstly, electrochemical stimulation enhances the fungal metabolic activity. The bioelectric field in MFCs (typically 0.2–0.5 V/cm) induces physiological changes in fungi, including increased respiration rates and upregulated metabolic pathways, which could be validated and predicted via numerical simulation [52]. Similar to a reconstructed thylakoid membrane [53], fungi could enhance ATP synthesis under electric fields, as the polarized cell membrane facilitates proton motive force generation, driving energy-intensive enzymatic reactions. This metabolic boost is critical for sustaining extracellular enzyme production, such as laccases and CYPs, which are essential for herbicide degradation.
In M. verrucaria-augmented MFCs, the metabolic flux through the tricarboxylic acid (TCA) cycle could increase significantly compared to non-electrified systems [19], correlating with higher NADH/NAD+ ratios and accelerated electron donation to oxidative enzymes. Similarly, fungal peroxidase activity might be increased when exposed to a 0.3 V/cm MFC field, attributed to improved redox balancing via direct electron transfer to the anode [54].
Secondly, electrochemical stimulation accelerates electron transfer. The anode in MFCs acts as an efficient electron sink [55], diverting electrons generated during bacterial/fungal oxidation of herbicides away from competing pathways. This reduces energy losses and lowers the Ea of degradation reactions. However, there is still a lack of reports that the fungi integration into MFCs decreases the Ea for herbicide degradation [56]. The direct electron transfer (DET) mechanism, facilitated by fungal hyphae acting as “biowires”, further optimizes this process [24]. Fusarium hyphae, for example, transport electrons from intracellular CYP-mediated agrochemical oxidation to the anode surface, bypassing diffusional limitations [57]. Beyond physical conduction, molecular-scale redox potential alignment governs these biowire functions. Specifically, c-type cytochromes (e.g., OmcS with ERAP = −0.212 V) enable DET when ERAP > −0.408 V [58], creating thermodynamic windows for fungal–electrode mutualism. Table 1 categorizes fungal applications in MFCs based on their localization (anode/cathode) and system configuration (single/dual chamber). In contrast to dual-chamber MFCs with strictly anaerobic anodic environments, single-chamber systems lack a physical membrane and, therefore, allow limited oxygen diffusion from the cathode or atmosphere. This creates a microaerobic niche near the anode, which selectively supports the survival and activity of electroactive fungi and facultative bacteria. As summarized in Table 1, aerobic fungi, such as Trametes versicolor and Ganoderma lucidum, exhibit preferential activity at the cathode, where oxygen functions as the terminal electron acceptor through laccase-mediated oxygen reduction reactions (oxygen reduction reaction (ORR): O2 + 4H+ + 4e− → 2H2O). While their direct electron transfer (DET) capability at the anode remains theoretically postulated, empirical evidence suggests that such functionality requires synergistic cocultivation with anaerobic electroactive bacteria (e.g., Shewanella oneidensis). This interspecies collaboration has been experimentally validated in dual-chamber MFC configurations [45], wherein the spatial segregation of cathodic aerobic fungal metabolism from anodic anaerobic zones effectively circumvents thermodynamic incompatibilities. Key examples include cathode-located Ganoderma lucidum for laccase-mediated oxygen reduction (Table 1) and anode-associated Exophiala dermatitidis for direct electron transfer under microaerobic conditions (Table 1), demonstrating the adaptability of fungi across electrochemical niches.

Table 1.
Fungal performance and mechanisms in microbial fuel cells.
Conductive materials in electrodes, such as carbon nanotubes or biochar [51,68,69], amplify this effect by providing high-surface-area pathways for electron shuttling. Similar to those in wastewater MFCs [70], graphene-modified anodes would increase the pollutant degradation, as compared to conventional graphite electrodes.
Thirdly, the electrochemical stimulation could reduce the Ea. The bioelectric field lowers Ea by stabilizing the transition states and reducing the energy barrier for bond cleavage [71]. For haloxyfop-P degradation by M. verrucaria, the Ea decreased from 58 kJ/mol to 42 kJ/mol in MFCs, as the electric field polarized the C-F bond, making it more susceptible to enzymatic attack [19]. Yet, how fungal bioaugmentation decreases Ea and increases pollutant degradation in MFCs calls for extensive studies of various systems.
Electrochemical impedance spectroscopy (EIS) analyses reveal that MFCs reduce the charge transfer resistance (Rct) by 60–70% [72], indicating more efficient electron flow during enzymatic catalysis [19]. This aligns with Arrhenius equation-derived kinetics, wherein a lower Ea correlates with exponential increases in reaction rates.
2.1.3. Microbial/Biotic Synergy
The added fungi alter the soil microbiota composition [73], enriching electroactive genera (e.g., Enterobacter, Bacillus) and sulfur/iron-cycling bacteria, which further degrade intermediates [74]. The bioelectric field also enriches electroactive bacteria (e.g., Geobacter, Shewanella) near fungal hyphae, creating synergistic consortia [23]. In fungi-augmented MFCs, it is highly possible that electroactive bacteria, e.g., Geobacter sulfurreducens [75], utilize fungal-secreted redox mediators (e.g., phenazines) to enhance extracellular electron transfer, achieving better pollutant degradation. Separately, carbonized fungal mycelia (e.g., Flammulina velutipes, Table 1) serve as structural scaffolds for electroactive bacteria, independent of metabolic interactions. This cross-kingdom interaction highlights the role of MFCs in fostering microbial cooperation.
Shifting interactions among bacteria, fungi, and archaea enhance the removal of antibiotics and antibiotic resistance genes during soil bioelectrochemical remediation [76]. In MFCs, metolachlor could render more complex relations, but a weaker connection strength, among soil microorganisms [77].
2.2. Fungi-Augmented MFC Remediation of Organic Pollutant-Contaminated Soils
2.2.1. Pollutant Removal Efficiency
MFCs integrated with fungi, bacteria, archaea, or algae have demonstrated remarkable efficiency in degrading diverse organic pollutants in contaminated soils [78,79,80]. Below, we highlight key studies showcasing the performance of these systems, with a focus on fungal contributions and supplementary examples from other microbial domains. Table 2 compares four treatment methods, namely electrokinetic remediation, non-electrode microbial treatment, microbial fuel cells (MFCs), and indigenous microorganism treatment under open-circuit conditions, in terms of their advantages and disadvantages, removal efficiencies, power generation performance (for some), treatment times, and references when degrading florpyrauxifen-benzyl and haloxyfop-P. In trials with Taxus rhizosphere soils, M. verrucaria-augmented MFCs achieved 97.9% haloxyfop-P removal within 60 days, outperforming standalone fungal or electrokinetic methods [19,22]. The bioelectric field (0.3 V/cm) enhanced fungal laccase and CYP activity, enabling the rapid dehalogenation of C-F bonds. The system generated a power density (PD) of 9.3 μW/cm2, demonstrating dual functionality in terms of remediation and energy recovery. In light of the strong PAH degradation activity of P. chrysosporium [35,81], MFCs augmented by white rot fungi might achieve a higher removal rate for carcinogenic PAHs. Fungal lignin peroxidase and manganese peroxidase generate free radicals to oxidize PAHs [82], while the anode facilitates electron transfer from enzymatic reactions. The Ea may decrease, accelerating mineralization. The significant azo dye decolorization by Trametes versicolor [83] is also inspiring, enabling the development of T. versicolor MFCs against textile-contaminated soils, so as to achieve optimal decolorization of dyes within a shorter period. Given that a microcurrent stimulated the activity of the microbial electron transfer chain [84], laccase activity may increase under a bioelectric field, driven by enhanced electron shuttling via fungal hyphae.

Table 2.
Comparison of treatment methods for herbicide removal.
Mechanistically, fungal MFC systems are dominated by enzymatic degradation (laccases, peroxidases), coupled with DET via hyphal networks (Table 3), bacterial systems rely more on extracellular electron transfer (EET) pathways (e.g., cytochromes, nanowires) for pollutant oxidation [57], while algal systems combine biosorption with cathodic oxygen reduction, enhancing pollutant immobilization and transformation [78]. Therefore, different MFC organisms are complementary and inter-supplementary. For example, the petroleum hydrocarbon degradation by the Geobacter MFC was salient [86]. Electrogenic bacteria coupled hydrocarbon oxidation to EET, reducing Ea, which could be further improved by the addition of fungi. In treating chlorpyrifos and dyes, both fungal MFCs and bacterial MFCs displayed high removal rates [87]. Esterase activity could be upregulated by the bioelectric field, hydrolyzing ester bonds to form non-toxic metabolites. A Chlorella vulgaris algal MFC showed its advantage in regard to removing carbon and nitrogen from swine wastewater [88]. Algal biomass acted as both a bioaccumulator and a biocathode catalyst [78]. The enhanced extracellular polysaccharide production of Scenedesmus MFCs [89] could facilitate pollutant adsorption and conversion.

Table 3.
Comparing putative mechanisms of fungal MFC, bacterial MFC, and algal MFC.
2.2.2. Power Generation
In fungal fuel cells, Trametes versicolor, Ganoderma lucidum, Galactomyces reessii, Aspergillus spp., Kluyveromyces marxianus, and Hansenula anomala were reported to generate electricity of 1200 mW/m3, 207 mW/m2, 1163 mW/m3, 438 mW/m3, 850,000 mW/m3, and 2900 mW/m3, respectively [24]. Fungal bioaugmentation increased the MFC PD to 9.3 μW/cm2, driven by enhanced electron flux from haloxyfop-P oxidation [19]. The bioelectric field (0.3 V/cm) could enhance fungal laccase activity, accelerating electron transfer from haloxyfop-P oxidation to the anode. This dual functionality highlights the synergy between pollutant degradation and energy recovery [19]. MFCs integrated with fungi, bacteria, or algae not only remediate organic pollutants, but also generate bioelectricity through microbial metabolic activities. The power output of these systems is closely linked to the efficiency of pollutant degradation, as electrons released during the enzymatic oxidation of contaminants are captured by the anode. The case studies of fungal MFCs are still scarce. It is expected that the addition of the PAH degradation fungi, P. chrysosporium, would increase the power generation of soil MFCs during PAH degradation. Fungal peroxidases oxidized PAHs into quinones [90], which acted as redox mediators to shuttle electrons to the anode. The addition of fungi could help the MFC system maintain a stable voltage for more days, correlating with better PAH removal. The addition of the dye decolorization fungus, T. versicolor [84], is expected to enhance the electrogenesis of MFCs during the treatment of azo dyes, driven by the laccase-mediated oxidation of phenolic intermediates [91]. The hyphal network facilitates DET, reducing the internal resistance of fungal MFCs, as compared to bacterial MFCs.
