Synergistic Effects of Trichoderma harzianum and Light Quality on Photosynthetic Carbon Metabolism and Growth in Tomato Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions, Plant Materials, and Treatments
2.2. Measurement of Plant Growth Parameters
2.3. Determination of Colonization Rate of T. harzianum in Tomato Roots
number of root segments) × 100%.
2.4. Measurement of Root Indices in Tomato Plants
2.5. Measurement of Soil Enzyme Activities in Rhizosphere Soil and Root Activity in Tomato Plants
2.6. Measurement of Photosynthetic Parameters in Tomato Plants
2.7. Measurement of Photosynthetic Pigment Content in Tomato Plants
2.8. Measurement of Chlorophyll Fluorescence Parameters
2.9. Measurement of Photosynthetic Enzyme Activity in Tomato Leaves
2.10. Measurement of Carbon Metabolism Indices and Related Enzyme Activities in Leaves and Roots of Tomato Plants
2.11. Determination of Gene Expression Levels
2.12. Statistical Analysis
3. Results
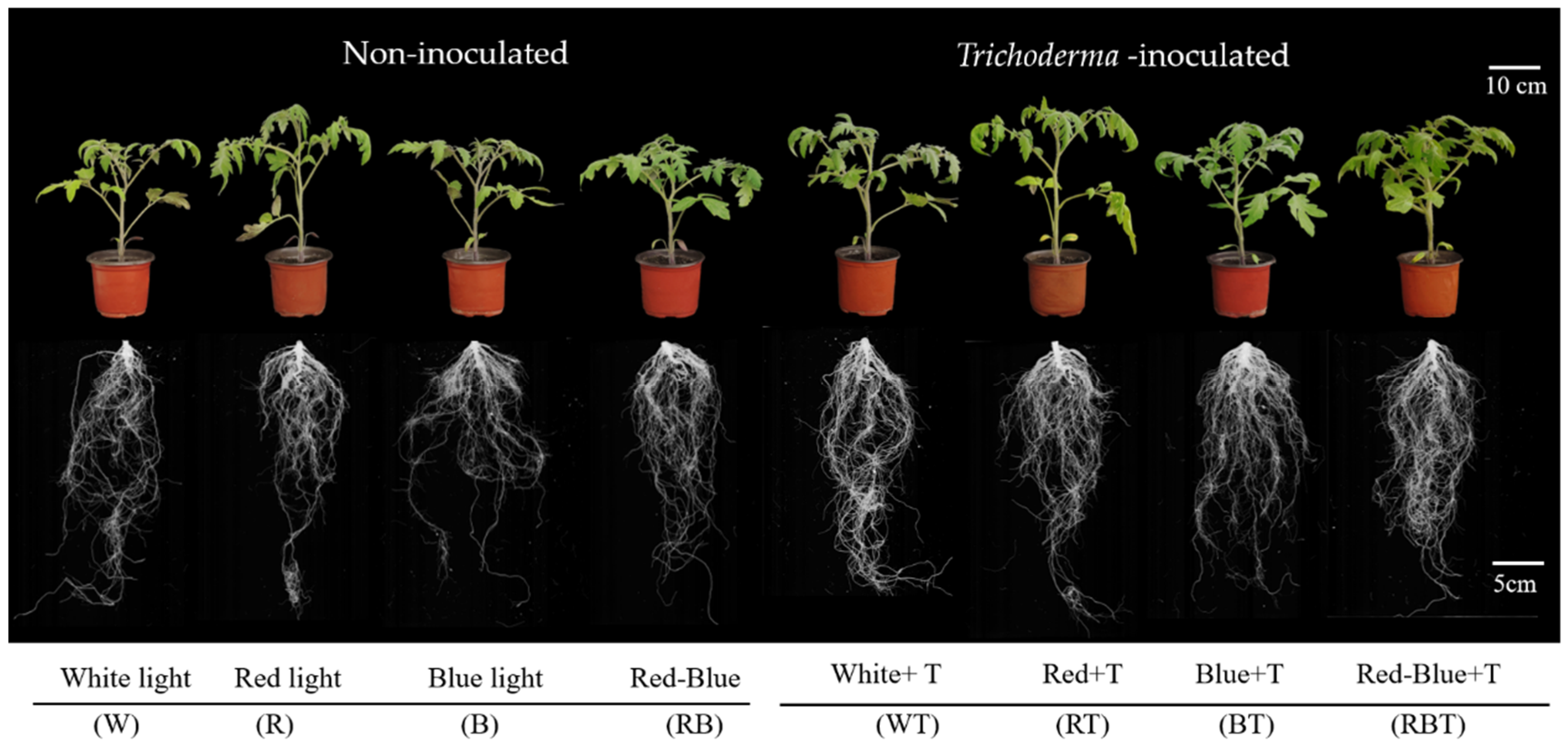
3.1. Effects of T. inoculation and Light Quality on Growth and Biomass Accumulation in Tomato Seedlings
3.2. Light Quality Affects Root Colonization with T. harzianum and Various Root Indexes
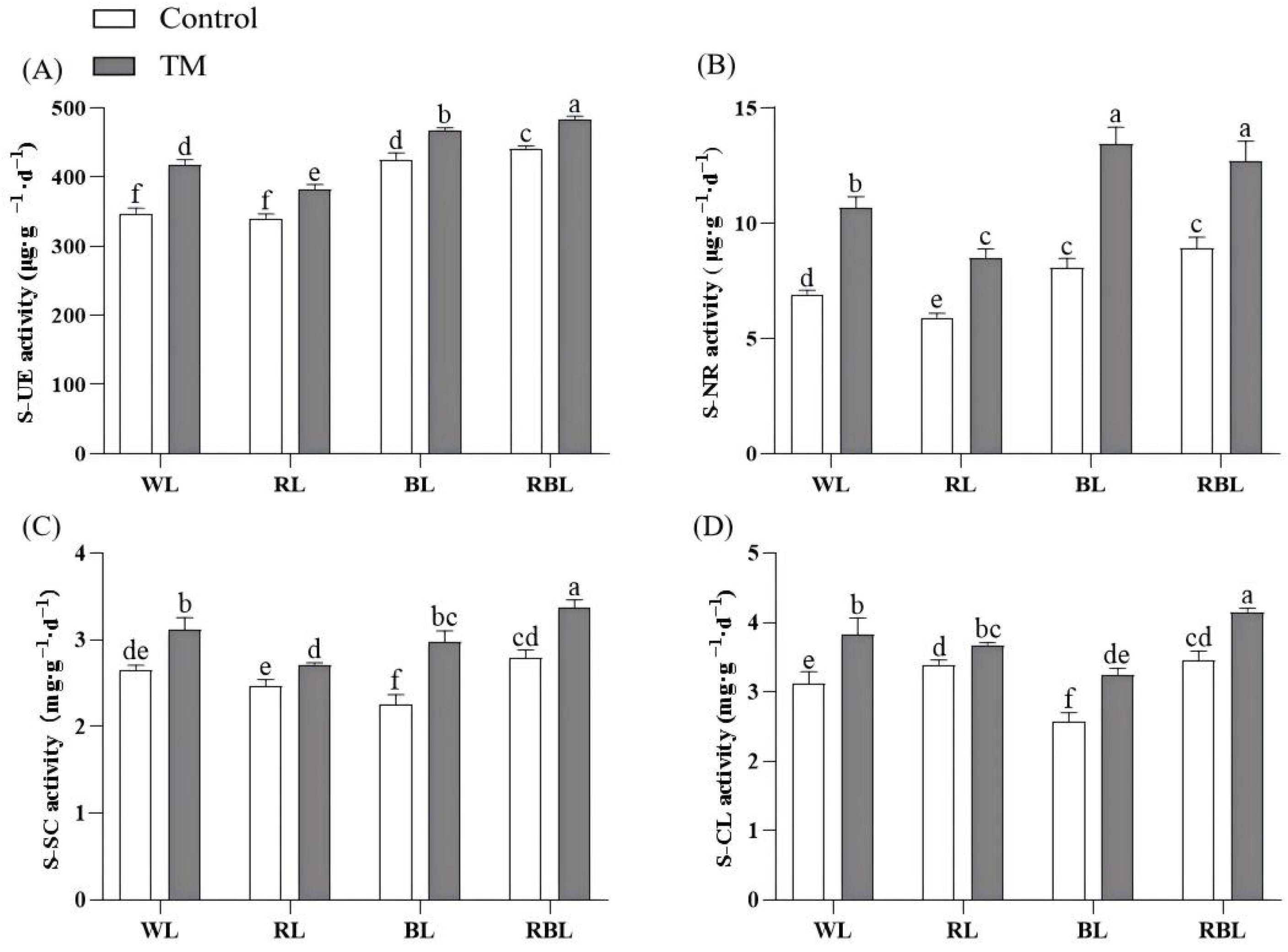
3.3. T. harzianum Inoculation and Light Quality Affect Soil Enzyme Activity and Root Activity
3.4. T. harzianum Inoculation and Light Quality Alter Photosynthetic Gas Exchange Parameters
3.5. Effects of T. harzianum Inoculation and Light Quality on Photosynthetic Pigment Contents
3.6. Effects of T. harzianum Inoculation Under Different Spectral Conditions on Chlorophyll Fluorescence Parameters
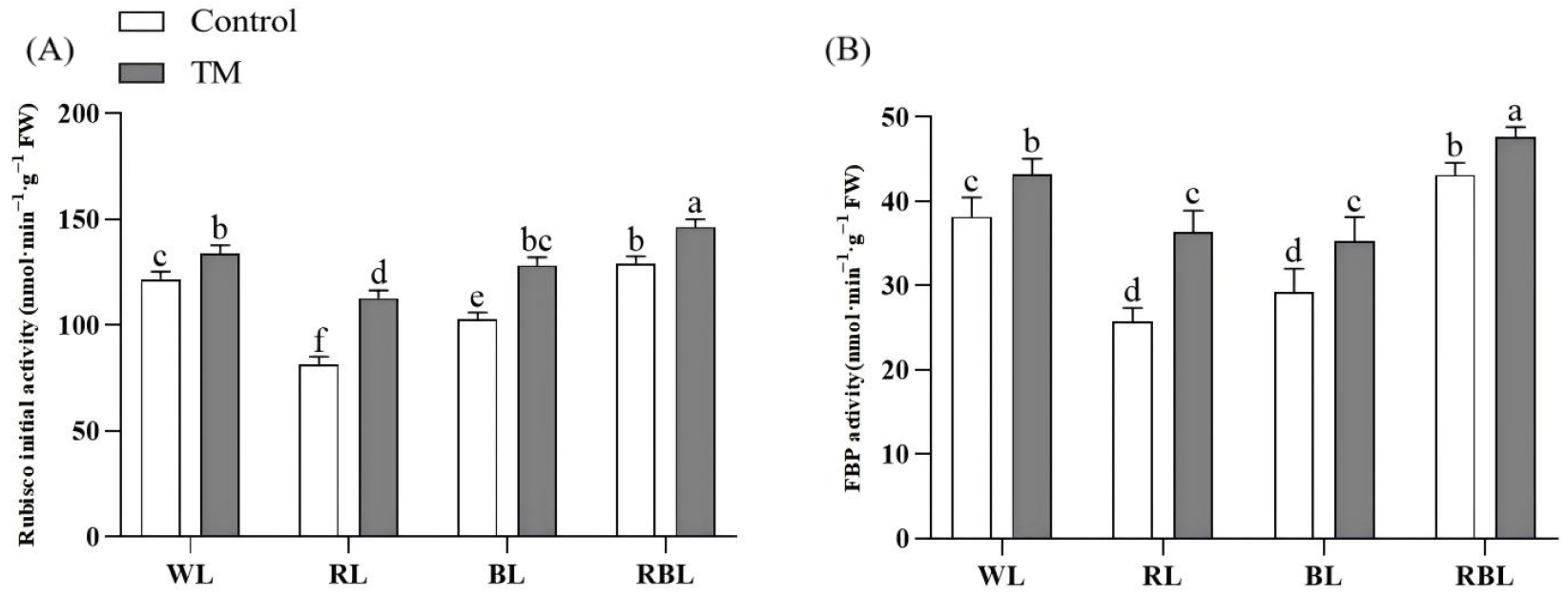
3.7. Effects of T. harzianum Inoculation and Light Quality on Photosynthetic Enzyme Activity in Tomato Leaves
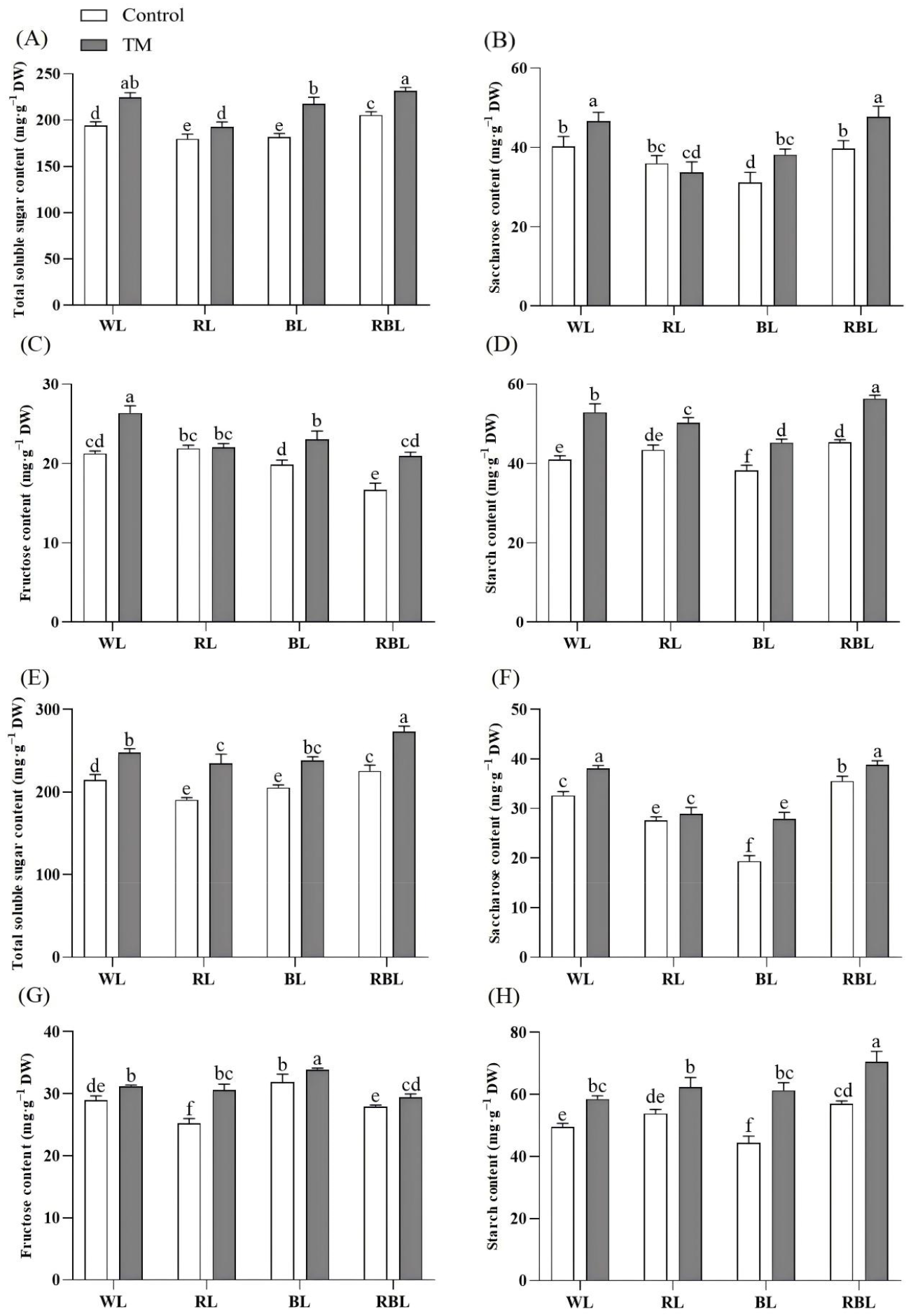
3.8. Effects of T. harzianum Inoculation and Light Quality on Total Soluble Sugar, Sucrose, Fructose, and Starch Content in Tomato Leaves and Roots
3.9. Effects of T. harzianum Inoculation and Light Quality on Sucrose Synthase (SS) Activity and Sucrose Phosphate Synthase (SPS) Activity in Tomato Leaves and Roots
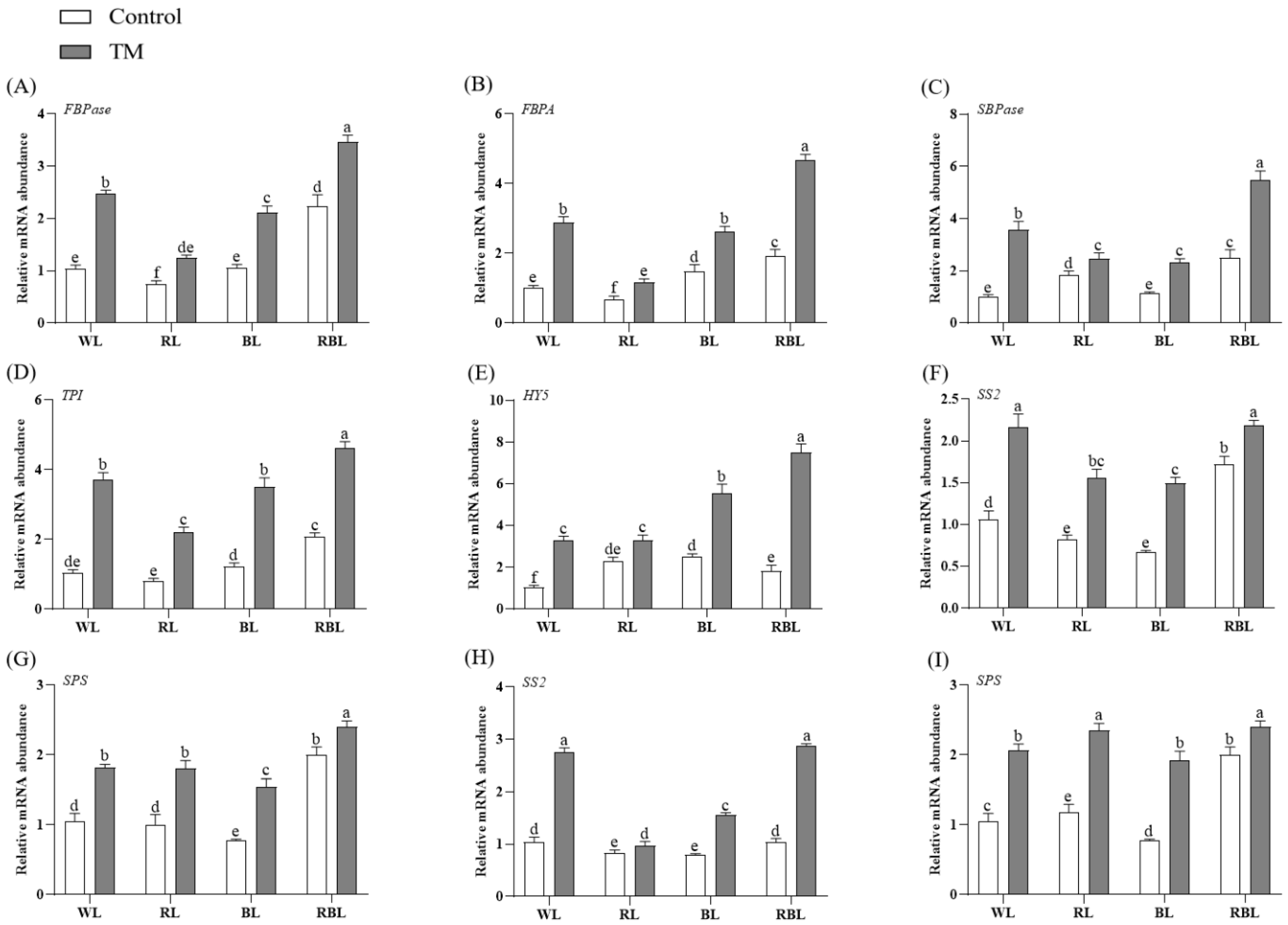
3.10. Effects of T. harzianum Inoculation and Light Quality on the Expression of Calvin Cycle Genes, HY5, and Carbon Metabolic Enzyme Genes
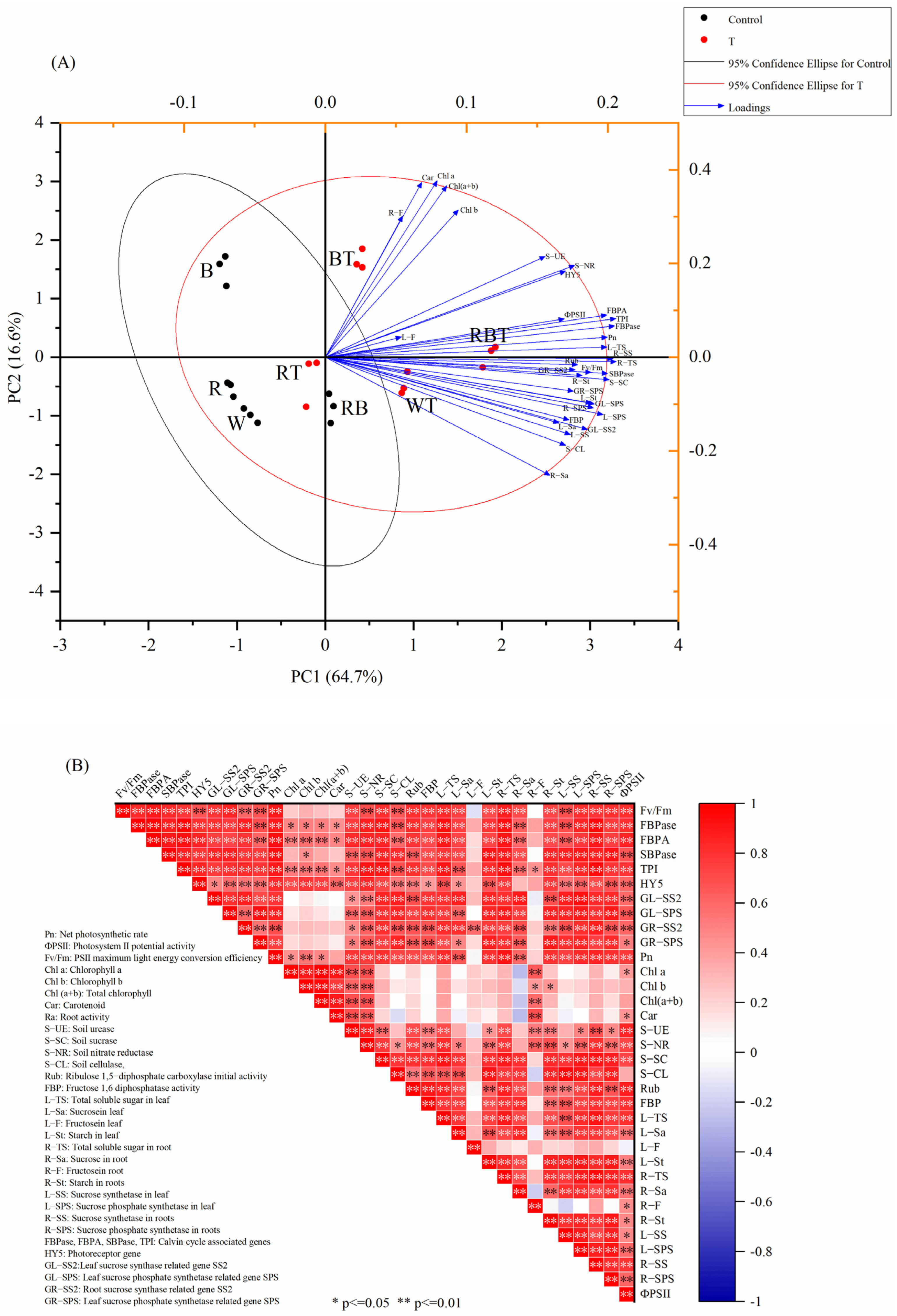
3.11. Principal Component Analysis and Pearson Correlation Analysis
4. Discussion
4.1. Effects of Light Spectra and T. harzianum Inoculation on the Biomass of Tomato Seedlings
4.2. Effects of the Spectrum on the Colonization of the Tomato Roots by T. harzianum and the Analysis of the Influence of the Colonization Rate on Plant Growth
4.3. Effects of Inoculation with T. harzianum Under Different Spectral Conditions on Photosynthesis
4.4. Sugar Metabolism in Tomato Leaves and Roots as Influenced by T. harzianum Inoculation Under Different Spectral Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TPI | Triose-3-phosphate isomerase |
| FBPA | Fructose-1,6-bisphosphate aldolase |
| SBPase | Sedoheptulose-1,7-bisphosphatase |
| FBPase | Fructose-1,6-bisphosphate phosphatase |
| ΦPSII | Photosystem II potential activity |
| Fv/Fm | Maximal photochemical efficiency of PSII |
| Rubisco | Ribulose-1,5-bisphosphate carboxylase |
| SS | Sucrose synthetase |
| SPS | Sucrose phosphate synthetase |
| Pn | Net photosynthetic rate |
| Tr | Transpiration rate |
| Gs | Stomatal conductance |
| Ci | Intracellular CO2 concentration |