Mechanistic insights suggest that there are differences between the three MFC systems (Table 3). Fungal systems are dominated by enzymatic oxidation (e.g., laccases, peroxidases) and DET via conductive hyphae, minimizing energy losses; bacterial systems rely on EET pathways (e.g., cytochromes, nanowires) for long-range electron transport [57,92], whereas algal systems promote both anodic pollutant oxidation and cathodic oxygen reduction, leveraging photosynthetic activity [93]. Supplementarily, Geobacter utilized cytochromes for EET [94], coupling hydrocarbon oxidation to current production. In degrading azo dye and chlorpyrifos, the maximal MFC potential was 635 and 706 mV in bacterial and fungal systems, with a corresponding PD of 224.01 and 276.9 mW m−2, respectively [87]. The maximum electron transfer of 122 and 27.35 mA and current densities of 13.8 and 10.66 mA cm−2 in the cathodic compartment were reported, where the degradation of pollutants was accomplished. In regard to real wastewater, the maximum PD was 519.49 mW m−2. Esterase-hydrolyzed chlorpyrifos was found to release electrons [95], which could be transferred via flavin-mediated EET pathways [57]. Biosorption by algal MFCs parallels electrogenesis and pollutant removal [96]. Algal photosynthesis at the cathode enhances oxygen reduction [97], complementing anodic pollutant oxidation. It would be intriguing to study the performance of hybrid MFC systems integrating exogenous fungi, bacteria, and algae.
2.2.3. Soil Health Restoration
MFCs not only detoxify soils, but also rejuvenate their biological and physicochemical integrity [80], paving the way for sustainable agricultural practices. MFCs integrated with fungi, bacteria, or algae not only degrade organic pollutants, but also restore soil health by stabilizing its physicochemical properties, enhancing enzymatic activity, and revitalizing microbial communities [98]. Below, we discuss key studies demonstrating the role of MFCs in soil health restoration, with examples spanning multiple microbial domains. Fungi-augmented MFCs maintained the soil pH stability and increased the dehydrogenase activity [19], critical for medicinal plant growth. In M. verrucaria-augmented MFCs treating haloxyfop-P-contaminated soils, the system maintained the soil pH within a narrow range (7.0–7.9) over 60 days, compared to the non-MFC controls, wherein the pH fluctuated between 6.2 and 8.5. The fungal secretion of organic acids buffered soil acidity [99], while the bioelectric field minimized redox potential swings, preventing metal ion leaching [100]. Fungal MFCs could increase the soil dehydrogenase activity in contaminated soils, promoting the removal of organic pollutants by enhancing microbial metabolic activity [101], so as to maintain soil health. Fungal peroxidases could degrade pollutants into humic precursors, stimulating microbial carbon cycling [39,102].
Fungal MFC systems stabilize soil pH via organic acid secretion and enhance edaphic enzyme activity (e.g., dehydrogenase, urease) through pollutant mineralization (Table 3). Bacterial systems promote nutrient cycling via siderophores and extracellular polymeric substances (EPSs) [103,104]. Algal systems enrich soil organic carbon (SOC) and improve the soil structure through photosynthetic biomass deposition [105,106]. A bioelectric field accelerates the conversion of carbon and nitrogen in soil MFCs [107], which might be beneficial to soil health. MFC bacteria could drive nutrient cycling to restore the soil multifunctionality [108]. Geobacter MFCs could enhance carbon/nitrogen fixation [109,110]; diverse phosphate-solubilizing microbial taxa could be added into MFCs to enhance soil phosphorus cycling [111]. Electrogenic bacteria were found to secrete siderophores [104], mobilizing iron-bound phosphates [112]. On the other hand, algal MFCs could also be useful for organic matter enrichment [113]. Chlorella vulgaris MFCs increased the SOC in polluted soils [114]. Algal biomass decomposition produced labile carbon, fostering heterotrophic microbial growth [115], and algal activity increased the bacterial abundance and directly stimulated the fungal production rates in the short term. In contrast, the role of archaea in MFCs is less studied. However, recent studies suggest methanotrophic archaea (e.g., Methanobacterium) may play a unique role in MFCs. These archaea utilize the methane as an electron donor, redirecting electrons from methanogenesis to the anode via direct interspecies electron transfer (DIET) [116,117]. This process not only suppresses methane emissions in waterlogged soils, but also enhances current generation by bypassing traditional methanogenic pathways. The anode acted as an electron acceptor, diverting electrons from methanogenesis to current generation [117]. The diverse functional characteristics of the above taxa are conducive to the rehabilitation of contaminated soil.
3. Applications in Medicinal Plant Cultivation
Medicinal plants (e.g., T3axus, Panax ginseng) are highly sensitive to herbicide/pesticide residues [118,119], which disrupt secondary metabolite synthesis. MFCs integrated with fungi, bacteria, or algae can offer transformative potential for medicinal plant cultivation, by detoxifying contaminated soils and enhancing soil fertility [78,120,121]. These systems not only remove organic pollutants, but also improve soil health, directly benefiting the yield and quality of medicinal plants. Below, we discuss specific applications and their impact on medicinal plant production.
3.1. Fungal MFCs for Pollutant Degradation and Phytometabolite Enhancement
Fungal MFCs offer dual benefits. First, soil detoxification and the rapid degradation of herbicides/pesticides (e.g., chlorfluazuron) prevent their uptake by plants, ensuring compliance with safety standards for herbal products. Second, nutrient cycling could be facilitated by fungal MFCs [24]. Fungal MFCs could stimulate nitrogen fixation and phosphorus solubilization [122], improving soil fertility for medicinal crop cultivation. Medicinal plants, such as Panax, are highly sensitive to herbicide/pesticide residues [14,123], which inhibit the synthesis of bioactive compounds. However, there is a lack of field trials, and whether fungi-augmented MFCs could achieve high pollutant removal within a certain number of days in cultivated soils is difficult to predict. By degrading agrochemicals, fungal MFCs prevented toxin uptake by plants, possibly resulting in an increase in the medicinal compound content compared to untreated soils. The bioelectric field may stimulate fungal laccase activity [124] (e.g., via redox environment modulation), hypothesized to accelerate pollutant mineralization, while concurrently maintaining soil pH stability (7.0–7.9) through field-driven ion migration. This pH range is critical for ginseng root development [125]. Further mechanistic studies are required to validate the field–enzyme–pH interplay.
3.2. Synergistic Systems: Fungi–Bacteria–Algae Consortia
Taxus species, valued for their taxol production, require nitrogen-rich soils [126]. Geobacter MFCs could enhance nitrogen fixation in contaminated soils through EET pathways [110]. Electrogenic bacteria have been found to secrete siderophores [104], mobilizing iron-bound phosphates and improving phosphorus availability [111], which potentially increase the taxol yield by promoting root biomass and secondary metabolite biosynthesis. Integrating MFCs with conservation tillage practices [127] further reduces soil erosion, preserving microbial diversity, essential for nutrient cycling. Medicinal crops often face herbicide contamination in industrial regions, which has shown adverse effects on root and shoot growth [128]. Chlorella MFCs favor biosorption and subsequent transformation of pollutants, while generating bioelectricity [114]. Algal photosynthesis at the cathode enriches SOC [129], fostering heterotrophic microbial activity and improving the soil structure. This could be conducive to higher phytometabolite content, due to reduced oxidative stress in plants [130].
Given that in MFCs, bacteria, fungi, and algae often fight side by side [19,96], it is assumed that hybrid MFCs combining fungi, bacteria, and algae could demonstrate synergistic effects in medicinal plant cultivation. Fungi degraded PAHs via peroxidase radicals [131], bacteria hydrolyzed organophosphate and carbamate pesticides [132], while algae immobilized heavy metals [133], making hybrid MFCs particularly suitable for treating mixed contaminated soil. Such systems hopefully increase the contents of high-valued phytometabolites in various parts of medicinal plants, while restoring soil dehydrogenase activity, an index of soil health [101]. The integration of MFCs with conservation tillage [127] and optimized nitrogen management [22] would enhance their efficacy. For example, reduced tillage preserves fungal hyphal networks [82], while MFC-driven nutrient cycling complements precise nitrogen applications [134], minimizing fertilizer overuse. This approach aligns with sustainable agriculture goals, ensuring the growth of high-quality medicinal products compliant with international safety standards. MFCs bridge soil remediation and medicinal plant agronomy, offering a dual solution in regard to environmental sustainability and high-value crop production.
4. Challenges and Solutions
4.1. Existing Limitations
The xeno-fungusphere framework, while promising for agricultural remediation and medicinal plant conservation, faces several critical limitations that hinder its scalability and efficacy in field applications. These challenges stem from both intrinsic biological constraints and extrinsic environmental factors, requiring interdisciplinary solutions to advance the technology.
4.1.1. Field Heterogeneity and Electrochemical Inconsistencies
Soil heterogeneity poses a major barrier to uniform bioelectric field distribution in xeno-fungusphere MFCs. Variations in soil texture, moisture, and organic content disrupt electrical conductivity [135], leading to localized “dead zones”, where fungal hyphae and electroactive bacteria fail to establish functional networks. For instance, clay-rich soils exhibit higher ionic resistance compared to sandy soils [136,137], reducing electron flux between electrodes and limiting fungal enzymatic activity. This heterogeneity destabilizes the xeno-fungusphere’s synergistic microbial interactions, as fungi require spatially continuous hyphal networks to act as bioelectrochemical highways. Therefore, field trials might demonstrate that regions with low conductivity (<0.1 S/m) achieved undesirably low degradation, in contrast to the high level of removal of pollutants in homogeneous laboratory setups [19,138].
Beyond physical heterogeneity, thermodynamic incompatibilities further destabilize the fungal–electrochemical synergy. Aerobic fungi rely on oxygen as the terminal electron acceptor (O2/H2O, +0.82 V vs. SHE (standard hydrogen electrode)), whereas typical MFC anodic potentials range from −0.3 V to +0.1 V (e.g., acetate oxidation at −0.28 V vs. SHE), creating a redox potential mismatch that impedes direct electron transfer [38]. This thermodynamic disparity and the fluctuating redox conditions in heterogeneous soils alter fungal metabolism [139], suppressing laccase and CYP expression, particularly in low-conductivity zones, wherein the electron flux is already compromised by soil heterogeneity. To address these dual challenges, adaptive electrode designs must reconcile spatial variability with thermodynamic constraints. For example, carbonized fungal mycelia (e.g., Flammulina velutipes) act as conductive scaffolds, reducing the interfacial charge transfer resistance to 2.2 Ω and enabling electron tunneling across heterogeneous soils [62]. Similarly, the genetic engineering of S. cerevisiae to express pyranose dehydrogenase bypasses oxygen dependency via methylene blue mediators, aligning the fungal redox activity with anode potentials [65]. In single-chamber MFCs, limited oxygen diffusion creates microaerobic niches that favor fungal adaptability, which impacts fungi transfer electrons in two ways: (1) Electron transfer is realized directly, through redox-active fungal proteins or through chemical mediators facilitating the electron transport. (2) Fungi cells also generate electrons from decomposing organic matter [56]. For instance, Exophiala dermatitidis utilizes membrane-bound dehydrogenases for direct electron transfer under microaerobic anode conditions, achieving 70% dye removal and reaching a maximum output voltage of 248 mV, that are thermodynamically sufficient for current generation [59]. Similar mediator-free electron transfer has also been observed in Hansenula anomala, which employs outer-membrane oxidoreductases, such as ferricyanide reductase and lactate dehydrogenase, to communicate directly with the anode surface [58]. This mediator-free mechanism aligns with observations in aeration tank-adapted single-chamber MFCs, wherein microaerobic conditions (0.2 mg O2 L⁻1) sustain stable cell voltages of 200 mV [140], demonstrating that oxygen-tolerant fungi can maintain redox activity, even under fluctuating oxygen regimes.
4.1.2. Fungal Survival, Ecological Competition, and Metabolic Bottlenecks
The viability of exogenous fungi within the xeno-fungusphere is compromised under field conditions, due to competition with indigenous microbiota and abiotic stressors. While fungi like Myrothecium verrucaria exhibit robust enzymatic activity in controlled environments, their survival rates drop sharply after 15 days in non-sterile soils [19]. Native microbes often outcompete introduced fungi for nutrients [141], while predatory microfauna (e.g., nematodes) directly disrupt hyphal networks [142]. Additionally, abiotic factors, such as UV exposure and temperature extremes, degrade fungal extracellular enzymes [143,144], reducing their catalytic efficiency. To enhance ecological integration, future strategies could leverage native fungal strains or engineer chimeric consortia that mimic natural soil microbiomes, ensuring compatibility within the xeno-fungusphere.
The partial degradation of recalcitrant agrochemicals generates toxic intermediates [145,146] that accumulate in the xeno-fungusphere, inhibiting both microbial activity and plant growth. For instance, dehalogenation products of haloxyfop-P could reduce soil dehydrogenase activity and stunt root development [22]. These intermediates also destabilize the xeno-fungusphere by altering the pH and redox gradients, disrupting fungal–bacterial electron transfer. Hybrid systems integrating phytoremediation [147] could intercept these metabolites, as hyperaccumulator plants uptake and sequester toxins, while the xeno-fungusphere focuses on primary degradation. However, plant–fungal competition for nutrients must be carefully managed to avoid undermining the bioelectrochemical synergy.
4.1.3. Scalability, Economic Barriers, and Knowledge Gaps
Scaling xeno-fungusphere MFCs from the lab to the field remains economically and technically challenging. High-performance electrodes (e.g., graphene-coated anodes; [148]) improve electron transfer, but are cost prohibitive for large-scale deployment. A 1-hectare fungal MFC system using graphene would incur material costs exceeding USD 12,000, rendering it impractical for smallholder farms [149]. Additionally, maintaining consistent fungal inoculum production at scale requires sterile bioreactors [150], further increasing operational costs. Low-cost alternatives, such as biochar electrodes derived from agricultural waste [151], show promise, but exhibit higher internal resistance (~250 Ω vs. 80 Ω for graphene), reducing the PD substantially [152]. Economic viability also depends on balancing the energy output with the remediation efficiency; current systems generate < 10 μW/cm2, insufficient to offset the installation costs.
Moreover, microbial synergy remains a riddle. The xeno-fungusphere’s success hinges on poorly understood cross-kingdom interactions. While electroactive bacteria (e.g., Geobacter) and fungi could collaborate in lab settings [57,153], their dynamics in complex soils are unpredictable. For example, fungal hyphae may inadvertently shield bacteria from the bioelectric field, reducing EET efficiency [23]. Conversely, bacterial siderophores could inhibit fungal growth by sequestering iron [104]. Omics-driven studies are needed to map the metabolic dependencies and antagonisms within the xeno-fungusphere, enabling the design of stable, self-regulating consortia.
4.1.4. Regulatory and Farmer Adoption Challenges
The regulatory frameworks for bioelectrochemical remediation are underdeveloped, particularly for medicinal plant systems. Residual fungal spores or engineered strains in regard to herbal products [154,155] may face stringent biosecurity restrictions, delaying commercialization. Additionally, farmers lack technical expertise to operate MFCs, perceiving them as overly complex compared to conventional methods. Demonstrating tangible benefits, such as increased ginsenoside yields in Panax crops, through pilot projects will be critical for adoption.
Therefore, the xeno-fungusphere concept must evolve to address field heterogeneity, microbial survivability, intermediate toxicity, and economic constraints. Interdisciplinary innovations in material science, synthetic ecology, and policy design will be essential to unlock its full potential for sustainable agriculture.
4.2. Strategies for Improvement
The xeno-fungusphere framework, while facing significant challenges, offers ample opportunities for optimization through interdisciplinary innovation. We propose targeted strategies to enhance its functionality, scalability, and integration into agricultural systems, with a focus on leveraging fungal–electrochemical synergies and addressing existing limitations.
4.2.1. Strain Engineering for Enhanced Fungal Resilience and Catalytic Efficiency
The success of xeno-fungusphere MFCs hinges on the adaptability and enzymatic prowess of exogenous fungi. Advances in genetic and metabolic engineering can be harnessed to develop stress-tolerant fungal hybrids, tailored for field conditions [156]. For instance, CRISPR–Cas9 editing [157] could optimize Myrothecium verrucaria to overexpress laccases and CYPs under bioelectric stimulation, enhancing the degradation of halogenated herbicides like haloxyfop-P [19]. Codon optimization and molecular chaperone-assisted expression in Aspergillus strains [50] could further stabilize enzyme production when subject to fluctuating soil pH and temperature. Additionally, ARTP mutagenesis may generate fungal mutants with superior hyphal conductivity [158], enabling efficient electron transfer across heterogeneous soil matrices.
To mitigate ecological competition, synthetic ecology approaches could engineer chimeric consortia that mimic natural soil microbiomes [159]. For example, introducing native fungal strains (e.g., Talaromyces), alongside electroactive bacteria (Geobacter), would foster cross-kingdom symbiosis within the xeno-fungusphere. Such consortia could leverage fungal hyphae as “bioelectrochemical highways,” while the bacteria utilize redox mediators (e.g., phenazines) for EET, creating self-sustaining pollutant degradation networks.
4.2.2. Hybrid Systems Integrating Phytoremediation and MFCs
To address intermediate toxicity and nutrient competition, xeno-fungusphere MFCs can be synergized with phytoremediation. Plants with remediation capacity [147,160] can be strategically planted to intercept toxic metabolites (e.g., fluorochloropyridinyl acid) generated during partial herbicide degradation. These plants sequester pollutants in their biomass, preventing phytotoxicity, while the xeno-fungusphere focuses on primary degradation. The bioelectric field in MFCs may further enhance plant–microbe interactions by stimulating root exudate secretion, which recruits beneficial bacteria to support fungal activity [161].
In medicinal plant cultivation, hybrid systems could combine Panax with fungal MFCs to simultaneously detoxify soils and boost ginsenoside synthesis. Fungal-secreted organic acids [99] would buffer the soil pH, while electrochemically enhanced laccase activity accelerates herbicide mineralization, reducing oxidative stress in plants [162]. The real-time monitoring of the soil redox potential and enzyme activity via Internet of Things (IoTs) sensors [163] could dynamically adjust MFC voltage gradients, ensuring optimal synergy between phytoremediation and bioelectrochemical processes.
4.2.3. Low-Cost, Conductive Electrodes from Agricultural Waste
The scalability of xeno-fungusphere MFCs is constrained by the high cost of advanced electrodes (e.g., graphene). To address this, biochar derived from crop residues (e.g., rice husks, corn stover) offers a sustainable alternative [152]. Pyrolyzed at 500–700 °C, biochar exhibits moderate conductivity (~100 S/m) and a high surface area, facilitating electron shuttling between fungal hyphae and the anode [51]. Functionalizing biochar with iron oxides (e.g., magnetite) could further reduce Rct, mimicking the performance of graphene at a fraction of the cost [151].
Modular electrode designs, such as 3D-printed carbon scaffolds [164] embedded with fungal spores, could adapt to soil heterogeneity. These scaffolds would provide structural support for hyphal networks in clay-rich soils, while ensuring continuous electron flow. Field trials in Taxus farms could be used to validate the efficacy of such electrodes, correlating biochar porosity with the degradation kinetics of herbicides/pesticides.
4.2.4. Long-Term Field Pilots, Farmer-Centric Optimization, and Circular Economy Integration
Transitioning xeno-fungusphere MFCs from the lab to the field requires robust, large-scale trials in diverse agricultural settings. Collaborations with medicinal plant farms in regions like Jilin (China) or Kerala (India) could test the system’s durability under real-world conditions. Key parameters to optimize include: (1) soil moisture, namely maintaining 60–80% water-holding capacity to sustain fungal enzyme activity without flooding the anode; (2) organic amendments, namely adding compost to boost electroactive microbial populations, while avoiding competition with fungi; and (3) crop rotation, namely aligning MFC operation with planting cycles to minimize the disturbance to hyphal networks.
Farmer training programs and simplified MFC kits (e.g., “plug-and-play” modules) would enhance adoption. Demonstrating tangible benefits, such as 20–30% increases in Panax yields or reduced costs, could incentivize smallholders to adopt the technology [127]. In regard to policy support, governments and agricultural agencies must play a pivotal role in promoting xeno-fungusphere MFCs. Subsidies for biochar electrode production or tax incentives for organic medicinal plant growers could lower the barriers to entry. Regulatory frameworks should streamline approvals for fungal inoculants [165], ensuring compliance with biosafety standards for herbal products. Circular economy models could link MFCs to biogas digesters or solar-powered irrigation systems. For example, electricity generated from haloxyfop-P degradation [19] could power soil sensors or LED grow lights, creating self-sustaining agroecological systems. Algal MFC hybrids might further close nutrient loops by converting CO2 from biogas into biomass for organic fertilizers [71].
By integrating these strategies, the xeno-fungusphere framework can evolve into a cornerstone of sustainable agriculture, balancing ecological remediation, energy recovery, and high-value crop production. Interdisciplinary collaboration, spanning synthetic ecology, materials science, and agronomy, will be essential to realize its full potential.