| S−UE | Soil urease |
| S−NR | Soil nitrate reductase |
| S−SC | Soil sucrase |
| S−CL | Soil cellulase |
| Chl a | Chlorophyll a |
| Chl b | Chlorophyll b |
| Chl(a + b) | Total chlorophyll |
| Car | Carotenoids |
References
- Arcel, M.M.; Lin, X.; Huang, J.; Wu, J.; Zheng, S. The application of LED illumination and intelligent control in plant factory, a new direction for modern agriculture: A review. J. Phys. Conf. Ser. 2021, 1732, 012178. [Google Scholar] [CrossRef]
- Fan, P.; Zhang, J.; Gao, L.; Wang, M.; Kong, H.; He, S. Exploring the frontier of plant phase separation: Current insights and future prospects. New Crops 2024, 1, 100026. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Liu, J.; Qin, R. Light absorption and growth response of Dunaliella under different light qualities. J. Appl. Phycol. 2020, 32, 1041–1052. [Google Scholar] [CrossRef]
- Zhen, S.; Bugbee, B. Far-red photons have equivalent efficiency to traditional photosynthetic photons: Implications for redefining photosynthetically active radiation. Plant Cell Environ. 2020, 43, 1259–1272. [Google Scholar] [CrossRef]
- Li, Y.; Xin, G.; Liu, C.; Shi, Q.; Yang, F.; Wei, M. Effects of red and blue light on leaf anatomy, CO2 assimilation and the photosynthetic electron transport capacity of sweet pepper (Capsicum annuum L.) seedlings. BMC Plant Biol. 2020, 20, 318. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Effects of light quality on growth and phytonutrient accumulation of herbs under controlled environments. Horticulturae 2017, 3, 36. [Google Scholar] [CrossRef]
- Han, P.-P.; Shen, S.-G.; Guo, R.-J.; Zhao, D.-X.; Lin, Y.-H.; Jia, S.-R.; Yan, R.-R.; Wu, Y.-K. ROS is a factor regulating the increased polysaccharide production by light quality in the edible cyanobacterium Nostoc flagelliforme. J. Agric. Food Chem. 2019, 67, 2235–2244. [Google Scholar] [CrossRef]
- Bononi, L.; Chiaramonte, J.B.; Pansa, C.C.; Moitinho, M.A.; Melo, I.S. Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth. Sci. Rep. 2020, 10, 2858. [Google Scholar] [CrossRef]
- Shi, X.; Zhao, Y.; Xu, M.; Ma, L.; Adams, J.M.; Shi, Y. Insights into plant–microbe interactions in the rhizosphere to promote sustainable agriculture in the new crops era. New Crops 2024, 1, 100004. [Google Scholar] [CrossRef]
- Kabir, A.H.; Baki, M.Z.I.; Ahmed, B.; Mostofa, M.G. Current, faltering, and future strategies for advancing microbiome-assisted sustainable agriculture and environmental resilience. New Crops 2024, 1, 100013. [Google Scholar] [CrossRef]
- Kou, C.; Song, F.; Li, D.; Xu, H.; Zhang, S.; Yang, W.; Shi, W.; Gao, Z. A necessary considering factor for crop resistance: Precise regulation and effective utilization of beneficial microorganisms. New Crops 2024, 1, 100023. [Google Scholar] [CrossRef]
- Singh, A.; Shukla, N.; Kabadwal, B.; Tewari, A.; Kumar, J. Review on plant-Trichoderma-pathogen interaction. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2382–2397. [Google Scholar] [CrossRef]
- Harman, G.; Doni, F.; Khadka, R.B.; Uphoff, N. Endophytic strains of Trichoderma increase plants’ photosynthetic capability. J. Appl. Microbiol. 2021, 130, 529–546. [Google Scholar] [CrossRef]
- Begum, N.; Wang, L.; Ahmad, H.; Akhtar, K.; Roy, R.; Khan, M.I.; Zhao, T. Co-inoculation of arbuscular mycorrhizal fungi and the plant growth-promoting rhizobacteria improve growth and photosynthesis in tobacco under drought stress by up-regulating antioxidant and mineral nutrition metabolism. Microb. Ecol. 2021, 83, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Liu, Z.; Liu, B.; Wang, Y.; Wang, J. The effect of Trichoderma biofertilizer on the quality of flowering Chinese cabbage and the soil environment. Sci. Hortic. 2020, 262, 109069. [Google Scholar] [CrossRef]