5. Conclusions and Future Perspectives
The xeno-fungusphere framework integrates fungal bioaugmentation with bioelectrochemical systems to simultaneously remediate agrochemical-contaminated soils and recover energy. By leveraging the enzymatic versatility of fungi and their ability to form “bioelectrochemical highways” within MFCs, this framework achieves efficient degradation of recalcitrant agrochemicals, while maintaining soil health for medicinal plant cultivation. Key advancements include the electrochemical enhancement of fungal enzymatic activity (e.g., laccases, CYPs) and the creation of synergistic microbial consortia that bridge fungal, bacterial, and algal functionalities. However, scaling this technology requires interdisciplinary innovation, aligned with global sustainability goals.
Future xeno-fungusphere systems could incorporate IoT sensors to enable real-time monitoring of soil parameters, such as pH, redox potential, and pollutant concentrations. These sensors would dynamically adjust the bioelectric field intensity (0.2–0.5 V/cm) to optimize fungal enzymatic activity and electron transfer efficiency. For instance, machine learning algorithms could predict herbicide degradation kinetics [166] based on soil conductivity and microbial community dynamics, enabling the adaptive control of MFC operational parameters. Such smart systems would not only enhance the remediation efficiency, but also reduce energy waste, aligning with precision agriculture principles [167]. On the other hand, the integration of xeno-fungusphere MFCs into circular agricultural systems holds immense potential. Electricity generated from pollutant degradation could power low-energy devices like soil moisture sensors or LED grow lights in medicinal plant greenhouses. Additionally, coupling MFCs with biogas digesters could convert organic waste into renewable energy, while algal MFC hybrids might repurpose CO2 emissions into biomass for organic fertilizers. This closed-loop approach minimizes external inputs and transforms agrochemical-contaminated soils into hubs for resource recovery.
The widespread adoption of xeno-fungusphere technology requires robust policy frameworks and financial incentives. Governments could subsidize biochar electrode production from agricultural waste, reducing the reliance on costly materials like graphene. Certification programs for “MFC-remediated” medicinal plants, ensuring compliance with international safety standards, would incentivize farmers to adopt this technology. Pilot projects in regions like genuine traditional Chinese medicine producing areas in China, where medicinal crops face severe agrochemical contamination [168], could demonstrate yield/quality improvements and catalyze commercial interest. Despite progress, critical knowledge gaps persist. Long-term field trials are needed to assess the ecological stability of xeno-fungusphere consortia under varying climatic conditions. Omics-driven studies should unravel the metabolic dependencies between fungi and electroactive bacteria, guiding the design of self-regulating microbial networks. Furthermore, genetic engineering tools like CRISPR could optimize fungal stress tolerance and enzymatic output, ensuring resilience in heterogeneous soils. Finally, interdisciplinary collaboration, spanning synthetic ecology, materials science, and agronomy, will be essential to scale this technology in order to have a global impact.
In conclusion, the xeno-fungusphere paradigm bridges environmental remediation and agroecological innovation. By addressing technical, economic, and regulatory barriers, it promises to safeguard medicinal plant quality, reduce the reliance on synthetic agrochemicals, and contribute to the green energy transition, ultimately fostering a sustainable future for agriculture.
Author Contributions
Conceptualization, D.-C.H. and P.X.; methodology and validation, D.-C.H., X.L. and Y.W.; resources, D.-C.H. and P.X.; visualization, Y.W. and J.L.; data curation, D.-C.H. and C.L.; writing—original draft preparation, review and editing, D.-C.H. and X.L.; supervision, D.-C.H. and P.X.; project administration, D.-C.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (41977048), the Scientific Research Funds Project of Liaoning Education Department (JDL2019012), and the China Scholarship Council (202108210156).
Data Availability Statement
The data is available upon request.
Acknowledgments
The authors thank the editor and anonymous review experts for providing helpful suggestions to improve the quality of this manuscript.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript: MFCs—microbial fuel cells; CYPs—cytochrome P450s; PAHs—polycyclic aromatic hydrocarbons; AOPP—aryloxyphenoxypropionate; Ea—activation energy; ARTP—atmospheric and room-temperature plasma; TCA—tricarboxylic acid; DET—direct electron transfer; EIS—electrochemical impedance spectroscopy; Rct—charge transfer resistance; PD—power density; EET—extracellular electron transfer; EPS—extracellular polymeric substances; SOC—soil organic carbon; IoTs—Internet of Things.
References
- Hao, D.C.; Li, X.J.; Xiao, P.G.; Wang, L.F. The utility of electrochemical systems in microbial degradation of polycyclic aromatic hydrocarbons: Discourse, diversity and design. Front. Microbiol. 2020, 11, 557400. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Yang, D.; Li, S.; Yu, K.; Yan, W.; Xu, H. Research progress on coupling and stacking systems to enhance power generation performance of microbial fuel cell. J. Environ. Sci. 2025, 154, 784–804. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Jin, H.; Tian, W.; Niu, Z.; Zhang, J.; Wang, T.; Zhou, M. The enhanced effect and mechanism of endogenous sigma factor RpoF on bioelectricity generation in Pseudomonas aeruginosa–inoculated Microbial fuel cells (MFCs). Biosens. Bioelectron. 2025, 278, 117380. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Yu, J.; Nguyen, V.K.; Park, S.; Kim, J.; Lee, T. Understanding complete ammonium removal mechanism in single–chamber microbial fuel cells based on microbial ecology. Sci. Total Environ. 2021, 764, 144231. [Google Scholar] [CrossRef]
- Case, N.T.; Gurr, S.J.; Fisher, M.C.; Blehert, D.S.; Boone, C.; Casadevall, A.; Chowdhary, A.; Cuomo, C.A.; Currie, C.R.; Denning, D.W.; et al. Fungal impacts on Earth’s ecosystems. Nature 2025, 638, 49–57. [Google Scholar] [CrossRef]
- Rezaei, Z.; Moghimi, H. Fungal–bacterial consortia: A promising strategy for the removal of petroleum hydrocarbons. Ecotoxicol. Environ. Saf. 2024, 280, 116543. [Google Scholar] [CrossRef]
- Hao, D.C.; Song, S.M.; Cheng, Y.; Qin, Z.Q.; Ge, G.B.; An, B.L.; Xiao, P.G. Functional and transcriptomic characterization of a dye–decolorizing fungus from Taxus rhizosphere. Pol. J. Microbiol. 2018, 67, 417–430. [Google Scholar] [CrossRef]
- Harms, H.; Schlosser, D.; Wick, L.Y. Untapped potential: Exploiting fungi in bioremediation of hazardous chemicals. Nat. Rev. Microbiol. 2011, 9, 177–192. [Google Scholar] [CrossRef]
- Dinakarkumar, Y.; Ramakrishnan, G.; Gujjula, K.R.; Vasu, V.; Balamurugan, P.; Murali, G. Fungal bioremediation: An overview of the mechanisms, applications and future perspectives. Environ. Chem. Ecotoxicol. 2024, 6, 293–302. [Google Scholar] [CrossRef]
- Schröder, U.; Harnisch, F.; Angenent, L.T. Microbial electrochemistry and technology: Terminology and classification. Energy. Environ. Sci. 2015, 8, 513–519. [Google Scholar] [CrossRef]
- Devi, O.Z.; Mishra, S.; Alam, S.; Thakur, L.K. Single–quad gas chromatography estimation of a few selected pesticides as residues in spices of the National Capital Region of Delhi. Environ. Monit. Assess. 2025, 197, 432. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.Z.; Liu, S.Q.; Han, B.X.; Zhou, T.; Wang, X.; Liu, D.H.; Yang, Y.; Guo, L.P. Development goals and strategies of ecological agriculture of Chinese materia medica. Zhongguo Zhong Yao Za Zhi 2025, 50, 42–47. [Google Scholar] [PubMed]
- Jiang, X.; Wang, H.; Huang, Y.; Jin, H.; Ding, J.; Ma, L.; Zheng, L. A novel microbial agent reduces soil paclobutrazol residue, enhances enzyme activities and increases Ophiopogon japonicus production. PeerJ 2025, 13, e19008. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Xiong, Y.; Ma, T.; Wang, Q.; Song, M.; Zhao, Q.; Zhang, N.; Guo, J.; Wang, Y.; Hou, Z.; et al. Dissipation and risk assessment of propaquizafop in ginseng under field conditions. J. Agric. Food Chem. 2024, 72, 6613–6624. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Bellisai, G.; Bernasconi, G.; Brancato, A.; Cabrera, L.C.; Castellan, I.; Del Aguila, M.; Ferreira, L.; Giner, G.; Greco, L.; et al. Targeted review of maximum residues levels (MRLs) for haloxyfop–P. EFSA J. 2022, 20, e07658. [Google Scholar]
- Gałczyńska, M.; Gamrat, R.; Puc, M. Honey varieties vs metal and pesticide content–literature review and own research. Ann. Agric. Environ. Med. 2025, 32, 9–19. [Google Scholar] [CrossRef]
- García, G.; Carlin, M.; Cano, R.J. Holobiome harmony: Linking environmental sustainability, agriculture, and human health for a thriving planet and one health. Microorganisms 2025, 13, 514. [Google Scholar] [CrossRef]
- Hao, D.C.; Wang, F.; Li, C.; Wang, Y.; Xue, J.; Xiao, P.G. Fungal bioaugmentation enhanced herbicide removal via soil microbial fuel cell: Taking Myrothecium verrucaria and haloxyfop–P as an example. Sci. Total Environ. 2025, 958, 178012. [Google Scholar] [CrossRef]
- Gomathi, S.; Ambikapathy, V.; Panneerselvam, A. Chapter 5—Biodegradation and bioaugmentation of pesticides using potential fungal species. In Plant–Microbe Interaction–Recent Advances in Molecular and Biochemical Approaches 2: Agricultural Aspects of Microbiome Leading to Plant Defence; Academic Press: Cambridge, MA, USA, 2023; pp. 79–94. [Google Scholar]
- Swathy, K.; Vivekanandhan, P.; Yuvaraj, A.; Sarayut, P.; Kim, J.S.; Krutmuang, P. Biodegradation of pesticide in agricultural soil employing entomopathogenic fungi: Current state of the art and future perspectives. Heliyon 2023, 10, e23406. [Google Scholar] [CrossRef]
- Hao, D.C.; Luan, Y.Y.; Wang, Y.X.; Xiao, P.G. Unveiling Nitrogen Fertilizer in Medicinal Plant Cultivation. Agronomy 2024, 14, 1647. [Google Scholar] [CrossRef]
- Thapa, B.S.; Kim, T.; Pandit, S.; Song, Y.E.; Afsharian, Y.P.; Rahimnejad, M.; Kim, J.R.; Oh, S.E. Overview of electroactive microorganisms and electron transfer mechanisms in microbial electrochemistry. Bioresour. Technol. 2022, 347, 126579. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Mubeen, M.; Ali, I.; Iftikhar, Y.; Sohail, M.A.; Sajid, A.; Kumar, A.; Solanki, M.K.; Kumar Divvela, P.; Zhou, L. Harnessing fungal bio–electricity: A promising path to a cleaner environment. Front. Microbiol. 2024, 14, 1291904. [Google Scholar] [CrossRef] [PubMed]
- Shraddha; Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial sources, production, purification, and potential biotechnological applications. Enzyme Res. 2011, 2011, 217861. [Google Scholar] [CrossRef]
- Ayala Schimpf, A.R.; Ortellado, L.E.; Gamarra, M.D.; Fonseca, M.I.; Zapata, P.D. In vitro and computational response of differential catalysis by Phlebia brevispora BAFC 633 laccase in interaction with 2,4–D and chlorpyrifos. Int. J. Mol. Sci. 2024, 25, 12527. [Google Scholar] [CrossRef]
- Chan-Cupul, W.; Heredia-Abarca, G.; Rodríguez-Vázquez, R. Atrazine degradation by fungal co–culture enzyme extracts under different soil conditions. J. Environ. Sci. Health B 2016, 51, 298–308. [Google Scholar] [CrossRef]
- Coleman, T.; Podgorski, M.N.; Doyle, M.L.; Scaffidi–Muta, J.M.; Campbell, E.C.; Bruning, J.B.; De Voss, J.J.; Bell, S.G. Cytochrome P450–catalyzed oxidation of halogen–containing substrates. J. Inorg. Biochem. 2023, 244, 112234. [Google Scholar] [CrossRef]
- Guo, J.; Kong, L.; Tian, L.; Han, Y.; Teng, C.; Ma, H.; Tao, B. Molecular docking and mutation sites of CYP57A1 enzyme with Fomesafen. Pestic. Biochem. Physiol. 2025, 209, 106328. [Google Scholar] [CrossRef]
- Sánchez, C. Fusarium as a promising fungal genus with potential application in bioremediation for pollutants mitigation: A review. Biotechnol. Adv. 2024, 77, 108476. [Google Scholar] [CrossRef]
- Song, H.; Chen, W.J.; Chen, S.F.; Zhu, X.; Mishra, S.; Ghorab, M.A.; Bhatt, P.; Chen, S. Removal of chlorimuron–ethyl from the environment: The significance of microbial degradation and its molecular mechanism. Chemosphere 2024, 366, 143456. [Google Scholar] [CrossRef]
- Bempelou, E.D.; Vontas, J.G.; Liapis, K.S.; Ziogas, V.N. Biodegradation of chlorpyrifos and 3,5,6–trichloro–2–pyridinol by the epiphytic yeasts Rhodotorula glutinis and Rhodotorula rubra. Ecotoxicology 2018, 27, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, Z.; Hussain, S.; Imran, M.; Mahmood, F.; Shahzad, T.; Ahmed, Z.; Azeem, F.; Muzammil, S. Perspectives of using fungi as bioresource for bioremediation of pesticides in the environment: A critical review. Environ. Sci. Pollut. Res. Int. 2016, 23, 16904–16925. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Hao, D.C.; Hu, W.L.; Song, S.; Li, S.Y.; Ge, G.B. Transcriptome profile of polycyclic aromatic hydrocarbon–degrading fungi isolated from Taxus rhizosphere. Curr. Sci. 2019, 116, 1218–1228. [Google Scholar] [CrossRef]
- Wang, C.; Sun, H.; Li, J.; Li, Y.; Zhang, Q. Enzyme activities during degradation of polycyclic aromatic hydrocarbons by white rot fungus Phanerochaete chrysosporium in soils. Chemosphere 2009, 77, 733–738. [Google Scholar] [CrossRef]
- Kwatra, N.; Abraham, J. Mycoremediation of pretilachlor and its metabolite by Aspergillus ficuum. J. Environ. Sci. Health B 2023, 58, 489–499. [Google Scholar] [CrossRef]
- Xu, T. Study on the Electrical Characteristics of Glucose Oxidase and Its Mixed Solution in Electromagnetic Environment. Master’s Thesis, Henan Normal University, Zhengzhou, China, 2021. [Google Scholar]
- Du, B.; Gu, M.Q.; Hu, Z.H.; Zhan, X.M.; Wu, G.X. Thermodynamic analysis of extracellular electron transfer during ethanol oxidation in anaerobic digestion systems. Biomass Bioenergy 2023, 172, 106755. [Google Scholar] [CrossRef]
- He, Y.Y.; Lin, R.; Yu, X.; Ma, Y.; Li, J.; Xie, L. Simultaneous enhancement on lignocellulose degradation and humic acid formation using the electric field coupled with an iron anode in the co–composting of food waste and agricultural waste. Chem. Eng. J. 2023, 475, 145846. [Google Scholar] [CrossRef]
- McGillivray, A.M.; Gow, N.A. Applied electrical fields polarize the growth of mycelial fungi. J. Gen. Microbiol. 1986, 132, 2515–2525. [Google Scholar] [CrossRef][Green Version]
- Jadhav, D.A.; Park, S.G.; Pandit, S.; Yang, E.; Ali Abdelkareem, M.; Jang, J.K.; Chae, K.J. Scalability of microbial electrochemical technologies: Applications and challenges. Bioresour. Technol. 2022, 345, 126498. [Google Scholar] [CrossRef]
- Daccò, C.; Girometta, C.; Asemoloye, M.D.; Carpani, G.; Picco, A.M.; Tosi, S. Key fungal degradation patterns, enzymes and their applications for the removal of aliphatic hydrocarbons in polluted soils: A review. Int. Biodeterior. Biodegrad. 2020, 147, 104866. [Google Scholar] [CrossRef]
- Banerjee, S.; Gupta, N.; Pramanik, K.; Gope, M.; GhoshThakur, R.; Karmakar, A.; Gogoi, N.; Hoque, R.R.; Mandal, N.C.; Balachandran, S. Microbes and microbial strategies in carcinogenic polycyclic aromatic hydrocarbons remediation: A systematic review. Environ. Sci. Pollut. Res. Int. 2024, 31, 1811–1840. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Li, Y.; Zhao, X.; Zhang, X.; Zhao, Q.; Wang, X.; Li, Y.T. Restructured fungal community diversity and biological interactions promote metolachlor biodegradation in soil microbial fuel cells. Chemosphere 2019, 221, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Sarma, H.; Bhattacharyya, P.N.; Jadhav, D.A.; Pawar, P.; Thakare, M.; Pandit, S.; Mathuriya, A.S.; Prasad, R. Fungal–mediated electrochemical system: Prospects, applications and challenges. Curr. Res. Microb. Sci. 2021, 2, 100041. [Google Scholar] [CrossRef] [PubMed]
- Puissant, J.; Jones, B.; Goodall, T.; Mang, D.; Blaud, A.; Gweon, H.S.; Malik, A.; Jones, D.L.; Clark, I.M.; Hirsch, P.R.; et al. The pH optimum of soil exoenzymes adapt to long term changes in soil pH. Soil Biol. Biochem. 2019, 138, 107601. [Google Scholar] [CrossRef]
- Zheng, Q.; Hu, Y.; Zhang, S.; Noll, L.; Böckle, T.; Dietrich, M.; Herbold, C.W.; Eichorst, S.A.; Woebken, D.; Richter, A.; et al. Soil multifunctionality is affected by the soil environment and by microbial community composition and diversity. Soil. Biol. Biochem. 2019, 136, 107521. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, S. Long–term phosphorus addition alters soil enzyme kinetics with limited impact on their temperature sensitivity in an alpine meadow. Sci. Total Environ. 2024, 957, 177569. [Google Scholar] [CrossRef]
- Patwardhan, S.B.; Deolikar, R.; Nag, M.; Lahiri, D.; Jadhav, D.A.; Ray, R.R.; Pandit, S. Chapter 10—Influence of operational parameters on the performance of microbial fuel cells. In Development in Wastewater Treatment Research and Processes: Bioelectrochemical Systems for Wastewater Management; Elsevier: Amsterdam, The Netherlands, 2023; pp. 153–189. [Google Scholar]
- Huang, J.; Wang, J.; Chen, L.; He, J.; Wu, Y.; Cui, X.; Mei, M.; Li, Y. Improved expression of Cerrena unicolor Laccase in Aspergillus niger via combined strategies and its applications. Biochem. Eng. J. 2024, 209, 109371. [Google Scholar] [CrossRef]
- Zhang, W.T.; Xu, D.; Zhao, Y.; Gao, D.; Xie, Z.; Zhang, X.; Wu, B.; Huang, T.; Peng, L.L. Enhancing electricity generation and pollutant degradation in microbial fuel cells using cyanobacteria–derived biochar electrodes. Bioresour. Technol. 2025, 418, 132000. [Google Scholar] [CrossRef]
- Mohammadi, F.; Vakili-Nezhaad, G.R.; Al-Rawahi, N.; Gholipour, S. Microbial electrochemical systems for bioelectricity generation: Current state and future directions. Result Eng. 2023, 20, 101619. [Google Scholar] [CrossRef]
- Chang, L.; Cui, H.; Liu, W.; Zhang, Y.P.; Zhang, L. Electrodriven ATP synthesis via a reconstructed thylakoid membrane. Angew. Chem. Int. Ed. Engl. 2025, 64, e202421120. [Google Scholar] [CrossRef]