- Yu, Z.; Fischer, R. Light sensing and responses in fungi. Nat. Rev. Microbiol. 2019, 17, 25–36. [Google Scholar] [CrossRef]
- Li, Y.; Sun, T.; Guo, D.; Gao, J.; Zhang, J.; Cai, F.; Fischer, R.; Shen, Q.; Yu, Z. Comprehensive analysis of the regulatory network of blue-light-regulated conidiation and hydrophobin production in Trichoderma guizhouense. Environ. Microbiol. 2021, 23, 6241–6256. [Google Scholar] [CrossRef]
- Missbach, K.; Flatschacher, D.; Bueschl, C.; Samson, J.M.; Leibetseder, S.; Marchetti-Deschmann, M.; Zeilinger, S.; Schuhmacher, R. Light-induced changes in secondary metabolite production of Trichoderma atroviride. J. Fungi 2023, 9, 785. [Google Scholar] [CrossRef]
- Stappler, E.; Walton, J.D.; Beier, S.; Schmoll, M. Abundance of secreted proteins of Trichoderma reesei is regulated by light of different intensities. Front. Microbiol. 2017, 8, 2586. [Google Scholar] [CrossRef]
- Tester, M.; Morris, C. The penetration of light through soil. Plant Cell Environ. 1987, 10, 281–286. [Google Scholar] [CrossRef]
- Tan, B.; Li, Y.; Deng, D.; Pan, H.; Zeng, Y.; Tan, X.; Zhuang, W.; Li, Z. Rhizosphere inoculation of Nicotiana benthamiana with Trichoderma harzianum TRA1-16 in controlled environment agriculture: Effects of varying light intensities on the mutualism-parasitism interaction. Front. Plant Sci. 2022, 13, 989155. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleki, A.K.; Pirdashti, H.; Yaghoubian, Y.; Abbasian, A.; Ghadirnezhad Shiade, S.R. Endophytic fungi improve growth and yield of wheat (Triticum aestivum L.) under limited light conditions. Gesunde Pflanz. 2023, 75, 1517–1529. [Google Scholar] [CrossRef]
- Hristozkova, M.; Geneva, M.; Stancheva, I.; Velikova, V. LED spectral composition effects on mycorrhizal symbiosis formation with tomato plants. Appl. Soil Ecol. 2017, 120, 189–196. [Google Scholar] [CrossRef]
- Ali, M.Y.; Sina, A.A.I.; Khandker, S.S.; Neesa, L.; Tanvir, E.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional composition and bioactive compounds in tomatoes and their impact on human health and disease: A review. Foods 2020, 10, 45. [Google Scholar] [CrossRef]
- Lima, G.P.P.; Gómez, H.A.G.; Seabra Junior, S.; Maraschin, M.; Tecchio, M.A.; Borges, C.V. Functional and nutraceutical compounds of tomatoes as affected by agronomic practices, postharvest management, and processing methods: A mini review. Front. Nutr. 2022, 9, 868492. [Google Scholar] [CrossRef] [PubMed]
- Maureira, F.; Rajagopalan, K.; Stöckle, C.O. Evaluating tomato production in open-field and high-tech greenhouse systems. J. Clean. Prod. 2022, 337, 130459. [Google Scholar] [CrossRef]
- Gatahi, D.M. Challenges and opportunities in tomato production chain and sustainable standards. Int. J. Hortic. Sci. Technol. 2020, 7, 235–262. [Google Scholar]
- Zhou, H.; Beynon-Davies, R.; Carslaw, N.; Dodd, I.C.; Ashworth, K. Yield, resource use efficiency or flavour: Trade-offs of varying blue-to-red lighting ratio in urban plant factories. Sci. Hortic. 2022, 295, 110802. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G. Plant factory as a resource-efficient closed plant production system. In Plant Factory; Elsevier: Amsterdam, The Netherlands, 2016; pp. 69–90. [Google Scholar]