- Sáez–Jiménez, V.; Baratto, M.C.; Pogni, R.; Rencoret, J.; Gutiérrez, A.; Santos, J.I.; Martínez, A.T.; Ruiz–Dueñas, F.J. Demonstration of lignin–to–peroxidase direct electron transfer: A transient–state kinetics, directed mutagenesis, EPR, and NMR study. J. Biol. Chem. 2015, 290, 23201–23213. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Ibrahim, M.N.; Guerrero-Barajas, C. Modern trend of anodes in microbial fuel cells (MFCs): An overview. Environ. Technol. Innovat. 2021, 23, 101579. [Google Scholar] [CrossRef]
- Sekrecka-Belniak, A.; Toczyłowska-Mamińska, R. Fungi-based microbial fuel cells. Energies 2018, 11, 2827. [Google Scholar] [CrossRef]
- Zhao, J.T.; Li, F.; Cao, Y.; Zhang, X.; Chen, T.; Song, H.; Wang, Z. Microbial extracellular electron transfer and strategies for engineering electroactive microorganisms. Biotechnol. Adv. 2021, 53, 107682. [Google Scholar] [CrossRef]
- Mahadevan, A.; Gunawardena, D.A.; Fernando, S. Biochemical and Electrochemical Perspectives of the Anode of a Microbial Fuel Cell. In Technology and Application of Microbial Fuel Cells; InTech: London, UK, 2014. [Google Scholar]
- Cuesta-Zedeño, L.F.; Batista-García, R.A.; Gunde-Cimerman, N.; Amabilis-Sosa, L.E.; Ramirez-Pereda, B. Utilizing black yeast for sustainable solutions: Pioneering clean energy production and wastewater treatment with Exophiala dermatitidis. Process Biochem. 2024, 147, 630–643. [Google Scholar] [CrossRef]
- Christwardana, M.; Kwon, Y. Yeast and carbon nanotube based biocatalyst developed by synergetic effects of covalent bonding and hydrophobic interaction for performance enhancement of membraneless microbial fuel cell. Bioresour. Technol. 2017, 225, 175–182. [Google Scholar] [CrossRef]
- Gal, I.; Schlesinger, O.; Amir, L.; Alfonta, L. Yeast surface display of dehydrogenases in microbial fuel-cells. Bioelectrochemistry 2016, 112, 53–60. [Google Scholar] [CrossRef]
- Tian, Y.; Li, C.; Liang, D.D.; Xie, T.; He, W.; Li, D.; Feng, Y. Fungus-sourced filament-array anode facilitates Geobacter en-richment and promotes anodic bio-capacitance improvement for efficient power generation in microbial fuel cells. Sci. Total Environ. 2022, 838, 155926. [Google Scholar] [CrossRef]
- Lin, T.; Bai, X.; Hu, Y.; Li, B. Synthetic Saccharomyces cerevisiae-Shewanella oneidensis Consortium Enables Glucose-Fed High-Performance Microbial Fuel Cell. AIChE J. 2017, 63, 1830–1838. [Google Scholar] [CrossRef]
- Islam, M.A.; Ethiraj, B.; Cheng, C.K.; Prasad, R.; Khan, M.M. Enhanced current generation using mutualistic interaction of yeast-bacterial coculture in dual chamber microbial fuel cell. Ind. Eng. Chem. Res. 2018, 57, 813–821. [Google Scholar] [CrossRef]
- Fernández de Dios, M.Á.; González del Campo, A.; Fernández, F.J.; Rodrigo, M.; Pazos, M.; Sanromán, M.Á. Bacterial–fungal interactions enhance power generation in microbial fuel cells and drive dye decolourisation by an ex situ and in situ elec-tro-Fenton process. Bioresour. Technol. 2013, 148, 39–46. [Google Scholar] [CrossRef]
- Chaijak, P.; Sukkasem, C.; Lertworapreecha, M.; Boonsawang, P.; Wijasika, S.; Sato, C. Enhancing electricity generation using laccase-based microbial fuel cell with yeast Galactomyces reessii on cathode. J. Microbiol. Biotechnol. 2018, 28, 999–1005. [Google Scholar] [CrossRef]
- Lai, C.-Y.; Wu, C.-H.; Meng, C.-T.; Lin, C.-W. Decolorization of azo dye and generation of electricity by microbial fuel cell with laccase-producing white-rot fungus on cathode. Appl. Energy 2017, 188, 392–398. [Google Scholar] [CrossRef]
- Yazdi, A.A.; D’Angelo, L.; Omer, N.; Windiasti, G.; Lu, X.; Xu, J. Carbon nanotube modification of microbial fuel cell electrodes. Biosens. Bioelectron. 2016, 85, 536–552. [Google Scholar] [CrossRef]
- Prado, A.; Berenguer, R.; Esteve-Núñez, A. Electroactive biochar outperforms highly conductive carbon materials for biodegrading pollutants by enhancing microbial extracellular electron transfer. Carbon 2019, 146, 597–609. [Google Scholar] [CrossRef]
- Mkilima, T.; Zharkenov, Y.; Abduova, A.; Kudaibergenov, N.; Fazylov, K.; Toleubayeva, S.; Kirgizbayeva, K.; Zhumadilov, I.; Jaxymbetova, M.; Zhapparova, A. Bioelectrochemical degradation of pollutants in wastewater using a dual–chamber microbial fuel cell with graphene–modified electrodes and electroactive bacteria. Case Studi. Chem. Environ. Eng. 2025, 11, 101184. [Google Scholar] [CrossRef]
- Xu, X.; Wei, W.; Zhou, Y.; Liu, J.; Gao, C.; Hu, G.; Li, X.; Wen, J.; Liu, L.; Wu, J.; et al. Customizing biocatalysts by reducing ΔG‡: Integrating ground–state destabilization and transition–state stabilization. Chem Catalysis 2025, 5, 101323. [Google Scholar] [CrossRef]
- Ha, P.T.; Moon, H.; Kim, B.H.; Ng, H.Y.; Chang, I.S. Determination of charge transfer resistance and capacitance of microbial fuel cell through a transient response analysis of cell voltage. Biosens. Bioelectron. 2010, 25, 1629–1634. [Google Scholar] [CrossRef]
- Hao, D.C.; Zhang, C.R.; Xiao, P.G. The first Taxus rhizosphere microbiome revealed by shotgun metagenomic sequencing. J. Basic Microbiol. 2018, 58, 501–512. [Google Scholar] [CrossRef]
- Aftab, Q.; Wang, X.; Lu, J.; Tariq, M.; Liu, Y. Advancing soil microbial fuel cells: Exploring bioelectrogenesis mechanisms for integration into environmental bioremediation. Renew. Sustain. Energy Rev. 2025, 214, 115495. [Google Scholar] [CrossRef]
- Yang, G.Q.; Xia, X.; Nie, W.; Qin, B.; Hou, T.; Lin, A.; Yao, S.; Zhuang, L. Bidirectional extracellular electron transfer pathways of Geobacter sulfurreducens biofilms: Molecular insights into extracellular polymeric substances. Environ. Res. 2024, 245, 118038. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.D.; Li, X.J.; Li, Y.; Sun, Y.; Zhang, X.; Weng, L.; Ren, T.; Li, Y.T. Shifting interactions among bacteria, fungi and archaea enhance removal of antibiotics and antibiotic resistance genes in the soil bioelectrochemical remediation. Biotechnol. Biofuels 2019, 12, 160. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, L.; Li, X.J.; Xu, H.; Weng, L.; Yang, L.; Li, Y.T. Response of soil bacterial and fungal community structure succession to earthworm addition for bioremediation of metolachlor. Ecotoxicol. Environ. Saf. 2020, 189, 109926. [Google Scholar] [CrossRef] [PubMed]
- Elshobary, M.E.; Zabed, H.M.; Yun, J.; Zhang, G.; Qi, X.H. Recent insights into microalgae–assisted microbial fuel cells for generating sustainable bioelectricity. Int. J. Hydrogen Energy 2021, 46, 3135–3159. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, S.; Tang, R.; Qiao, C.; Yang, M.; You, Z.; Shao, S.; Wu, D.; Yu, H.; Zhang, J.; et al. Recent advances in enrichment, isolation, and bio–electrochemical activity evaluation of exoelectrogenic microorganisms. Biotechnol. Adv. 2023, 66, 108175. [Google Scholar] [CrossRef]
- Chhachhiya, N.; Tiwari, A.; Sharma, R.S.; Rai, P.K.; Anand, S.; Mishra, V. Transformative potential of optimized microbial fuel cell designs and materials for eco–friendly management of hazardous chemical waste. J. Water Process Eng. 2025, 69, 106647. [Google Scholar] [CrossRef]
- Hayasaka, M.; Hamajima, L.; Yoshida, Y.; Mori, R.; Kato, H.; Suzuki, H.; Tsurigami, R.; Kojima, T.; Kato, M.; Shimizu, M. Phenanthrene degradation by a flavoprotein monooxygenase from Phanerodontia chrysosporium. Appl. Environ. Microbiol. 2025, 91, e0157424. [Google Scholar] [CrossRef]
- Kadri, T.; Rouissi, T.; Kaur Brar, S.; Cledon, M.; Sarma, S.; Verma, M. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by fungal enzymes: A review. J. Environ. Sci. 2017, 51, 52–74. [Google Scholar] [CrossRef]
- Fayyaz, I.; Saddick, S.; Mahmood, R.T.; Asad, M.J.; Hussain, M.A.; Hu, J.; Awais, M.; Khan, M.I.; Saydaxmetova, S. Biodegradation of Azo and disperse dyes by Trametes versicolor: Process optimization and MnP enzyme dynamics. Results Eng. 2025, 25, 103980. [Google Scholar] [CrossRef]
- Lan, B.; Liu, C.; Wang, S.; Jin, Y.; Yadav, A.K.; Srivastava, P.; Yuan, S.; Hu, C.; Zhu, G. Enhanced electron transfer for the improvement of nitrogen removal efficiency and N2O reduction at low temperatures. Water Res. 2025, 272, 122993. [Google Scholar] [CrossRef]
- Hao, D.C.; Zheng, Y.W.; Wang, F.; Han, L.; Zhang, Z. Fungal Electrochemical Remediation of Herbicide-contaminated Soil: Preliminary Study on Degradation Kinetics. Biotechnol. Bull. 2024, 40, 261–272. [Google Scholar]
- Fatehbasharzad, P.; Aliasghari, S.; Tabrizi, I.S.; Khan, J.A.; Boczkaj, G. Microbial fuel cell applications for removal of petroleum hydrocarbon pollutants: A review. Water Resour. Industry 2022, 28, 100178. [Google Scholar] [CrossRef]
- Raqba, R.; Rafaqat, S.; Ali, N.; Munis, M.F. Biodegradation of Reactive Red 195 azo dye and chlorpyrifos organophosphate along with simultaneous bioelectricity generation through bacterial and fungal based biocathode in microbial fuel cell. J. Water Process Eng. 2022, 50, 103177. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Zhou, M. A photosynthetic algal microbial fuel cell for treating swine wastewater. Environ. Sci. Pollut. Res. Int. 2019, 26, 6182–6190. [Google Scholar] [CrossRef]