- Fylladitakis, E.D. Controlled LED lighting for horticulture: A review. Open J. Appl. Sci. 2023, 13, 175–188. [Google Scholar] [CrossRef]
- Ofori, P.A.; Owusu-Nketia, S.; Opoku-Agyemang, F.; Agbleke, D.; Amissah, J.N. Greenhouse tomato production for sustainable food and nutrition security in the tropics. In Tomato-From Cultivation to Processing Technology; IntechOpen: London, UK, 2022. [Google Scholar]
- Marie, T. Growing Tomato in Controlled Environments Under Continuous Light Requires Dynamic LEDs to Entrain the Circadian Rhythm, Adjust Canopy Architecture, and Balance Photostasis. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2024. [Google Scholar]
- Hikosaka, S.; Iyoki, S.; Hayakumo, M.; Goto, E. Effects of light intensity and amount of supplemental LED lighting on photosynthesis and fruit growth of tomato plants under artificial conditions. J. Agric. Meteorol. 2013, 69, 93–100. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, Z.; Liu, J.; Xu, M.; Shi, Y.; Hou, L.; Zhang, Y. Effects of silicon and phototrophic bacteria on the growth of tomato seedlings and the physicochemical properties of the cultivation substrate under low light. Chin. J. Eco-Agric. 2025, 33, 1–13. [Google Scholar]
- Boedijn, K. Trypan blue as a stain for fungi. Stain. Technol. 1956, 31, 115–116. [Google Scholar] [CrossRef]
- Phillips, J.; Hayman, D. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158-IN118. [Google Scholar] [CrossRef]
- Sun, S.; Yang, Z.; Song, Z.; Wang, N.; Guo, N.; Niu, J.; Liu, A.; Bai, B.; Ahammed, G.J.; Chen, S. Silicon enhances plant resistance to Fusarium wilt by promoting antioxidant potential and photosynthetic capacity in cucumber (Cucumis sativus L.). Front. Plant Sci. 2022, 13, 1011859. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Sun, M.; Shi, A.; Di, Q.; Chen, R.; Jin, D.; Li, Y.; Yu, X.; Chen, S.; He, C. The application of tomato plant residue compost and plant growth-promoting rhizobacteria improves soil quality and enhances the ginger field soil bacterial community. Agronomy 2022, 12, 1741. [Google Scholar] [CrossRef]
- Zhang, Z.Q.W. The Experimental Guide for Plant Physiology; Higher Education Press: Beijing, China, 2003. [Google Scholar]
- Geng, L.; Fu, Y.; Peng, X.; Yang, Z.; Zhang, M.; Song, Z.; Guo, N.; Chen, S.; Chen, J.; Bai, B. Biocontrol potential of Trichoderma harzianum against Botrytis cinerea in tomato plants. Biol. Control 2022, 174, 105019. [Google Scholar] [CrossRef]
- Shyam, R.; Aery, N.C. Effect of cerium on growth, dry matter production, biochemical constituents and enzymatic activities of cowpea plants [Vigna unguiculata (L.) Walp.]. J. Soil Sci. Plant Nutr. 2012, 12, 1–14. [Google Scholar] [CrossRef]
- Tang, W.; Baskin, C.C.; Baskin, J.M.; Nan, Z. Plastic film mulching improves seed germination, seedling development and potential for perenniality of Vicia unijuga under subalpine climate conditions. Crop Pasture Sci. 2020, 71, 592–609. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Q.; Liu, W.; Li, B.; Shao, M.; Zhang, Y. Effects of red/blue versus white LED light of different intensities on the growth and organic carbon and autotoxin secretion of hydroponic lettuce. Hortic. Environ. Biotechnol. 2022, 63, 195–205. [Google Scholar] [CrossRef]
- Machado-Rosa, T.A.; Barbosa, E.T.; de Carvalho Cortes, M.V.B.; Lobo Jr, M. Microtiter method for quantitative assay of IAA from fungal isolates, demonstrated with Trichoderma. Rhizosphere 2023, 25, 100666. [Google Scholar] [CrossRef]
- Liu, F.; Huang, L.-J.; Wang, P.-C.; Zhao, L.-L. Physiological adaptation mechanism of Paspalum notatum in seedling stage to different phosphorus concentrations. Acta Agrestia Sin. 2021, 29, 684. [Google Scholar]
- Guangshu, M.; Xiao, L.; Mei, L.; Mingxin, L.; Yurong, C.; Hua, L. Effect of Trichoderma on cucumber damping-off and physiological characteristics. Chin. J. Biol. Control 2021, 37, 277. [Google Scholar]
- Song, X.; Li, H.; Song, J.; Chen, W.; Shi, L. Biochar/vermicompost promotes Hybrid Pennisetum plant growth and soil enzyme activity in saline soils. Plant Physiol. Biochem. 2022, 183, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Neemisha; Sharma, S. Soil enzymes and their role in nutrient cycling. In Structure and Functions of Pedosphere; Springer: Berlin/Heidelberg, Germany, 2022; pp. 173–188. [Google Scholar]
- Li, Y.; Feng, H.; Chen, J.; Lu, J.; Wu, W.; Liu, X.; Li, C.; Dong, Q.g.; Siddique, K.H. Biochar incorporation increases winter wheat (Triticum aestivum L.) production with significantly improving soil enzyme activities at jointing stage. Catena 2022, 211, 105979. [Google Scholar] [CrossRef]
- Mao, T.; Jiang, X. Changes in microbial community and enzyme activity in soil under continuous pepper cropping in response to Trichoderma hamatum MHT1134 application. Sci. Rep. 2021, 11, 21585. [Google Scholar] [CrossRef]
- Poveda, J. Trichoderma as biocontrol agent against pests: New uses for a mycoparasite. Biol. Control 2021, 159, 104634. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Lou, X.; Lu, Y.; Huang, H.; Yang, Q.; Zhang, Z.; Zhao, W.; Li, Z.; Liu, H.; Du, S. Suitable light combinations enhance cadmium accumulation in Bidens pilosa L. by regulating the soil microbial communities. Environ. Exp. Bot. 2023, 205, 105128. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Najeeb, S.; Chen, J.; Wang, R.; Zhang, J.; Hou, J.; Liu, T. Insights into the molecular mechanism of Trichoderma stimulating plant growth and immunity against phytopathogens. Physiol. Plant. 2023, 175, e14133. [Google Scholar] [CrossRef]
- Ozimek, E.; Jaroszuk-Ściseł, J.; Bohacz, J.; Korniłłowicz-Kowalska, T.; Tyśkiewicz, R.; Słomka, A.; Nowak, A.; Hanaka, A. Synthesis of indoleacetic acid, gibberellic acid and ACC-deaminase by Mortierella strains promote winter wheat seedlings growth under different conditions. Int. J. Mol. Sci. 2018, 19, 3218. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, Y.; Xu, B. Mechanisms of the IAA and ACC-deaminase producing strain of Trichoderma longibrachiatum T6 in enhancing wheat seedling tolerance to NaCl stress. BMC Plant Biol. 2019, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Halifu, S.; Deng, X.; Song, X.; Song, R. Effects of two Trichoderma strains on plant growth, rhizosphere soil nutrients, and fungal community of Pinus sylvestris var. mongolica annual seedlings. Forests 2019, 10, 758. [Google Scholar] [CrossRef]
- Roso, R.; Nunes, U.; Müller, C.; Paranhos, J.; Lopes, S.; Dornelles, S.; Bertagnolli, C.; Huth, C.; Forte, C.; Menegaes, J. Light quality and dormancy overcoming in seed germination of Echium plantagineum L. (Boraginaceae). Braz. J. Biol. 2020, 81, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Knyazeva, I.V.; Panfilova, O.; Vershinina, O.; Smirnov, A.A.; Dorokhov, A.S.; Kahramanoğlu, I. The Effect of Nighttime LED Lighting on Tomato Growth, Yield, and Nutrient Content of Fruits. Horticulturae 2024, 10, 1259. [Google Scholar] [CrossRef]