- Angelaalincy, M.; Senthilkumar, N.; Karpagam, R.; Kumar, G.G.; Ashokkumar, B.; Varalakshmi, P. Enhanced extracellular polysaccharide production and self–sustainable electricity generation for PAMFCs by Scenedesmus sp. SB1. ACS Omega 2017, 2, 3754–3765. [Google Scholar] [CrossRef]
- Karich, A.; Ullrich, R.; Scheibner, K.; Hofrichter, M. Fungal unspecific peroxygenases oxidize the majority of organic EPA priority pollutants. Front. Microbiol. 2017, 8, 1463. [Google Scholar] [CrossRef]
- Ma, H.L.; Kermasha, S.; Gao, J.; Borges, R.M.; Yu, X. Laccase–catalyzed oxidation of phenolic compounds in organic media. J. Mol. Catal. B Enzym. 2009, 57, 89–95. [Google Scholar] [CrossRef]
- Portela, P.C.; Shipps, C.C.; Shen, C.; Srikanth, V.; Salgueiro, C.A.; Malvankar, N.S. Widespread extracellular electron transfer pathways for charging microbial cytochrome OmcS nanowires via periplasmic cytochromes PpcABCDE. Nat. Commun. 2024, 15, 2434. [Google Scholar] [CrossRef]
- Yahampath Arachchige Don, C.D.; Babel, S. Chapter 1—Integration of bioelectrochemical and algal systems for bioproducts generation. In Algae Based Bioelectrochemical Systems for Carbon Sequestration, Carbon Storage, Bioremediation and Bioproduct Generation; Academic Press: Cambridge, MA, USA, 2024; pp. 1–19. [Google Scholar]
- Ueki, T. Cytochromes in extracellular electron transfer in Geobacter. Appl. Environ. Microbiol. 2021, 87, e03109-20. [Google Scholar] [CrossRef]
- Wang, B.; Wu, S.; Chang, X.; Chen, J.; Ma, J.; Wang, P.; Zhu, G. Characterization of a novel hyper–thermostable and chlorpyrifos–hydrolyzing carboxylesterase EstC: A representative of the new esterase family XIX. Pestic. Biochem. Physiol. 2020, 170, 104704. [Google Scholar] [CrossRef]
- Enamala, M.K.; Dixit, R.; Tangellapally, A.; Singh, M.; Dinakarrao, S.M.; Chavali, M.; Pamanji, S.R.; Ashokkumar, V.; Kadier, A.; Chandrasekhar, K. Photosynthetic microorganisms (Algae) mediated bioelectricity generation in microbial fuel cell: Concise review. Environ. Technol. Innovat. 2020, 19, 100959. [Google Scholar] [CrossRef]
- Gajda, I.; Stinchcombe, A.; Greenman, J.; Melhuish, C.; Ieropoulos, I. Algal ‘lagoon’ effect for oxygenating MFC cathodes. Int. J. Hydrogen Energy 2014, 39, 21857–21863. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, C.; Zhao, X.; Wang, Y.; Li, Z.; Zhou, Y.; Ren, G. Algae–Bacteria cooperated microbial ecosystem: A self–circulating semiartificial photosynthetic purifying strategy. Sci. Total Environ. 2023, 905, 167187. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, R.; Nwangburuka, C.; Oboirien, B. Origins, roles and fate of organic acids in soils: A review. S. Afr. J. Bot. 2017, 108, 393–406. [Google Scholar] [CrossRef]
- Chen, X.D.; Li, X.J.; Li, Y.; Zhao, L.; Sun, Y.; Rushimisha, I.E.; Han, T.; Weng, L.; Lin, X.; Li, Y.T. Bioelectric field drives ion migration with the electricity generation and pollutant removal. Environ. Technol. Innovat. 2021, 24, 101901. [Google Scholar] [CrossRef]
- Kaur, J.; Kaur, G. Dehydrogenase activity as a biological indicator of soil health. Chem. Sci. Rev. Lett. 2021, 10, 326–329. [Google Scholar]
- He, Y.Y.; Chen, W.; Xiang, Y.; Zhang, Y.; Xie, L. Unveiling the effect of PFOA presence on the composting process: Roles of oxidation stress, carbon metabolism, and humification process. J. Hazard. Mater. 2024, 479, 135682. [Google Scholar] [CrossRef]
- Vandana; Priyadarshanee, M.; Das, S. Bacterial extracellular polymeric substances: Biosynthesis and interaction with environmental pollutants. Chemosphere 2023, 332, 138876. [Google Scholar] [CrossRef]
- Schalk, I.J. Bacterial siderophores: Diversity, uptake pathways and applications. Nat. Rev. Microbiol. 2025, 23, 24–40. [Google Scholar] [CrossRef]
- Guo, S.; Wang, P.; Wang, X.; Zou, M.; Liu, C.; Hao, J. Microalgae as biofertilizer in modern agriculture. In Microalgae Biotechnology for Food, Health and High Value Products; Alam, M., Xu, J.L., Wang, Z., Eds.; Springer: Singapore, 2020; pp. 397–411. [Google Scholar]
- Yang, L.L.; Song, X.; Feng, Y.; Qiu, X.; Yan, X.; Ruan, R.; Cheng, P. Improving soil structure and function for continuous cropping of sweet melon using microalgae–based biological fertilizer. Algal Res. 2025, 85, 103868. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.J.; Zhao, X.; Chen, X.; Zhou, B.; Weng, L.; Li, Y.T. Bioelectric field accelerates the conversion of carbon and nitrogen in soil bioelectrochemical systems. J. Hazard. Mater. 2020, 388, 121790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; He, G.; Geng, Y.; Chen, C.; Zhou, J.; Li, Z.; Feng, J.; Diao, Y.; Yang, L.; et al. Rhizosphere bacterial community structure and nutrient cycling genes jointly drive the soil multifunctionality of Phoebe bournei young plantations under potassium fertilizer. Glob. Ecol. Conserv. 2025, 58, e03473. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, X.C.; Ding, R.; Xu, K.; Tremblay, P.L. The hidden chemolithoautotrophic metabolism of Geobacter sulfurreducens uncovered by adaptation to formate. ISME J. 2020, 14, 2078–2089. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.Y.; Liu, X.; Zhang, Z.; Wang, X.; Rensing, C.; Zhou, S. Anode respiration–dependent biological nitrogen fixation by Geobacter sulfurreducens. Water Res. 2022, 208, 117860. [Google Scholar] [CrossRef]
- Liang, J.L.; Liu, J.; Jia, P.; Yang, T.T.; Zeng, Q.W.; Zhang, S.C.; Liao, B.; Shu, W.S.; Li, J.T. Novel phosphate–solubilizing bacteria enhance soil phosphorus cycling following ecological restoration of land degraded by mining. ISME J. 2020, 14, 1600–1613. [Google Scholar] [CrossRef]
- Sulu–Gambari, F.; Seitaj, D.; Meysman, F.J.; Schauer, R.; Polerecky, L.; Slomp, C.P. Cable bacteria control iron–phosphorus dynamics in sediments of a coastal hypoxic basin. Environ. Sci. Technol. 2016, 50, 1227–1233. [Google Scholar] [CrossRef]
- Wang, Y.F.; Wang, W.; Qi, X.; Li, D.; Liu, Y.; Song, X.; Cao, X. Magnetite–equipped algal–rich sediments for microbial fuel cells: Remediation of sediment organic matter pollution and mechanisms of remote electron transfer. Sci. Total Environ. 2024, 912, 169545. [Google Scholar] [CrossRef]
- Huarachi-Olivera, R.; Dueñas-Gonza, A.; Yapo-Pari, U.; Vega, P.; Romero-Ugarte, M.; Tapia, J.; Molina, L.; Lazarte-Rivera, A.; Pacheco-Salazar, D.G.; Esparza, M. Bioelectrogenesis with microbial fuel cells (MFCs) using the microalga Chlorella vulgaris and bacterial communities. Electron. J. Biotechnol. 2018, 31, 34–43. [Google Scholar] [CrossRef]
- Halvorson, H.M.; Barry, J.R.; Lodato, M.B.; Findlay, R.H.; Francoeur, S.N.; Kuehn, K.A. Periphytic algae decouple fungal activity from leaf litter decomposition via negative priming. Funct. Ecol. 2019, 33, 188–201. [Google Scholar] [CrossRef]
- Glodowska, M.; Welte, C.U.; Kurth, J.M. Chapter Four—Metabolic potential of anaerobic methane oxidizing archaea for a broad spectrum of electron acceptors. In Advances in Microbial Physiology; Academic Press: Cambridge, MA, USA, 2022; Volume 80, pp. 157–201. [Google Scholar]
- Ouboter, H.T.; Mesman, R.; Sleutels, T.; Postma, J.; Wissink, M.; Jetten, M.S.M.; Ter Heijne, A.; Berben, T.; Welte, C.U. Mechanisms of extracellular electron transfer in anaerobic methanotrophic archaea. Nat. Commun. 2024, 15, 1477. [Google Scholar] [CrossRef]
- Tripathy, V.; Basak, B.B.; Varghese, T.S.; Saha, A. Residues and contaminants in medicinal herbs—A review. Phytochem. Lett. 2015, 14, 67–78. [Google Scholar] [CrossRef]
- Peng, B.; Xie, Y.; Lai, Q.; Liu, W.; Ye, X.; Yin, L.; Zhang, W.; Xiong, S.; Wang, H.; Chen, H. Pesticide residue detection technology for herbal medicine: Current status, challenges, and prospects. Anal Sci. 2024, 40, 581–597. [Google Scholar] [CrossRef]
- Pandya, R.S.; Kaur, T.; Bhattacharya, R.; Bose, D.; Saraf, D. Harnessing microorganisms for bioenergy with microbial fuel cells: Powering the future. Water–Energy Nexus 2024, 7, 1–12. [Google Scholar] [CrossRef]
- Rathinavel, N.; Samuel, J.O.; Veleeswaran, A.; Nallathambi, S.; Ponnuchamy, K.; Muthusamy, G.; Raja, R.; Ramalingam, K.R.; Alagarsamy, A. Exploring the key factors enhancing the microbial fuel cell performance. Process Safety Environ. Protect. 2025, 193, 385–402. [Google Scholar] [CrossRef]
- Qiu, Q.; Bender, S.F.; Mgelwa, A.S.; Hu, Y. Arbuscular mycorrhizal fungi mitigate soil nitrogen and phosphorus losses: A meta–analysis. Sci. Total Environ. 2022, 807 Pt 1, 150857. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhang, J.; Wang, Y.; Luo, Z.; Li, X.; Wang, Y.; Luo, J.; Yang, M.H. Unveiling the contamination patterns of neonicotinoid insecticides: Detection, distribution, and risk assessment in Panax notoginseng across plant parts. J. Agric. Food Chem. 2024, 72, 17834–17846. [Google Scholar] [CrossRef]
- Lin, C.W.; Lai, C.; Liu, S.; Chen, Y.; Alfanti, L. Enhancing bioelectricity generation and removal of copper in microbial fuel cells with a laccase–catalyzed biocathode. J. Clean. Prod. 2021, 298, 126726. [Google Scholar] [CrossRef]
- Hong, S.B.; Piao, S.L.; Chen, A.; Liu, Y.; Liu, L.; Peng, S.; Sardans, J.; Sun, Y.; Peñuelas, J.; Zeng, H. Afforestation neutralizes soil pH. Nat. Commun. 2018, 9, 520. [Google Scholar] [CrossRef]