- Kuleshova, T.; Udalova, O.; Balashova, I.; Anikina, L.; Kononchuk, P.Y.; Mirskaya, G.; Dubovitskaya, V.; Vertebny, V.; Khomyakov, Y.V.; Panova, G. Features of the lighting spectrum influence on the productivity and biochemical composition of test fruit and leaf vegetable crops. Tech. Phys. 2024, 69, 296–304. [Google Scholar] [CrossRef]
- Soltani, S.; Arouiee, H.; Salehi, R.; Nemati, S.H.; Moosavi-Nezhad, M.; Gruda, N.S.; Aliniaeifard, S. Morphological, phytochemical, and photosynthetic performance of grafted tomato seedlings in response to different LED light qualities under protected cultivation. Horticulturae 2023, 9, 471. [Google Scholar] [CrossRef]
- El-Sheekh, M.; Morsy, H.; Hassan, L. Assessment of the optimum growth medium and the effect of different light intensities on growth and photosynthetic pigments of Chlorella vulgaris and Scenedesmus arvernensis. Egypt. J. Bot. 2020, 60, 395–404. [Google Scholar] [CrossRef]
- Zhao, J.; Thi, L.T.; Park, Y.G.; Jeong, B.R. Light quality affects growth and physiology of Carpesium triste Maxim. cultured in vitro. Agriculture 2020, 10, 258. [Google Scholar] [CrossRef]
- Liang, Y.; Kang, C.; Kaiser, E.; Kuang, Y.; Yang, Q.; Li, T. Red/blue light ratios induce morphology and physiology alterations differently in cucumber and tomato. Sci. Hortic. 2021, 281, 109995. [Google Scholar] [CrossRef]
- Oljira, A.M.; Hussain, T.; Waghmode, T.R.; Zhao, H.; Sun, H.; Liu, X.; Wang, X.; Liu, B. Trichoderma enhances net photosynthesis, water use efficiency, and growth of wheat (Triticum aestivum L.) under salt stress. Microorganisms 2020, 8, 1565. [Google Scholar] [CrossRef]
- Lu, H.; Zhou, P.; Li, F.; Wang, Y.; Gu, J.; Wang, Y.; Sun, S.; Zhang, M.; Wang, X. Trichoderma guizhouense NJAU4742 augments morphophysiological responses, nutrient availability and photosynthetic efficacy of ornamental Ilex verticillata. Tree Physiol. 2024, 44, tpae033. [Google Scholar] [PubMed]
- Nie, R.; Wei, X.; Jin, N.; Su, S.; Chen, X. Response of photosynthetic pigments, gas exchange and chlorophyll fluorescence parameters to light quality in Phoebe bournei seedlings. Plant Growth Regul. 2024, 103, 675–687. [Google Scholar]
- Li, Z.; Chen, Q.; Xin, Y.; Mei, Z.; Gao, A.; Liu, W.; Yu, L.; Chen, X.; Chen, Z.; Wang, N. Analyses of the photosynthetic characteristics, chloroplast ultrastructure, and transcriptome of apple (Malus domestica) grown under red and blue lights. BMC Plant Biol. 2021, 21, 483. [Google Scholar] [CrossRef]
- Fiorini, L.; Guglielminetti, L.; Mariotti, L.; Curadi, M.; Picciarelli, P.; Scartazza, A.; Sarrocco, S.; Vannacci, G. Trichoderma harzianum T6776 modulates a complex metabolic network to stimulate tomato cv. Micro-Tom growth. Plant Soil 2016, 400, 351–366. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar]
- Gupta, R.; Singh, M.; Khan, B.R. Photosynthetic electron transport rate and root dynamics of finger millet in response to Trichoderma harzianum. Plant Signal. Behav. 2022, 17, 2146373. [Google Scholar]
- Li, X.; Wang, S.; Chen, X.; Cong, Y.; Cui, J.; Shi, Q.; Liu, H.; Diao, M. The positive effects of exogenous sodium nitroprusside on the plant growth, photosystem II efficiency and Calvin cycle of tomato seedlings under salt stress. Sci. Hortic. 2022, 299, 111016. [Google Scholar] [CrossRef]
- Qu, L.; Xu, Y.; Ji, D.; Chen, C.; Xie, C. Cloning and expression analysis of the cytosolic fructose-1,6-bisphosphatase gene from Pyropia haitanensis. J. Appl. Oceanogr. 2015, 34, 402–410. [Google Scholar]
- Subramaniam, S.; Zainudin, N.A.I.M.; Aris, A.; Hasan, Z.A.E. Role of Trichoderma in plant growth promotion. In Advances in Trichoderma Biology for Agricultural Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 257–280. [Google Scholar]
- Stein, O.; Granot, D. An overview of sucrose synthases in plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar]
- Wang, Y.; Yuan, J.; Li, S.; Hui, L.; Li, Y.; Chen, K.; Meng, T.; Yu, C.; Leng, F.; Ma, J. Comparative analysis of carbon and nitrogen metabolism, antioxidant indexes, polysaccharides and lobetyolin changes of different tissues from Codonopsis pilosula co-inoculated with Trichoderma. J. Plant Physiol. 2021, 267, 153546. [Google Scholar] [CrossRef]
- Esparza-Reynoso, S.; Ruíz-Herrera, L.F.; Pelagio-Flores, R.; Macías-Rodríguez, L.I.; Martínez-Trujillo, M.; López-Coria, M.; Sánchez-Nieto, S.; Herrera-Estrella, A.; López-Bucio, J. Trichoderma atroviride-emitted volatiles improve growth of Arabidopsis seedlings through modulation of sucrose transport and metabolism. Plant Cell Environ. 2021, 44, 1961–1976. [Google Scholar] [CrossRef] [PubMed]
- Apriyanto, A.; Compart, J.; Fettke, J. A review of starch, a unique biopolymer–Structure, metabolism and in planta modifications. Plant Sci. 2022, 318, 111223. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xin, G.; Wei, M.; Shi, Q.; Yang, F.; Wang, X. Carbohydrate accumulation and sucrose metabolism responses in tomato seedling leaves when subjected to different light qualities. Sci. Hortic. 2017, 225, 490–497. [Google Scholar] [CrossRef]


| Gene | GenBank Accession No. | Sequence of Forward Primers | Sequence of Reverse Primers |
|---|---|---|---|
| FBPase | XM_004246317.1 | CAAGTGGAAGGACTGGGATT | CACAGCAGCATCAATGTTTG |
| FBPA | AF332957.1 | AGAGTACCTCAAGGCATGG | GAAGCTCCGACGTACTTTCC |
| SBPase | NM_001247656.1 S | ATACACCGGAGGAATGGTGC | ACGGATTGTTTCCCATCGCT |
| TPI | XM_004236698.1 | AGCTTATGAGCCTGTTTGGG | ATTTGCTTGAAGCCATTTCC |
| HY5 | NM_001247891.2 | AAATGCAAGAGCAAGCGACG | TCCAGAAGCTGACGTTGTCC |
| SS2 | XM_016036481.4 | CGATCATGGCCGACCCTAAA | TTTCCTTGCGCCACATACCT |
| SPS | NM_001246991.2 | GGTCTACGCAAGGCTGTCAT | GGTGCTGCTACATTCCTCGT |
| actin | U60478.1 | GTCCTCTTCCAGCCATCCAT | ACCACTGAGCACAATGTTACCG |
| Treatment | Plant Height (cm) | Stem Thickness (mm) | Whole Plant Fresh Weight (g) | Shoot Dry Weight (g) | Root Dry Weight (g) | Seedling Strength Index |
|---|---|---|---|---|---|---|
| W | 22.10 ± 0.36 c | 4.54 ± 0.09 d | 12.60 ± 0.60 d | 1.35 ± 0.03 d | 0.23 ± 0.01 d | 0.48 ± 0.01 d |
| WT | 24.07 ± 0.67 b | 4.74 ± 0.06 c | 14.68 ± 0.35 b | 1.51 ± 0.04 ab | 0.28 ± 0.01 b | 0.52 ± 0.01 b |
| R | 29.53 ± 0.25 a | 5.21 ± 0.06 a | 13.61 ± 0.16 c | 1.41 ± 0.02 cd | 0.20 ± 0.01 e | 0.40 ± 0.01 e |
| RT | 30.17 ± 0.31 a | 5.30 ± 0.05 a | 14.50 ± 0.22 b | 1.46 ± 0.02 bc | 0.25 ± 0.01 c | 0.48 ± 0.01 d |
| B | 22.10 ± 0.36 c | 4.57 ± 0.06 d | 12.46 ± 0.24 d | 1.26 ± 0.02 e | 0.18 ± 0.01 f | 0.41 ± 0.01 e |
| BT | 24.50 ± 0.30 b | 4.72 ± 0.05 c | 14.14 ± 0.21 bc | 1.35 ± 0.03 d | 0.25 ± 0.01 c | 0.49 ± 0.01 cd |
| RB | 22.27 ± 0.47 c | 4.73 ± 0.06 c | 14.25 ± 0.14 bc | 1.39 ± 0.05 d | 0.24 ± 0.01 cd | 0.50 ± 0.01 c |
| RBT | 24.43 ± 0.21 b | 4.98 ± 0.09 b | 15.52 ± 0.43 a | 1.54 ± 0.02 a | 0.30 ± 0.01 a | 0.56 ± 0.01 a |
| Treatment | Root Colonization Rate (%) | Length (cm) | SA (cm2) | PA (cm2) | VOL (cm3) | Ntips (pcs) | Nforks (pcs) |
|---|---|---|---|---|---|---|---|
| W | — | 346.98 ± 11.70 cd | 36.55 ± 1.63 c | 10.30 ± 0.94 c | 0.31 ± 0.02 cd | 1945.50 ± 43.44 c | 1299.50 ± 17.50 c |