- Pan, L.; Li, Y.; Zhao, W.; Sui, Y.; Yang, N.; Liu, L.; Liu, Y.; Tang, Z.; Mu, L. Metabolomics analysis of different diameter classes of Taxus chinensis reveals that the resource allocation is related to carbon and nitrogen metabolism. BMC Plant Biol. 2024, 24, 383. [Google Scholar] [CrossRef]
- Hao, D.C.; Li, C.X.; Xiao, P.G.; Xie, H.T.; Bao, X.L.; Wang, L.F. Conservation Tillage in Medicinal Plant Cultivation in China: What, Why, and How. Agronomy 2023, 13, 1890. [Google Scholar] [CrossRef]
- Hammami, H.; Eslami, S.V. Physiological and growth responses of milk thistle (Silybum marianum (L.) Gaertn.) to soil–applied herbicides. J. Environ. Manag. 2024, 365, 121420. [Google Scholar] [CrossRef]
- Shukla, M.; Kumar, S. Algal growth in photosynthetic algal microbial fuel cell and its subsequent utilization for biofuels. Renew. Sustain. Energy Rev. 2018, 82 Pt 1, 402–414. [Google Scholar] [CrossRef]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Expt. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef]
- Chanika, E.; Georgiadou, D.; Soueref, E.; Karas, P.; Karanasios, E.; Tsiropoulos, N.G.; Tzortzakakis, E.A.; Karpouzas, D.G. Isolation of soil bacteria able to hydrolyze both organophosphate and carbamate pesticides. Bioresour. Technol. 2011, 102, 3184–3192. [Google Scholar] [CrossRef]
- Chugh, M.; Kumar, L.; Shah, M.P.; Bharadvaja, N. Algal Bioremediation of heavy metals: An insight into removal mechanisms, recovery of by–products, challenges, and future opportunities. Energy Nexus 2022, 7, 100129. [Google Scholar] [CrossRef]
- Cai, S.; Zhao, X.; Yan, X.Y. Towards precise nitrogen fertilizer management for sustainable agriculture. Earth Crit. Zone 2025, 2, 100026. [Google Scholar] [CrossRef]
- Turkeltaub, T.; Wang, J.; Cheng, Q.; Jia, X.; Zhu, Y.; Shao, M.; Binley, A. Soil moisture and electrical conductivity relationships under typical Loess Plateau land covers. Vadose Zone J. 2022, 21, e20174. [Google Scholar] [CrossRef]
- Tahir, S.; Marschner, P. Clay addition to sandy soil—Influence of clay type and size on nutrient availability in sandy soils amended with residues differing in C/N ratio. Pedosphere 2017, 27, 293–305. [Google Scholar] [CrossRef]
- Liu, X.D.; Tournassat, C.; Grangeon, S.; Kalinichev, A.G.; Takahashi, Y.; Fernandes, M.M. Molecular–level understanding of metal ion retention in clay–rich materials. Nat. Rev. Earth Environ. 2022, 3, 461–476. [Google Scholar] [CrossRef]
- Farkas, D.; Proctor, K.; Kim, B.; Avignone Rossa, C.; Kasprzyk-Hordern, B.; Di Lorenzo, M. Assessing the impact of soil microbial fuel cells on atrazine removal in soil. J. Hazard. Mater. 2024, 478, 135473. [Google Scholar] [CrossRef]
- Zhang, Z.; Furman, A. Soil redox dynamics under dynamic hydrologic regimes—A review. Sci. Total Environ. 2021, 763, 143026. [Google Scholar] [CrossRef]
- Cha, J.; Choi, S.; Yu, H.; Kim, H.; Kim, C. Directly applicable microbial fuel cells in aeration tank for wastewater treatment. Bioelectrochem. 2010, 78, 72–79. [Google Scholar] [CrossRef]
- Wang, C.; Kuzyakov, Y. Mechanisms and implications of bacterial–fungal competition for soil resources. ISME J. 2024, 18, wrae073. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Li, H.; Wang, R.; Zhang, K.Q.; Xu, J. Fungi–nematode interactions: Diversity, ecology, and biocontrol prospects in agriculture. J. Fungi. 2020, 6, 206. [Google Scholar] [CrossRef]
- Wong, H.J.; Mohamad-Fauzi, N.; Rizman-Idid, M.; Convey, P.; Alias, S.A. Protective mechanisms and responses of micro–fungi towards ultraviolet–induced cellular damage. Polar Sci. 2019, 20 Pt 1, 19–34. [Google Scholar] [CrossRef]
- Alster, C.J.; Allison, S.D.; Johnson, N.G.; Glassman, S.I.; Treseder, K.K. Phenotypic plasticity of fungal traits in response to moisture and temperature. ISME Commun. 2021, 1, 43. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Gill, J.P.K.; Datta, S.; Singh, S.; Dhaka, V.; Kapoor, D.; Wani, A.B.; Dhanjal, D.S.; Kumar, M.; et al. Herbicide glyphosate: Toxicity and microbial degradation. Int. J. Environ. Res. Public Health 2020, 17, 7519. [Google Scholar] [CrossRef]
- Huang, Y.P.; Li, Z.; Yao, K.; Chen, C.; Deng, C.; Fang, Y.; Li, R.; Tian, H. Suppressing toxic intermediates during photocatalytic degradation of glyphosate by controlling adsorption modes. Appl. Catal. B Environ. 2021, 299, 120671. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Yang, C.; Shahzad, M.W.; Yan, Y.; Dai, L.; Lu, W.; Chen, W.; He, X.; Xu, B.B.; et al. A hierarchical multimetal oxides@graphene fabric electrode with high energy density and robust cycling performance for flexible supercapacitors. Nano Lett. 2025, 25, 4485–4493. [Google Scholar] [CrossRef]
- Aiswaria, P.; Mohamed, S.N.; Singaravelu, D.L.; Brindhadevi, K.; Pugazhendhi, A. A review on graphene/graphene oxide supported electrodes for microbial fuel cell applications: Challenges and prospects. Chemosphere 2022, 296, 133983. [Google Scholar]
- Gomes, D.G.; Coelho, E.; Silva, R.; Domingues, L.; Teixeira, J.A. 8—Bioreactors and engineering of filamentous fungi cultivation. In Current Developments in Biotechnology and Bioengineering: Filamentous Fungi Biorefinery; Elsevier: Amsterdam, The Netherlands, 2023; pp. 219–250. [Google Scholar]
- Chakraborty, I.; Sathe, S.M.; Dubey, B.K.; Ghangrekar, M.M. Waste–derived biochar: Applications and future perspective in microbial fuel cells. Bioresour. Technol. 2020, 312, 123587. [Google Scholar] [CrossRef]
- Huggins, T.; Wang, H.; Kearns, J.; Jenkins, P.; Ren, Z.J. Biochar as a sustainable electrode material for electricity production in microbial fuel cells. Bioresour. Technol. 2014, 157, 114–119. [Google Scholar] [CrossRef]
- Logan, B.E.; Rossi, R.; Ragab, A.; Saikaly, P.E. Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Microbiol. 2019, 17, 307–319. [Google Scholar] [CrossRef]
- Liu, C.M.; Qin, J.; Dou, X.; Yang, M.H.; Sun, X.B. Extrinsic harmful residues in Chinese herbal medicines: Types, detection, and safety evaluation. Chin. Herb. Med. 2018, 10, 117–136. [Google Scholar] [CrossRef]
- Zhang, F.; Hao, X.; Liu, J.; Hou, H.; Chen, S.L.; Wang, C. Herbal Multiomics Provide insights into gene discovery and bioproduction of triterpenoids by engineered microbes. J. Agric. Food Chem. 2025, 73, 47–65. [Google Scholar] [CrossRef]
- Garg, S.; Kim, M.; Romero-Suarez, D. Current advancements in fungal engineering technologies for Sustainable Development Goals. Trends Microbiol. 2025, 33, 285–301. [Google Scholar] [CrossRef]
- Schuster, M.; Kahmann, R. CRISPR–Cas9 genome editing approaches in filamentous fungi and oomycetes. Fungal Genet. Biol. 2019, 130, 43–53. [Google Scholar] [CrossRef]
- Ottenheim, C.; Nawrath, M.; Wu, J. Microbial mutagenesis by atmospheric and room–temperature plasma (ARTP): The latest development. Bioresour. Bioprocess. 2018, 5, 12. [Google Scholar] [CrossRef]
- Jansson, J.K.; McClure, R.; Egbert, R.G. Soil microbiome engineering for sustainability in a changing environment. Nat. Biotechnol. 2023, 41, 1716–1728. [Google Scholar] [CrossRef]
- Tripathi, S.; Singh, V.K.; Srivastava, P.; Singh, R.; Devi, R.S.; Kumar, A.; Bhadouria, R. Chapter 4—Phytoremediation of organic pollutants: Current status and future directions. In Abatement of Environmental Pollutants: Trends and Strategies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 81–105. [Google Scholar]
- Tong, Y.Y.; Zheng, X.; Hu, Y.; Wu, J.; Liu, H.; Deng, Y.; Lv, W.; Yao, H.; Chen, J.; Ge, T. Root exudate–mediated plant–microbiome interactions determine plant health during disease infection. Agri. Ecosyst. Environ. 2024, 370, 109056. [Google Scholar] [CrossRef]
- Singh, S.; Tiwari, S. Chapter 10—Responses of plants to herbicides: Recent advances and future prospectives. In Plant Life Under Changing Environment: Responses and Management; Springer: Berlin/Heidelberg, Germany, 2020; pp. 237–250. [Google Scholar]
- Shahab, H.; Naeem, M.; Iqbal, M.; Aqeel, M.; Ullah, S.S. IoT–driven smart agricultural technology for real–time soil and crop optimization. Smart Agric. Technol. 2025, 10, 100847. [Google Scholar] [CrossRef]
- Chang, Y.Y.; Cao, Q.; Venton, B.J. 3D printing for customized carbon electrodes. Curr. Opin. Electrochem. 2023, 38, 101228. [Google Scholar] [CrossRef]
- Ghorui, M.; Chowdhury, S.; Balu, P.; Burla, S. Arbuscular mycorrhizal inoculants and its regulatory landscape. Heliyon 2024, 10, e30359. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, B.; Yang, L.; Zhuang, J.; Chen, X. Annual atrazine residue estimation in Chinese agricultural soils by integrated modeling of machine learning and mechanism–based models. J. Hazard. Mater. 2024, 472, 134539. [Google Scholar] [CrossRef]
- Kumar, V.; Zadokar, A.; Kumar, P.; Sharma, R.; Sharma, R.; Siddiqui, M.W.; Irfan, M.; Chandora, R. Advancing medicinal plant agriculture: Integrating technology and precision agriculture for sustainability. PeerJ 2025, 13, e19058. [Google Scholar] [CrossRef]
- Luo, H.; Zhao, Y.; Hua, H.; Zhang, Y.; Zhang, X.; Fang, Q.; Li, Q.; Zhang, Y.; Tan, P.; Yang, A.; et al. Research progress on quality assurance of genuine Chinese medicinal in Sichuan. Chin. Med. 2021, 16, 19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).