| WT | 44.67 | 403.77 ± 8.72 ab | 44.29 ± 1.91 b | 15.16 ± 1.24 ab | 0.37 ± 0.02 ab | 2317.50 ± 36.50 b | 1472.00 ± 54.06 b |
| R | — | 253.25 ± 15.15 e | 29.09 ± 1.66 f | 7.76 ± 0.14 d | 0.26 ± 0.05 d | 1556.50 ± 21.50 e | 994.00 ± 89.00 e |
| RT | 40.00 | 301.02 ± 50.37 de | 34.95 ± 1.08 cd | 11.13 ± 0.45 c | 0.32 ± 0.03 bc | 1958.33 ± 59.14 cd | 1235.00 ± 34.51 cd |
| B | — | 279.97 ± 13.04 e | 30.08 ± 0.99 ef | 10.77 ± 1.22 c | 0.26 ± 0.02 d | 1616.50 ± 57.50 e | 1042.00 ± 26.00 e |
| BT | 53.33 | 367.99 ± 21.82 bc | 41.81 ± 1.33 b | 13.31 ± 1.00 b | 0.38 ± 0.03 ab | 2208.50 ± 30.50 d | 1185.00 ± 22.52 d |
| RB | — | 357.50 ± 9.83 bc | 32.84 ± 2.69 de | 10.27 ± 1.29 c | 0.31 ± 0.02 cd | 1994.00 ± 21.93 b | 1434.00 ± 21.00 b |
| RBT | 50.00 | 431.64 ± 29.18 a | 49.98 ± 1.51 a | 15.82 ± 0.61 a | 0.43 ± 0.04 a | 2342.33 ± 50.30 a | 1778.33 ± 32.72 a |
| Treatment | Pn (μmol·m−2·s−1) | Gs (mmol·m−2·s−1) | Ci (μmol·mol−1) | Tr (mmol·m−2·s−1) |
|---|---|---|---|---|
| W | 9.57 ± 0.21 e | 0.15 ± 0.01 d | 593.35 ± 7.51 cd | 4.74 ± 0.09 f |
| WT | 12.33 ± 0.47 b | 0.23 ± 0.02 ab | 561.35 ± 5.39 e | 8.00 ± 0.15 b |
| R | 10.20 ± 0.2 de | 0.19 ± 0.01 c | 683.35 ± 12.50 a | 6.41 ± 0.23 d |
| RT | 11.30 ± 0.3 c | 0.24 ± 0.02 a | 580.56 ± 7.60 d | 8.54 ± 0.11 a |
| B | 10.37 ± 0.31 d | 0.19 ± 0.01 c | 651.89 ± 9.01 b | 7.45 ± 0.13 c |
| BT | 12.00 ± 0.2 bc | 0.21 ± 0.01 b | 592.17 ± 4.60 cd | 7.93 ± 0.04 b |
| RB | 12.03 ± 0.32 bc | 0.13 ± 0.01 d | 603.54 ± 5.33 c | 5.10 ± 0.04 e |
| RBT | 15.10 ± 0.7 a | 0.24 ± 0.01 a | 589.04 ± 5.14 cd | 8.01 ± 0.10 b |
| Treatment | Chl a (mg·g−1 FW) | Chl b (mg·g−1 FW) | Chl (a + b)(mg·g−1 FW) | Car (mg·g−1 FW) |
|---|---|---|---|---|
| W | 0.88 ± 0.02 e | 0.36 ± 0.01 e | 1.24 ± 0.03 e | 0.17 ± 0.01 e |
| WT | 1.13 ± 0.03 c | 0.45 ± 0.02 cd | 1.58 ± 0.05 c | 0.21 ± 0.01 cd |
| R | 1.04 ± 0.06 cd | 0.47 ± 0.03 cd | 1.51 ± 0.03 cd | 0.19 ± 0.02 de |
| RT | 1.08 ± 0.11 cd | 0.44 ± 0.05 cd | 1.52 ± 0.16 cd | 0.20 ± 0.01 d |
| B | 1.28 ± 0.06 ab | 0.50 ± 0.01 bc | 1.77 ± 0.06 b | 0.24 ± 0.01 ab |
| BT | 1.39 ± 0.02 a | 0.58 ± 0.03 a | 1.97 ± 0.06 a | 0.25 ± 0.02 a |
| RB | 0.99 ± 0.02 de | 0.40 ± 0.04 de | 1.39 ± 0.13 de | 0.19 ± 0.01 de |
| RBT | 1.26 ± 0.02 b | 0.56 ± 0.06 ab | 1.82 ± 0.07 ab | 0.23 ± 0.01 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Xu, Q.; Qin, C.; Geng, L.; Yan, Z.; Li, H.; Ahammed, G.J.; Chen, S. Synergistic Effects of Trichoderma harzianum and Light Quality on Photosynthetic Carbon Metabolism and Growth in Tomato Plants. Agronomy 2025, 15, 1362. https://doi.org/10.3390/agronomy15061362
Wang N, Xu Q, Qin C, Geng L, Yan Z, Li H, Ahammed GJ, Chen S. Synergistic Effects of Trichoderma harzianum and Light Quality on Photosynthetic Carbon Metabolism and Growth in Tomato Plants. Agronomy. 2025; 15(6):1362. https://doi.org/10.3390/agronomy15061362
Chicago/Turabian StyleWang, Ningyu, Qihui Xu, Congrui Qin, Lijiahong Geng, Zhenglin Yan, Haolong Li, Golam Jalal Ahammed, and Shuangchen Chen. 2025. "Synergistic Effects of Trichoderma harzianum and Light Quality on Photosynthetic Carbon Metabolism and Growth in Tomato Plants" Agronomy 15, no. 6: 1362. https://doi.org/10.3390/agronomy15061362
APA StyleWang, N., Xu, Q., Qin, C., Geng, L., Yan, Z., Li, H., Ahammed, G. J., & Chen, S. (2025). Synergistic Effects of Trichoderma harzianum and Light Quality on Photosynthetic Carbon Metabolism and Growth in Tomato Plants. Agronomy, 15(6), 1362. https://doi.org/10.3390/agronomy15061362






